1. Introduction
Histologically, the skin consists of the epidermis,
dermis and subcutis. The epidermis, which is the outermost layer of
the skin, protects the human body from the external environment
(1,2). The epidermis is divided into four
layers (stratum basale, stratum spinosum, stratum granulosum and
stratum corneum) as a result of the process of epidermal
differentiation. This creates an epidermal barrier at the level of
the stratum corneum, the uppermost layer, to prevent dehydration
and moisture loss. The epidermis also prevents external antigens
from entering the skin and is a defense against ultraviolet (UV)
rays (3–6). The epidermal barrier plays important
roles in skin aging, dermatitis, psoriasis and atopic dermatitis,
and is the subject of intense research (7–10).
Generally, the epidermal barrier is formed by the
multiple actions of lipids produced in the lamellar bodies of the
stratum granulosum during the process of keratinocyte
differentiation, which involves terminally differentiated
corneocytes and corneodesmosomes that connect keratinocytes
(11,12). These lipids create a multilamellar
barrier between corneocytes, both increasing the adhesion and
hindering the movement of material between cells, thus creating an
epidermal barrier. The major lipids that form the multilamellar
barrier of the skin consist of 50% ceramide, 25% cholesterol and
15% fatty acids (FAs) (11).
Ceramides, also known to act as moderators of cellular physiology,
are sphingolipids which are composed of FAs connected to
sphingosine (12,13).
2. Biosynthesis and structure of ceramides
and their derivatives
Ceramides are primarily synthesized in the
endoplasmic reticulum (ER) of the stratum spinosum within the
epidermis. They are transferred out of cells through lamellar
bodies created in the stratum granulosum and create a multilamellar
barrier between the corneocytes of the stratum corneum (14–18). Ceramides are chemically composed
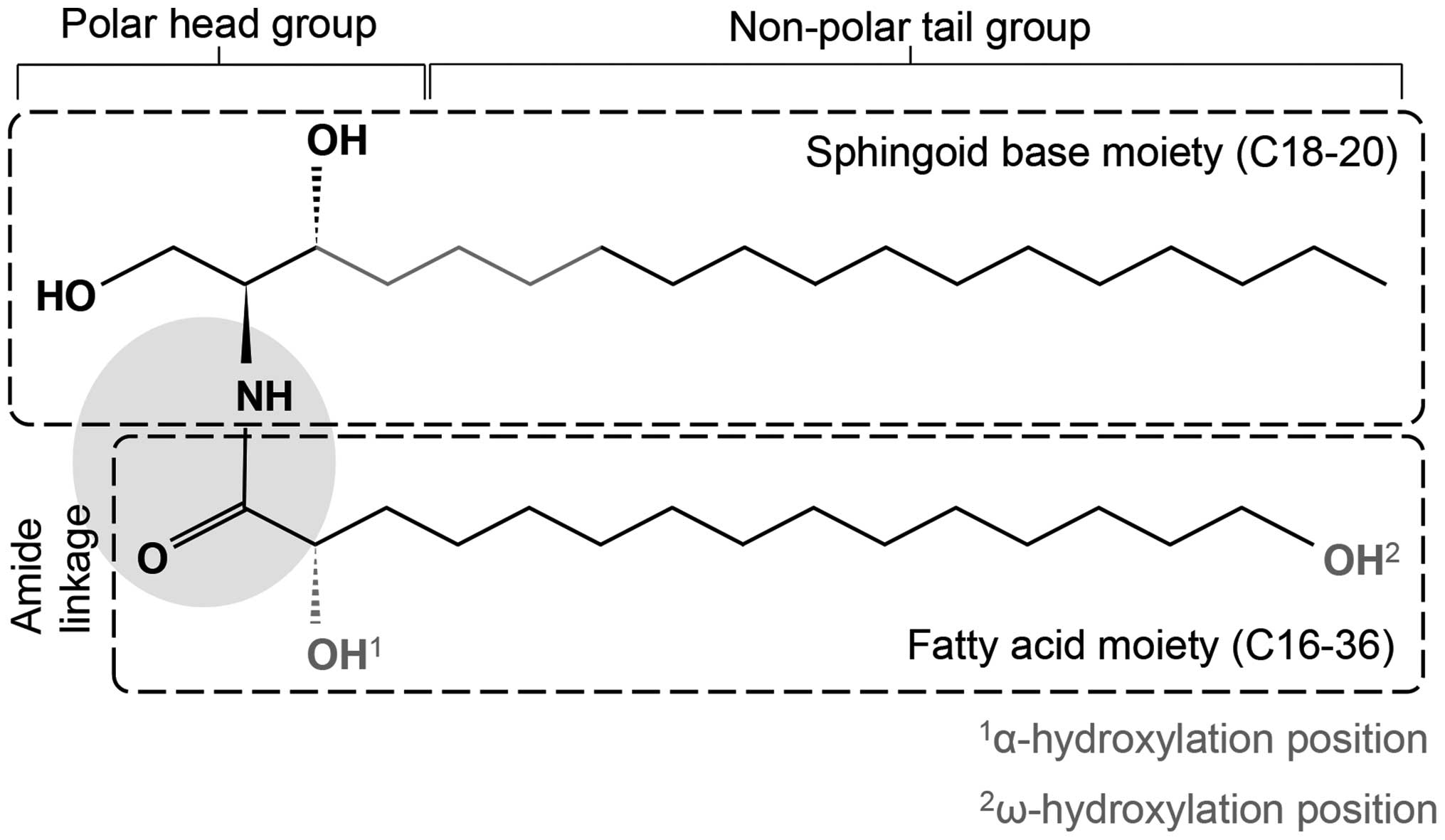
of a sphingoid base, which is a long-chain amino alcohol
[long-chain base (LCB)], and a FA joined by an amide bond (Fig. 1). The sphingoid base may consist
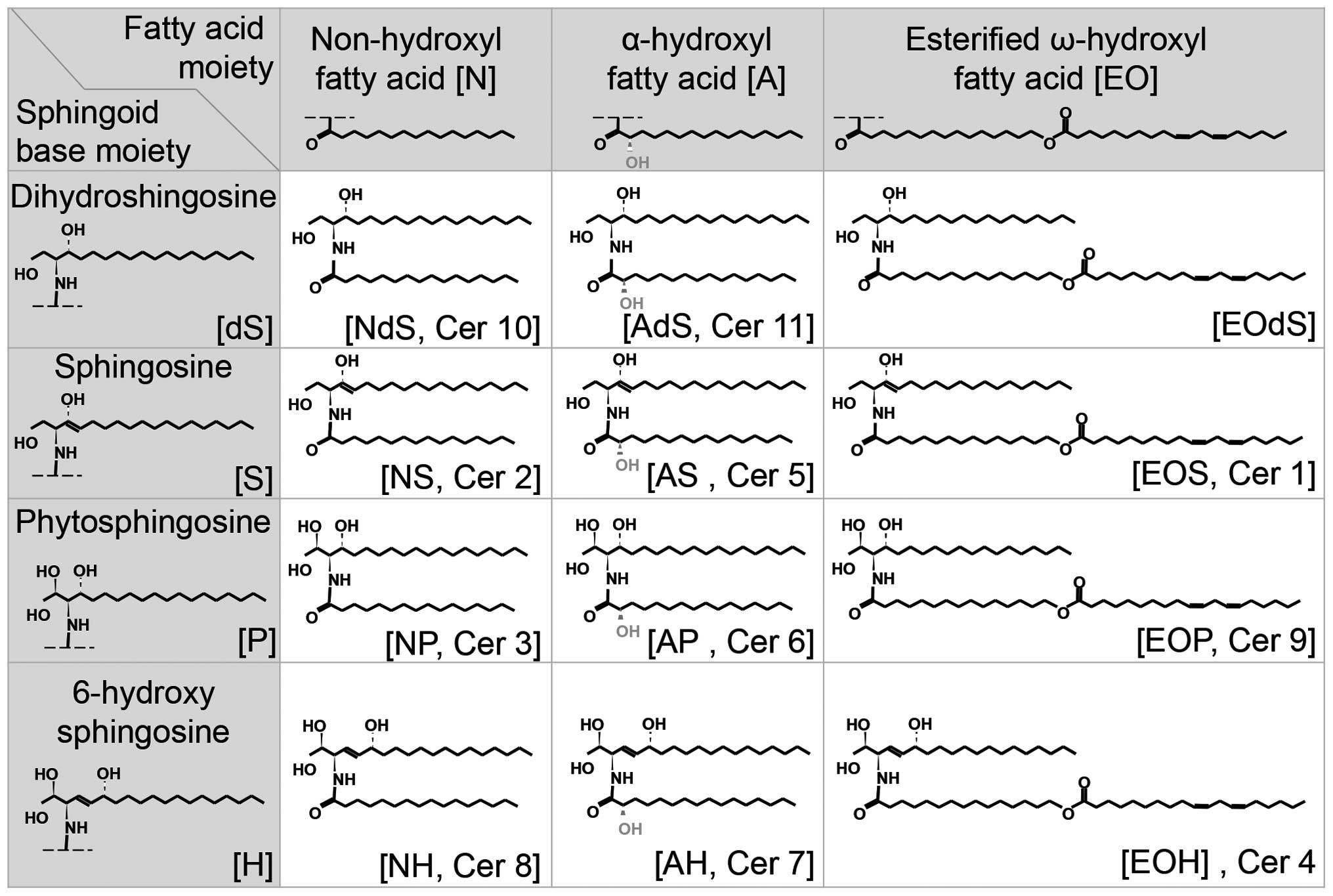
of dihydrosphingosine (dS), sphingosine (S), phytosphingosine (P)
or 6-hydroxy sphingosine (H) (19,20). The FA may be a non-hydroxyl FA
(N), an α-hydroxyl FA (A), or an esterified ω-hydroxyl FA (EO).
Thus, various ceramides are created by different combinations of
these two types of molecules (Fig.
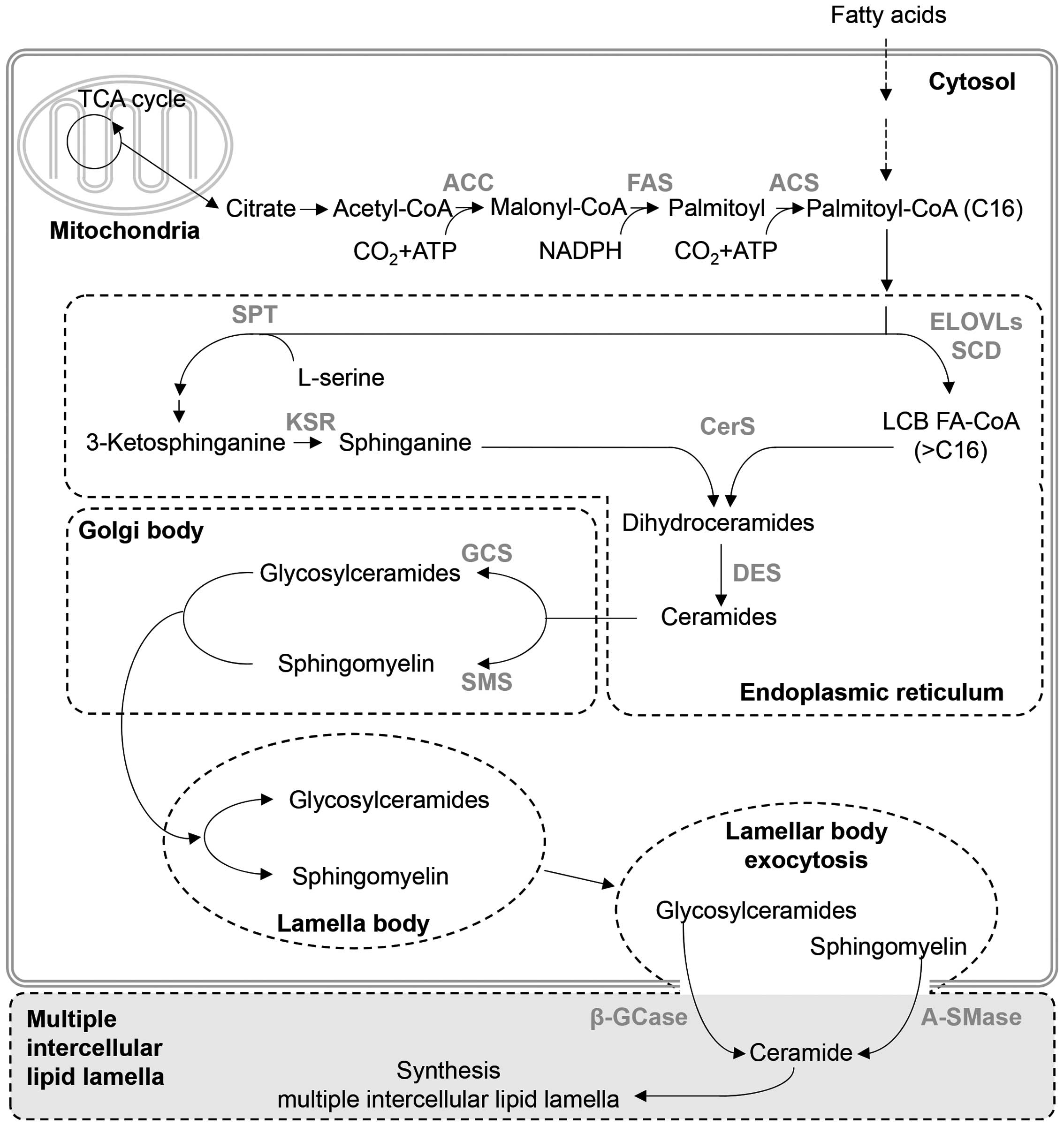
2). Ceramides undergo biosynthesis through various mechanisms,
and the most common synthetic pathway is the de novo
pathway, which is the most important biosynthetic mechanism for
creating an epidermal barrier (Fig.
3). The de novo pathway can be divided into pathways
that produce the sphingoid base and the FA.
The first step of the pathway responsible for
sphingoid base synthesis uses palmitoyl-CoA and L-serine (17,18). Initially, 3-ketosphinganine
(ketodihydrosphingosine) reacts with palmitoyl-CoA and L-serine by
serine palmitoyl transferase. The synthesized 3-ketosphinganine is
deoxygenated by 3-ketosphinganine reductase, producing sphinganine
(dihydrosphingosine) (17,18).
The resulting long-chain amino alcohol of sphinganine has 18 carbon
atoms; however, ceramides with 12–28 carbon atoms have been found
in the stratum corneum (21–24).
Using acetyl-CoA carboxylase, FA synthase, and
acyl-CoA synthetase, the FA synthesis pathway combines acetyl-CoA
from citrate in the TCA cycle, malonyl-CoA and palmitoyl to
synthesize palmitoyl-CoA with 16 carbon atoms. Then, after a
condensation reaction by 3-ketoacyl-CoA synthase [elongation of
very long chain FAs (ELOVL) protein] of palmitoyl-CoA, a reduction
reaction by 3-keto-acyl-CoA reductase, a dehydration reaction by
3-hydroxyacyl-CoA dehydratase and a reduction reaction by
2,3-enoyl-CoA reductase, the carbon number of the FA is increased
by 2. Therefore, the length and saturation of the FA are determined
by ELOVL proteins. For example, ELOVL6 creates C16 and C16:1,
ELOVL1 creates C18-C24, ELOVL4 creates C24 or above, ELOVL3 creates
C18-C24 and C18:1-C24:1, ELOVL7 creates C18-C22, ELOVL5 creates
polyunsaturated C18-C20, and ELOVL2 creates polyunsaturated C20-C24
FAs (25,26). In particular, ELOVL1, 3 and 4 are
principally found in the epidermis (27,28). The FAs subjected to long-chain
elongation undergo hydroxylation at the α- or ω-position, and the
ω-hydroxylation of FAs involves ω-esterification with linoleic acid
to produce ultra-long chain (ULC) FAs that have 28–38 carbon atoms
(14,29). Aside from creating ULC-ceramides,
the ω-hydroxyl group also connects proteins and ceramides through
ω-esterification to the side chain of glutamate in cornified
envelope protein (30). Moreover,
1-O-acylceramides, in which very long-chain acyl residues are
connected to the N- and O-positions of ceramide, have also been
discovered (31).
The sphingosine base and FA are combined to produce
dihydroceramides by N-acylation which is catalyzed by ceramide
synthase (CerS)1–6 (32).
Finally, C4 and C5 are unsaturated by dihydroceramide
Δ4-desaturase, creating ceramides (16–18). There are six types of CerS (CerS1
to CerS6) that produce different types of ceramides (21,33). CerS3 and CerS4 are highly
concentrated in the skin. CerS3 levels are elevated during
keratinocyte differentiation and it has been found to be mutated in
congenital ichthyosis (34,35) Moreover, alopecia occurs in mice as
a result of a lack of CerS4 (36). Therefore, CerS3 and CerS4 are
expected to play significant roles in creating an epidermal barrier
in human skin. Accordingly, NP (ceramide 3) and EOH (ceramide 4),
created by CerS3 and CerS4, and long-chain ceramides with 18–26
carbons are known to be the major components of the epidermal
barrier (29,33,37). The amount of total lipids in the
stratum corneum is low in patients with atopy and dry skin, and
ceramide levels are also low (38,39). Decreases in EOS (ceramide 1)
levels are known to convert the orthorhombic structure of the
epidermal barrier to a hexagonal gel structure, thus increasing
moisture loss from the skin (40–42). The ceramides known to play
important roles in the lamellar structure of the skin are EOS, NP
and EOH, among which EOS is known to be an essential component in
creating the lamellar structure (14). Ceramides produced in the ER are
converted into glucosylceramides and sphingomyelin (SM) by
glucosylceramide synthase and SM synthase (SMS), respectively, and
are translocated to the Golgi complex to create the lamellar body
(43). These compounds then exit
the cell and are converted back into ceramides by
β-glucocerebrosidase and acid sphingomyelinase (A-SMase), creating
the multilamellar barrier.
Aside from the biosynthetic mechanism through which
ceramides are produced, ceramides and their derivatives are
synthesized by the SM and catabolic pathways (44) and used as intracellular
messengers. The SM pathway synthesizes ceramides through the
hydrolysis of SM by sphingomyelinase (SMase), and the typical
SMases which play a role in this mechanism are epidermal A-SMase
and neutral SMase (45,46). By contrast, to synthesize SM, SMS
uses ceramide. Moreover, the catabolic pathway uses ceramidase to
produce derivatives of sphingosine and sphingosine-1-phosphate
(S1P) to produce sphingosine from ceramide, and synthesizes
ceramides from sphingosine in the reverse direction through CerS
(44). Moreover, S1P is created
when sphingosine is phosphorylated by sphingosine kinase, and
sphingosine may be regenerated when S1P is dephosphorylated by S1P
phosphatase. Ceramides and their derivatives act as different
cellular messengers, which are repeatedly synthesized and degraded
through reversible processes by multiple enzymes.
3. Intracellular and extracellular functions
of ceramides
Ceramides and their derivatives act as intra- and
extracellular messengers in the epidermal barrier (9,12).
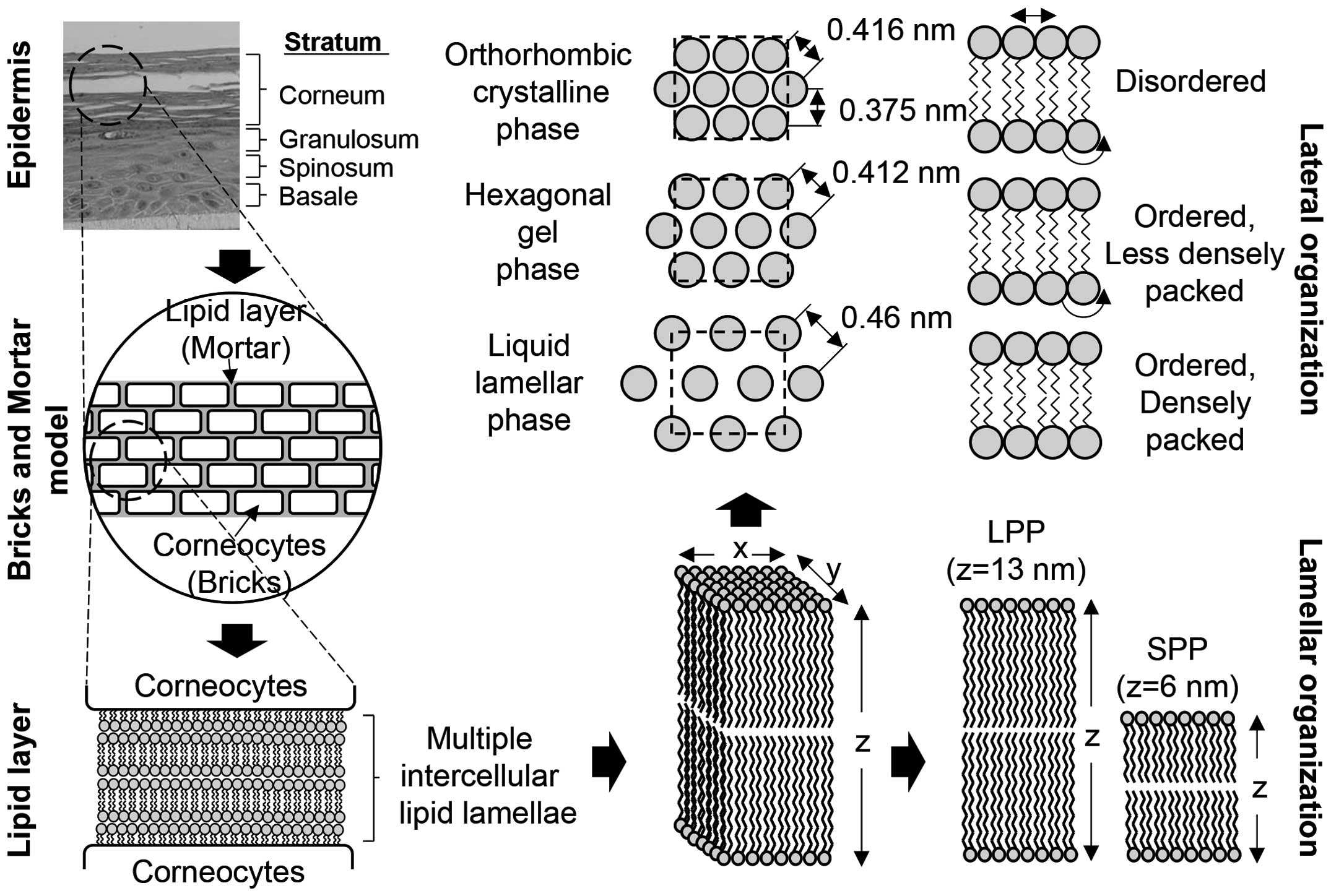
Lipids that form the multiple intercellular lipid lamellae may be
used to illustrate the structure of the epidermal barrier, either
by a two compartment model or a bricks and mortar model (Fig. 4).
With regard to the detailed lipid structure and
layout of multiple intercellular lipid lamellae, the structure of
lipids in the stratum corneum was analyzed by X-ray diffraction in
the 1950s and 1960s, and the structure of lamellae was determined
by electron microscopy in the 1960s (47,48). Structural analysis revealed that
the lamellar structure had unique 13-nm intervals (long periodicity
phase), which disappeared when the temperature rose above 70°C and
was regained at temperatures below 25°C, proving that the structure
is reversible (49–51). A structure of 6-nm intervals
(short periodicity phase) was observed in certain types of
ceramides (52). Moreover,
lamellae have three different structures according to the layout of
the head group and the packing of the alkyl group: orthorhombic,
hexagonal gel and liquid lamellar (53). As a result of analyzing wide-angle
X-ray diffraction results, these structures show only the peak
orthorhombic patterns of 0.375 and 0.416 nm, and the peak liquid
lamellar pattern of 0.46 nm. At 45°C, the peak orthorhombic and
liquid lamellar patterns disappear, and only the 0.412 nm peak
hexagonal gel pattern appears, which becomes one phase when the
temperature rises (52,53). If the temperature rises to 70°C
and the motility of the alkyl chain increases, the peak liquid
lamellar pattern of 0.46 nm is observed (39). The epidermal barrier structure
consists of liquid lamellar < hexagonal gel < orthorhombic,
according to structural differences.
Intracellular ceramides act as second messengers for
various processes (apoptosis, cell growth, differentiation,
senescence, diabetes, insulin resistance, inflammation,
neurodegenerative disorders or atherosclerosis) (54–57). Ceramides which play roles in
intracellular signal transduction are produced using the
aforementioned de novo pathway, which participates in
different reactions according to the specific isoforms and activity
of CerS (58). Most ceramides
induce cellular apoptosis or growth arrest. For example,
C18-ceramide is created by CerS1, which induces cell growth
inhibition and apoptosis. However, there is an exceptional case in
which the activity of CerS6 increases, rescuing the cells from ER
stress and apoptosis by creating acyl-C16-ceramide (59).
One synthetic mechanism produces ceramides through
the hydrolysis of SM (Fig. 5).
Six types of SMases have been discovered in mammals: four types of
neutral SMases, one A-SMase, and one alkaline SMase. Ceramides used
in intracellular signal transduction are primarily created by
neutral SMases existing in the ER and plasma membrane (60). Another mechanism synthesizes
ceramides from S1P using S-1-phosphate phosphatase and CerS. An
additional mechanism produces ceramides through the phosphorylation
of ceramide-1-phosphate phosphatase (CPP) generated from
ceramide-1-phosphate. Most ceramides created in this way inhibit
cell growth and induce apoptosis (61–64). Thus, intracellular ceramides are
known to be increased by inducing apoptosis through TNF-α, Fas,
radiation, and antitumor agents, and by the conversion of SM into
ceramides in cell membranes or lysosomes. Moreover, ceramides are
known to induce apoptosis by activating intracellular c-Jun
N-terminal kinase (JNK)/stress-activated protein kinase (SAPK),
protein kinase C (PKC)δ/ε and caspase-3 (CASP3)-like protease
signals, and to reduce the phosphorylation of Ser473 of AKT by
activating PKCζ and PP2A, thus inhibiting cell growth (65,66). Multiple mechanisms exist as the
long carbon chain of ceramides make it structurally difficult for
ceramides to pass through cell membranes. Thus, there is a clear
distinction between ceramides that function in the multilamellar
barrier outside the cell and ceramides that function as a second
messenger inside the cell. Moreover, each mechanism is a reversible
pathway, thus, S1P and ceramide-1-phosphate play opposing roles in
cell growth inhibition and apoptosis.
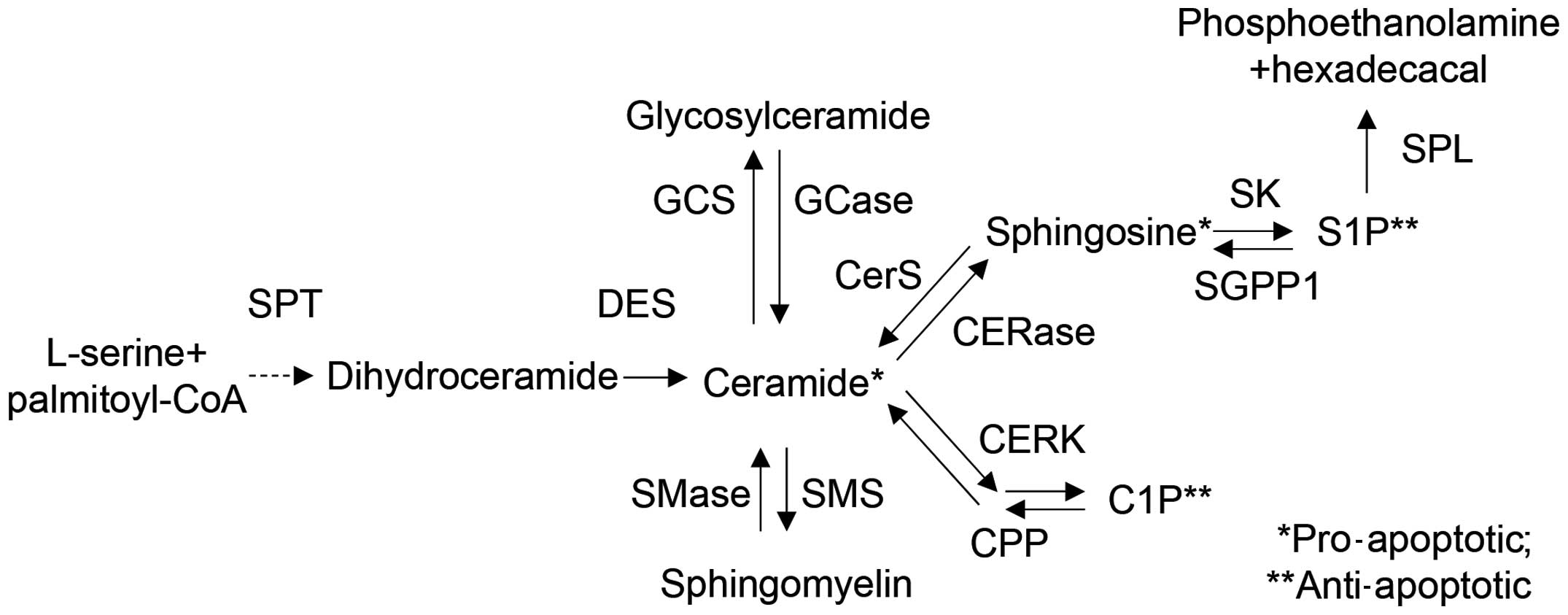 | Figure 5Overview of sphingolipid metabolism
and related enzymes. Ceramides are synthesized from L-serine and
palmitoyl-CoA by serine palmitoyl transferase (SPT). The generated
ceramides are metabolized into glycosylceramide, sphingomyelin,
sphingosine, sphingosine-1-phosphate (S1P), and
ceramide-1-phosphate (C1P) by various enzymes, such as ceramide
synthase (CerS), dihydroceramide Δ4-desaturase (DES), ceramide
kinase (CERK), ceramide-1-phosphate phosphatase (CPP),
sphingomyelin synthase (SMS), sphingomyelinase (SMase), ceramidase
(CERase), sphingosine kinase (SK), S1P phosphatase (SGPP1), and S1P
lyase (SPL). Each metabolite functions as a signaling molecule in
apoptosis, cell growth, differentiation, senescence, diabetes,
insulin resistance, inflammation, neurodegenerative disorders or
atherosclerosis. |
A study reported that for keratinocytes in the
epidermis, intracellular ceramides induce the apoptosis of cells
exposed to UVA radiation, thereby controlling the expression of
ICAM1 by mediating AP2 activity (67). Moreover, processed short-chain
ceramides may permeate into cells and induce apoptosis and
differentiation, and reduce proliferation by activating apoptosis
signal-regulating kinase 1 (ASK-1), p38 and caspase-14 in cells
(68–70). Moreover, glucosylceramide and S1P,
derivatives of ceramide, also induce keratinocyte differentiation
(71). Furthermore, in
melanocytes, AKT phosphorylation is reduced by short-chain
ceramide, thereby decreasing melanocyte growth and increasing
melanin synthesis (72).
4. Conclusion
Ceramides and their derivatives form the lamellar
barrier of the skin and facilitate the differentiation of
keratinocytes, thereby creating the epidermal barrier. Thus, they
limit the movement of material through the skin, maintain skin
moisture by preventing dehydration and prevent microbes and
allergens from entering tissues from the outside. As a consequence,
impaired ceramide synthesis damages the barrier function of the
epidermis, making it impossible for the skin to control moisture
levels. External microbes and allergens may then enter the tissues
and dehydrate the skin, causing inflammation and resulting in such
cutaneous diseases as atopic dermatitis or psoriasis. Accordingly,
it is crucial that the skin controls the type and amount of
ceramides produced in the skin. Ceramides perform a number of
functions inside cells, creating signals associated with apoptosis,
proliferation and differentiation. The metabolism of ceramides may
suppress apoptosis. Therefore, through the synthesis and metabolic
conversion of ceramides, it is possible to control the apoptosis,
proliferation and differentiation of skin cells and the formation
of the skin barrier.
Acknowledgments
The present study was supported by the KU Research
Professor Program (H.-J.C.) of Konkuk University. This study was
also supported by grants from the Ministry of Science, ICT and
Future Planning (grant no. 20110028646), the Korean Health
Technology R&D Project, Ministry of Health and Welfare (grant
no. HN13C0075), and the Ministry of Oceans and Fisheries, Republic
of Korea (grant no. OF123321).
References
|
1
|
Natarajan VT, Ganju P, Ramkumar A, Grover
R and Gokhale RS: Multifaceted pathways protect human skin from UV
radiation. Nat Chem Biol. 10:542–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rawlings AV and Harding CR: Moisturization
and skin barrier function. Dermatol Ther (Heidelb). 17(Suppl 1):
43–48. 2004. View Article : Google Scholar
|
|
3
|
Wertz PW: Current understanding of skin
biology pertinent to skin penetration: skin biochemistry. Skin
Pharmacol Physiol. 26:217–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Del Rosso JQ and Levin J: Clinical
relevance of maintaining the structural and functional integrity of
the stratum corneum: why is it important to you? J Drugs Dermatol.
10(Suppl): s5–s12. 2011.PubMed/NCBI
|
|
5
|
Jacobi OT: About the mechanism of moisture
regulation in the horny layer of the skin. Proc Sci Sect Toilet
Goods Assoc. 31:22–24. 1959.
|
|
6
|
Blank IH: Factors which influence the
water content of the stratum corneum. J Invest Dermatol.
18:433–440. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elias PM: Epidermal lipids, barrier
function, and desquamation. J Invest Dermatol. 80(Suppl): 44s–49s.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feingold KR: Thematic review series: skin
lipids. The role of epidermal lipids in cutaneous permeability
barrier homeostasis. J Lipid Res. 48:2531–2546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feingold KR and Elias PM: Role of lipids
in the formation and maintenance of the cutaneous permeability
barrier. Biochim Biophys Acta. 1841:280–294. 2014. View Article : Google Scholar
|
|
10
|
Elias PM and Wakefield JS: Mechanisms of
abnormal lamellar body secretion and the dysfunctional skin barrier
in patients with atopic dermatitis. J Allergy Clin Immunol.
134:781–791.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Candi E, Schmidt R and Melino G: The
cornified envelope: a model of cell death in the skin. Nat Rev Mol
Cell Biol. 6:328–340. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Smeden J, Janssens M, Gooris GS and
Bouwstra JA: The important role of stratum corneum lipids for the
cutaneous barrier function. Biochim Biophys Acta. 1841:295–313.
2014. View Article : Google Scholar
|
|
13
|
Feingold KR: The regulation and role of
epidermal lipid synthesis. Adv Lipid Res. 24:57–82. 1991.PubMed/NCBI
|
|
14
|
Coderch L, López O, de la Maza A and Parra
JL: Ceramides and skin function. Am J Clin Dermatol. 4:107–129.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi MJ and Maibach HI: Role of ceramides
in barrier function of healthy and diseased skin. Am J Clin
Dermatol. 6:215–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogretmen B and Hannun YA: Biologically
active sphingolipids in cancer pathogenesis and treatment. Nat Rev
Cancer. 4:604–616. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arana L, Gangoiti P, Ouro A, Trueba M and
Gómez-Muñoz A: Ceramide and ceramide 1-phosphate in health and
disease. Lipids Health Dis. 9:152010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mimeault M, Hauke R and Batra SK: Recent
advances on the molecular mechanisms involved in the drug
resistance of cancer cells and novel targeting therapies. Clin
Pharmacol Ther. 83:673–691. 2008. View Article : Google Scholar
|
|
19
|
Robson KJ, Stewart ME, Michelsen S, Lazo
ND and Downing DT: 6-Hydroxy-4-sphingenine in human epidermal
ceramides. J Lipid Res. 35:2060–2068. 1994.PubMed/NCBI
|
|
20
|
t'Kindt R, Jorge L, Dumont E, Couturon P,
David F, Sandra P and Sandra K: Profiling and characterizing skin
ceramides using reversed-phase liquid chromatography-quadrupole
time-of-flight mass spectrometry. Anal Chem. 84:403–411. 2012.
View Article : Google Scholar
|
|
21
|
Masukawa Y, Narita H, Shimizu E, Kondo N,
Sugai Y, Oba T, Homma R, Ishikawa J, Takagi Y, Kitahara T, et al:
Characterization of overall ceramide species in human stratum
corneum. J Lipid Res. 49:1466–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pruett ST, Bushnev A, Hagedorn K, Adiga M,
Haynes CA, Sullards MC, Liotta DC and Merrill AH Jr: Biodiversity
of sphingoid bases ('sphingosines') and related amino alcohols. J
Lipid Res. 49:1621–1639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farwanah H, Pierstorff B, Schmelzer CE,
Raith K, Neubert RH, Kolter T and Sandhoff K: Separation and mass
spectrometric characterization of covalently bound skin ceramides
using LC/APCI-MS and Nano-ESI-MS/MS. J Chromatogr B Analyt Technol
Biomed Life Sci. 852:562–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stewart ME and Downing DT: Free
sphingosines of human skin include 6-hydroxysphingosine and
unusually long-chain dihydrosphingosines. J Invest Dermatol.
105:613–618. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nugteren DH: The enzymic chain elongation
of fatty acids by rat-liver microsomes. Biochim Biophys Acta.
106:280–290. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guillou H, Zadravec D, Martin PG and
Jacobsson A: The key roles of elongases and desaturases in
mammalian fatty acid metabolism: insights from transgenic mice.
Prog Lipid Res. 49:186–199. 2010. View Article : Google Scholar
|
|
27
|
Jakobsson A, Westerberg R and Jacobsson A:
Fatty acid elongases in mammals: their regulation and roles in
metabolism. Prog Lipid Res. 45:237–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Breiden B and Sandhoff K: The role of
sphingolipid metabolism in cutaneous permeability barrier
formation. Biochim Biophys Acta. 1841:441–452. 2014. View Article : Google Scholar
|
|
29
|
Jennemann R, Rabionet M, Gorgas K, Epstein
S, Dalpke A, Rothermel U, Bayerle A, van der Hoeven F, Imgrund S,
Kirsch J, et al: Loss of ceramide synthase 3 causes lethal skin
barrier disruption. Hum Mol Genet. 21:586–608. 2012. View Article : Google Scholar
|
|
30
|
Rabionet M, Gorgas K and Sandhoff R:
Ceramide synthesis in the epidermis. Biochim Biophys Acta.
1841:422–434. 2014. View Article : Google Scholar
|
|
31
|
Marekov LN and Steinert PM: Ceramides are
bound to structural proteins of the human foreskin epidermal
cornified cell envelope. J Biol Chem. 273:17763–17770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wertz PW and Downing DT: Ceramides of pig
epidermis: structure determination. J Lipid Res. 24:759–765.
1983.PubMed/NCBI
|
|
33
|
Mizutani Y, Mitsutake S, Tsuji K, Kihara A
and Igarashi Y: Ceramide biosynthesis in keratinocyte and its role
in skin function. Biochimie. 91:784–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eckl KM, Tidhar R, Thiele H, Oji V,
Hausser I, Brodesser S, Preil ML, Onal-Akan A, Stock F, Müller D,
et al: Impaired epidermal ceramide synthesis causes autosomal
recessive congenital ichthyosis and reveals the importance of
ceramide acyl chain length. J Invest Dermatol. 133:2202–2211. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holleran WM, Takagi Y and Uchida Y:
Epidermal sphingolipids: metabolism, function, and roles in skin
disorders. FEBS Lett. 580:5456–5466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ebel P, Imgrund S, Vom Dorp K, Hofmann K,
Maier H, Drake H, Degen J, Dörmann P, Eckhardt M, Franz T and
Willecke K: Ceramide synthase 4 deficiency in mice causes lipid
alterations in sebum and results in alopecia. Biochem J.
461:147–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wertz PW: Lipids and barrier function of
the skin. Acta Derm Venereol Suppl (Stockh). 208:7–11. 2000.
View Article : Google Scholar
|
|
38
|
Farwanah H, Raith K, Neubert RH and
Wohlrab J: Ceramide profiles of the uninvolved skin in atopic
dermatitis and psoriasis are comparable to those of healthy skin.
Arch Dermatol Res. 296:514–521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Imokawa G, Abe A, Jin K, Higaki Y,
Kawashima M and Hidano A: Decreased level of ceramides in stratum
corneum of atopic dermatitis: an etiologic factor in atopic dry
skin? J Invest Dermatol. 96:523–526. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Houben E, Holleran WM, Yaginuma T, Mao C,
Obeid LM, Rogiers V, Takagi Y, Elias PM and Uchida Y:
Differentiation-associated expression of ceramidase isoforms in
cultured keratinocytes and epidermis. J Lipid Res. 47:1063–1070.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bouwstra JA, Gooris GS, Dubbelaar FE,
Weerheim AM, Ijzerman AP and Ponec M: Role of ceramide 1 in the
molecular organization of the stratum corneum lipids. J Lipid Res.
39:186–196. 1998.PubMed/NCBI
|
|
42
|
Bouwstra JA, Gooris GS, Dubbelaar FE and
Ponec M: Phase behavior of stratum corneum lipid mixtures based on
human ceramides: the role of natural and synthetic ceramide 1. J
Invest Dermatol. 118:606–617. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hanada K: Intracellular trafficking of
ceramide by ceramide transfer protein. Proc Jpn Acad Ser B Phys
Biol Sci. 86:426–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ponnusamy S, Meyers-Needham M, Senkal CE,
Saddoughi SA, Sentelle D, Selvam SP, Salas A and Ogretmen B:
Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in
the regulation of cell death and drug resistance. Future Oncol.
6:1603–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jensen JM, Förl M, Winoto-Morbach S, Seite
S, Schunck M, Proksch E and Schütze S: Acid and neutral
sphingomyelinase, ceramide synthase, and acid ceramidase activities
in cutaneous aging. Exp Dermatol. 14:609–618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jensen JM, Fölster-Holst R, Baranowsky A,
Schunck M, Winoto-Morbach S, Neumann C, Schütze S and Proksch E:
Impaired sphingomyelinase activity and epidermal differentiation in
atopic dermatitis. J Invest Dermatol. 122:1423–1431. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Swanbeck G and Thyresson N: A study of the
state of aggregation of the lipids in normal and psoriatic horny
layer. Acta Derm Venereol. 42:445–447. 1962.PubMed/NCBI
|
|
48
|
Swanbeck G and Thyresson N: An x-ray
diffraction study of scales from different dermatoses. Acta Derm
Venereol. 41:289–296. 1961.PubMed/NCBI
|
|
49
|
Breathnach AS, Goodman T, Stolinski C and
Gross M: Freeze-fracture replication of cells of stratum corneum of
human epidermis. J Anat. 114:65–81. 1973.PubMed/NCBI
|
|
50
|
Breathnach AS: Aspects of epidermal
ultrastructure. J Invest Dermatol. 65:2–15. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
White SH, Mirejovsky D and King GI:
Structure of lamellar lipid domains and corneocyte envelopes of
murine stratum corneum. An X-ray diffraction study. Biochemistry.
27:3725–3732. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schreiner V, Gooris GS, Pfeiffer S,
Lanzendörfer G, Wenck H, Diembeck W, Proksch E and Bouwstra J:
Barrier characteristics of different human skin types investigated
with X-ray diffraction, lipid analysis, and electron microscopy
imaging. J Invest Dermatol. 114:654–660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Moore DJ, Rerek ME and Mendelsohn R: Lipid
domains and orthorhombic phases in model stratum corneum: evidence
from Fourier transform infrared spectroscopy studies. Biochem
Biophys Res Commun. 231:797–801. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hannun YA: Functions of ceramide in
coordinating cellular responses to stress. Science. 274:1855–1859.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ruvolo PP: Intracellular signal
transduction pathways activated by ceramide and its metabolites.
Pharmacol Res. 47:383–392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ballou LR, Laulederkind SJ, Rosloniec EF
and Raghow R: Ceramide signalling and the immune response. Biochim
Biophys Acta. 1301:273–287. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Geilen CC, Wieder T and Orfanos CE:
Ceramide signalling: regulatory role in cell proliferation,
differentiation and apoptosis in human epidermis. Arch Dermatol
Res. 289:559–566. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Uchida Y: Ceramide signaling in mammalian
epidermis. Biochim Biophys Acta. 1841:453–462. 2014. View Article : Google Scholar :
|
|
59
|
Senkal CE, Ponnusamy S, Bielawski J,
Hannun YA and Ogretmen B: Antiapoptotic roles of
ceramide-synthase-6-generated C16-ceramide via selective regulation
of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J.
24:296–308. 2010. View Article : Google Scholar :
|
|
60
|
Tonnetti L, Veri MC, Bonvini E and
D'Adamio L: A role for neutral sphingomyelinase-mediated ceramide
production in T cell receptor-induced apoptosis and
mitogen-activated protein kinase-mediated signal transduction. J
Exp Med. 189:1581–1589. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gómez-Muñoz A: Ceramide-1-phosphate: a
novel regulator of cell activation. FEBS Lett. 562:5–10. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gómez-Muñoz A, Kong JY, Salh B and
Steinbrecher UP: Ceramide-1-phosphate blocks apoptosis through
inhibition of acid sphingomyelinase in macrophages. J Lipid Res.
45:99–105. 2004. View Article : Google Scholar
|
|
63
|
Gomez-Muñoz A, Martin A, O'Brien L and
Brindley DN: Cell-permeable ceramides inhibit the stimulation of
DNA synthesis and phospholipase D activity by phosphatidate and
lysophosphatidate in rat fibroblasts. J Biol Chem. 269:8937–8943.
1994.PubMed/NCBI
|
|
64
|
Gómez-Muñoz A, Kong JY, Parhar K, Wang SW,
Gangoiti P, González M, Eivemark S, Salh B, Duronio V and
Steinbrecher UP: Ceramide-1-phosphate promotes cell survival
through activation of the phosphatidylinositol 3-kinase/protein
kinase B pathway. FEBS Lett. 579:3744–3750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bourbon NA, Sandirasegarane L and Kester
M: Ceramide-induced inhibition of Akt is mediated through protein
kinase Czeta: implications for growth arrest. J Biol Chem.
277:3286–3292. 2002. View Article : Google Scholar
|
|
66
|
Schubert KM, Scheid MP and Duronio V:
Ceramide inhibits protein kinase B/Akt by promoting
dephosphorylation of serine 473. J Biol Chem. 275:13330–13335.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Grether-Beck S, Bonizzi G, Schmitt-Brenden
H, Felsner I, Timmer A, Sies H, Johnson JP, Piette J and Krutmann
J: Non-enzymatic triggering of the ceramide signalling cascade by
solar UVA radiation. EMBO J. 19:5793–5800. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wakita H, Tokura Y, Yagi H, Nishimura K,
Furukawa F and Takigawa M: Keratinocyte differentiation is induced
by cell-permeant ceramides and its proliferation is promoted by
sphingosine. Arch Dermatol Res. 286:350–354. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sayama K, Hanakawa Y, Shirakata Y,
Yamasaki K, Sawada Y, Sun L, Yamanishi K, Ichijo H and Hashimoto K:
Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular
inducer of keratinocyte differentiation. J Biol Chem. 276:999–1004.
2001. View Article : Google Scholar
|
|
70
|
Jiang YJ, Kim P, Uchida Y, Elias PM, Bikle
DD, Grunfeld C and Feingold KR: Ceramides stimulate caspase-14
expression in human keratinocytes. Exp Dermatol. 22:113–118. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ma nggau M, K i m DS, Ruwisch L, Vogler R,
Kor ting HC, Schäfer-Kor ting M and Kleuser B:
1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from
apoptosis by the formation of sphingosine-1-phosphate. J Invest
Dermatol. 117:1241–1249. 2001. View Article : Google Scholar
|
|
72
|
Kim DS, Kim SY, Moon SJ, Chung JH, Kim KH,
Cho KH and Park KC: Ceramide inhibits cell proliferation through
Akt/PKB inactivation and decreases melanin synthesis in Mel-Ab
cells. Pigment Cell Res. 14:110–115. 2001. View Article : Google Scholar : PubMed/NCBI
|