Introduction
The cornea is an immunologically privileged area,
and keratoplasty is one of the most successful forms of solid
tissue transplantation that serves as the only clinical therapy for
blindness (1). Approximately
36,000 cases of corneal transplantation surgery are performed
annually in the USA and about 3,000 cases in China each year
(2,3). Despite steady improvements in the
graft survival rate, immune-mediated graft rejection remains the
major cause of human corneal allograft failure, particularly
following high-risk keratoplasty or a second keratoplasty (4,5).
Currently, glucocorticoids, cyclosporin A and tacrolimus are the
main clinical treatment strategies for corneal graft rejection;
however, treatment is limited by the non-specific inhibition of
immune defense function. Hence, specific local therapeutic
approaches are expected to prolong the survival time of corneal
grafts.
CD4+ T-cells play an important role in
corneal graft rejection (6).
Activated naive CD4+ T-helper cells (Th0) differentiate
into Th1, Th2 or Th3 cell subsets depending on the different kinds
of cytokines within the graft microenvironment. The Th cells were
then activated by recognizing alloantigens to secrete a series of
cytokines including IL-10, interferon-γ (INF-γ) and transforming
growth factor-β (TGF-β) (7).
Interleukin (IL)-35, a heterodimer composed of p35
and Epstein-Barr virus-induced gene 3 (EBI3) subunits, belongs to
the IL-12 family and exerts anti-inflammatory and immunomodulatory
effects (8). It is predominantly
expressed by thymus-derived and peripheral
CD4+CD25+Foxp3+ regulatory T
(Treg) cells (9). Previous
findings have demonstrated that the elevated serum IL-35 level was
associated with the inflammatory response in multiple inflammatory
diseases such as acute pancreatitis (10), collagen-induced arthritis (CIA)
(11), periodontal inflammation
(12), allergic rhinitis and
asthma (13) as well as coronary
artery disease. In vitro studies demonstrated that animals
without functional IL-35 exhibited enhanced inflammatory immune
responses and were more likely to develop diseases, such as liver
fibrosis, inflammatory bowel disease and models of lethal
autoimmune disease (14–17). Furthermore, reduced IL-35 levels
are associated with rejection following allogeneic hematopoietic
stem cell transplantation (10),
and IL-35 therapy may inhibit cardiac allograft rejection in mice
(18). Jin et al
demonstrated that the expression of IL-35 in human placental
trophoblasts may prevent matrix immune rejection induced by fetal
antigens (19). However, the
effect of IL-35 on other corneal graft rejection-related cytokines
in the eyes has not been substantiated.
In the present study, we successfully injected a
pcDNA3.1-IL-35 plasmid into the mouse vitreous cavity to observe
whether IL-35 affected the expression of corneal graft
rejection-related cytokines. Our results showed that intravitreal
injection of pcDNA3.1-IL-35 plasmid is safe for mouse eyes.
Furthermore, enhanced levels of IL-35 may suppress pro-inflammatory
cytokine expression and increase anti-inflammatory cytokine
expression.
Materials and methods
Animals
A total of 72 specific pathogen-free (SPF) female
BALB/c mice, aged 6–10 weeks old and weighing between 15–18 g were
purchased from the Medical Laboratory Animal Center of Sun Yat-sen
University (Guangzhou, China). This study was approved by the
Ethics Committee of Sun Yat-sen University. All animal experiments
were performed in accordance with the Guidelines of Institutional
Animal Care and Use Committee at Sun Yat-sen University.
Intravitreal injection of pcDNA3.1-IL-35
plasmid
The pcDNA3.1-IL-35 plasmid harboring IL-35-coding
sequences was constructed by Guangzhou Vipotion Biotechnology Co.,
Ltd. (Guangzhou, China). Each mouse was deeply anesthetized by an
intraperitoneal injection of 4.3% chloral hydrate (China National
Medicines Corporation, Ltd., Beijing, China) and mydriasis was
induced with tropicamide eye drops (Shenyang Xingqi Pharmaceutical
Co., Ltd., Shenyang, China). To induce superficial anesthesia of
the eye, 0.5% tetracaine hydrochloride (National Institutes for
Food and Drug Control, Beijing, China) was subsequently used. A 33
g Hamilton microinjector was used to puncture the vitreous cavity
at a 45° angle to the transection of the lens and 1 µl (1
µg/µl) pcDNA3.1-IL-35 plasmid was injected into
vitreous cavity. Subsequently, tobramycin (Qilu Pharmaceutical Co.,
Ltd., Jinan, China) was applied to the conjunctival sac. Equal
volumes of pcDNA3.1 plasmid and PBS were injected in parallel as a
control. Ocular status (pre-mydriasis and post-mydriasis) was
examined by slit-lamp biomicroscopy 1, 2 and 4 weeks after
injection and 6–8 mice in each group were then sacrificed by
cervical dislocation. The mouse eyes were enucleated for subsequent
experiments.
Enzyme-linked immunosorbent assay
(ELISA)
The enucleated eyes in each mouse were lysed
according to a previously published method (20). Thereafter, ELISA was peformed
using a mouse ELISA kit for IL-35 (USCN Business Co., Ltd., Wuhan,
China) strictly according to the manufacturer's instructions, with
five replicates for each testing point including blank wells and
untreated controls (0 week). Optical density (OD) values were
assessed at 450 nm by a microplate reader (Biotek, Winooski, VT,
USA).
Western blot analysis
The enucleated eyes from each mouse were lysed and
the total proteins were extracted as previously described (20), and the protein concentration was
quantified using a bicinchoninic acid (BCA) protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Proteins (40
µg) in each sample were loaded and separated using sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA). The membranes were then blocked with
5% non-fat milk overnight and probed with anti-p35 (ab66064),
anti-IL-10 (ab134742), anti-TFG-β (ab190503), anti-IFN-γ (ab24324),
anti-IL-12 (ab89889) and anti-IL-17 (ab77171) primary antibodies
(1:500 dilution; all from Abcam, Cambridge, MA, USA) at 4°C
overnight, followed by incubation with secondary anti-rabbit
IgG-HRP antibody (A0208) or anti-mouse IgG-HRP antibody (A0216)
(1:5000 dilution; Beyotime Institute of Biotechnology) at 37°C for
45 min after washing twice with TBST. The target bands were
visualized using enhanced chemiluminescence (ECL) solution (Qihai
Biotec, Shanghai, China) and analyzed using Gel-Pro-Analyzer
software (Media Cybernetics, Bethesda, MD, USA). β-actin served as
an internal control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the enucleated eyes
using an RNA extraction kit (Tiangen Biotech, Beijing, China) and
then reverse transcribed into cDNA. RT-qPCR reactions were
performed in an Exicycler™ 96 (Bioneer, Daejeon, Korea) using
SYBR-Green Master mix (Solarbio, Beijing, China) according to the
following protocol: initial denaturation at 95°C for 5 min, 30
cycles consisting of 95°C for 20 sec, 60°C for 20 sec, and 72°C for
30 sec, and final elongation at 4°C for 5 min. The primer sequences
were as follows: p35, 5′-GACCTGGACCCTGAGATTGTGAA-3′ (sense) and
5′-GGTCCCTGTGCAGCACGTTA-3′ (antisense); and β-actin,
5′-GGAGATTACTGCCCTGGCTCCTA-3′ (sense) and
5′-GACTCATCGTACTCCTGCTTGCTG-3′ (antisense). Relative mRNA
expression was calculated using the 2−ΔΔCT method and
β-actin was used as an internal control.
Hematoxylin and eosin (H&E)
staining
Corneal and retinal tissues were fixed in 10%
formaldehyde for 24 h, dehydrated with a graded series of ethanol
(70% for 2 h, 80% overnight, 90% for 2 h and 100% for 2 h) and
embedded in dimethylbenzene-paraffin. Sections (5 µm) were
cut, placed onto slides and dried in a 70°C chamber for 40 min.
After being dewaxed with ethanol, the sections were soaked with
hematoxylin (Solarbio), water and 1% hydrochloric ethanol at room
temperature for 5 min, 5 min, and 3 sec respectively, and rinsed
with water for 20 min. The slices were subsequently stained with
eosin for 3 min, and dehydrated through a graded series of ethanol
(75, 85, 95% each for 2 min, and 100% twice for 5 min). The stained
tissues were then permeabilized twice with xylene for 10 min and
mounted with resinene and observed under a microscope (DP73;
Olympus, Tokyo, Japan) and images were captured.
Immunofluorescence double staining
The 5 µm slices were fixed in 4% formaldehyde
for 15 min, washed with PBS three times, and blocked with 10%
bovine serum albumin (BSA) at room temperature for 1 h. Thereafter,
the cells were co-incubated with p35 (sc-821; 1:50 dilution; Santa
Cruz Biotechnology, Santa Cruz, CA, USA) and EBI3 primary
antibodies (sc-166158; 1:250 dilution; Santa Cruz Biotechnology) at
4°C overnight and subsequently incubated with FITC-labeled goat
anti-rabbit IgG (H+L) (A0562) and Cy3-labeled goat anti-mouse IgG
(H+L) (A0516) secondary antibodies (Beyotime Institute of
Biotechnology) at a dilution of 1:200 for 1 h at room temperature.
Unbound antibodies in each step were removed by washing with PBS.
The coverslips were mounted inversely onto slides with 95% glycerol
and observed under a laser scanning confocal microscope (Olympus)
and images were captured.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 software. All data are reported as the means ± standard
deviation (SD). The differences between groups were analyzed by
one-way analysis of variance (ANOVA). A p-value <0.05 was
considered to indicate a statistically significant difference.
Results
Intravitreal injection of pcDNA3.1-IL-35
plasmid increases intraocular level of IL-35
To explore the function of IL-35 in the eyes of
mice, the pcDNA3.1-IL-35 plasmid encoding IL-35 was injected into
the vitreous cavity of BALB/c mice, and then the expression level
of IL-35 was detected by ELISA, RT-qPCR and western blot analysis.
The results of the ELISA showed that the intraocular IL-35 level
was significantly elevated at 1 week after injection with the
pcDNA3.1-IL-35 plasmid compared with pcDNA3.1 injection (Fig. 1A; p<0.01) and in fact, peaked
after 1 week (20.43±1.032 pg/eyeball). Moreover, the mRNA levels of
intraocular p35 at 1, 2 and 4 weeks after injection with the
pcDNA3.1-IL-35 plasmid were 3.53-, 2.39 and 2.14-fold (Fig. 1B; p<0.001) higher,
respectively, than those injected with with respective pcDNA3.1.
The protein levels of intraocular p35 at 1, 2 and 4 weeks after
IL-35 plasmid injection were 2.25-, 1.94- and 1.79-fold higher,
respectively, (Fig. 1B and C;
p<0.01) than those in the presence of pcDNA3.1 alone. These data
suggest the successful expression of the pcDNA3.1-IL-35 plasmid in
the mouse eyes.
Intravitreal injection of pcDNA3.1-IL-35
plasmid is safe to ocular tissues
To determine the effect of an intravitreal injection
of pcDNA3.1-IL-35 plasmid on intraocular tissues, slit-lamp and
H&E staining were employed to evaluate the physiological
changes in the cornea and retina. As shown in Fig. 2A, there were no differences
between the mouse eyes injected with pcDNA3.1-IL-35 plasmid and the
control. The cornea exhibited transparency without obvious edema,
the aqueous humor was clear, iris vessels were clearly visible, and
the size and location of the pupil was normal. There was no
intraocular hemorrhage, retinal detachment, or neovascularization
during the 4 week period following plasmid injection. Further
H&E staining revealed no abnormalities of the cornea in all
groups except for the loose arrangement of corneal stroma collagen
fibers which disappeared at 4 weeks after injection (Fig. 2B). There were no apparent
abnormalities in the retinal pigment epithelium, retinal cells,
cell layer or bipolar cells, indicating that the method of
intravitreal injection of pcDNA3.1-IL-35 plasmid is safe to ocular
tissues.
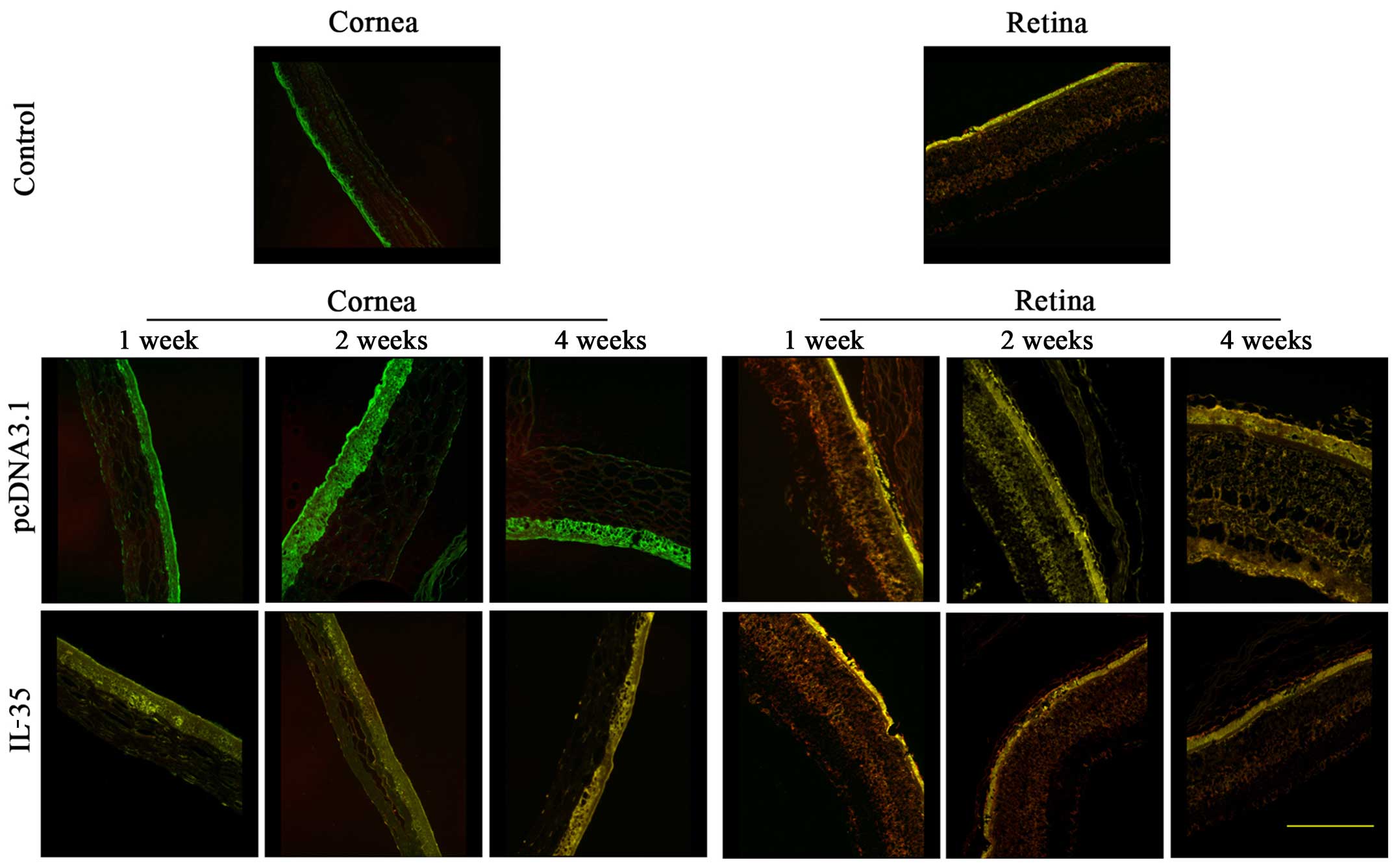
IL-35 is uniformly distributed on corneal
epithelial cells
IL-35 is formed by pairing IL-12α (also known as
p35) and EBI3. As IL-35 shares the p35 subunit with IL-12 and
shares the EBI3 subunit with IL-27 (8), we employed the immunofluorescence
double staining assay to identify the distribution of intraocular
IL-35. As shown in Fig. 3, there
was no IL-35 expression in the corneal epithelial cells of the
control- or pcDNA3.1-injected mice, as evidenced by the positively
stained p35 antibody and negatively stained EBI3 antibody.
Moreover, we observed coexpressed p35 (green) and EBI3 (red) on
corneal epithelial cells from the pcDNA3.1-IL-35 plasmid-injected
mice, and their localizations were matched, indicating the
successful expression of IL-35, and that the expression of IL-35 in
the corneal epithelial cells lasted for at least 4 weeks. On the
other hand, the expression of IL-35 was predominantly distributed
in the retinal epithelium in both the control and pcDNA3.1-IL-35
plasmid-injected mice. The above results demonstrate that IL-35 is
expressed on mouse corneal epithelial cells following intravitreal
injection with the pcDNA3.1-IL-35 plasmid.
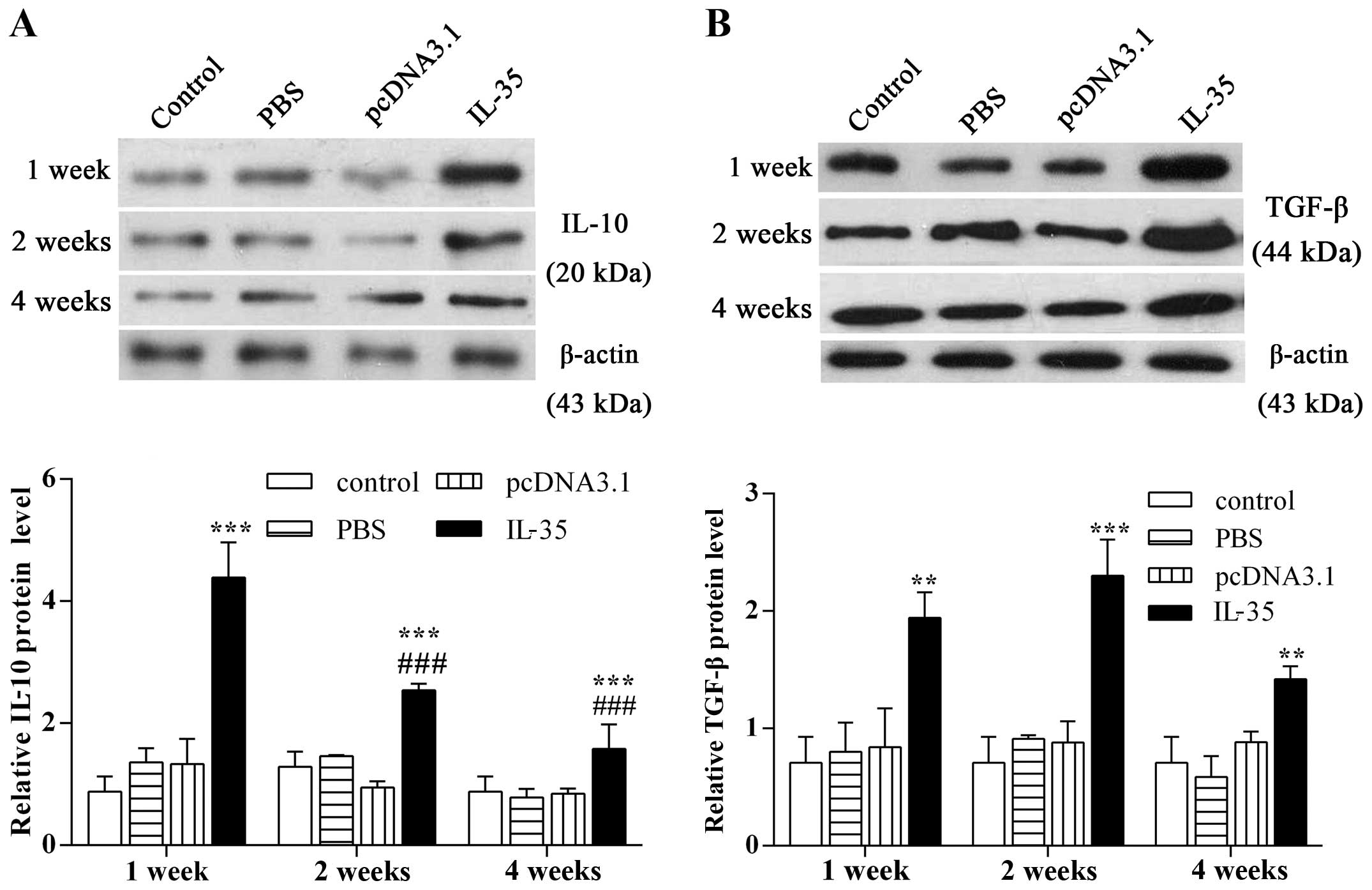
Elevated IL-35 level enhances the
expression of intraocular IL-10 and TGF-β
We conducted western blot analysis to examine the
effect of IL-35 on the expression of anti-inflammatory cytokines.
The results showed that the expression of IL-10 was notably
enhanced by 5.0-fold compared with the control 1 week following an
intravitreal injection of pcDNA3.1-IL-35 plasmid (Fig. 4A; p<0.001). The increased IL-10
level then faded markedly at 2 and 4 weeks after injection
(p<0.001). Moreover, an intravitreal injection of pcDNA3.1-IL-35
plasmid resulted in a significant increase of TGF-β expression
(Fig. 4B; p<0.01), which
reached a peak at 2 weeks (p<0.001). The above results indicate
that an intravitreal injection of pcDNA3.1-IL-35 plasmid in mice
upregulates the expression of anti-inflammatory cytokines.
Elevated IL-35 level suppresses the
expression of INF-γ, IL-12 and IL-17
Western blot analysis was performed to observe the
effect of IL-35 on the expression of pro-inflammatory cytokines.
The results showed that an intravitreal injection of pcDNA3.1-IL-35
plasmid induced a sharp decline in the expression of INF-γ 2 weeks
after injection (Fig. 5A;
p<0.05). Simultaneously, the expression of IL-12 was apparently
decreased by 1.91-fold compared with the control at 2 weeks after
the pcDNA3.1-IL-35 plasmid injection (Fig. 5B; p<0.05). In addition, a
marked downregulation of IL-17 was observed at 4 weeks after the
pcDNA3.1-IL-35 plasmid injection (Fig. 5C; p<0.05). Taken together,
these findings demonstrate that elevated IL-35 levels inhibit the
expression of pro-inflammatory cytokines.
Discussion
The anti-inflammatory cytokine IL-35 is able to
inhibit rejection following organ transplantation. However, the
role of IL-35 in corneal graft rejection remains unclear, and the
effect of IL-35 on corneal graft rejection-related cytokines has
not been fully elucidated. Herein, we found that increased IL-35
expression promoted the expression of anti-inflammatory cytokines
and reduced the expression of pro-inflammatory cytokines following
an intravitreal injection of pcDNA3.1-IL-35 plasmid, which was also
proved to be safe to use in ocular tissues as there were no
abnormalities observed in the mouse corneal and retinal tissues.
Collectively, these findings demonstrate that an intravitreal
injection of plasmid harboring IL-35 may be a potential approach
for reducing the risk of corneal allograft rejection.
The immunosuppressive effect of IL-35 is not only
characterized by the suppression of effector T cell (Teff)
responses and Teff proliferation, but is also manifested in the
induction of Tregs to generate and propagate infectious tolerance,
and these Tregs in turn secrete IL-35 to enhance the
immunosuppressive effect (14,15,21). Detailed studies have identified
that Tregs play an essential role in protecting individuals against
graft rejection and prolonging survival time, including corneal
transplantation (22,23). In view of these findings, we
hypothesized that IL-35 may contribute to the inhibition of corneal
allograft rejection. Moreover, plasmid injection does not result in
genomic integration, therefore the expression of foreign genes in
the tissue cells is temporary, and the cells will gradually lose
the plasmid over time (24).
Previous studies examined the hydrodynamic tail vein injection of
plasmid DNA; however, efficient expression was only detected in the
mouse liver (25–27). Other studies found that an
intravitreal injection of plasmid DNA led to expression in the
cornea (28–30). Thus, we preliminarily detected
alterations in the expression of relevant cytokines after injecting
the pcDNA3.1-IL-35 plasmid into the vitreous cavity of mice. We
detected that the expression of IL-35 lasted for at least 4 weeks
in corneal epithelial cells, which is concurrent with the
occurrence time of an alloantigen-induced inflammatory reaction
following corneal allograft surgery.
IL-10 and TGF-β are secreted by a variety of cell
types with graft tolerance and anti-inflammatory properties
(31). IL-10 may block the
production of IL-12 and downregulate MHC class II expression on
monocytes (32). TGF-β regulates
the proliferation, differentiation and apoptosis of several immune
cells (33). Jin et al
proved that IL-35 boosted the proliferation of Tregs by increasing
the expression of IL-10 and TGF-β, which was important for the
establishment and maintenance of maternal-fetal tolerance during
early pregnancy (19). In this
study, we demonstrated that the expression of IL-10 and TGF-β were
significantly increased in the eyes following an intravitreal
injection of pcDNA3.1-IL-35 plasmid, which was consistent with the
previous findings of Jin et al. The elevated levels of IL-10
and TGF-β are closely associated with immune tolerance to the
corneal allograft (34–36). Klebe et al reported that
transferred ovine IL-10-cDNA reduced the incidence of corneal graft
rejection and prolonged corneal allograft survival (37). Wang et al found that TGF-β
plays an important role in the conversion of Tregs from T-helper
(Th)17 cells and thereby affects the Treg-Th17 balance to
facilitate immunological tolerance following allogenic corneal
transplantation (38). Hence, we
demonstrated that the exogenous injection of IL-35 upregulated the
expression of the graft tolerance-related cytokines, IL-10 and
TGF-β.
INF-γ is a potent, pro-inflammatory cytokine
responsible for strengthening the Th1 immune response, and IL-12 is
another pro-inflammatory cytokine capable of promoting the
proliferation of Th1-type cells (39). Recent evidence suggested that
IL-12 stimulated IFN-γ production by NK and T cells (40), and the increased production of
IL-12 and IFN-γ may be predictive of corneal graft rejection
(41,42). In our study, we detected the
downregulation of IL-12 and IFN-γ in pcDNA3.1-IL-35
plasmid-injected eyes, indicating the presence of an
anti-inflammatory environment around the cornea. Additionally,
IL-17, a potent pro-inflammatory cytokine, mediates tissue
inflammation by inducing chemokine expression and leukocyte
infiltration (43). The IL-17
antagonist prolonged the median survival time of non-vascularized
and vascularized cardiac allograft patients and improved the
survival rate of corneal allogeneic transplantation (44–46). Chen et al observed that the
anti-IL-17 mAb suppressed the expression of IFN-γ and IL-12 p40
significantly in transplanted splenocytes (46,47). Similarly, we found a notable
decline in IL-17 expression 4 weeks after IL-35 injection and more
significantly reduced IFN-γ and IL-12 levels at the same time
point, suggesting that the decreased expression of IL-17 may
further reduce IFN-γ and IL-12 levels. Taken together, these
findings demonstrated that an intravitreal injection of
pcDNA3.1-IL-35 plasmid downregulated the expression of the
pro-inflammatory cytokines IL-12, IL-17 and IFN-γ.
There are some limitations in our study. We explored
the effect of elevated IL-35 expression on corneal allograft
rejection-related cytokines in healthy mice without establishing a
model of corneal allograft rejection. In our next study, we will
detect IL-35 distribution and pathological changes in other ocular
tissues following an intravitreal injection of pcDNA3.1-IL-35
plasmid in a murine model of corneal allograft transplantation.
The findings of the present study suggest that an
intravitreal injection of IL-35 plasmid suppresses the expression
of pro-inflammatory cytokines and promotes the expression of
anti-inflammatory cytokines. Our data preliminarily identified the
effects of IL-35 on corneal allograft rejection-related cytokines,
and suggest that IL-35 may serve as a potential target for gene
therapy of corneal allograft rejection.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81371065).
References
|
1
|
Qin Q, Shi Y, Zhao Q, Luo D, Chen Y, Wu J
and Zhao M: Effects of CD25siRNA gene transfer on high-risk rat
corneal graft rejection. Graefes Arch Clin Exp Ophthalmol.
253:1765–1776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan Q, Xu Q, Boylan NJ, Lamb NW, Emmert
DG, Yang JC, Tang L, Heflin T, Alwadani S, Eberhart CG, et al:
Corticosteroid-loaded biodegradable nanoparticles for prevention of
corneal allograft rejection in rats. J Control Release. 201:32–40.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Liao C, Gao M, Belin MW, Wang M,
Yu H and Yu J: Efficacy and safety of corneal transplantation using
corneas from foreign donors versus domestic donors: a prospective,
randomized, controlled trial. J Ophthalmol. 2015:1782892015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paunicka KJ, Mellon J, Robertson D,
Petroll M, Brown JR and Niederkorn JY: Severing corneal nerves in
one eye induces sympathetic loss of immune privilege and promotes
rejection of future corneal allografts placed in either eye. Am J
Transplant. 15:1490–1501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Wang L and Zhang L: Cyclosporine
nanomicelle eye drop: a novel medication for corneal graft
transplantation treatment. Biol Pharm Bull. 38:893–900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pleyer U, Milani JK, Dukes A, Chou J, Lutz
S, Rückert D, Thiel HJ and Mondino BJ: Effect of topically applied
anti-CD4 monoclonal antibodies on orthotopic corneal allografts in
a rat model. Invest Ophthalmol Vis Sci. 36:52–61. 1995.PubMed/NCBI
|
|
7
|
Niederkorn JY: Immunology and
immunomodulation of corneal transplantation. Int Rev Immunol.
21:173–196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olson BM, Sullivan JA and Burlingham WJ:
Interleukin 35: a key mediator of suppression and the propagation
of infectious tolerance. Front Immunol. 4:3152013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Käser T, Müllebner A, Hartl RT, Essler SE,
Saalmüller A and Catharina Duvigneau J: Porcine T-helper and
regulatory T cells exhibit versatile mRNA expression capabilities
for cytokines and co-stimulatory molecules. Cytokine. 60:400–409.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang YL, Zhou XY, Guo XY and Tu JW:
Association between serum interleukin-35 levels and severity of
acute pancreatitis. Int J Clin Exp Med. 8:7430–7434.
2015.PubMed/NCBI
|
|
11
|
Filková M, Vernerová Z, Hulejová H,
Prajzlerová K, Veigl D, Pavelka K, Vencovský J and Šenolt L:
Pro-inflammatory effects of interleukin-35 in rheumatoid arthritis.
Cytokine. 73:36–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Köseoğlu S, Sağlam M, Pekbağrıyanık T,
Savran L and Sütçü R: Level of interleukin-35 in gingival
crevicular fluid, saliva, and plasma in periodontal disease and
health. J Periodontol. 86:964–971. 2015. View Article : Google Scholar
|
|
13
|
Ding LF, Chen Q, Li L, Liu JM, Zhang GP,
Zhu XH, Wu AM, Ke JW, Dai YL and Wu CX: Effects of sublingual
immunotherapy on serum IL-17 and IL-35 levels in children with
allergic rhinitis or asthma. Zhongguo Dang Dai Er Ke Za Zhi.
16:1206–1210. 2014.In Chinese. PubMed/NCBI
|
|
14
|
Collison LW, Chaturvedi V, Henderson AL,
Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ,
Brown SA, et al: IL-35-mediated induction of a potent regulatory T
cell population. Nat Immunol. 11:1093–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collison LW, Workman CJ, Kuo TT, Boyd K,
Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS and Vignali DA:
the inhibitory cytokine IL-35 contributes to regulatory T-cell
function. Nature. 450:566–569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsuda M, Zhang W, Yang GX, Tsuneyama K,
Ando Y, Kawata K, Park O, Leung PS, Coppel RL, Ansari AA, et al:
Deletion of interleukin (IL)-12p35 induces liver fibrosis in
dominant-negative TGFβ receptor type II mice. Hepatology.
57:806–816. 2013. View Article : Google Scholar
|
|
17
|
Wirtz S, Billmeier U, Mchedlidze T,
Blumberg RS and Neurath MF: Interleukin-35 mediates mucosal immune
responses that protect against T-cell-dependent colitis.
Gastroenterology. 141:1875–1886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo H, Wang W, Zhao N, He X, Zhu L and
Jiang X: Inhibiting cardiac allograft rejection with interleukin-35
therapy combined with decitabine treatment in mice. Transpl
Immunol. 29:99–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin E, Wang C, Hu Q, Jin G and Li S: The
regular distribution and expression pattern of immunosuppressive
cytokine IL-35 in mouse uterus during early pregnancy. Rom J
Morphol Embryol. 55:1353–1361. 2014.
|
|
20
|
Ali TK, Matragoon S, Pillai BA, Liou GI
and El-Remessy AB: Peroxynitrite mediates retinal neurodegeneration
by inhibiting nerve growth factor survival signaling in
experimental and human diabetes. Diabetes. 57:889–898. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niedbala W, Wei XQ, Cai B, Hueber AJ,
Leung BP, McInnes IB and Liew FY: IL-35 is a novel cytokine with
therapeutic effects against collagen-induced arthritis through the
expansion of regulatory T cells and suppression of Th17 cells. Eur
J Immunol. 37:3021–3029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He Y, Jie Y, Wang B, Zeng H, Zhang Y and
Pan Z: Adoptive transfer of donor corneal antigen-specific
regulatory T cells can prolong mice corneal grafts survival.
Cornea. 29(Suppl 1): S25–S31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rossmanith W, Chabicovsky M, Herkner K and
Schulte-Hermann R: Cellular gene dose and kinetics of gene
expression in mouse livers transfected by high-volume tail-vein
injection of naked DNA. DNA Cell Biol. 21:847–853. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maruyama H, Higuchi N, Nishikawa Y, Kameda
S, Iino N, Kazama JJ, Takahashi N, Sugawa M, Hanawa H, Tada N, et
al: High-level expression of naked DNA delivered to rat liver via
tail vein injection. J Gene Med. 4:333–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu F, Song Y and Liu D:
Hydrodynamics-based transfection in animals by systemic
administration of plasmid DNA. Gene Ther. 6:1258–1266. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andrianaivo F, Lecocq M, Wattiaux-De
Coninck S, Wattiaux R and Jadot M: Hydrodynamics-based transfection
of the liver: entrance into hepatocytes of DNA that causes
expression takes place very early after injection. J Gene Med.
6:877–883. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang Y, Zhang Q and Steinle JJ:
Intravitreal injection of IGFBP-3 restores normal insulin signaling
in diabetic rat retina. PLoS One. 9:e937882014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon KC, Ahn KY, Lee JH, Chun BJ, Park SW,
Seo MS, Park YG and Kim KK: Lipid-mediated delivery of
brain-specific angiogenesis inhibitor 1 gene reduces corneal
neovascularization in an in vivo rabbit model. Gene Ther.
12:617–624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sonoda S, Tachibana K, Uchino E, Okubo A,
Yamamoto M, Sakoda K, Hisatomi T, Sonoda KH, Negishi Y, Izumi Y, et
al: Gene transfer to corneal epithelium and keratocytes mediated by
ultrasound with microbubbles. Invest Ophthalmol Vis Sci.
47:558–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Masli S, Turpie B, Hecker KH and Streilein
JW: Expression of thrombospondin in TGFbeta-treated APCs and its
relevance to their immune deviation-promoting properties. J
Immunol. 168:2264–2273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Torres PF, De Vos AF, van der Gaag R,
Martins B and Kijlstra A: Cytokine mRNA expression during
experimental corneal allograft rejection. Exp Eye Res. 63:453–461.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song SS, Yuan PF, Chen JY, Fu JJ, Wu HX,
Lu JT and Wei W: TGF-β favors bone marrow-derived dendritic cells
to acquire tolerogenic properties. Immunol Invest. 43:360–369.
2014. View Article : Google Scholar
|
|
34
|
Zhou L, Zhu X, Tan J, Wang J and Xing Y:
Effect of recombinant adeno-associated virus mediated transforming
growth factor-beta1 on corneal allograft survival after high-risk
penetrating keratoplasty. Transpl Immunol. 28:164–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Enzmann V, Hollborn M, Wiedemann P and
Kohen L: Minor influence of the immunosuppressive cytokines IL-10
and TGF-beta on the proliferation and apoptosis of human retinal
pigment epithelial (RPE) cells in vitro. Ocul Immunol Inflamm.
9:259–266. 2001. View Article : Google Scholar
|
|
36
|
Li B, Tian L, Diao Y, Li X, Zhao L and
Wang X: Exogenous IL-10 induces corneal transplantation immune
tolerance by a mechanism associated with the altered Th1/Th2
cytokine ratio and the increased expression of TGF-β. Mol Med Rep.
9:2245–2250. 2014.PubMed/NCBI
|
|
37
|
Klebe S, Sykes PJ, Coster DJ, Krishnan R
and Williams KA: Prolongation of sheep corneal allograft survival
by ex vivo transfer of the gene encoding interleukin-10.
Transplantation. 71:1214–1220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Wang W, Xu J, Wu S and Le Q:
All-trans retinoid acid promotes allogeneic corneal graft survival
in mice by regulating Treg-Th17 balance in the presence of TGF-β.
BMC Immunol. 16:172015. View Article : Google Scholar
|
|
39
|
Klebe S, Coster DJ, Sykes PJ, Swinburne S,
Hallsworth P, Scheerlinck JP, Krishnan R and Williams KA:
Prolongation of sheep corneal allograft survival by transfer of the
gene encoding ovine IL-12-p40 but not IL-4 to donor corneal
endothelium. J Immunol. 175:2219–2226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gee K, Guzzo C, Che Mat NF, Ma W and Kumar
A: The IL-12 family of cytokines in infection, inflammation and
autoimmune disorders. Inflamm Allergy Drug Targets. 8:40–52. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
King WJ, Comer RM, Hudde T, Larkin DF and
George AJ: Cytokine and chemokine expression kinetics after corneal
transplantation. Transplantation. 70:1225–1233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maier P, Heizmann U, Böhringer D, Kern Y
and Reinhard T: Predicting the risk for corneal graft rejection by
aqueous humor analysis. Mol Vis. 17:1016–1023. 2011.PubMed/NCBI
|
|
43
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang JL, Subbotin VM, Antonysamy MA,
Troutt AB, Rao AS and Thomson AW: Interleukin-17 antagonism
inhibits acute but not chronic vascular rejection. Transplantation.
72:348–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Antonysamy MA, Fanslow WC, Fu F, Li W,
Qian S, Troutt AB and Thomson AW: Evidence for a role of IL-17 in
alloimmunity: a novel IL-17 antagonist promotes heart graft
survival. Transplant Proc. 31:931999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen X, Zhao S, Tang X, Ge H and Liu P:
Neutralization of mouse interleukin-17 bioactivity inhibits corneal
allograft rejection. Mol Vis. 17:2148–2156. 2011.PubMed/NCBI
|
|
47
|
Chen H, Wang W, Xie H, Xu X, Wu J, Jiang
Z, Zhang M, Zhou L and Zheng S: A pathogenic role of IL-17 at the
early stage of corneal allograft rejection. Transpl Immunol.
21:155–161. 2009. View Article : Google Scholar : PubMed/NCBI
|