Introduction
Neutrophils are the first line of the innate immune
defense against microbes, and play a critical role in maintaining
intestinal homeostasis (1).
Neutrophils contain several antimicrobial peptides that function in
microbial killing, such as bactericidal/permeability-increasing
protein, cathelicidin and four defensins, namely human neutrophil
peptide 1 to 4 (HNP-1 to -4) (2).
HNP-1, HNP-2 and HNP-3 have almost identical amino
acid sequences (3). Both HNP-1
and HNP-3 are composed of 30 amino acid residues, with only one
additional amino acid residue at the N-terminus as compared to
HNP-2: alanine for HNP-1 and aspartate for HNP-3. HNP-4 is a
33-residue-long peptide with an amino acid sequence distinct from
those of other HNPs (4).
HNPs have been shown to exert antimicrobial effects
against Gram-negative and Gram-positive bacteria, fungi and
enveloped viruses (5–7). For example, HNP-1 has been shown to
inhibit the growth of Staphylococcus aureus (S.
aureus) and Escherichia coli (E. coli) under
low-salt conditions with minimum inhibitory concentration ranges of
2.2–7.9 and 0.7–3.7 µg/ml, respectively (8).
In addition to their antimicrobial activity, HNPs
exert immunomodulatory effects that depend on the concentration
range. At low concentrations, HNP-1 and HNP-2 demonstrate
chemotactic activity for monocytes, naive T cells and immature
dendritic cells, with a peak response at 10–20 ng/ml (9,10).
At intermediate concentrations, HNP-1-3 enhance the proliferation
of epithelial cells, fibroblasts and tumor cells with a peak
response at 5–15 µg/ml (11–14). At high concentrations (>50
µg/ml), HNPs are cytotoxic to epithelial cells and tumor
cells (12–14).
The concentration of HNP-1-3 within neutrophils is
very high, at almost 6 mg/ml (15). By contrast, the plasma
concentrations of HNPs are substantially below the levels required
to mediate direct antimicrobial activities. The total
concentrations of HNP-1-3 in the plasma of healthy individuals have
been reported to be around 40–100 ng/ml when measured with
enzyme-linked immunosorbent assays (ELISAs) (16–18) and around 200–400 ng/ml when
measured with radioimmunoassays (19,20). However, the plasma concentrations
of HNP-1-3 can increase as high as 170 µg/ml during severe
infections (16).
Neutrophil infiltration into the mucosa is a
pathological hallmark of inflammatory bowel disease, particularly
ulcerative colitis (21). We have
previously demonstrated that the plasma concentrations of HNP-1-3
in patients with active ulcerative colitis are significantly higher
than those in healthy subjects or those with inactive ulcerative
colitis, Crohn's disease or infectious enterocolitis, which may
reflect the infiltration and activation of neutrophils in the
intestinal mucosa of patients with active ulcerative colitis
(18). To elucidate the
pathological role of HNPs in intestinal inflammation, we selected a
murine experimental model since mouse neutrophils lack homologs of
HNPs (22). Our previous study
using mice demonstrated that the intraperitoneal administration of
high-dose synthetic HNP-1 (100 µg/day) aggravated dextran
sulfate sodium (DSS)-induced colitis (14). This result suggested that high
concentrations of HNPs may be a pathogenic factor in ulcerative
colitis. However, the role of low physiological concentrations of
HNPs in the intestinal tract remains largely unknown.
In the present study, we examined the effects of the
mild transgenic overexpression of HNP-1 and the intraperitoneal
injection of low-dose synthetic HNP-1 in DSS-induced colitis in
order to determine the effects of low concentrations of HNPs on
intestinal inflammation.
Materials and methods
Reagents
Synthetic HNP-1 was purchased from Peptide Institute
(Osaka, Japan). Purified native HNPs from human neutrophils were
purchased from Athens Research and Technology (Athens, GA, USA).
These peptides were dissolved in phosphate-buffered saline (PBS).
RPMI-1640 medium, fetal bovine serum (FBS),
penicillin-streptomycin, PBS, Hank's balanced salt solution (HBSS),
EDTA and TRIzol reagent were obtained from Life Technologies
(Carlsbad, CA, USA). Dithiothreitol (DTT) was obtained from Wako
Pure Chemical Industries (Osaka, Japan). DSS (molecular weight,
50,000 Da) was obtained from Ensuiko Sugar Refining Co. (Yokohama,
Japan). Collagenase D and DNase I were obtained from Roche
(Mannheim, Germany). Percoll was obtained from GE Healthcare
(Little Chalfont, UK).
Animals
The generation of HNP-1 transgenic mice has been
described elsewhere (23).
Briefly, an HNP-1 cDNA fragment encoding the entire open reading
frame (nucleotides 90–387) was subcloned into a pCAGGS expression
vector, which contains the cytomegalovirus early enhancer element
and chicken β-actin promoter (CAG promoter). The CAG-HNP-1 fragment
was isolated and microinjected into the fertilized eggs of C57BL/6N
mice to produce HNP-1 transgenic mice. Five HNP-1 transgenic mice
were used in this study. Specific-pathogen-free male C57BL/6N and
BALB/c mice were obtained from Kyudo Co. (Saga, Japan). We used 22
C57BL/6N mice, which were also used as the wild-type mice, and 12
BALB/c mice in this study. All the mice were used at 8 weeks of
age. All animal protocols were approved by the Ethics Committee of
the Kagoshima University Graduate School of Medical and Dental
Sciences, Kagoshima, Japan.
ELISA
Plasma HNP-1 levels were measured using a human
HNP-1-3 ELISA kit (Hycult Biotechnology, Uden, Netherlands)
according to the manufacturer's instructions, and analyzed in
duplicate using a microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA) at 450 nm. The concentration of HNP-1 in the
plasma was calculated according to a standard curve.
Induction and assessment of colitis
Experimental colitis was induced in mice by
administering DSS in their drinking water ad libitum; 2.5%
DSS was administered for 5 days to HNP-1 transgenic and C57BL/6N
mice, while 2% DSS was administered for 7 days to the BALB/c
mice.
PBS or 5 µg of HNP-1 was intraperitoneally
administered to DSS-treated C57BL/6N mice from days 1 to 5 and
DSS-treated BALB/c mice from days 1 to 7.
A disease activity index (DAI) was based on clinical
scores for weight loss, stool consistency and bleeding, as
previously described (24). Each
clinical parameter was scored on a scale from 0 to 4, and the
parameter values were summed.
On the last day of the experiment, the mice were
sacrificed by cervical dislocation and colon tissues were collected
for histological scoring and mRNA analysis. For histological
assessment, the sections of the colon were fixed in 10% buffered
formalin, embedded in paraffin and stained with hematoxylin and
eosin. Histological scoring was based on a previously described
method (25). In brief, colon
damage was categorized into 5 groups as follows: grade 0, normal;
grade 1, loss of one-third of the crypts; grade 2, loss of
two-thirds of the crypts; grade 3, lamina propria covered with a
single epithelial layer with mild inflammatory cell infiltration;
grade 4, erosions and marked inflammatory cell infiltration. All
scores were obtained in a blinded manner by 2 investigators.
Preparation of colonic lamina propria
mononuclear cells (LPMCs)
Colonic LPMCs from the C57BL/6N mice were isolated
using a modified protocol as previously described (26). Briefly, the isolated colons were
washed with calcium- and magnesium-free HBSS and dissected into
small sections. The tissues were incubated in HBSS containing 2 mM
DTT for 30 min at 37°C and then incubated in HBSS containing 5 mM
EDTA for 15 min at 37°C. The sections were collected and digested
with HBSS containing 1 mg/ml collagenase D and 0.1 mg/ml DNase I
for 60 min at 37°C. The cell suspension was subjected to Percoll
gradient centrifugation.
Stimulation of colonic LPMCs by
heat-killed E. coli
The macrophages of colonic LPMCs were enriched via
their adherence to plastic surfaces. Colonic LPMCs were seeded at a
concentration of 2.0×106 cells/ml in a 24-well plate and
incubated in RPMI-1640 medium supplemented with 10% FBS and
penicillin-streptomycin at 37°C for 2 h. The cells were then washed
twice with PBS to remove unattached cells. With this protocol,
approximately 70% of the adherent cells were F4/80-positive
macrophages. The attached cells were then incubated in serum-free
medium without or with heat-killed E. coli 0111:B4
(InvivoGen, San Diego, CA, USA) at 1×108 CFU/ml and
various concentrations of HNP-1 or native HNPs (0.1–100
µg/ml) for 6 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the colon tissues and
colonic LPMCs using TRIzol reagent according to the manufacturer's
instructions and stored at −80°C. Equal amounts of total RNA were
reverse transcribed using the PrimeScript RT reagent kit (Takara
Bio, Otsu, Japan). Synthesized cDNA was amplified using SYBR Premix
Ex Taq II (Takara Bio) and analyzed with the StepOnePlus Real-Time
PCR system and StepOne Software version 2.0 (Applied Biosystems,
Foster City, CA, USA). The primers for interleukin (IL)-1β (Primer
Set ID: MA025939), IL-6 (MA104898), tumor necrosis factor (TNF)-α
(MA117190), IL-10 (MA118529), monocyte chemoattractant protein
(MCP)-1 (CCL2; MA108953) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) (MA050371) were purchased from Takara Bio.
The cycling conditions were as follows: one cycle at 95°C for 30
sec followed by 40 cycles each at 95°C for 5 sec and 60°C for 30
sec. To normalize the amount of total RNA present in each reaction,
the GAPDH gene was used as an internal standard.
Cell viability assay
The viability of the colonic LPMCs was determined by
Cell Count Reagent SF (Nacalai Tesque, Kyoto, Japan) based on the
WST-8 assay. LPMCs (1×105 cells/ml) were grown in
96-well plates for 24 h and then treated without or with
heat-killed E. coli at 5×106 CFU/ml and various
concentrations of HNP-1 or native HNPs (0.1–100 µg/ml) in
serum-free medium. After 24 h, 10 µl of Cell Count Reagent
SF were added to each well, followed by incubation for 1 h. The
absorbance of each well was measured at 450 nm with a reference
wavelength of 620 nm using a microplate reader (Bio-Rad
Laboratories).
Statistical analysis
Data were analyzed for statistical differences using
the Mann-Whitney U test or Student's t-test with SPSS 15.0J
software (SPSS, Chicago, IL, USA). The results are expressed as the
means ± SD. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Mild transgenic overexpression of HNP-1
reduces the susceptibility to DSS-induced colitis
The HNP-1 transgenic mice expressing human HNP-1
driven by the CAG promoter exhibited plasma HNP-1 concentrations of
46.12±21.87 ng/ml, which are similar to the physiological
concentrations in human plasma, and did not develop spontaneous
colitis. No immunoreactive HNP-1 was detected in the plasma of the
wild-type C57BL/6N mice.
To examine the effect of low concentrations of HNP-1
in experimental murine colitis, we first induced acute DSS colitis
by the addition of 2.5% DSS to the drinking water of HNP-1
transgenic and wild-type mice for 5 days. The HNP-1 transgenic mice
exhibited significantly milder colitis than the wild-type mice in
response to DSS. The DAI scores on the last day of the experiment
were significantly lower in the HNP-1 transgenic mice than in the
wild-type mice (4.60±0.55 vs. 6.20±0.45, P=0.006) (Fig. 1A). The histological findings of
the colonic tissues correlated well with clinical scores.
Histological examination of the distal colons of the wild-type mice
revealed erosion, disappearance of glandular epithelium and marked
inflammatory cell infiltration. By contrast, the colon specimens of
the HNP-1 transgenic mice showed reduced tissue damage and
inflammatory cell numbers (Fig.
1B). The histological scores reflecting colon damage were
significantly lower in the HNP-1 transgenic mice than in the
wild-type mice (1.60±0.55 vs. 3.60±0.55, P=0.007) (Fig. 1C). In parallel with the
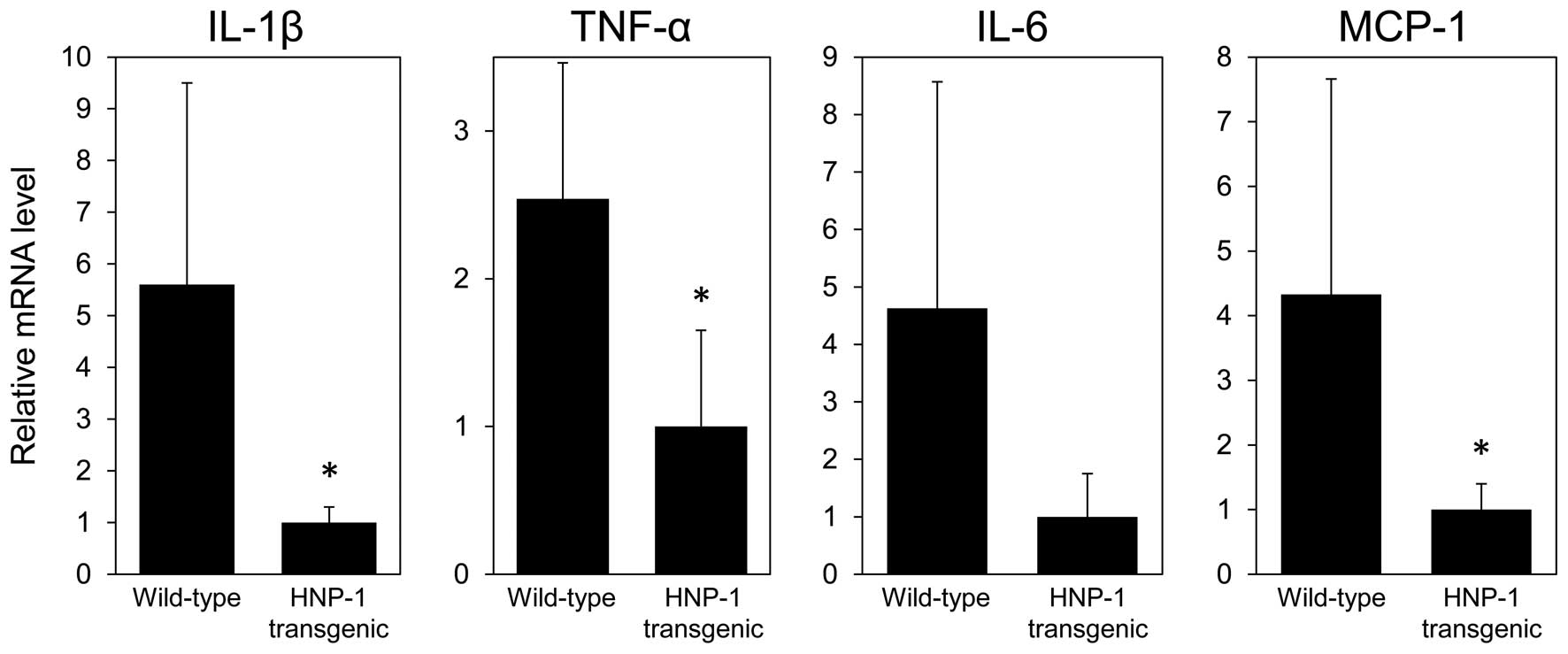
histological findings, the mRNA levels of IL-1β, TNF-α and MCP-1 in
the colon tissues of the HNP-1 transgenic mice were significantly
lower than in those of the wild-type mice (Fig. 2). The IL-6 mRNA levels in the
colon tissues tended to be lower in the HNP-1 transgenic mice than
in the wild-type mice, although the difference was not
statistically significant. These results suggest that low
concentrations of HNP-1 may play an anti-inflammatory role in
intestinal inflammation.
Intraperitoneal injection of low-dose
HNP-1 ameliorates DSS-induced colitis
To rule out the possibility of the developmental or
compensatory effects of HNP-1 overexpression in transgenic mice, we
then injected synthetic HNP-1 into normal mice. We measured plasma
HNP-1 levels following intraperitoneal injections of various
concentrations of synthetic HNP-1. When we injected single doses of
5 µg of HNP-1 into 3 C57BL/6N mice, the plasma HNP-1 levels
at 1, 3 and 6 h following administration were 32.43±14.53,
45.02±3.19 and 18.30±7.05 ng/ml, respectively. Since the peak level
of plasma HNP-1 was similar to the plasma concentration of HNP-1 in
the HNP-1 transgenic mice, we used 5 µg of HNP-1 in the
subsequent experiments.
The C57BL/6N mice were administered 2.5% DSS in
their drinking water for 5 days, and injected with HNP-1 (5
µg/day) or PBS intraperitoneally from days 1 to 5. On day 5,
the HNP-1-treated mice had lower DAI scores compared with the
PBS-treated mice although the difference was not statistically
significant (5.83±0.98 vs. 7.17±1.17, P=0.058) (Fig. 3A, left panel). The histological
examination of the colon tissue sections from the HNP-1-treated
mice revealed a significant reduction in inflammatory cell
infiltration and the preservation of epithelial integrity (Fig. 3B, left panels). The HNP-1-treated
mice had significantly lower histological scores than the
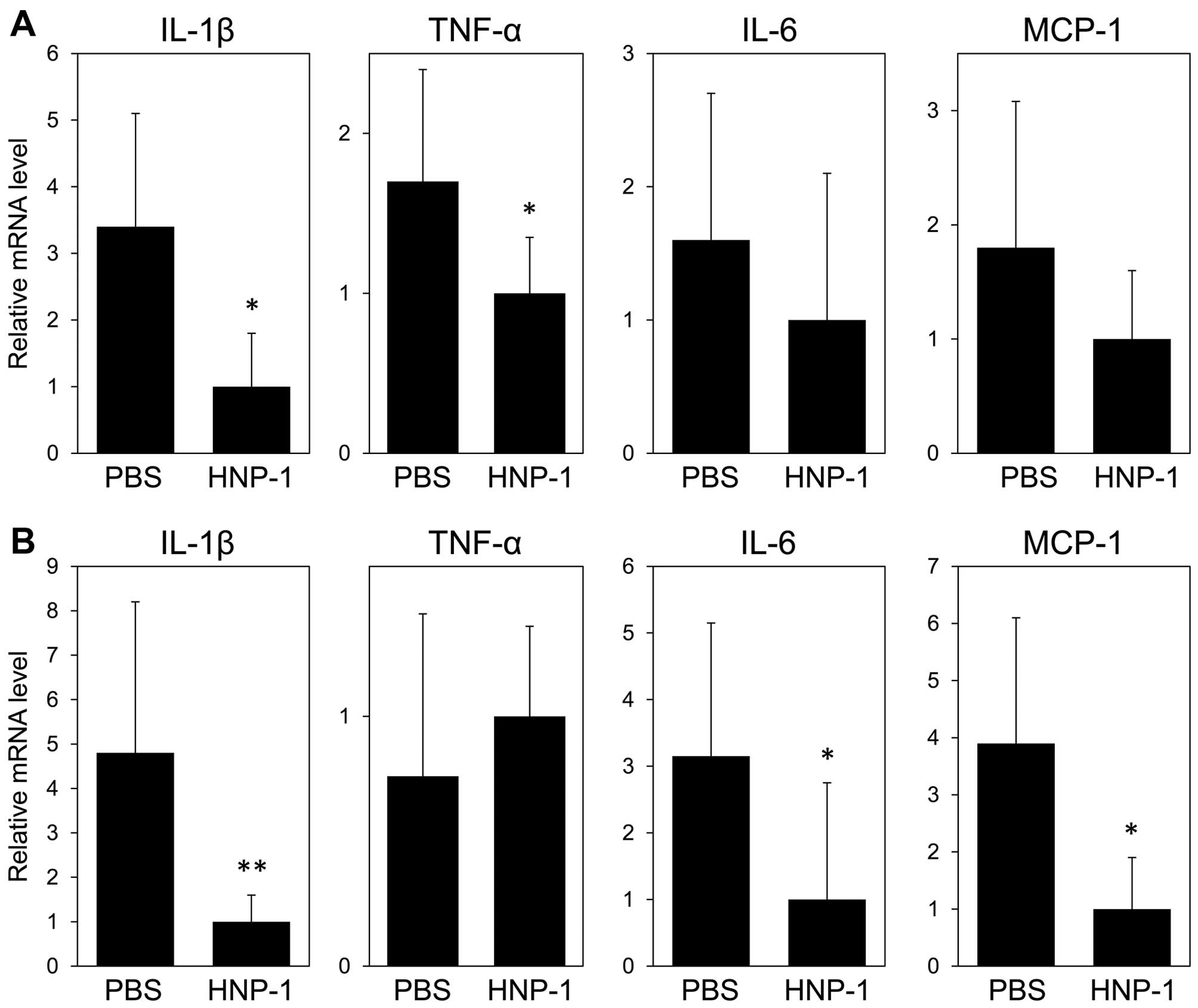
PBS-treated mice (2.33±0.52 vs. 3.83±0.41, P=0.004) (Fig. 3C, left panel). The mRNA expression
levels of IL-1β and TNF-α in the colon tissues from the
HNP-1-treated mice were significantly decreased compared to those
of the PBS-treated mice (Fig.
4A). The mRNA expression levels of IL-6 and MCP-1 were lower in
the HNP-1-treated mice compared with the PBS-treated mice, although
no statistically significant difference was observed.
The genetic background of mouse strains may
differentially impact antimicrobial defense. For example, C57BL/6
mice have 5 strain-specific Paneth cell α-defensins that have not
been identified in other inbred strains (27). To exclude the possibility that the
immunomodulatory effect of HNP-1 results from the synergistic
interaction between HNP-1 and C57BL/6N-specific antimicrobial
peptides, we also examined the effect of HNP-1 on DSS-induced
colitis in BALB/c mice. Colitis was induced in the BALB/c mice by
the oral administration of 2% DSS in their drinking water for 7
days. The mice were injected intraperitoneally with HNP-1 (5
µg/day) or PBS from days 1 to 7. The HNP-1-treated BALB/c
mice had significantly lower DAI scores (4.17±2.23 vs. 8.83±2.14,
P=0.006) (Fig. 3A, right panel),
less histological damage (Fig.
3B, right panel) and significantly lower histological score s
(1.83±0.98 vs. 3.50±0.55, P=0.011) (Fig. 3C, right panels) than the
PBS-treated mice. The mRNA expression levels of IL-1β, IL-6 and
MCP-1 in the colon tissues of the HNP-1-treated mice were
significantly lower than those of the PBS-treated mice, although no
statistically significant differences were observed in the TNF-α
mRNA levels between the PBS- and HNP-1-treated mice (Fig. 4B). Thus, as well as the mild
transgenic overexpression of HNP-1, the exogenous administration of
low-dose HNP-1 also ameliorated DSS-induced colitis regardless of
the mouse strain.
Low concentrations of HNP have no
significant effect on the expression of pro- and anti-inflammatory
cytokines in colonic LPMCs activated with heat-killed E. coli
Treatment of DSS-induced colitis with high-dose
HNP-1 has been shown to increase colonic levels of
macrophage-derived cytokines such as IL-1β (14). To determine whether intestinal
macrophages are involved in the HNP-1-mediated amelioration of
DSS-induced colitis, in this study, we investigated the effect of
HNP-1 on the expression of cytokines associated with macrophage
activation in vitro (Fig.
5A). The activation of macrophage-enriched LPMCs with
heat-killed E. coli resulted in a significant increase in
the mRNA levels of IL-1β, TNF-α, IL-6 and IL-10. Incubation with a
low concentration of HNP-1 (0.1 µg/ml) had no significant
effect on the expression levels of these cytokines. These results
suggest that the anti-inflammatory effect of low-dose HNP-1 on
DSS-induced colitis may not be exerted by direct action on
intestinal macrophages. An intermediate concentration of HNP-1 (10
µg/ml) significantly increased the IL-6 and IL-10 expression
levels. On the contrary, a high concentration of HNP-1 (100
µg/ml) significantly decreased the expression levels of
IL-1β, TNF-α and IL-10. This decrease may not be caused by cell
death, since the high concentration of HNP-1 enhanced the
proliferation of activated LPMCs (Fig. 5B).
It is uncertain whether synthetic HNP-1 has the same
structural and functional characteristics as native HNP-1.
Moreover, neutrophils secrete HNP-1 together with other HNP
isoforms. Therefore, we additionally examined the effects on
activated LPMCs of native HNPs purified from human neutrophils
(Fig. 5C). Native HNPs and
synthetic HNP-1 had very similar effects on cytokine expression in
activated LPMCs, with the exception of IL-1β. As with synthetic
HNP-1, a low concentration of native HNPs had no significant effect
on the expression of any of the four cytokines examined. An
intermediate concentration of native HNPs significantly increased
the expression of IL-6 and IL-10, while a high concentration
decreased the expression levels of IL-1β, TNF-α and IL-10 without
causing significant cytotoxicity (Fig. 5D). Hence, synthetic HNP-1 is
thought to have similar immunological properties to the native
HNPs. The one exception is that an intermediate concentration of
native HNPs significantly increased IL-1β expression, indicating
that HNPs other than HNP-1 may increase IL-1β.
Discussion
This study demonstrated that low physiological
concentrations of HNP-1 ameliorated intestinal inflammation in
DSS-induced colitis. In comparison, our previous study demonstrated
that high concentrations of HNP-1 aggravated this inflammation
(14). The amelioration of
colitis by low-dose HNP-1 may be explained by its indirect
antimicrobial activity.
It has been demonstrated that the intestinal flora
is involved in the pathogenesis of DSS-induced colitis, as well as
human inflammatory bowel disease (28). However, local colonic
concentrations of HNP-1 in transgenic mice may not reach levels
that cause direct antimicrobial activity, since HNP-1 protein
concentrations in the neutrophils of HNP-1 transgenic mice (<1
ng/mg protein) are much lower than those in human neutrophils
(23). It was previously shown
that the intravenous injection of low-dose HNP-1 (4 ng to 4
µg) resulted in local leukocyte accumulation and markedly
reduced bacterial numbers in the infected peritoneal cavity. As the
administration of low-dose HNP-1 did not exert antibacterial
effects in leukocytopenic mice, leukocyte accumulation appears to
be essential for the antibacterial effect (29). Therefore, low HNP-1 concentrations
in HNP-1 transgenic mice or HNP-1-treated mice are likely to exert
indirect antimicrobial effects via a chemotactic effect.
An early study investigating the immunological
effects of HNPs on cytokine production demonstrated an increased
production of TNF-α and IL-1β in human monocytes activated with
S. aureus or phorbol myristate acetate. However, the TNF-α
production peaked at a very low concentration of HNP
(10−9 M; 3.4 ng/ml) and declined at higher
concentrations (30). Several
recent independent studies have demonstrated that HNPs have
anti-inflammatory properties. HNP-1 has been shown to block the
ATP-induced IL-1β release from lipopolysaccharide (LPS)-activated
human monocytes (31). In another
study, HNP-1 and HNP-2 attenuated IL-6 and keratinocyte-derived
chemokine responses to recombinant hemagglutinin B from
Porphyromonas gingivalis (rHagB) (32). HNP-1-3 reduced the production of
several pro-inflammatory cytokines, including TNF-α, from LPS- or
CD40L/interferon-γ-stimulated human monocyte-derived macrophages.
In addition, the systemic administration of HNP-1-3 protected mice
in a murine model of peritonitis (33). In the present study, a low
concentration of HNP-1 (0.1 µg/ml) had no significant effect
on the expression levels of IL-1β, TNF-α, IL-6 or IL-10 in
activated LPMCs.
It is noteworthy that an intermediate concentration
of HNP-1 (10 µg/ml) significantly increased the mRNA levels
of IL-6 and IL-10. IL-6 is ordinarily considered to be a
pro-inflammatory cytokine, but it also has a regenerative effect on
intestinal epithelial cells (34). IL-10 produced by intestinal
macrophages limits inflammation by maintaining Foxp3 expression in
Tregs (35). Therefore, the
intermediate concentration of HNPs may accelerate recovery from
inflammation by intestinal epithelial repair and promotion of Treg
function.
By contrast, a high concentration of HNP-1 (100
µg/ml) decreased the expression levels of IL-1β, TNF-α and
IL-10 in activated LPMCs without causing cytotoxicity. Thus,
high-dose HNP-1 may lead to the aggravation of DSS-induced colitis
by direct cytotoxicity and the induction of chemokines in
epithelial cells. High concentrations of HNP-1 reduce the
proliferation and viability of intestinal epithelial cells
(14). Additionally, HNP-1
induces IL-8 production from intestinal epithelial cells in a
dose-dependent manner, which may stimulate additional neutrophil
accumulation in the intestine (36).
The human cathelicidin LL-37, which is released from
activated neutrophils and epithelial cells, also has dose-dependent
effects on inflammatory responses. At modest concentrations as low
as 1 µg/ml, LL-37 reduces the production of TNF-α by
LPS-treated macrophages. By contrast, concentrations of LL-37
>20 µg/ml induce the production of chemokines MCP-1 and
IL-8 in macrophages and lung epithelial cells (37). Furthermore, similar to HNPs,
low-to-intermediate concentrations of LL-37 induce cell
proliferation and migration, and high concentrations of LL-37 have
cytotoxic effects in bronchial epithelial cells (38).
Thus, it is clear that the neutrophil antimicrobial
peptides HNPs and LL-37 have a variety of immunomodulatory
functions, which may vary depending on local inflammatory
conditions. When encountering pathogens, neutrophils phagocytose
and digest the invading microorganisms, and release antimicrobial
peptides, such as HNPs and LL-37. High local concentrations of
these peptides exert potent antimicrobial effects, induce
epithelial cells to produce IL-8 which stimulates the infiltration
of more neutrophils into tissue, and have cytolytic activity which
may promote wound debridement. After the inflammation subsides,
decreased local concentrations of HNPs and LL-37 may facilitate a
return to homeostasis via the downregulation of the
pro-inflammatory response and the promotion of epithelial wound
repair by the induction of epithelial proliferation, migration and
differentiation (38,39).
HNP-1 and LL-37 have also been shown to suppress
neutrophil apoptosis in a dose-dependent manner, resulting in
prolonged neutrophil survival (40). The colonic expression of LL-37, as
well as HNPs, has been shown to be increased in ulcerative colitis
(18,41). In patients with ulcerative
colitis, neutrophil apoptosis is delayed, and intestinal
neutrophils express high levels of survivin, which protects cells
from various apoptotic stimuli (42,43). Corticosteroids, commonly used in
patients with ulcerative colitis, are known to induce apoptosis in
a wide range of cells, but they cause a dose-dependent inhibition
of neutrophil apoptosis (44).
There has recently been increasing evidence that neutrophil
apoptosis and the subsequent clearance by macrophages are essential
for the control of infection and the resolution of the inflammatory
response (45). The phagocytosis
of apoptotic neutrophils also reprograms macrophages to an
anti-inflammatory phenotype. Failure to properly remove
neutrophils, for instance due to delayed apoptosis, contributes to
prolonged tissue injury. In this context, the removal of activated
neutrophils by cytapheresis, which may lead to a decrease in the
neutrophil antimicrobial peptides, HNP-1 and LL-37, is considered
to be a reasonable and effective therapy for the treatment of
ulcerative colitis. Indeed, cytapheresis has been shown to be
useful in treating patients with steroid-refractory or
steroid-dependent ulcerative colitis (46).
In conclusion, in this study, we demonstrated the
biphasic dose-dependent immunomodulatory effect of HNP-1 on
DSS-induced colitis. In contrast to the aggravation of colitis by
high-dose HNP-1, low-dose HNP-1 ameliorates colitis accompanied
with the reduced colonic expression of pro-inflammatory cytokines.
Low concentrations of HNPs may contribute to the maintenance of
intestinal homeostasis.
Acknowledgments
We would like to thank Ms. Yuko Morinaga for
providing technical assistance.
References
|
1
|
Fournier BM and Parkos CA: The role of
neutrophils during intestinal inflammation. Mucosal Immunol.
5:354–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levy O: Antimicrobial proteins and
peptides: Anti-infective molecules of mammalian leukocytes. J
Leukoc Biol. 76:909–925. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selsted ME, Harwig SS, Ganz T, Schilling
JW and Lehrer RI: Primary structures of three human neutrophil
defensins. J Clin Invest. 76:1436–1439. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilde CG, Griffith JE, Marra MN, Snable JL
and Scott RW: Purification and characterization of human neutrophil
peptide 4, a novel member of the defensin family. J Biol Chem.
264:11200–11203. 1989.PubMed/NCBI
|
|
5
|
Ganz T, Selsted ME, Szklarek D, Harwig
SSL, Daher K, Bainton DF and Lehrer RI: Defensins. Natural peptide
antibiotics of human neutrophils. J Clin Invest. 76:1427–1435.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lehrer RI, Ganz T, Szklarek D and Selsted
ME: Modulation of the in vitro candidacidal activity of human
neutrophil defensins by target cell metabolism and divalent
cations. J Clin Invest. 81:1829–1835. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daher KA, Selsted ME and Lehrer RI: Direct
inactivation of viruses by human granulocyte defensins. J Virol.
60:1068–1074. 1986.PubMed/NCBI
|
|
8
|
Turner J, Cho Y, Dinh NN, Waring AJ and
Lehrer RI: Activities of LL-37, a cathelin-associated antimicrobial
peptide of human neutrophils. Antimicrob Agents Chemother.
42:2206–2214. 1998.PubMed/NCBI
|
|
9
|
Territo MC, Ganz T, Selsted ME and Lehrer
R: Monocyte-chemotactic activity of defensins from human
neutrophils. J Clin Invest. 84:2017–2020. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang D, Chen Q, Chertov O and Oppenheim
JJ: Human neutrophil defensins selectively chemoattract naive T and
immature dendritic cells. J Leukoc Biol. 68:9–14. 2000.PubMed/NCBI
|
|
11
|
Murphy CJ, Foster BA, Mannis MJ, Selsted
ME and Reid TW: Defensins are mitogenic for epithelial cells and
fibroblasts. J Cell Physiol. 155:408–413. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aarbiou J, Ertmann M, van Wetering S, van
Noort P, Rook D, Rabe KF, Litvinov SV, van Krieken JH, de Boer WI
and Hiemstra PS: Human neutrophil defensins induce lung epithelial
cell proliferation in vitro. J Leukoc Biol. 72:167–174.
2002.PubMed/NCBI
|
|
13
|
Müller CA, Markovic-Lipkovski J, Klatt T,
Gamper J, Schwarz G, Beck H, Deeg M, Kalbacher H, Widmann S,
Wessels JT, et al: Human alpha-defensins HNPs-1, -2, and -3 in
renal cell carcinoma: Influences on tumor cell proliferation. Am J
Pathol. 160:1311–1324. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hashimoto S, Uto H, Kanmura S, Sakiyama T,
Oku M, Iwashita Y, Ibusuki R, Sasaki F, Ibusuki K, Takami Y, et al:
Human neutrophil peptide-1 aggravates dextran sulfate
sodium-induced colitis. Inflamm Bowel Dis. 18:667–675. 2012.
View Article : Google Scholar
|
|
15
|
Lehrer RI: Questions and answers about
defensins. Clin Infect Dis. 25:1141–1142. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panyutich AV, Panyutich EA, Krapivin VA,
Baturevich EA and Ganz T: Plasma defensin concentrations are
elevated in patients with septicemia or bacterial meningitis. J Lab
Clin Med. 122:202–207. 1993.PubMed/NCBI
|
|
17
|
Albrethsen J, Møller CH, Olsen J, Raskov H
and Gammeltoft S: Human neutrophil peptides 1, 2 and 3 are
biochemical markers for metastatic colorectal cancer. Eur J Cancer.
42:3057–3064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanmura S, Uto H, Numata M, Hashimoto S,
Moriuchi A, Fujita H, Oketani M, Ido A, Kodama M, Ohi H and
Tsubouchi H: Human neutrophil peptides 1–3 are useful biomarkers in
patients with active ulcerative colitis. Inflamm Bowel Dis.
15:909–917. 2009. View Article : Google Scholar
|
|
19
|
Mukae H, Iiboshi H, Nakazato M, Hiratsuka
T, Tokojima M, Abe K, Ashitani J, Kadota J, Matsukura S and Kohno
S: Raised plasma concentrations of α-defensins in patients with
idiopathic pulmonary fibrosis. Thorax. 57:623–628. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashitani J, Mukae H, Hiratsuka T, Nakazato
M, Kumamoto K and Matsukura S: Elevated levels of α-defensins in
plasma and BAL fluid of patients with active pulmonary
tuberculosis. Chest. 121:519–526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer PB and Lehrer RI: Mouse
neutrophils lack defensins. Infect Immun. 60:3446–3447.
1992.PubMed/NCBI
|
|
23
|
Ibusuki R, Uto H, Arima S, Mawatari S,
Setoguchi Y, Iwashita Y, Hashimoto S, Maeda T, Tanoue S, Kanmura S,
et al: Transgenic expression of human neutrophil peptide-1 enhances
hepatic fibrosis in mice fed a choline-deficient, L-amino
acid-defined diet. Liver Int. 33:1549–1556. 2013.PubMed/NCBI
|
|
24
|
Murthy SN, Cooper HS, Shim H, Shah RS,
Ibrahim SA and Sedergran DJ: Treatment of dextran sulfate
sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis
Sci. 38:1722–1734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
26
|
Kamada N, Hisamatsu T, Okamoto S, Sato T,
Matsuoka K, Arai K, Nakai T, Hasegawa A, Inoue N, Watanabe N, et
al: Abnormally differentiated subsets of intestinal macrophage play
a key role in Th1-dominant chronic colitis through excess
production of IL-12 and IL-23 in response to bacteria. J Immunol.
175:6900–6908. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shanahan MT, Tanabe H and Ouellette AJ:
Strain-specific polymorphisms in Paneth cell α-defensins of C57BL/6
mice and evidence of vestigial myeloid α-defensin pseudogenes.
Infect Immun. 79:459–473. 2011. View Article : Google Scholar
|
|
28
|
Perše M and Cerar A: Dextran sodium
sulphate colitis mouse model: Traps and tricks. J Biomed
Biotechnol. 2012:7186172012. View Article : Google Scholar
|
|
29
|
Welling MM, Hiemstra PS, van den Barselaar
MT, Paulusma-Annema A, Nibbering PH, Pauwels EK and Calame W:
Antibacterial activity of human neutrophil defensins in
experimental infections in mice is accompanied by increased
leukocyte accumulation. J Clin Invest. 102:1583–1590. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaly YV, Paleolog EM, Kolesnikova TS,
Tikhonov II, Petratchenko EV and Voitenok NN: Neutrophil
alpha-defensin human neutrophil peptide modulates cytokine
production in human monocytes and adhesion molecule expression in
endothelial cells. Eur Cytokine Netw. 11:257–266. 2000.PubMed/NCBI
|
|
31
|
Shi J, Aono S, Lu W, Ouellette AJ, Hu X,
Ji Y, Wang L, Lenz S, van Ginkel FW, Liles M, et al: A novel role
for defensins in intestinal homeostasis: Regulation of IL-1β
secretion. J Immunol. 179:1245–1253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kohlgraf KG, Ackermann A, Lu X, Burnell K,
Bélanger M, Cavanaugh JE, Xie H, Progulske-Fox A and Brogden KA:
Defensins attenuate cytokine responses yet enhance antibody
responses to Porphyromonas gingivalis adhesins in mice. Future
Microbiol. 5:115–125. 2010. View Article : Google Scholar :
|
|
33
|
Miles K, Clarke DJ, Lu W, Sibinska Z,
Beaumont PE, Davidson DJ, Barr TA, Campopiano DJ and Gray M: Dying
and necrotic neutrophils are anti-inflammatory secondary to the
release of α-defensins. J Immunol. 183:2122–2132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuhn KA, Manieri NA, Liu TC and
Stappenbeck TS: IL-6 stimulates intestinal epithelial proliferation
and repair after injury. PLoS One. 9:e1141952014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murai M, Turovskaya O, Kim G, Madan R,
Karp CL, Cheroutre H and Kronenberg M: Interleukin 10 acts on
regulatory T cells to maintain expression of the transcription
factor Foxp3 and suppressive function in mice with colitis. Nat
Immunol. 10:1178–1184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ibusuki K, Sakiyama T, Kanmura S, Maeda T,
Iwashita Y, Nasu Y, Sasaki F, Taguchi H, Hashimoto S, Numata M, et
al: Human neutrophil peptides induce interleukin-8 in intestinal
epithelial cells through the P2 receptor and ERK1/2 signaling
pathways. Int J Mol Med. 35:1603–1609. 2015.PubMed/NCBI
|
|
37
|
Scott MG, Davidson DJ, Gold MR, Bowdish D
and Hancock RE: The human antimicrobial peptide LL-37 is a
multifunctional modulator of innate immune responses. J Immunol.
169:3883–3891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shaykhiev R, Beisswenger C, Kändler K,
Senske J, Püchner A, Damm T, Behr J and Bals R: Human endogenous
antibiotic LL-37 stimulates airway epithelial cell proliferation
and wound closure. Am J Physiol Lung Cell Mol Physiol.
289:L842–L848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aarbiou J, Verhoosel RM, Van Wetering S,
De Boer WI, Van Krieken JH, Litvinov SV, Rabe KF and Hiemstra PS:
Neutrophil defensins enhance lung epithelial wound closure and
mucin gene expression in vitro. Am J Respir Cell Mol Biol.
30:193–201. 2004. View Article : Google Scholar
|
|
40
|
Nagaoka I, Suzuki K, Niyonsaba F, Tamura H
and Hirata M: Modulation of neutrophil apoptosis by antimicrobial
peptides. ISRN Microbiol. 2012:3457912012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schauber J, Rieger D, Weiler F, Wehkamp J,
Eck M, Fellermann K, Scheppach W, Gallo RL and Stange EF:
Heterogeneous expression of human cathelicidin hCAP18/LL-37 in
inflammatory bowel diseases. Eur J Gastroenterol Hepatol.
18:615–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brannigan AE, O'Connell PR, Hurley H,
O'Neill A, Brady HR, Fitzpatrick JM and Watson RW: Neutrophil
apoptosis is delayed in patients with inflammatory bowel disease.
Shock. 13:361–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Altznauer F, Martinelli S, Yousefi S,
Thürig C, Schmid I, Conway EM, Schöni MH, Vogt P, Mueller C, Fey
MF, et al: Inflammation-associated cell cycle-independent block of
apoptosis by survivin in terminally differentiated neutrophils. J
Exp Med. 199:1343–1354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liles WC, Dale DC and Klebanoff SJ:
Glucocorticoids inhibit apoptosis of human neutrophils. Blood.
86:3181–3188. 1995.PubMed/NCBI
|
|
45
|
Amulic B, Cazalet C, Hayes GL, Metzler KD
and Zychlinsky A: Neutrophil function: From mechanisms to disease.
Annu Rev Immunol. 30:459–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sacco R, Romano A, Mazzoni A, Bertini M,
Federici G, Metrangolo S, Parisi G, Nencini C, Giampietro C,
Bertoni M, et al: Granulocytapheresis in steroid-dependent and
steroid-resistant patients with inflammatory bowel disease: A
prospective observational study. J Crohn's Colitis. 7:e692–e697.
2013. View Article : Google Scholar
|