Introduction
Ossification of the ligamentum flavum (OLF) of the
spine is characterized by ectopic bone formation in the ligament
flavum and is highly prevalent in the population of East Asia,
including the Japanese and Chinese populations (1–4).
Epidemiological studies have demonstrated that OLF frequently
occurs in the thoracic spine. Thoracic OLF (TOLF) progresses
insidiously over a long period of time, resulting in devastating
spinal cord injury that invariably leads to serious myelopathy.
Many factors contribute to OLF, including genetic background,
dietary habits, metabolic abnormalities and mechanical stress
(5–7).
TOLF most often affects one or two levels of the
spine and presents mainly in the lower thoracic spine (T10-T12)
(8), which is a mobile transition
region that may be more prone to degeneration owing to the high
tensile forces in the posterior column. Axial mechanical overload
and the consequent increase in repetitive tensile strain on
ligamentum tissues contribute to TOLF (9). Additionally, we have previously
found that mechanical stress induces the osteogenic differentiation
of cells from patients with TOLF (6,10).
Thus, local abnormal mechanical stress is thought to contribute to
the progression of TOLF. However, there are also many cases of
multiple-level TOLF in immobile, as well as mobile segments that
differ from single-level lesions in terms of disease progression
and clinical outcomes (11–13). On the basis of the whole clinical
condition, we hypothesized that TOLF involving multiple levels
extensively and TOLF in the circumscribed region may have a
different pathogenesis. Probably on the account of the differences
in genetic background, the osteogenic differentiation potency may
be intrinsically greater in multiple-level TOLF than in
single-level TOLF.
During osteogenic differentiation, transcription
factors, such as osterix and Runt-related transcription factor-2
(Runx2) and osteogenesis-related genes, such as bone morphogenetic
protein (BMP)2 regulate the expression of osteoblast markers,
including alkaline phosphatase (ALP), osteopontin (OPN), and
osteocalcin (OCN) among others (7,14–16). Susceptibility genes, including
collagen type VI alpha1 (COL6A1), BMP4 and major histocompatibility
complex, class II, DQ alpha1 (HLA-DQA1) have also been shown to be
associated with the occurrence of TOLF (17,18).
To date, single- and multiple-level TOLF have not
been investigated as separate conditions and there have been no
comparative studies examining differences in osteogenic
differentiation potency and related gene expression, at least to
the best of our knowledge. Only a similar and preliminary research
was conducted on the ossification of the posterior longitudinal
ligament (OPLL) (19). Thus, this
was addressed in the present study using spinal ligament samples
obtained from patients with single- and multiple-level TOLF in
which osteogenic differentiation was induced by the application of
cyclic mechanical stress.
Materials and methods
Clinical diagnosis and spinal ligament
samples
The diagnosis of TOLF or non-TOLF (i.e., other
thoracic diseases) was confirmed by pre-operative radiography,
computed tomography and magnetic resonance imaging (MRI) of the
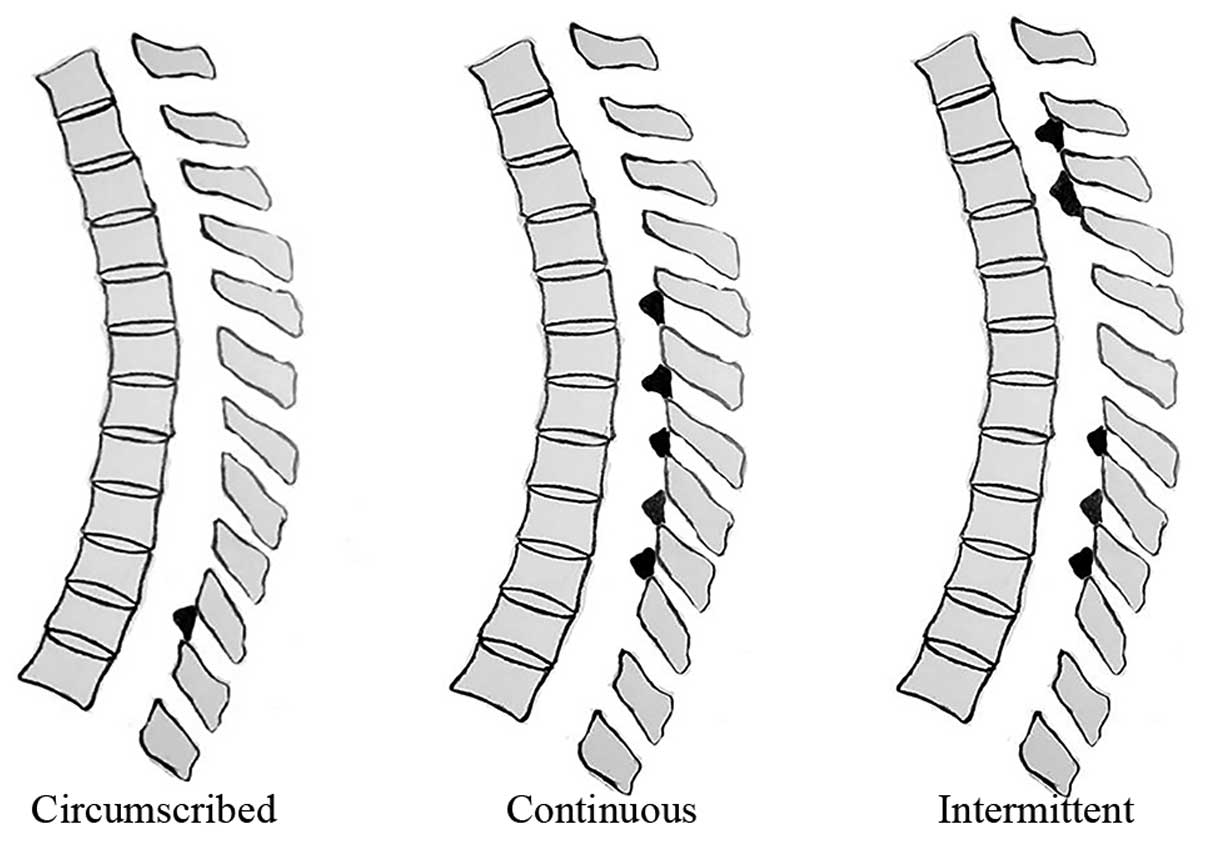
whole spine. We previously established a TOLF classification scheme
based on lesion distribution in MRI (20), as follows (Fig. 1): circumscribed (ossification in 2
or fewer adjacent levels), continuous (continuous ossification in 3
or more levels) and intermittent (intermittent distribution of
local or continuous ossification). To assess genetic differences in
the osteogenic differentiation potency among lesion types, a
comparative analysis of single- and multiple-level TOLF (1 and ≥5
levels, respectively) was performed. Non-TOLF specimens were
obtained from patients with other thoracic spine diseases, such as
trauma or disc herniation, who had no ossification in any spinal
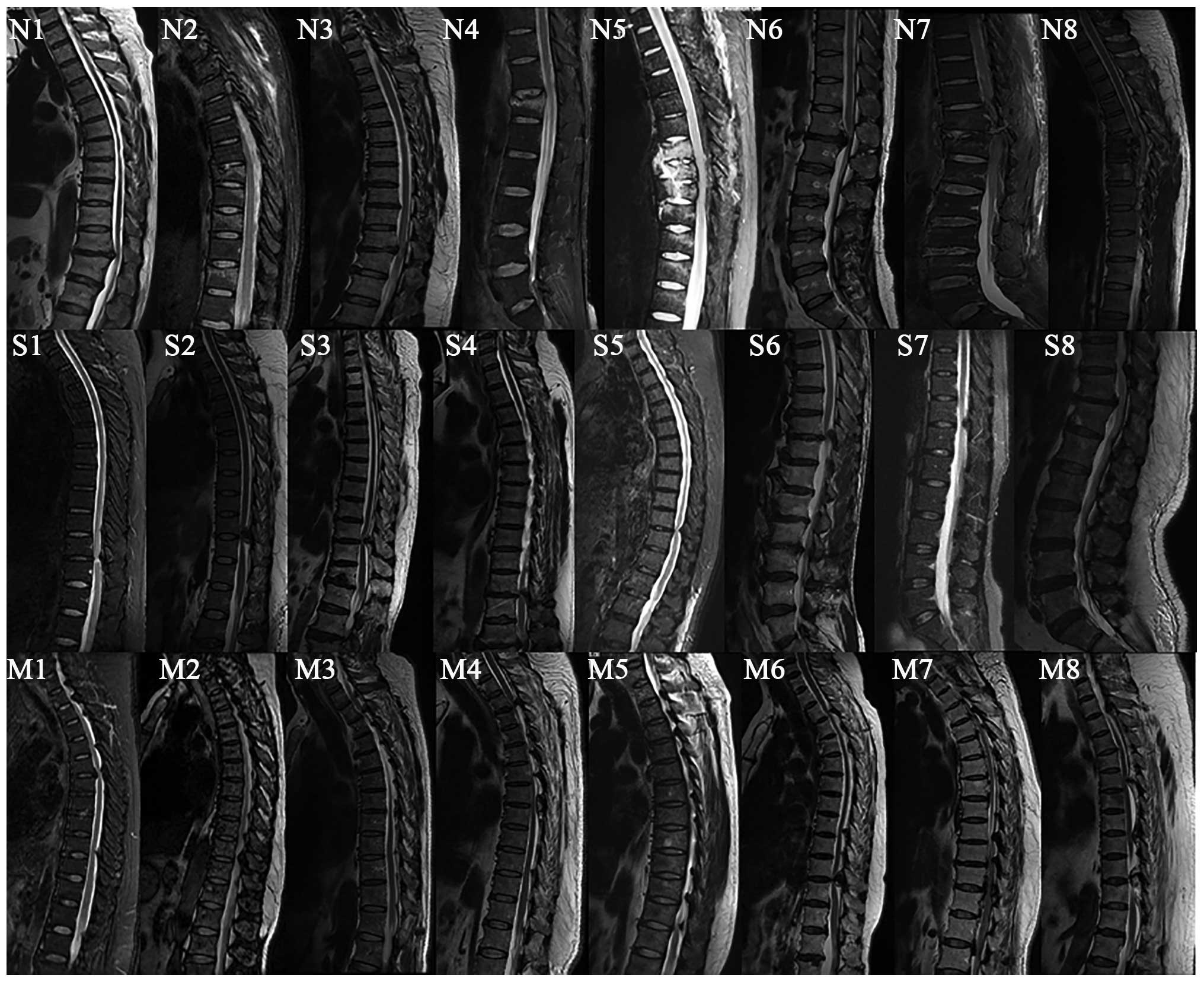
ligaments. Spinal ligament tissue specimens and associated clinical
diagnoses in this study are shown in Table I and Fig. 2. Single- and multiple-level TOLF
and non-TOLF ligaments were aseptically obtained from patients
during surgery and ligaments were obtained from non-ossified sites
to avoid any possible contamination of osteogenic cells. This study
was approved by the Ethics Committee of Peking University Third
Hospital (Beijing, China) and all patients provided written
informed consent prior to obtaining the samples.
 | Table IPatient information. |
Table I
Patient information.
| Groups | Sample no.
|
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|
| Non-TOLF |
| Age/gender | 62/M | 44/F | 58/M | 52/M | 44/M | 42/M | 52/M | 43/F |
| Diagnosis | T11-12 TDH | T8,10,12
fracture | T11-12 TDH | T12 fracture | T7-9 fracture | T12-L1 TDH | T12 fracture | T6-7 fracture |
| Single-level
TOLF |
| Age/gender | 54/M | 44/F | 65/F | 63/M | 69/F | 58/M | 43/F | 70/M |
| Ossification
level | T10-11 | T10-11 | T10-11 | T11-12 | T10-11 | T10-11 | T11-12 | T10-11 |
| Multiple-level
TOLF |
| Age/gender | 57/F | 59/F | 61/M | 68/M | 55/M | 59/F | 52/F | 58/F |
|
Classification | Intermittent | Intermittent | Continuous | Continuous | Intermittent | Intermittent | Intermittent | Intermittent |
| Ossification
level | T2-4, T5-7,
T10-11 | T1-3,T8-11 | T1-8 | T2-8 | T2-5, T9-12 | T2-5,T8-11 | T2-6,T9-L1 | T2-3,6-7,8-12 |
Cell culture
Cell culture was conducted as previously described
(21). The ligament specimens
were minced into 0.5-mm3 sections and digested with
0.25% trypsin (Gibco, Grand Island, NY, USA) for 1 h at 37°C
followed by 200 U/ml type I collagenase (Sigma-Aldrich, St. Louis,
MO, USA) for 4 h at 37°C. The sections were maintained in low
glucose Dulbecco's Modified Eagle's Medium (Hyclone, Logan, UT,
USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin,
and 100 µg/ml streptomycin (all from Gibco) in a humidified
incubator at 37°C and 5% CO2. Cells derived from
explants were passaged by digestion with 0.25% trypsin. Passage 3
cells were used for the experiments. The morphology of the primary
cells was observed visually under a microscope (5221227/293232;
Leica, Wetzlar, Germany) and images were obtained under the
magnification of ×50, ×100 and ×200.
Application of mechanical stretch
The cells were trypsinized and placed on a silicon
chamber coated with type I collagen (Flexcell International,
Hillsborough, NC, USA) at a density of 10,000 cells/cm2
and after 3 days, the cells reached confluence. Firstly, the cells
derived from 6 patients (TOLF and non-TOLF patients) and were
subjected to cyclic mechanical stretch at 0, 5, 10, 15 and 20% by
using a equi-biaxial cell-stretching apparatus (Flexcell FX-4000
Tension Plus System; Flexcell International) at 0.5 Hz and 37°C in
a humidified atmosphere of 95% air and 5% CO2 for 12 and
24 h, and the optimal magnitude of stretch was determined according
to the result. The optimal magnitude of stretch was then applied to
cells from all the patients for 6, 12 and 24 h.
ALP activity assay
ALP activity was quantitatively analyzed using the
LabAssay ALP kit (Wako, Osaka, Japan) and by staining using a
commercial kit (Genmed, Shanghai, China). For the quantitative
assay, total cellular protein was isolated from the cultured cells
using radioimmunoprecipitation (RIPA) extraction buffer (Applygen
Technologies, Beijing, China) and the concentration was determined
using the bicinchoninic acid (BCA) method. The extracted protein
solution was analyzed for ALP activity according to the
manufacturer's instructions, with the activity reported relative to
the protein concentration (U/mg). The cells were stained for ALP
activity and ALP-positive cells were blue, while the counterstained
nuclei appeared red. Micrographs of 10 random fields were obtained
for analysis. Stained areas were measured using Image-Pro Plus 6.0
software (Media Cybernetics, Rockville, MD, USA), and the ratio of
stained cells to the number of total cells was determined.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cell monolayers
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), and 1 µg total RNA was reverse transcribed into cDNA
using the GoScript Reverse Transcription System (Promega Corp.,
Madison, WI, USA). The primers used for amplification are listed in
Table II. qPCR was performed in
triplicate using SYBR-Green SuperReal PreMix Plus [Tiangen Biotech
(Beijing) Co., Ltd., Beijing, China] and the iQ5 PCR system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction
conditions were as follows: 95°C for 10 min, and 40 cycles of 95°C
for 15 sec and 60°C for 1 min. Data are represented as cycle
threshold (Ct) values. The RNA levels in different samples were
compared using the 2−ΔΔCt method and were normalized to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels.
 | Table IIPrimers used for RT-qPCR. |
Table II
Primers used for RT-qPCR.
| Gene | Primer
sequence | Product size
(bp) | Annealing
temperature (°C) |
|---|
| GAPDH | Forward:
5′-CAGGAGGCATTGCTGATGAT-3′
Reverse: 5′-GAAGGCTGGGGCTCATTT-3′ | 126 | 60 |
| ALP | Forward:
5′-AAGGACGCTGGGAAATCTGT-3′
Reverse: 5′-GGGCATCTCGTTGTCTGAGT-3′ | 117 | 57 |
| BMP2 | Forward:
5′-TCAAGCCAAACACAAACAGC-3′
Reverse: 5′-GGAGCCACAATCCAGTCATT-3′ | 105 | 55 |
| Osterix | Forward:
5′-GAGGTTCACTCGTTCGGATG-3′
Reverse: 5′-TGGTGTTTGCTCAGGTGGT-3′ | 120 | 56 |
| Runx2 | Forward:
5′-CCGTCCATCCACTCTACCAC-3′
Reverse: 5′-ATGAAATGCTTGGGAACTGC-3′ | 139 | 56 |
| OCN | Forward:
5′-CTCACACTCCTCGCCCTATT-3′
Reverse: 5′-CGCCTGGGTCTCTTCACTAC-3′ | 143 | 58 |
| OPN | Forward:
5′-GCCGTGGGAAGGACAGTTAT-3′
Reverse: 5′-GCTCATTGCTCTCATCATTGG-3′ | 114 | 56 |
Western blot analysis
Total cellular protein was isolated from the
cultured cells using RIPA extraction buffer and the concentration
was determined by BCA assay. A total of 30 µg protein per
well was separated by 10–15% SDS-polyacrylamide gel electrophoresis
and transferred onto nitrocellulose membranes, which were blocked
for 2 h at 24–25°C with 5% BSA/TBST and then incubated overnight at
4°C with the following primary antibodies: rabbit monoclonal
anti-BMP2 (1:1,000; ab183729), mouse monoclonal anti-osterix
(1:1,000; ab57335), rabbit polyclonal anti-OPN (1:1,000; ab181440)
and rabbit monoclonal anti-ALP (1:1,000; ab186422) (all from Abcam,
Cambridge, UK); and mouse monoclonal anti-GAPDH (1:2,000; BE0023;
Bioeasytech, Beijing, China). Immunoreactivity was detected with
IRDye 800cw-conjugated goat anti-rabbit/-mouse IgG (1:10,000;
LI-COR Biosciences, Lincoln, NE, USA) secondary antibodies and
visualized with an Odyssey infrared imaging system (LI-COR
Biosciences), with gray values analyzed using Odyssey v3.0
software.
Statistical analysis
In this study, the data are presented as the means ±
standard deviation and were analyzed using SPSS 20.0 software
(SPSS, Inc., Chicago, IL, USA). The mRNA expression data were
evaluated by one-way analysis of variance (ANOVA) with a post hoc
Dunnett's test (compared to 0 h in the non-TOLF group) and the post
hoc Tukey's test for multiple comparisons. Other data sets were
evaluated by ANOVA with a post hoc Tukey's test for multiple
comparisons. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphology of primary cells and
determination of the optimal mechanical stress level
The primary cells in all 3 groups were long and
spindle-shaped and exhibited fibrocyte-like adherent growth
(Fig. 3A). Following exposure to
different levels (0, 5, 10, 15 and 20%) of cyclic mechanical stress
for 12 or 24 h, osteogenic potency was evaluated with the ALP
activity assay. In the non-TOLF group, ALP activity remained
constant at different levels of cyclic mechanical stress and
periods of induction (Fig. 3B and
C). However, ALP activity was increased in the TOLF group,
particularly upon the application of 15% mechanical stress for 12 h
(Fig. 3B) and 24 h (Fig. 3C). We therefore applied a
mechanical stress of 15% for the subsequent experiments.
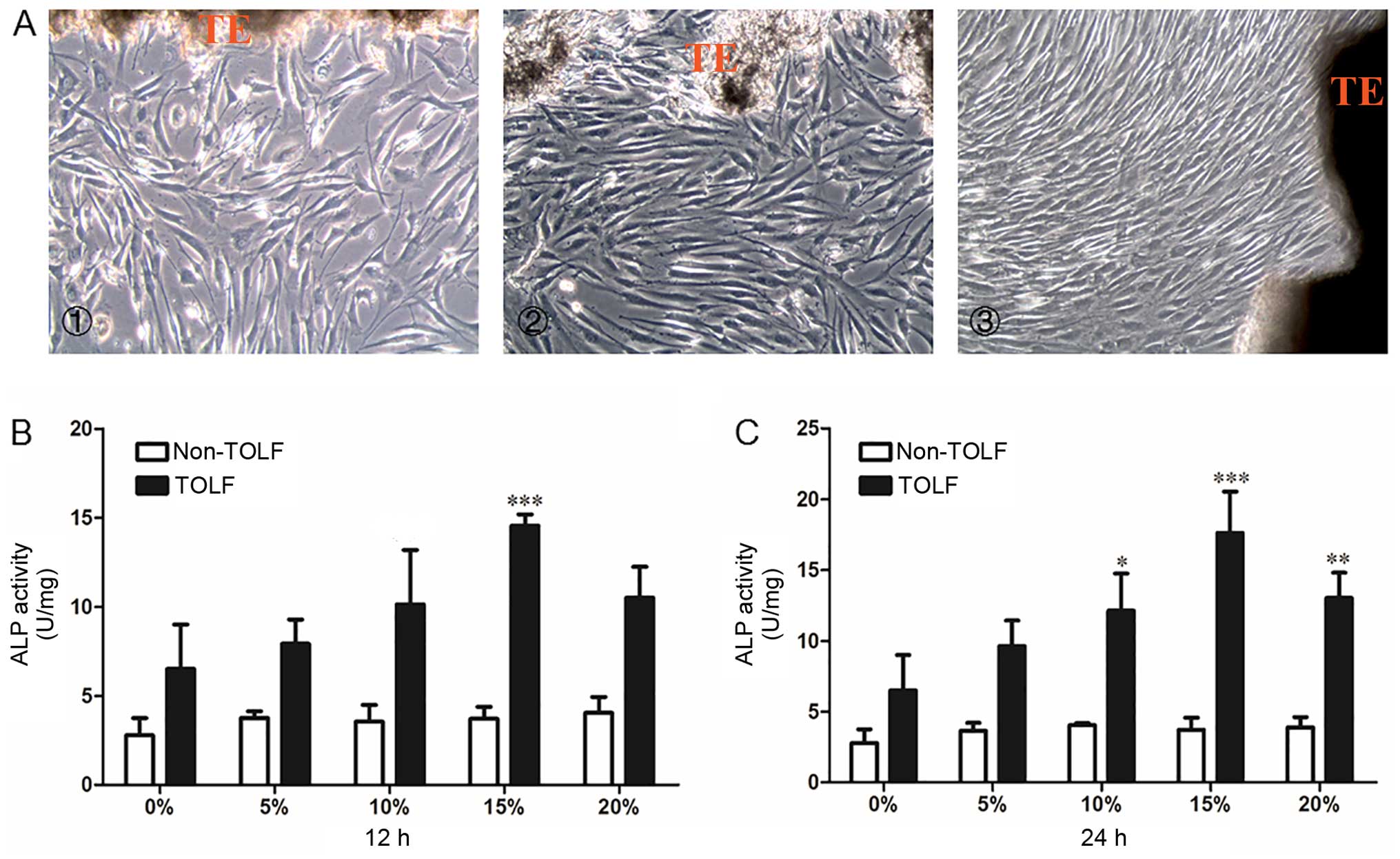 | Figure 3Morphology of primary ligament cells
and quantitative analysis of alkaline phosphatase (ALP) activity in
determination of optimal stress level. Morphology of primary
ligament cells. (A) Cell morphoology in the cells from patients in
the different groups: panel 1, non-TOLF gorup; panel 2,
single-level TOLF group; and panel 3, multiple-level TOLF goup
(×100 magnification). TE, tissue explant. (B and C) Quantitative
analysis of ALP activity in TOLF and non-TOLF groups under
different strengths of cyclic mechanical stress (0, 5, 10, 15 and
20%) for (B) 12 h and (C) 24 h (data are the means ± standard
deviation; n=3 patients/group). *P<0.05,
**P<0.01, ***P<0.001 vs. 0% in each
group. TOLF, thoracic ossification of ligament flavum. |
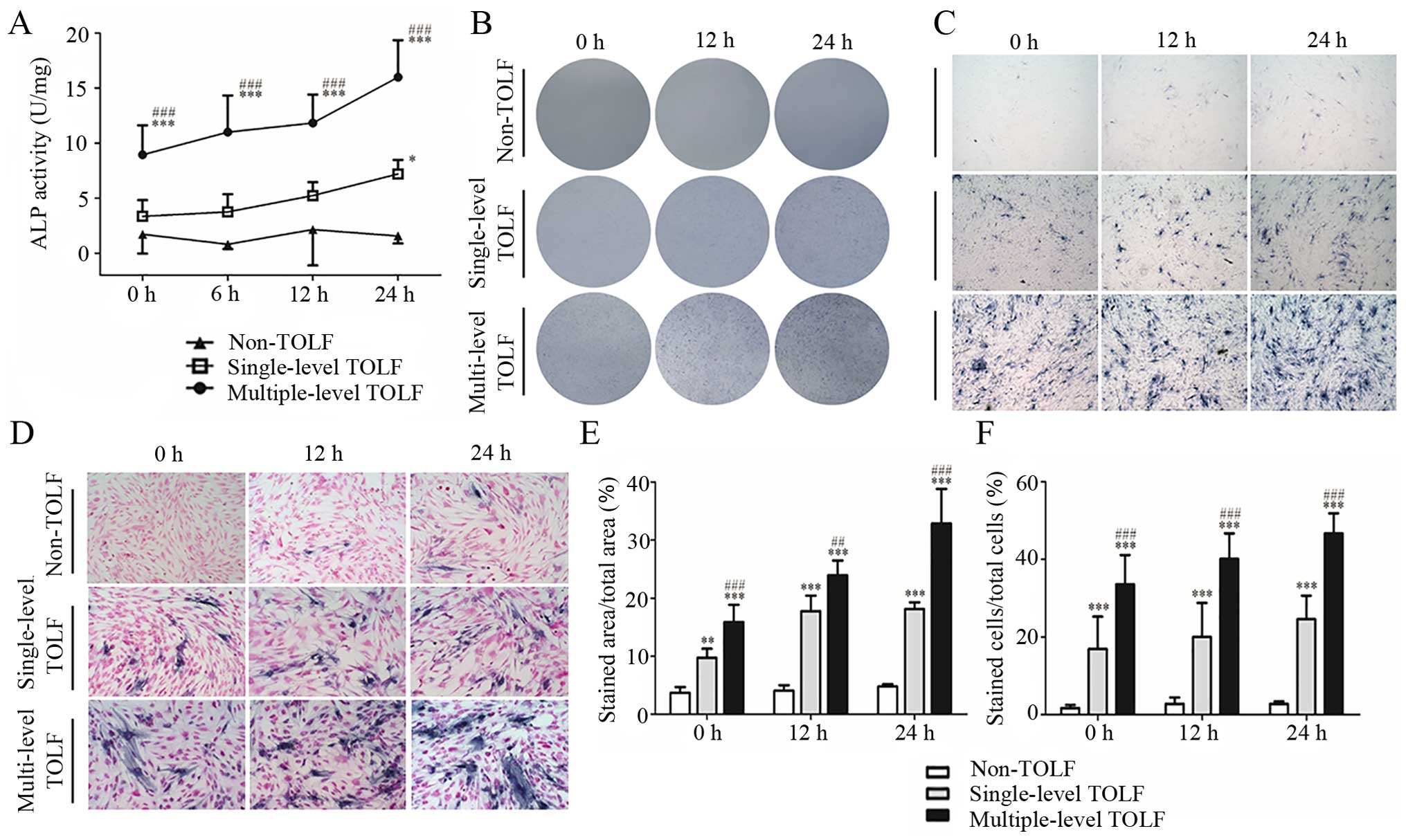
Changes in ALP activity induced by cyclic
mechanical stress
In the absence of cyclic mechanical stress, ALP
activity was higher in the multiple-level than in the single-level
TOLF and non-TOLF groups (P<0.001). The activity was slightly
higher in the single-level TOLF than in the non-TOLF group,
although the difference was not statistically significant. At
longer induction times, ALP activity increased in the 2 TOLF
groups, with a greater increase observed in the multiple-level
group (Fig. 4A).
There were almost no ALP-positive cells in the
non-TOLF group regardless of stress application. However, there
were many ALP-positive cells in the 2 TOLF groups even in the
absence of stress, and their numbers increased with the application
time, particularly in the multiple-level TOLF group (Fig. 4B–D). The semi-quantitative
analysis of the stained area and positive cells yielded similar
results (Fig. 4E and F).
Osteogenesis marker expression is
upregulated at the mRNA level by cyclic mechanical stress
The mRNA levels of osteogenesis markers, including
ALP, BMP2, osterix, Runx2, OCN and OPN were upregulated by cyclic
mechanical stress in the single- and multiple-level TOLF groups, as
determined by RT-qPCR. ALP expression was highest in the
multiple-level TOLF group, followed by the single-level and
non-TOLF groups at 0 h, and it increased over time in both TOLF
groups with the application of stress; at 24 h, the ALP expression
level was 2.1-fold higher in the multiple-level group than in the
single-level group (Fig. 5A).
Similar trends were observed for BMP2; at 24 h, the expression
level was 2.3-fold higher in the multiple-level group than in the
single-level group (Fig. 5B). The
mRNA expression of osterix was highest in the multiple-level TOLF
group at 0 h, followed by the single-level and non-TOLF groups,
although the differences between the groups were not statistically
significant. While the application of mechanical stress did not
affect the single-level group, the expression level increased in
the multiple-level TOLF group relative to the other 2 groups at 12
h, and was approximately 2.1-fold higher than that in the
single-level TOLF group at 24 h (Fig.
5C). In addition, Runx2 expression was higher in the TOLF
groups than in the non-TOLF group and it increased with stress
application; at 0 and 24 h, the Runx2 transcript level was higher
in the multiple-level group than in the single-level group
(Fig. 5D). Likewise, OCN and OPN
expression was higher in the TOLF groups than in the non-TOLF group
(Fig. 5E and F), and it was
increased by stress in the multiple-level TOLF group; OCN
expression was 1.8-fold higher in the multiple-level group than in
the single-level group at 24 h.
Pairwise comparisons among the 3 groups indicated
that the expression of ALP, BMP2, osterix and OPN was higher in the
multiple-level TOLF group than in the other 2 groups. OCN
expression was higher in the multiple-level TOLF group than in the
non-TOLF group, whereas there were no differences in Runx2
expression among the 3 groups. Moreover, the expression levels of
all markers were similar between single-level and non-TOLF groups
(Table III).
 | Table IIIVariance analysis of different curves
using ANOVA with Tukey's post-hoc test. |
Table III
Variance analysis of different curves
using ANOVA with Tukey's post-hoc test.
| Gene | Non-TOLF vs.
single-level TOLF groups | Non-TOLF vs.
multiple-level TOLF groups | Single-level TOLF
vs. multiple-level TOLF groups |
|---|
| ALP | ns | P<0.001 | P<0.01 |
| BMP-2 | ns | P<0.001 | P<0.001 |
| Osterix | ns | P<0.01 | P<0.01 |
| Runx2 | ns | ns | ns |
| OCN | ns | P<0.05 | ns |
| OPN | ns | P<0.001 | P<0.05 |
Osteogenic marker expression is
upregulated at the protein level by cyclic mechanical stress
The protein levels of osteogenic markers were
assessed by western blot analysis (Fig. 6A). ALP and osterix were more
highly expressed in the multiple-level TOLF group than in the other
2 groups, and their expression increased with the application of
mechanical stress to levels that were significantly higher at 12 h
(Fig. 6B and C). The expression
of both markers was higher in the single-level TOLF group than in
the non-TOLF group. The BMP2 and OPN levels were higher in the TOLF
groups than in the non-TOLF group. However, their levels in the
single-level TOLF group were not altered with stress application
(Fig. 6D and E).
Discussion
Many studies have demonstrated the critical role of
mechanical stress in the development of TOLF (7,15,22–25). We previously reported that
mechanical stress induced the osteogenic differentiation of
ligament cells derived from patients with TOLF, which in turn
promoted OLF, leading us to conclude that stress facilitated, but
did not initiate the development of TOLF (10). In the present study, we used
cyclic stress to induce osteogenesis in fibroblasts derived from
non-TOLF and single and multiple-level TOLF in order to assess
genetic differences in the osteogenic differentiation potency.
Although the morphology of ligament cells derived
from the 3 groups was similar, the cells differed in terms of
osteogenic potency, possibly due to cytogenetic differences.
Ligament cells from patients with TOLF already possess certain
osteoblast characteristics, including the upregulation of specific
markers, whereas cells from non-TOLF patients exhibit a fibroblast
phenotype (3,26–28). OCN and collagen type II are
expressed in cells derived from patients with OLF, but not cells
derived from non-OLF patients; cells derived from patients with OLF
would thus present an osteoblast and chondrocyte phenotype
(29). Another study reported
that stress induced the mineralization of cells from patients with
TOLF (10). These results
demonstrate that cells from patients with TOLF have a greater
potential for differentiation from fibroblasts to osteoblasts or
chondrocytes.
Clinically, OLF mainly occurs in the thoracic spine,
but rarely in the cervical and lumbar vertebrae that have a greater
range of motion, which possibly indicates that ligament cells from
patients with TOLF are sensitive to a certain range of cyclic
mechanical stress in osteogenic induction. In addition, the degree
of mechanical stress required for osteogenic induction has been
investigated in several studies (30–32). For example, an equi-biaxial
stretch chamber applying 9% mechanical stress was determined as
optimal for inducing the differentiation of cells from patients
with TOLF (10). On the other
hand, 20% stress has been applied to cells from patients with TOLF
and OPLL using the Flexcell FX-3000 and -4000 systems,
respectively, although these studies did not explore the optimal
range of mechanical stress (15,33). The differences between these
devices, as well as the reactivity of cells derived from different
tissues underscored the importance of exploring the optimal level
of mechanical stress in the present study. ALP is a sensitive and
specific indicator of the early stage of osteoblast differentiation
(34); we found that 15% stress
produced the highest ALP activity, indicating that this strength
effectively induced osteogenesis of ligament cells from patients
with TOLF. We also found that ALP activity was higher in the 2 TOLF
groups than in the non-TOLF group, with the highest activity
observed in the multiple-level group. Accordingly, a larger area of
positive ALP staining, corresponding to a higher osteogenic
potency, was observed in this group as compared to the single-level
TOLF group.
BMP2 is an osteogenic factor that has been
implicated in the ossification of the spinal ligament. BMP and its
receptors have been found to be widely expressed in OLF tissues,
but to be only partially expressed in the region connecting the
ligamentum flavum and vertebrae in non-OLF tissues (35), leading these investigators to
conclude that the aberrant expression of BMP and its receptors
contributes to OLF. Similarly, another study reported an abundance
of BMP2-positive fibroblasts distributed in areas of calcification
and ossification in TOLF (14).
In this study, we found that the BMP2 mRNA level was higher in the
multiple-level group than in the single-level and non-TOLF groups
at 0 h and that it increased with application of cyclic mechanical
stress. These results demonstrate that BMP2 plays an important role
in the osteogenic differentiation of cells from patients with TOLF
and that differences in expression may account for the distinct
prognoses of single- and multiple-level TOLF.
Runx2 is a transcription factor that regulates
osteoblast differentiation. In a previous study, Runx2 expression
was found to be higher in OLF than in the control and was mainly
expressed in hyperplastic and hypertrophic cartilage cells
(7). In another study, cyclic
tensile strain applied for 24 h increased Runx2 and OPN mRNA levels
in cells from OLF sections, whereas similar mechanical stress had
negligible effects on cells from non-OLF sections (15). In the present study, Runx2
expression was similar in the multiple- and single-level TOLF, and
higher than that in the non-TOLF group, indicating that Runx2
expression cannot be used as a marker for distinguishing the
osteogenic potency of single- and multiple-level TOLF.
Osterix is a transcription factor activated upon
mechanical stress that regulates the transcription of
osteogenesis-related genes. Our previous study showed that
mechanical stress induced osterix and Runx2 overexpression in TOLF
cells (10). It has also been
reported that osterix is more highly expressed in OLF- than in
non-OLF-derived cells and is mainly distributed in cartilage cells
located in the calcified cartilage and fibrocartilage layers
(7). Osterix has been shown to
promote osteoblast differentiation and mineralization at a
heterotopic site (36). In this
study, osterix expression was higher in the multiple-level group as
compared to the single-level TOLF group at 0 h and increased with
the application of mechanical stress in the former, but not in the
latter group, suggesting that osterix can serve as a marker for
multiple-level TOLF.
OCN is an extracellular matrix protein that reflects
the status of the process of osteogenesis (37). OPN is secreted as an adhesive
glycophosphoprotein and modulates matrix mineralization in
mechano-transduction (38).
Previous studies have reported that OCN and OPN expression increase
with the application of mechanical stress. This was confirmed by
the results presented in this study: at an early stage of
mechanical stress, the OCN and OPN levels were comparable in the 2
TOLF groups; however, over time, the levels were upregulated to a
greater degree in the multiple-level than in the singe-level group.
This indicates that the osteogenic differentiation of
multiple-level TOLF-derived cells is more active under the
application of cyclic mechanical stress.
Clinically, multiple-level TOLF differs from
single-level lesions in terms of disease progression and clinical
outcomes, and multiple-level TOLF has a poor prognosis and may
continue to develop after surgical resection (12,39,40), suggesting that the osteogenic
potency may be differ between them, which is consistent with our
experimental results. There were some limitations to this study.
Firstly, the mechanistic basis for differences in osteogenic
potency between multiple- and single-level TOLF requires more
detailed investigation, such as bioinformatics analysis of gene
expression profiles, and we aim to do this in future studies. In
addition, in the current study, we examined changes in osteogenic
potency and gene expression in cells from patients with TOLF in
response to mechanical stress in vitro, and did not consider
other types of stress that are present in vivo, including
compression, twisting and shear forces. Therefore, additional
studies using an in vivo TOLF model are required.
In conclusion, this study demonstrated that ligament
cells from patients with TOLF were sensitive to a certain range of
mechanical stress in osteogenic differentiation. Single- and
multiple-level TOLF differed in terms of osteogenic potency and
related gene expression under cyclic stress, with the latter
exhibiting a greater potency for osteogenic differentiation that
may be related to the different pathogenesis of them and contribute
to the different clinical outcome.
Abbreviations:
|
TOLF
|
thoracic ossification of ligamentum
flavum
|
|
ALP
|
alkaline phosphatase
|
|
BMP2
|
bone morphogenetic protein 2
|
|
OPN
|
osteopontin
|
|
OCN
|
osteocalcin
|
|
COL6A1
|
collagen type VI alpha1
|
|
HLA-DQA1
|
major histocompatibility complex,
class II, DQ α1
|
|
OPLL
|
ossification of posterior longitudinal
ligament
|
|
RIPA
|
radio-immunoprecipitation
|
|
BCA
|
bicinchoninic acid
|
|
Ct
|
cycle threshold
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
ANOVA
|
one-way analysis of variance
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81272031, 81071505 and
81572101). We acknowledge the assistance of Peking University Third
Hospital Central Laboratory with the technical guidance.
References
|
1
|
Sato T, Kokubun S, Tanaka Y and Ishii Y:
Thoracic myelopathy in the Japanese: Epidemiological and clinical
observations on the cases in Miyagi Prefecture. Tohoku J Exp Med.
184:1–11. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo JJ, Luk KD, Karppinen J, Yang H and
Cheung KM: Prevalence, distribution, and morphology of ossification
of the ligamentum flavum: A population study of one thousand seven
hundred thirty-six magnetic resonance imaging scans. Spine.
35:51–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kudo S, Ono M and Russell WJ: Ossification
of thoracic ligamenta flava. AJR Am J Roentgenol. 141:117–121.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lang N, Yuan HS, Wang HL, Liao J, Li M,
Guo FX, Shi S and Chen ZQ: Epidemiological survey of ossification
of the ligamentum flavum in thoracic spine: CT imaging observation
of 993 cases. Eur Spine J. 22:857–862. 2013. View Article : Google Scholar :
|
|
5
|
Mobbs RJ and Dvorak M: Ossification of the
ligamentum flavum: diet and genetics. J Clin Neurosci. 14:703–705.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan D, Chen Z, Chen Y and Shang Y:
Mechanistic roles of leptin in osteogenic stimulation in thoracic
ligament flavum cells. J Biol Chem. 282:29958–29966. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uchida K, Yayama T, Cai HX, Nakajima H,
Sugita D, Guerrero AR, Kobayashi S, Yoshida A, Chen KB and Baba H:
Ossification process involving the human thoracic ligamentum
flavum: Role of transcription factors. Arthritis Res Ther.
13:R1442011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okada K, Oka S, Tohge K, Ono K, Yonenobu K
and Hosoya T: Thoracic myelopathy caused by ossification of the
ligamentum flavum. Clinicopathologic study and surgical treatment.
Spine. 16:280–287. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maigne JY, Ayral X and Guérin-Surville H:
Frequency and size of ossifications in the caudal attachments of
the ligamentum flavum of the thoracic spine. Role of rotatory
strains in their development. An anatomic study of 121 spines. Surg
Radiol Anat. 14:119–124. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan D, Chen Z, Wang D, Guo Z, Qiang Q and
Shang Y: Osterix is a key target for mechanical signals in human
thoracic ligament flavum cells. J Cell Physiol. 211:577–584. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao R, Yuan W, Yang L, Shi G and Jia L:
Clinical features and surgical outcomes of patients with thoracic
myelopathy caused by multilevel ossification of the ligamentum
flavum. Spine J. 13:1032–1038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawaguchi Y, Yasuda T, Seki S, Nakano M,
Kanamori M, Sumi S and Kimura T: Variables affecting postsurgical
prognosis of thoracic myelopathy caused by ossification of the
ligamentum flavum. Spine J. 13:1095–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li F, Chen Q and Xu K: Surgical treatment
of 40 patients with thoracic ossification of the ligamentum flavum.
J Neurosurg Spine. 4:191–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yayama T, Uchida K, Kobayashi S, Kokubo Y,
Sato R, Nakajima H, Takamura T, Bangirana A, Itoh H and Baba H:
Thoracic ossification of the human ligamentum flavum:
Histopathological and immunohistochemical findings around the
ossified lesion. J Neurosurg Spine. 7:184–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai HX, Yayama T, Uchida K, Nakajima H,
Sugita D, Guerrero AR, Yoshida A and Baba H: Cyclic tensile strain
facilitates the ossification of ligamentum flavum through β-catenin
signaling pathway: In vitro analysis. Spine. 37:E639–E646. 2012.
View Article : Google Scholar
|
|
16
|
Kim HN, Min WK, Jeong JH, Kim SG, Kim JR,
Kim SY, Choi JY and Park BC: Combination of Runx2 and BMP2
increases conversion of human ligamentum flavum cells into
osteoblastic cells. BMB Rep. 44:446–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong Q, Ma X, Li F, Guo Z, Qi Q, Li W,
Yuan H, Wang Z and Chen Z: COL6A1 polymorphisms associated with
ossification of the ligamentum flavum and ossification of the
posterior longitudinal ligament. Spine. 32:2834–2838. 2007.
View Article : Google Scholar
|
|
18
|
Liu Y, Zhao Y, Chen Y, Shi G and Yuan W:
RUNX2 polymorphisms associated with OPLL and OLF in the Han
population. Clin Orthop Relat Res. 468:3333–3341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kudo H, Furukawa K, Yokoyama T, Ono A,
Numasawa T, Wada K, Tanaka S, Asari T, Ueyama K, Motomura S and Toh
S: Genetic differences in the osteogenic differentiation potency
according to the classification of ossification of the posterior
longitudinal ligament of the cervical spine. Spine. 36:951–957.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen ZQ, Dang GT, Liu XG and Cai QL: The
choice of treatment for ossification of ligamentum flavum of the
thoracic spine. Chin J Orthop. 19:197–200. 1999.In Chinese.
|
|
21
|
Yin X, Chen Z, Guo Z, Liu X and Yu H:
Tissue transglutaminase expression and activity in human ligamentum
flavum cells derived from thoracic ossification of ligamentum
flavum. Spine. 35:E1018–E1024. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahn DK, Lee S, Moon SH, Boo KH, Chang BK
and Lee JI: Ossification of the ligamentum flavum. Asian Spine J.
8:89–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsukamoto N, Maeda T, Miura H, Jingushi S,
Hosokawa A, Harimaya K, Higaki H, Kurata K and Iwamoto Y:
Repetitive tensile stress to rat caudal vertebrae inducing
cartilage formation in the spinal ligaments: A possible role of
mechanical stress in the development of ossification of the spinal
ligaments. J Neurosurg Spine. 5:234–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwasawa T, Iwasaki K, Sawada T, Okada A,
Ueyama K, Motomura S, Harata S, Inoue I, Toh S and Furukawa KI:
Pathophysiological role of endothelin in ectopic ossification of
human spinal ligaments induced by mechanical stress. Calcif Tissue
Int. 79:422–430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakatani T, Marui T, Hitora T, Doita M,
Nishida K and Kurosaka M: Mechanical stretching force promotes
collagen synthesis by cultured cells from human ligamentum flavum
via transforming growth factor-beta1. J Orthop Res. 20:1380–1386.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoshi K, Amizuka N, Sakou T, Kurokawa T
and Ozawa H: Fibroblasts of spinal ligaments pathologically
differentiate into chondrocytes induced by recombinant human bone
morphogenetic protein-2: Morphological examinations for
ossification of spinal ligaments. Bone. 21:155–162. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwasaki K, Furukawa KI, Tanno M, Kusumi T,
Ueyama K, Tanaka M, Kudo H, Toh S, Harata S and Motomura S:
Uni-axial cyclic stretch induces Cbfa1 expression in spinal
ligament cells derived from patients with ossification of the
posterior longitudinal ligament. Calcif Tissue Int. 74:448–457.
2004. View Article : Google Scholar
|
|
28
|
Ishida Y and Kawai S: Characterization of
cultured cells derived from ossification of the posterior
longitudinal ligament of the spine. Bone. 14:85–91. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong ZM and Chen JT: Phenotypic
characterization of ligamentum flavum cells from patients with
ossification of ligamentum flavum. Yonsei Med J. 50:375–379. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanno M, Furukawa KI, Ueyama K, Harata S
and Motomura S: Uniaxial cyclic stretch induces osteogenic
differentiation and synthesis of bone morphogenetic proteins of
spinal ligament cells derived from patients with ossification of
the posterior longitudinal ligaments. Bone. 33:475–484. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohishi H, Furukawa K, Iwasaki K, Ueyama K,
Okada A, Motomura S, Harata S and Toh S: Role of prostaglandin I2
in the gene expression induced by mechanical stress in spinal
ligament cells derived from patients with ossification of the
posterior longitudinal ligament. J Pharmacol Exp Ther. 305:818–824.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Furukawa K: Current topics in
pharmacological research on bone metabolism: Molecular basis of
ectopic bone formation induced by mechanical stress. J Pharmacol
Sci. 100:201–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, Wei P, Chen Y, Yang L, Jiang C,
Jiang P and Chen D: Down-regulated expression of vimentin induced
by mechanical stress in fibroblasts derived from patients with
ossification of the posterior longitudinal ligament. Eur Spine J.
23:2410–2415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pagani F, Francucci CM and Moro L: Markers
of bone turnover: Biochemical and clinical perspectives. J
Endocrinol Invest. 28(Suppl 10): 8–13. 2005.
|
|
35
|
Hayashi K, Ishidou Y, Yonemori K, Nagamine
T, Origuchi N, Maeda S, Imamura T, Kato M, Yoshida H, Sampath TK,
et al: Expression and localization of bone morphogenetic proteins
(BMPs) and BMP receptors in ossification of the ligamentum flavum.
Bone. 21:23–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fu H, Doll B, McNelis T and Hollinger JO:
Osteoblast differentiation in vitro and in vivo promoted by
Osterix. J Biomed Mater Res A. 83:770–778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Komaki M, Karakida T, Abe M, Oida S,
Mimori K, Iwasaki K, Noguchi K, Oda S and Ishikawa I: Twist
negatively regulates osteoblastic differentiation in human
periodontal ligament cells. J Cell Biochem. 100:303–314. 2007.
View Article : Google Scholar
|
|
38
|
Ishijima M, Tsuji K, Rittling SR,
Yamashita T, Kurosawa H, Denhardt DT, Nifuji A, Ezura Y and Noda M:
Osteopontin is required for mechanical stress-dependent signals to
bone marrow cells. J Endocrinol. 193:235–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Inamasu J and Guiot BH: A review of
factors predictive of surgical outcome for ossification of the
ligamentum flavum of the thoracic spine. J Neurosurg Spine.
5:133–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He S, Hussain N, Li S and Hou T: Clinical
and prognostic analysis of ossified ligamentum flavum in a Chinese
population. J Neurosurg Spine. 3:348–354. 2005. View Article : Google Scholar : PubMed/NCBI
|