Parkinson's disease (PD) is characterized by
neuropsychiatric symptoms such as depression and anxiety preceding
the onset of motor symptoms (1).
Major features of PD include the loss of dopaminergic neurons in
the substantia nigra and Lewy body depositions (2). It has been suggested that
mitochondrial dysfunction, oxidative stress and oxidative damage
underlie the pathogenesis of PD (3). Activity of substantia nigra
dopaminergic neurons is critical for striatal synaptic plasticity
and associative learning. The degeneration of dopaminergic neurons
leads to a disinhibition of the subthalamic nucleus thus increasing
excitatory projections to the substantia nigra. In consequence,
excessive activity causes excitotoxicity and oxidative stress
(3,4). Consequently, intracellular
accumulation of filamentous α-synuclein (α-syn) aggregates to form
Lewy bodies, a pathologic hallmark of PD (4). Lewy body disease is also a group of
neurodegenerative disorders characterized by α-syn accumulation
that includes Lewy body dementia and PD symptoms (5). Genetic defects, aging, and
environmental toxicants have been recognized as risk factors for
the development of these diseases. Although the pathogenesis is
still unclear, evidence suggests that oxidative stress plays a
central role in progession of the disease. In particular, reactive
oxygen species (ROS) may play an important role in inflammatory
processes (6). Cellular ROS
metabolism is definitely regulated by a variety of proteins
involved in the redox mechanism with the
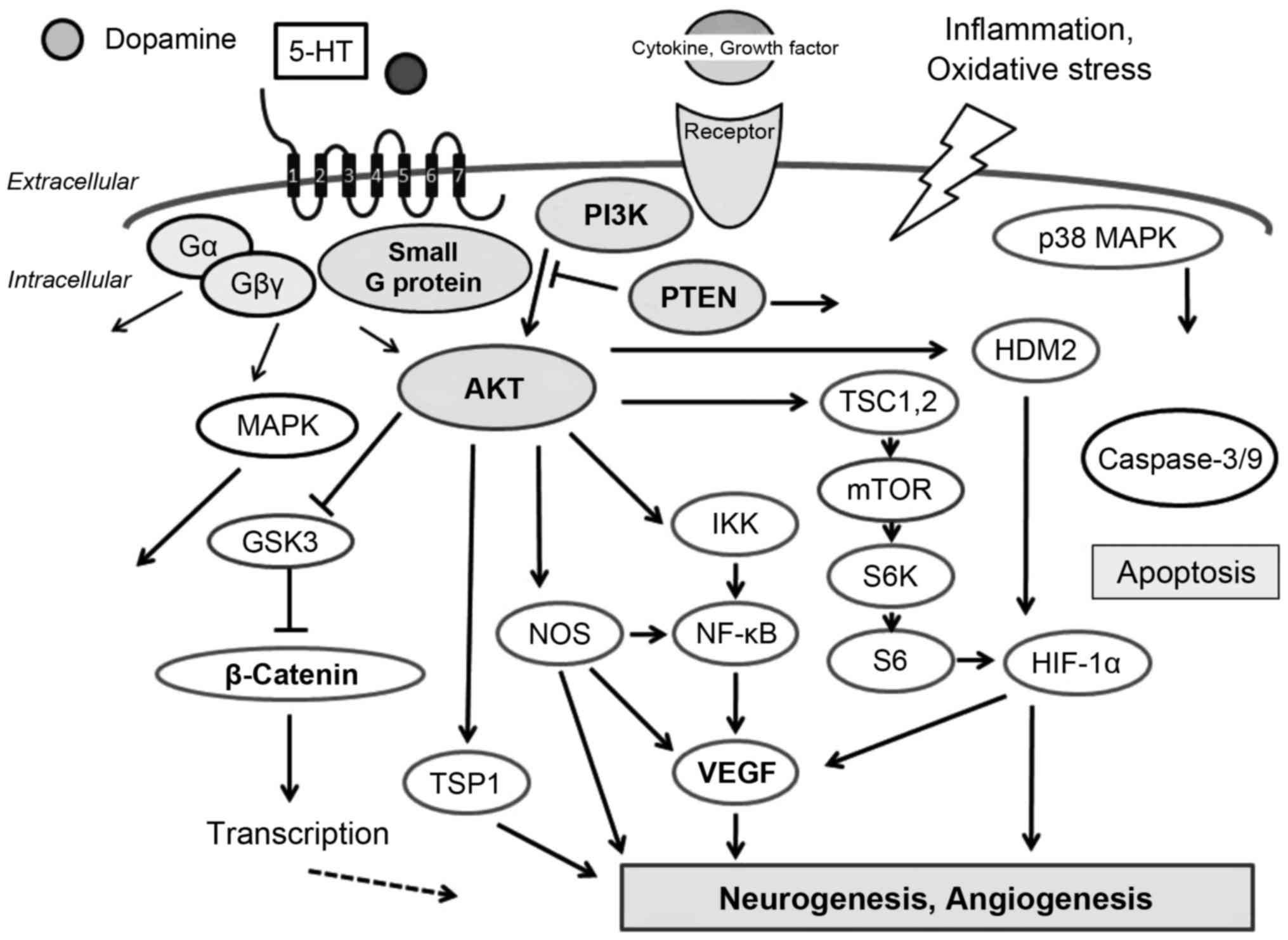
phosphatidyl-inositol-3-kinase (PI3K)/AKT signaling pathway
(7) (Fig. 1). Accordingly, the PI3K/AKT
pathway acts as a pivotal determinant of cell fate regarding
senescence and apoptosis, which is mediated by intracellular ROS
generation (7). In addition, ROS
activate PI3K/AKT and inactivate phosphatase and tensin homologue
deleted on chromosome ten (PTEN) (8,9).
High concentrations of ROS may induce cellular damage. However at
lower concentrations ROS may act as intracellular secondary
messengers. An excess amount of oxidative stress can lead to
crushing consequences in the nervous system during aging.
Therefore, both acute and chronic neurodegenerative disorders could
be mainly a result of oxidative stress (10). ROS regulation and inhibition of
the apoptotic pathway thereby protecting cells have been shown to
be controlled by the PI3K/AKT signaling pathway (11). The mechanism involved in PI3K/AKT
activation exhibits stimuli-specific variations.
G protein-coupled receptors (GPCRs) are a large
class of molecules involved in signal transduction across cell
membranes, which are the most common targets of
neuro-pharmacological drugs in the central nervous system (12,13). Stimulation of GPCRs leads to
activation of heterotrimeric G proteins and their intracellular
signaling pathways. In addition to the signaling via heterotrimeric
G proteins, GPCRs can also signal by interacting with various small
G proteins to regulate downstream effector pathways (14). Some small G proteins can associate
directly with GPCRs, and often modulate the GPCR signaling network.
It is becoming clear that these GPCRs are not only activated by
authentic agonists but that they also exhibit agonist-independent
intrinsic activity. In addition, a hallmark of GPCRs is their
ability to recognize and respond to chemically diverse ligands,
which efficiently activate PI3K/AKT signaling in numerous cell
types (Fig. 1). As mentioned
above, the PI3K/AKT signaling pathway transduces a signal
regulating a wide range of events involved in cell survival and
metabolism. Defective regulation of the PI3K/AKT pathway has been
linked to several diseases including cancer, diabetes,
atherosclerosis and neurodegenerative diseases (15,16) (Fig.
1). Knowledge concerning the interplay between GPCRs and
PI3K/AKT may contribute to improved treatment and prevention of
these diseases. However, regulation of the interplay appears to be
complex. Some PI3Ks can be activated by binding of the regulatory
subunit to specific tyrosine-phosphorylated domains in cell surface
receptors. In addition, Ras family proteins are important direct
activators of PI3Ks, interacting via an amino-terminal Ras-binding
domain (RBD) (17,18). Different PI3Ks could also be
activated in a receptor-specific manner and by distinct GTPases of
the Ras and Rho families (19).
This review summarizes current understanding of therapeutic GPCRs
and PI3K/AKT signaling for neurodegenerative diseases such as PD.
We also address the behavioral relevance of GPCRs and PI3K/AKT
signaling in PD.
GPCRs are integral membrane proteins that regulate
intracellular secondary messenger levels via the coupling of
activation by extracellular stimuli. For example, activation of
GPCRs starts a series of molecular events leading to GPCR
kinase-mediated receptor phosphorylation (20). In addition, GPCRs can stimulate
Ras and activate class I PI3Ks depending on RasGEF and RasGRP4
(21). Several effector molecules
for the small GTPases support cancer cell migration and invasion by
regulating the PI3K/AKT signaling pathway (22). Furthermore, it has been shown that
Rit and Rin subfamily Ras-related small GTPases are associated with
neuronal disorders such as PD (23). Members of the Rho GTPase family
have important roles in regulating several aspects of
cytoskeleton-based functions, including cell migration,
proliferation, and apoptosis. The Rho-associated coiled-coil
containing protein kinase (ROCK) is a serine/threonine kinase and a
major downstream effector of Rho GTPases (24). ROCK enhances actin/myosin
contraction (25). Furthermore,
ROCK activates caspase-dependent apoptosis signaling cascades
(26). PTEN has been identified
as a ROCK substrate that is also involved in cell death and
survival (27,28). ROCK phosphorylates PTEN and
stimulates its phosphatase activity. PTEN is a negative regulator
of the PI3K/AKT pathway by dephosphorylating the inositol
3′-phosphate group, which has important roles in cell survival and
apoptosis (Fig. 2). PTEN
decreases the AKT phosphorylation levels induced by ROCK
activation. Accordingly, ROCK appears to be involved in regulation
of PI3K/AKT signaling. Hence, inhibition of ROCK activation
attenuates apoptosis (29).
Furthermore, the Rho/ROCK/PTEN pathway may be a key regulatory step
in cell transformation, and thus plays an essential role in
Ras-induced tumorigenesis (30).
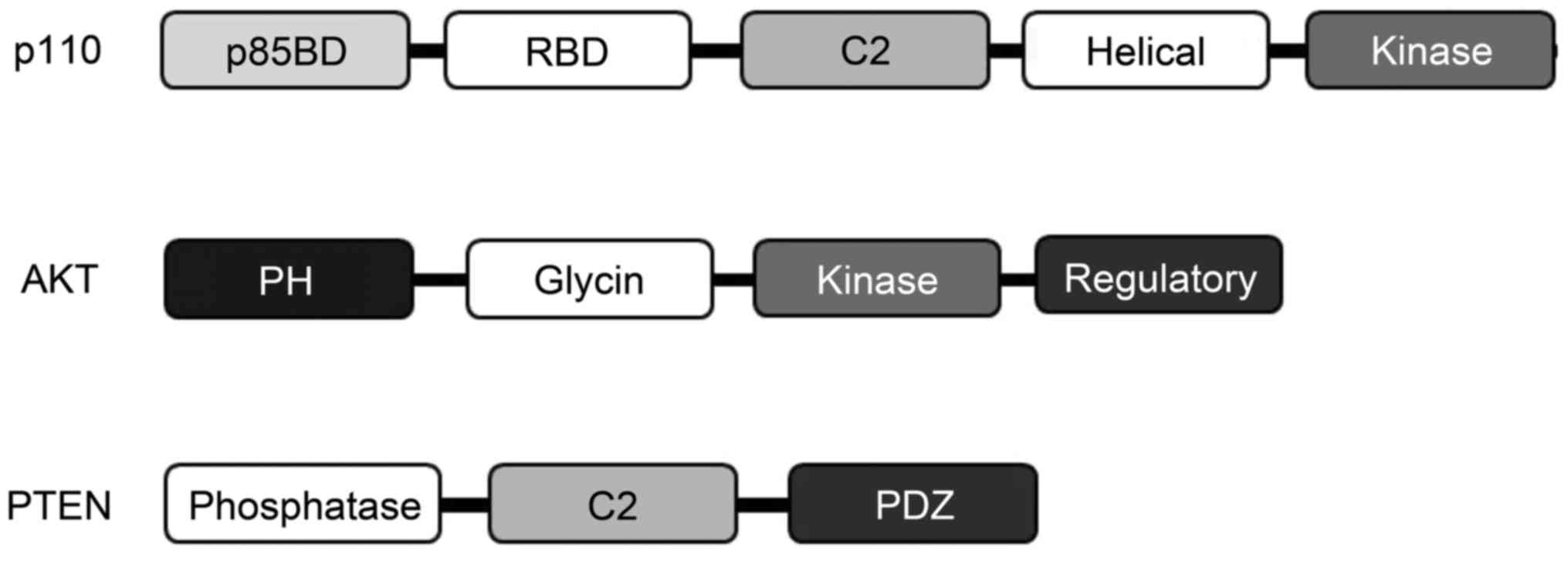
In mammals, four isoforms of the type I PI3K catalytic p110
subunits have been identified (Fig.
2). Activated Ras molecules bind directly to an N-terminal RBD
on p110 to appropriately activate lipid kinase activity of PI3K
following AKT activation (31).
There are three known AKT isoforms which play critical and diverse
roles in cells. A type of GPCR agonist could trigger the
pro-survival AKT signaling pathway and protect neurons (32) (Fig.
2). Notably, a novel role for AKT has been found in maintaining
neuronal serotonin (5-HT) receptor function (33). In addition, 5-HT activates the
PI3K/AKT signaling pathway in several cancer cell lines (34). Growing evidence suggests their
possible roles in the pathogenesis and treatment of PD (35,36). The serotonergic system may play a
significant role in the pathogenesis of PD.
Human 5-HT receptor is a seven-transmembrane-domain
GPCR, which activates adenylyl cyclase constitutively upon agonist
activation (37). A
pharmacological model for GPCR activation is the ternary complex
model in which GPCR exists in an equilibrium of dynamic
conformational states (38).
Through the GPCR, 5-HT activates the PI3K/AKT and MAPK signaling
pathways (34), which is an
important intermediate signaling process in the behavioral
functions of 5-HT receptors (39)
(Fig. 1). 5-HT also functions as
an angiokine to promote angiogenesis (40). In endothelial cells, 5-HT also
activates PI3K/AKT signaling via GPCRs similar to that observed
with VEGF (40). It has been
apparent that the interaction of 5-HT and dopamine plays a key role
in the behavior. 5-HT and dopamine levels decrease with age
(41). In addition, 5-HT has been
postulated as a key neuromodulator and neurotransmitter involved in
aggression and stress. 5-HT receptors may control dopaminergic
neuron activity in a region-dependent manner. Thus, alterations in
5-HT release and a loss of serotonergic neurons may be linked to PD
symptoms. Recent studies are focusing on agents involving
neurotransmitters including 5-HT receptors. In addition, among a
variety of proteins included in the GPCR family, serotonin 5-HT
receptors are attractive as important biological targets of PD
(42). It has been shown that the
role of small GTPases of the Rho family in morphogenic signaling
linked to 5-HT in neurons may control neuronal morphology and
motility (43). 5-HT receptors
are widely distributed in the central nervous system, especially in
the brain region and are essential for learning and cognition
(44). Among them, the basal
ganglia are an extremely organized network of subcortical nuclei
including the striatum and substantia nigra, which play a key role
in many functions such as emotion, cognition, and motor control.
These regions are critically involved in neurodegenerative diseases
including PD and Lewy body disease (45,46). Serotonergic neurons of the dorsal
raphe nucleus are excited at a steady rate during waking (47). Certain hallucinogens,
antipsychotics, and antidepressants function by targeting the 5-HT
receptor in addition to endogenous 5-HT. Through its traditional
activity as a GPCR and ligand-gated ion channel, the
neurotransmitter 5-HT has a complicated function in the modulation
of brain information processing. In addition, it can be speculated
that local microinjection of 5-HT would affect activity of the
corresponding neurons (48). 5-HT
can also exert intricate effects on the activity of midbrain
dopaminergic neurons mediated by its various receptor subtypes.
Dopamine-containing neurons in the brain receive an obvious
innervation from 5-HT originating in the raphe nuclei of the
brainstem (49). Therefore, the
significant role of 5-HT in central dopamine dysfunction has been
shown (50). Principal control of
the interaction between 5-HT and dopamine-containing neurons in the
brain appears to be mutually inhibitory. When dopamine innervation
in the striatum is critically low, the serotonergic system plays an
important role in the development of idiopathic PD (51). Patients with PD frequently develop
dementia, which is associated with neocortical deposition of α-syn
in Lewy bodies and Lewy neurites (52). Widespread deficits in serotonergic
and dopamine innervation of neocortical and basal ganglia regions
have been demonstrated in advanced PD (53). 5-HT has major roles in brain
diseases involving abnormal mood and cognition. Studies show that
5-HT receptor-antagonists have antipsychotic and antidepressant
properties, whereas agonist ligands possess cognition-enhancing and
hallucinogenic properties. In addition, the effects of a rapid
reduction in 5-HT function have shown a reduction in cognitive
status in dementia with Lewy bodies (54). Consequently, antidepressants may
be useful in treating depression in PD (55).
Therapeutic neuroprotective agents are currently
receiving increased attention for the treatment of PD patients
(32). For example, regrowth of
axons within the adult nigrostriatal projection which is
prominently affected in PD can be achieved by activation of
PI3K/AKT signaling (56). In an
attractive therapeutic approach, a GPCR and its agonist could
trigger the pro-survival PI3K/AKT signaling pathway and protect
neurons in in vivo and in vitro models against
neuronal toxicity. Hence, treatment with an AKT inhibitor was found
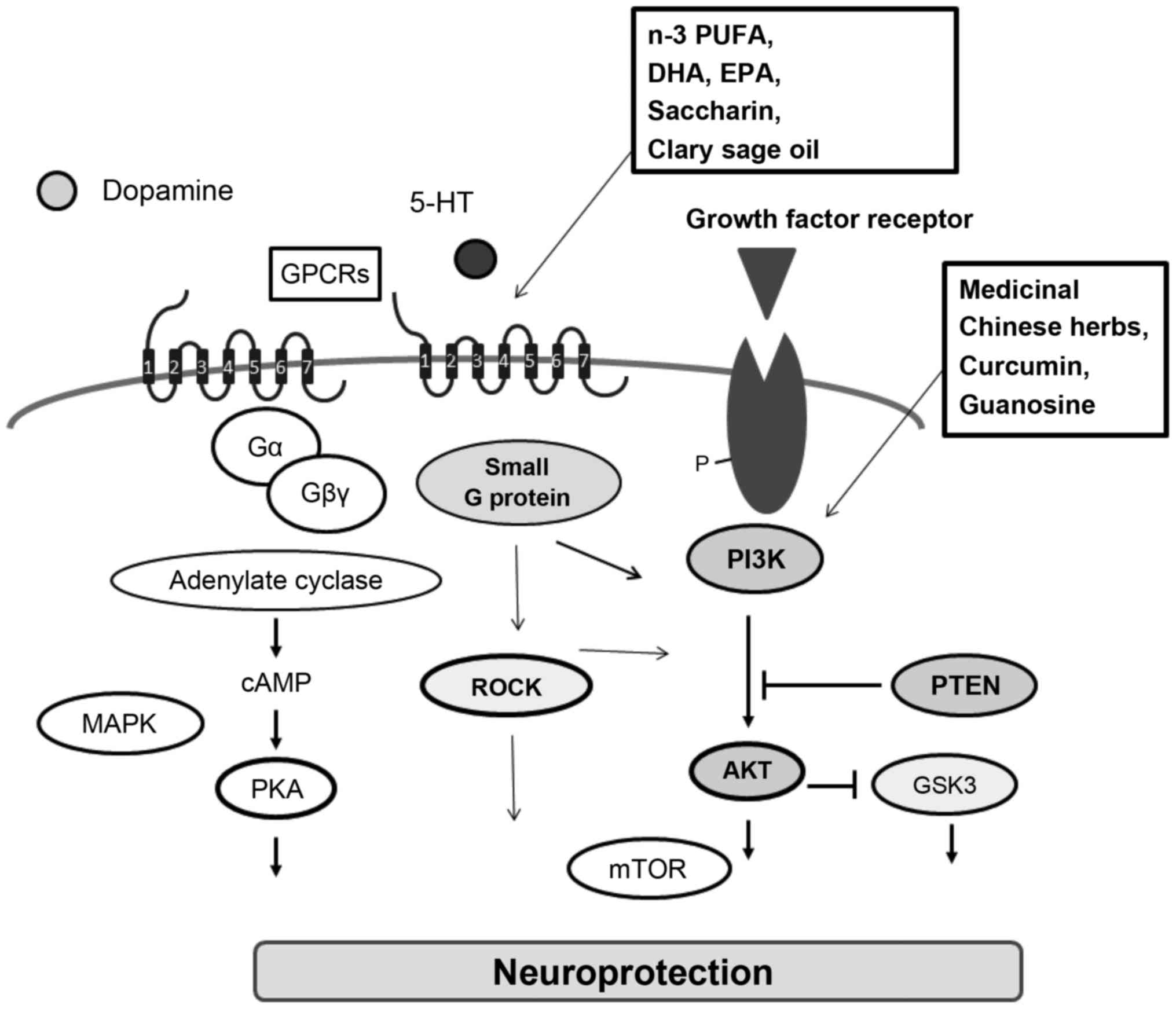
to block the neuroprotective effect (57). Medicinal Chinese herbs and its
active ingredients may play various neuroprotective roles,
including antioxidant, radical-scavenging, anti-inflammatory, and
antiapoptotic activity (Fig. 3).
For example, curcumin, which is a major active polyphenol component
extracted from the rhizomes of Curcuma longa, has been
reported to exert neuroprotective effects in an experimental model
of PD (58). Curcumin ameliorates
dopamine neuronal oxidative damage via activation of the PI3K/AKT
pathway (58) (Fig. 3). The effects of curcumin may also
be related to increased expression of PTEN (59). In addition, curcumin similarly
protects cardiomyocytes against high glucose-induced apoptosis via
the PI3K/AKT signaling pathway (60). Danshensu, a main hydrophilic
component of the Chinese Materia Medica Radix Salviae
Miltiorrhizae, has ROS scavenging and antioxidant activities via
activation of the PI3K/Akt signaling pathway (61). Puerarin, an active component of
Pueraria montana var. lobata is well-known for its
anti-oxidative and neuroprotective activities via modulation of the
PI3K/AKT pathway (62). In
addition, a novel synthetic squamosamide derivative from a Chinese
herb has been shown to have neuroprotective effects by activating
the PI3K/AKT signaling pathway in experimental PD models (63). Eucommia ulmoides Oliv. bark
attenuates oxidative stress through activation of PI3K/AKT, thereby
protecting cells from neuronal cell death (64). Tyrosol exerted a neuroprotective
effect via activation of the PI3K/AKT signaling pathway in a model
of PD (65). A series of oxicam
non-steroidal anti-inflammatory drugs have been shown to be
neuroprotective via activation of the PI3K/AKT signaling pathway
(66).
N-acetyl-5-hydroxytryptamines may also attenuate oxidative
cytotoxicity via activation of PI3K/AKT-dependent signaling
(67). Furthermore, previous
studies have shown the neuroprotective effects of
pramipexole-induced hypothermia via the PI3K/AKT signaling pathway
(68). Drynaria fortunei,
a Polydiaceae plant, exerts its cell protective effects via the
PI3K/AKT pathway (69). IGF-1 was
found to protect the nigrostriatal pathway in a progressive PD
model (70). This protection may
be preceded by activation of the pro-survival PI3K/AKT signaling
cascades. Guanosine was found to protect glial cells via the
PI3K/AKT signaling pathway (71).
In contrast, gallic acid, a polyphenol found in numerous fruits and
vegetables particularly in hickory nuts, downregulates AKT
phosphorylation but promotes PTEN expression (71).
Estradiol was previously found to have an
antidepressant-like effect. The antidepressant-like effect of
estradiol is due to estrogen receptor (ER)β activation, whereas
blockade of the effect of an SSRI by estradiol was mediated by ERα.
Estradiol shows a potential slowing of 5-HT clearance mediated by
ERβ (83). Maintaining a level of
endogenous estrogen in females may prevent women from developing PD
(84). Tocotrienols, members of
the vitamin E family, have antioxidant properties. Tocotrienols are
favorable candidates for neuroprotection in the pathogenesis of PD,
and exhibit not only antioxidant properties but also
signal-mediated action following ERβ/PI3K/AKT signaling (85). Related activation of ERβ may
reduce the progression of PD by precluding α-syn accumulation
(86). The α-syn, an
intrinsically disordered presynaptic 14 kDa protein whose
fibrillation is a critical step in the pathogenesis of PD, affects
serotonergic neuronal projections within the hippocampus (87). Inhibition of α-syn fibrillation is
brought about by a polyphenolic acid known as caffeic acid in a
dose-dependent manner (88).
Blocking PI3K/AKT signaling prevents the expression of α-syn and
attenuates neuroprotection (63).
The inhibitory activity of caffeic acid against α-syn fibrillation
may guide in the planning of novel therapeutic treatments for
PD.
Environmental exposures to toxic mediators such as
ROS may lead to neurodegenerative disorders that have shared
clinical findings with PD. It is critical to develop strategies to
ensure that healthy neurons remain alive following ROS attack
without using intricate medications. The precise identity and
functional prototypes of molecular intermediates leading to
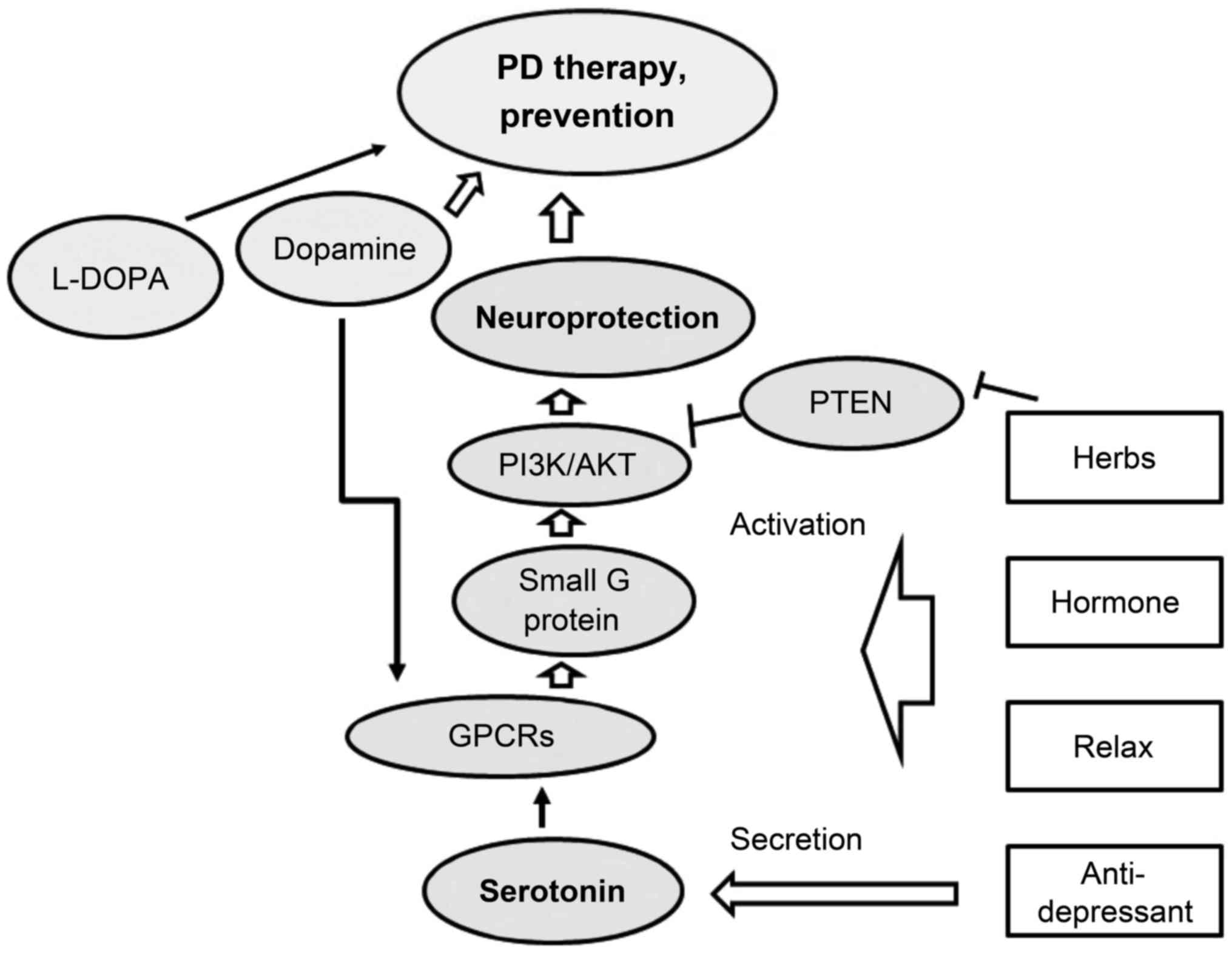
neuronal mortality remain to be deciphered. Recently, traditional
Chinese medicinal herbs have become popular as new approaches for
the prevention and treatment of PD and/or other neurodegenerative
diseases (Fig. 4). Functioning of
the PI3K/AKT pathway may ensure that neuro-defense is active in
order to render neuroprotection by preventing apoptosis and
neuro-inflammation. Herbs may facilitate the above process. In
addition, the recent development of selective ligands for 5-HT
receptors will not only allow a detailed understanding of this
protection but will lead to the development of new treatment
strategies, appropriate for neurodegenerative disorders including
PD. However, any therapeutic approach that limits itself to drugs
against a single pathological process is invalid. Accordingly,
combinations with various pharmacological properties are likely to
be more effective. We believe that increased knowledge of the
molecular details of the nature of the GPCR/PI3K/AKT signaling
interaction may lead to better insight into the overall
understanding of the function of GPCRs in neurodegenerative
disease. Future studies should focus on the availability of novel
treatments to improve the therapeutic efficacy in this field.
The present study was supported by JSPS KAKENHI
(grant nos. 24240098 and 26-12035).
|
1
|
Armento ME, Stanley MA, Marsh L, Kunik ME,
York MK, Bush AL and Calleo JS: Cognitive behavioral therapy for
depression and anxiety in Parkinson's disease: A clinical review. J
Parkinsons Dis. 2:135–151. 2012.PubMed/NCBI
|
|
2
|
Jagmag SA, Tripathi N, Shukla SD, Maiti S
and Khurana S: Evaluation of models of Parkinson's disease. Front
Neurosci. 9:5032016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo Y, Hoffer A, Hoffer B and Qi X:
Mitochondria: A therapeutic target for Parkinson's disease? Int J
Mol Sci. 16:20704–20730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giráldez-Pérez R, Antolín-Vallespín M,
Muñoz M and Sánchez-Capelo A: Models of α-synuclein aggregation in
Parkinson's disease. Acta Neuropathol Commun. 2:1762014. View Article : Google Scholar
|
|
5
|
Kim WS, Kågedal K and Halliday GM:
Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Ther.
6:732014. View Article : Google Scholar
|
|
6
|
Tokuhira N, Kitagishi Y, Suzuki M, Minami
A, Nakanishi A, Ono Y, Kobayashi K, Matsuda S and Ogura Y:
PI3K/AKT/PTEN pathway as a target for Crohn's disease therapy
(Review). Int J Mol Med. 35:10–16. 2015.
|
|
7
|
Nakanishi A, Wada Y, Kitagishi Y and
Matsuda S: Link between PI3K/AKT/PTEN pathway and NOX protein in
diseases. Aging Dis. 5:203–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang JS, Cho CY, Hong CC, Yan MD, Hsieh
MC, Lay JD, Lai GM, Cheng AL and Chuang SE: Oxidative stress
enhances Axl-mediated cell migration through an Akt1/Rac1-dependent
mechanism. Free Radic Biol Med. 65:1246–1256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo H, Yang Y, Duan J, Wu P, Jiang Q and
Xu C: PTEN-regulated AKT/FoxO3a/Bim signaling contributes to
reactive oxygen species-mediated apoptosis in selenite-treated
colorectal cancer cells. Cell Death Dis. 4:e4812013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maiese K, Chong ZZ, Wang S and Shang YC:
Oxidant stress and signal transduction in the nervous system with
the PI3-K, Akt, and mTOR cascade. Int J Mol Sci. 13:13830–13866.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma Y, Zhao P, Zhu J, Yan C, Li L, Zhang H,
Zhang M, Gao X and Fan X: Naoxintong protects primary neurons from
oxygen-glucose deprivation/reoxygenation induced injury through
PI3K-Akt signaling pathway. Evid Based Complement Alternat Med.
2016:58159462016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flor PJ and Acher FC: Orthosteric versus
allosteric GPCR activation: The great challenge of group-III
mGluRs. Biochem Pharmacol. 84:414–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bohn LM, Gainetdinov RR and Caron MG: G
protein-coupled receptor kinase/beta-arrestin systems and drugs of
abuse: Psychostimulant and opiate studies in knockout mice.
Neuromolecular Med. 5:41–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhattacharya M, Babwah AV and Ferguson SS:
Small GTP-binding protein-coupled receptors. Biochem Soc Trans.
32:1040–1044. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu CY, Yang YC, Li CC, Liu KL, Lii CK and
Chen HW: Andrographolide inhibits TNFα-induced ICAM-1 expression
via suppression of NADPH oxidase activation and induction of HO-1
and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1
pathways in human endothelial cells. Biochem Pharmacol. 91:40–50.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song S, Zhou F and Chen WR: Low-level
laser therapy regulates microglial function through Src-mediated
signaling pathways: Implications for neurodegenerative diseases. J
Neuroinflammation. 9:2192012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akagi T, Murata K, Shishido T and Hanafusa
H: v-Crk activates the phosphoinositide 3-kinase/AKT pathway by
utilizing focal adhesion kinase and H-Ras. Mol Cell Biol.
22:7015–7023. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ballou LM, Chattopadhyay M, Li Y, Scarlata
S and Lin RZ: Galphaq binds to p110alpha/p85alpha phosphoinositide
3-kinase and displaces Ras. Biochem J. 394:557–562. 2006.
View Article : Google Scholar :
|
|
19
|
Fritsch R, de Krijger I, Fritsch K, George
R, Reason B, Kumar MS, Diefenbacher M, Stamp G and Downward J: Ras
and Rho families of GTPases directly regulate distinct
phosphoinositide 3-kinase isoforms. Cell. 153:1050–1063. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dale LB, Bhattacharya M, Anborgh PH,
Murdoch B, Bhatia M, Nakanishi S and Ferguson SS: G protein-coupled
receptor kinase-mediated desensitization of metabotropic glutamate
receptor 1A protects against cell death. J Biol Chem.
275:38213–38220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suire S, Lécureuil C, Anderson KE,
Damoulakis G, Niewczas I, Davidson K, Guillou H, Pan D, Clark J,
Stephens L and Hawkins PT: GPCR activation of Ras and PI3Kc in
neutrophils depends on PLCβ2/β3 and the RasGEF RasGRP4. EMBO J.
31:3118–3129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu CL, Wang JZ, Xia XP, Pan CW, Shao XX,
Xia SL, Yang SX and Zheng B: Rab11-FIP2 promotes colorectal cancer
migration and invasion by regulating PI3K/AKT/MMP7 signaling
pathway. Biochem Biophys Res Commun. 470:397–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi GX, Cai W and Andres DA: Rit subfamily
small GTPases: Regulators in neuronal differentiation and survival.
Cell Signal. 25:2060–2068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Julian L and Olson MF: Rho-associated
coiled-coil containing kinases (ROCK): Structure, regulation, and
functions. Small GTPases. 5:e298462014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uehara R, Hosoya H and Mabuchi I: In vivo
phosphorylation of regulatory light chain of myosin II in sea
urchin eggs and its role in controlling myosin localization and
function during cytokinesis. Cell Motil Cytoskeleton. 65:100–115.
2008. View
Article : Google Scholar
|
|
26
|
Koyanagi M, Takahashi J, Arakawa Y, Doi D,
Fukuda H, Hayashi H, Narumiya S and Hashimoto N: Inhibition of the
Rho/ROCK pathway reduces apoptosis during transplantation of
embryonic stem cell-derived neural precursors. J Neurosci Res.
86:270–280. 2008. View Article : Google Scholar
|
|
27
|
Li G, Liu L, Shan C, Cheng Q, Budhraja A,
Zhou T, Cui H and Gao N: RhoA/ROCK/PTEN signaling is involved in
AT-101-mediated apoptosis in human leukemia cells in vitro and in
vivo. Cell Death Dis. 5:e9982014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang S and Kim HM: The RhoA-ROCK-PTEN
pathway as a molecular switch for anchorage dependent cell
behavior. Biomaterials. 33:2902–2915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song H and Gao D: Fasudil, a
Rho-associated protein kinase inhibitor, attenuates retinal
ischemia and reperfusion injury in rats. Int J Mol Med. 28:193–198.
2011.PubMed/NCBI
|
|
30
|
Man JH, Liang B, Gu YX, Zhou T, Li AL, Li
T, Jin BF, Bai B, Zhang HY, Zhang WN, et al: Gankyrin plays an
essential role in Ras-induced tumorigenesis through regulation of
the RhoA/ROCK pathway in mammalian cells. J Clin Invest.
120:2829–2841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rodriguez-Viciana P, Warne PH, Dhand R,
Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD and Downward J:
Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature.
370:527–532. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chu JM, Chen LW, Chan YS and Yung KK:
Neuroprotective effects of neurokinin receptor one in dopaminergic
neurons are mediated through Akt/PKB cell signaling pathway.
Neuropharmacology. 61:1389–1398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saunders C, Siuta M, Robertson SD, Davis
AR, Sauer J, Matthies HJ, Gresch PJ, Airey DC, Lindsley CW, Schetz
JA, et al: Neuronal ablation of p-Akt at Ser473 leads to altered
5-HT1A/2A receptor function. Neurochem Int. 73:113–121. 2014.
View Article : Google Scholar :
|
|
34
|
Dizeyi N, Hedlund P, Bjartell A, Tinzl M,
Austild-Taskén K and Abrahamsson PA: Serotonin activates MAP kinase
and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol
Oncol. 29:436–445. 2011. View Article : Google Scholar
|
|
35
|
Gil S, Park C, Lee J and Koh H: The roles
of striatal serotonin and L-amino-acid decarboxylase on
L-DOPA-induced dyskinesia in a hemiparkinsonian rat model. Cell Mol
Neurobiol. 30:817–825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mazzucchi S, Frosini D, Ripoli A,
Nicoletti V, Linsalata G, Bonuccelli U and Ceravolo R: Serotonergic
antidepressant drugs and L-dopa-induced dyskinesias in Parkinson's
disease. Acta Neurol Scand. 131:191–195. 2015.
|
|
37
|
Chan RJ, McBride AW and Crabb DW: Seven
transmembrane domain receptor subtypes identified in NG108-15 cells
by reverse transcription-polymerase chain reaction. Biochem Biophys
Res Commun. 205:1311–1317. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
González-Maeso J and Sealfon SC:
Agonist-trafficking and hallucinogens. Curr Med Chem. 16:1017–1027.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Polter AM, Yang S, Jope RS and Li X:
Functional significance of glycogen synthase kinase-3 regulation by
serotonin. Cell Signal. 24:265–271. 2012. View Article : Google Scholar
|
|
40
|
Zamani A and Qu Z: Serotonin activates
angiogenic phosphorylation signaling in human endothelial cells.
FEBS Lett. 586:2360–2365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yin JA, Liu XJ, Yuan J, Jiang J and Cai
SQ: Longevity manipulations differentially affect
serotonin/dopamine level and behavioral deterioration in aging
Caenorhabditis elegans. J Neurosci. 34:3947–3958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ivachtchenko AV and Ivanenkov YA: 5-HT(6)
receptor antagonists: A patent update. Part 1. Sulfonyl
derivatives. Expert Opin Ther Pat. 22:917–964. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ponimaskin E, Voyno-Yasenetskaya T,
Richter DW, Schachner M and Dityatev A: Morphogenic signaling in
neurons via neurotransmitter receptors and small GTPases. Mol
Neurobiol. 35:278–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang G and Stackman RW Jr: The role of
serotonin 5-HT2A receptors in memory and cognition. Front
Pharmacol. 6:2252015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miguelez C, Morera-Herreras T, Torrecilla
M, Ruiz-Ortega JA and Ugedo L: Interaction between the 5-HT system
and the basal ganglia: Functional implication and therapeutic
perspective in Parkinson's disease. Front Neural Circuits.
8:212014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cummings JL: Lewy body diseases with
dementia: Pathophysiology and treatment. Brain Cogn. 28:266–280.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Monti JM: The role of dorsal raphe nucleus
serotonergic and non-serotonergic neurons, and of their receptors,
in regulating waking and rapid eye movement (REM) sleep. Sleep Med
Rev. 14:319–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Monti JM and Jantos H: The roles of
dopamine and serotonin, and of their receptors, in regulating sleep
and waking. Prog Brain Res. 172:625–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Soiza-Reilly M and Commons KG:
Quantitative analysis of glutamatergic innervation of the mouse
dorsal raphe nucleus using array tomography. J Comp Neurol.
519:3802–3814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Di Giovanni G, Esposito E and Di Matteo V:
Role of serotonin in central dopamine dysfunction. CNS Neurosci
Ther. 16:179–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roussakis AA, Politis M, Towey D and
Piccini P: Serotonin-to-dopamine transporter ratios in Parkinson
disease: Relevance for dyskinesias. Neurology. 86:1152–1158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sekigawa A, Takamatsu Y, Sekiyama K and
Hashimoto M: Role of α-and β-synucleins in the axonal pathology of
Parkinson's disease and related synucleinopathies. Biomolecules.
5:1000–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Buddhala C, Loftin SK, Kuley BM, Cairns
NJ, Campbell MC, Perlmutter JS and Kotzbauer PT: Dopaminergic,
serotonergic, and noradrenergic deficits in Parkinson disease. Ann
Clin Transl Neurol. 2:949–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mace JL, Porter RJ, Dalrymple-Alford JC,
Collins C and Anderson TJ: Acute tryptophan depletion and Lewy body
dementias. Int Psychogeriatr. 28:1487–1491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Peña E, Mata M, López-Manzanares L, Kurtis
M, Eimil M, Martínez-Castrillo JC, Navas I, Posada IJ, Prieto C,
Ruíz-Huete C, et al: Antidepressants in Parkinson's disease.
Recommendations by the movement disorder study group of the
Neurological Association of Madrid. Neurologia. Mar 19–2016.Epub
ahead of print. PubMed/NCBI
|
|
56
|
Kim SR, Chen X, Oo TF, Kareva T, Yarygina
O, Wang C, During M, Kholodilov N and Burke RE: Dopaminergic
pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann
Neurol. 70:110–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lin CH, Lin HI, Chen ML, Lai TT, Cao LP,
Farrer MJ, Wu RM and Chien CT: Lovastatin protects neurite
degeneration in LRRK2-G2019S parkinsonism through activating the
Akt/Nrf pathway and inhibiting GSK3β activity. Hum Mol Genet.
25:1965–1978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cui Q, Li X and Zhu H: Curcumin
ameliorates dopaminergic neuronal oxidative damage via activation
of the Akt/Nrf2 pathway. Mol Med Rep. 13:1381–1388. 2016.
|
|
59
|
Li X, Xie W, Xie C, Huang C, Zhu J, Liang
Z, Deng F, Zhu M, Zhu W, Wu R, et al: Curcumin modulates
miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7
breast cancer cell proliferation. Phytother Res. 28:1553–1560.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yu W, Zha W, Ke Z, Min Q, Li C, Sun H and
Liu C: Curcumin protects neonatal rat cardiomyocytes against high
glucose-induced apoptosis via PI3K/Akt signalling pathway. J
Diabetes Res. 2016:41585912016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chong CM, Zhou ZY, Razmovski-Naumovski V,
Cui GZ, Zhang LQ, Sa F, Hoi PM, Chan K and Lee SM: Danshensu
protects against 6-hydroxydopamine-induced damage of PC12 cells in
vitro and dopaminergic neurons in zebrafish. Neurosci Lett.
543:121–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhu G, Wang X, Wu S, Li X and Li Q:
Neuroprotective effects of puerarin on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's
disease model in mice. Phytother Res. 28:179–186. 2014. View Article : Google Scholar
|
|
63
|
Bao XQ, Kong XC, Kong LB, Wu LY, Sun H and
Zhang D: Squamosamide derivative FLZ protected dopaminergic neuron
by activating Akt signaling pathway in 6-OHDA-induced in vivo and
in vitro Parkinson's disease models. Brain Res. 1547:49–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kwon SH, Ma SX, Hong SI, Kim SY, Lee SY
and Jang CG: Eucommia ulmoides Oliv. bark attenuates
6-hydroxydopamine-induced neuronal cell death through inhibition of
oxidative stress in SH-SY5Y cells. J Ethnopharmacol. 152:173–182.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dewapriya P, Himaya SW, Li YX and Kim SK:
Tyrosol exerts a protective effect against dopaminergic neuronal
cell death in in vitro model of Parkinson's disease. Food Chem.
141:1147–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tasaki Y, Yamamoto J, Omura T, Sakaguchi
T, Kimura N, Ohtaki K, Ono T, Suno M, Asari M, Ohkubo T, et al:
Meloxicam ameliorates motor dysfunction and dopaminergic
neurodegeneration by maintaining Akt-signaling in a mouse
Parkinson's disease model. Neurosci Lett. 521:15–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jin MC, Yoo JM, Sok DE and Kim MR:
Neuroprotective effect of N-acetyl-5-hydroxytryptamines on
glutamate-induced cytotoxicity in HT-22 cells. Neurochem Res.
39:2440–2451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ma J, Wang Z, Liu C, Shen H, Chen Z, Yin
J, Zuo G, Duan X, Li H and Chen G: Pramipexole-induced hypothermia
reduces early brain injury via PI3K/AKT/GSK3β pathway in
subarachnoid hemorrhage rats. Sci Rep. 6:238172016. View Article : Google Scholar
|
|
69
|
Kuo HC, Chang HC, Lan WC, Tsai FH, Liao JC
and Wu CR: Protective effects of Drynaria fortunei against
6-hydroxydopamine-induced oxidative damage in B35 cells via the
PI3K/AKT pathway. Food Funct. 5:1956–1965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Giuliani P, Ballerini P, Buccella S,
Ciccarelli R, Rathbone MP, Romano S, D'Alimonte I, Caciagli F, Di
Iorio P and Pokorski M: Guanosine protects glial cells against
6-hydroxydopamine toxicity. Adv Exp Med Biol. 837:23–33. 2015.
View Article : Google Scholar
|
|
71
|
He Z, Chen AY, Rojanasakul Y, Rankin GO
and Chen YC: Gallic acid, a phenolic compound, exerts
anti-angiogenic effects via the PTEN/AKT/HIF-1α/VEGF signaling
pathway in ovarian cancer cells. Oncol Rep. 35:291–297. 2016.
|
|
72
|
Caruso V, Le Grevés M, Shirazi Fard S,
Haitina T, Olszewski PK, Alsiö J, Schiöth HB and Fredriksson R: The
orphan G protein-coupled receptor gene GPR178 is evolutionary
conserved and altered in response to acute changes in food intake.
PLoS One. 10:e01220612015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Guixà-González R, Javanainen M,
Gómez-Soler M, Cordobilla B, Domingo JC, Sanz F, Pastor M, Ciruela
F, Martinez-Seara H and Selent J: Membrane omega-3 fatty acids
modulate the oligomerisation kinetics of adenosine A2A and dopamine
D2 receptors. Sci Rep. 6:198392016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Young G and Conquer J: Omega-3 fatty acids
and neuropsychiatric disorders. Reprod Nutr Dev. 45:1–28. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Young GS, Conquer JA and Thomas R: Effect
of randomized supplementation with high dose olive, flax or fish
oil on serum phospholipid fatty acid levels in adults with
attention deficit hyperactivity disorder. Reprod Nutr Dev.
45:549–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang RH, Lin J, Hou XH, Cao R, Yu F, Liu
HQ, Ji AL, Xu XN, Zhang L and Wang F: Effect of docosahexaenoic
acid on hippocampal neurons in high-glucose condition: Involvement
of PI3K/AKT/nuclear factor-κB-mediated inflammatory pathways.
Neuroscience. 274:218–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Simon BR, Parlee SD, Learman BS, Mori H,
Scheller EL, Cawthorn WP, Ning X, Gallagher K, Tyrberg B,
Assadi-Porter FM, et al: Artificial sweeteners stimulate
adipogenesis and suppress lipolysis independently of sweet taste
receptors. J Biol Chem. 288:32475–32489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Huang W, Zhao Y, Zhu X, Cai Z, Wang S, Yao
S, Qi Z and Xie P: Fluoxetine upregulates phosphorylated-AKT and
phosphorylated-ERK1/2 proteins in neural stem cells: Evidence for a
crosstalk between AKT and ERK1/2 pathways. J Mol Neurosci.
49:244–249. 2013. View Article : Google Scholar
|
|
79
|
Morandini L, Ramallo MR, Moreira RG, Höcht
C, Somoza GM, Silva A and Pandolfi M: Serotonergic outcome, stress
and sexual steroid hormones, and growth in a South American cichlid
fish fed with an L-tryptophan enriched diet. Gen Comp Endocrinol.
223:27–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Seol GH, Shim HS, Kim PJ, Moon HK, Lee KH,
Shim I, Suh SH and Min SS: Antidepressant-like effect of Salvia
sclarea is explained by modulation of dopamine activities in rats.
J Ethnopharmacol. 130:187–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lee KB, Cho E and Kang YS: Changes in
5-hydroxytryptamine and cortisol plasma levels in menopausal women
after inhalation of clary sage oil. Phytother Res. 28:1599–1605.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Qiu Y, Huang X, Huang L, Tang L, Jiang J,
Chen L and Li S: 5-HT(1A) receptor antagonist improves behavior
performance of delirium rats through inhibiting PI3K/Akt/mTOR
activation-induced NLRP3 activity. IUBMB Life. 68:311–319. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Benmansour S, Privratsky AA, Adeniji OS
and Frazer A: Signaling mechanisms involved in the acute effects of
estradiol on 5-HT clearance. Int J Neuropsychopharmacol.
17:765–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cui J, Shen Y and Li R: Estrogen synthesis
and signaling pathways during aging: From periphery to brain.
Trends Mol Med. 19:197–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nakaso K, Tajima N, Horikoshi Y, Nakasone
M, Hanaki T, Kamizaki K and Matsura T: The estrogen receptor
β-PI3K/Akt pathway mediates the cytoprotective effects of
tocotrienol in a cellular Parkinson's disease model. Biochim
Biophys Acta. 1842:1303–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Marwarha G, Rhen T, Schommer T and Ghribi
O: The oxysterol 27-hydroxycholesterol regulates α-synuclein and
tyrosine hydroxylase expression levels in human neuroblastoma cells
through modulation of liver X receptors and estrogen receptors -
relevance to Parkinson's disease. J Neurochem. 119:1119–1136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Deusser J, Schmidt S, Ettle B, Plötz S,
Huber S, Müller CP, Masliah E, Winkler J and Kohl Z: Serotonergic
dysfunction in the A53T alpha-synuclein mouse model of Parkinson's
disease. J Neurochem. 135:589–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fazili NA and Naeem A: Anti-fibrillation
potency of caffeic acid against an antidepressant induced
fibrillogenesis of human α-synuclein: Implications for Parkinson's
disease. Biochimie. 108:178–185. 2015. View Article : Google Scholar
|