Introduction
Acute pulmonary embolism (APE) is a disease caused
by various emboli blocking the pulmonary artery, leading to
pulmonary circulation disorder. The morbidity and fatality rate are
high in European and American countries, and have increased in
recent years. The morbidity in the United States during 1979–1999
was reported to be 0.4%, and ~150,000 individuals are hospitalized
due to APE every year. There was no obvious change in 20 years
(1). The epidemiology of APE is
difficult to confirm due to the lack of symptoms and proper
diagnosis. In 2004, according to the demographic statistics of
454.4 million people in 6 countries of the European Union, 317,000
deaths related to venous thrombus were reported. Among them, 34%
died of acute fat embolism, 59% died of APE (diagnosed by autopsy,
not alive), and only 7% were diagnosed with APE prior to death
(2). In China, of the 16,972,182
hospitalized patients, 18,206 patients had APE. The annual
occurrence rate is 0.1%, and the incidence is significantly higher
in males (0.2%) than in females (0.1%). The fatality decreased from
25.1% in 1997 to 8.7% in 2008 (3). APE has become a common
cardiovascular disease in China, seriously threatening human
health. Thus, interventions are urgently needed.
Previous research found that the levels of tumor
necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-8, CX3CL1,
CX3CR1, nuclear factor-κB (NF-κB), extracellular signal-regulated
kinase (ERK), PI3K/Akt, brain natriuretic peptide (BNP), troponin T
(TnT) and D-dimer (D2D) were significantly increased in APE rat
models (4–6). The increased CX3CL1 level in serum
was found to have a positive correlation with serum IL-8 and TNF-α,
and aspirin significantly inhibited all the aforementioned factors.
Meanwhile, it was also found that aspirin could inhibit
lipopolysaccharide and induce the expression of PI3K, Akt, ERK,
NF-κB, CX3CL1, matrix metallopeptidase-7 (MMP-7) and MMP-12 in
human bronchial epithelial cells (7). Therefore, it is believed that the
inflammatory response occurs in APE, CX3CL1/CX3CR1 significantly
increases, and TNF-α stimulates CX3CL1, and aspirin can inhibit the
aforementioned factors. However, the effect of the CX3CL1/CX3CR1
signaling pathway on the occurrence of APE remains unclear.
In the present study, CX3CL1-short hairpin RNA
(shRNA) adenovirus (AD) and CX3CL1-overexpression vector were
constructed. Reverse transcription-polymerase chain reaction
(RT-PCR), enzyme-linked immunosorbent assay (ELISA), laser confocal
scanning microscopy, and pulmonary artery pressure detection were
applied to explore the protective effect of aspirin on APE via the
CX3CL1/CX3CR1 signaling pathway in an animal model.
Materials and methods
Materials
Enteric-coated aspirin tablets were procured from
Nanjing Baijingyu Pharmaceutical Co., Ltd. (Jiangsu, China; drug
specifications: 25 mg × 100 pills/bottle, batch no. 141201).
Male Sprague-Dawley (SD) rats (200 g) were purchased
from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China)
and Vital River Laboratories Co., Ltd. (Beijing, China). The
feeding temperature was 20–25°C, and the humidity was 40–70%. After
a 6-day acclimation, the experiment was intiated. The study was
approved by the Ethics Committee of Zhejiang Chinese Medical
University.
Construction of the CX3CL1-overexpression vector and
shRNA was performed. Vector pHBAd-murine cytomegalo-virus
(MCMV)-green fluorescent protein (GFP) and vector pHBAd-U6-GFP
(Hanbio, Hangzhou, China) were used. Escherichia coli strain
DH5α (Tiangen, Beijing, China), restriction enzymes, T4 ligase
(both from Fermentas, Waltham, MA, USA), and plasmid DNA extraction
kit (CWBio, Beijing, China) were used.
Biological function experimental system (BL-420S)
and animal ventilator (HX-300) were obtained from Taimeng (Sichuan,
China). Multiskan spectrum microplate spectrophotometer (Spectra
Plus 384) was purchased from Molecular Devices (Sunnyvale, CA,
USA).
Hematoxylin and eosin staining (H&E) was used to
observe histopathological changes in the pulmonary tissue.
Thromboxane A2 (TXA2), troponin I type 3 (TNNI3),
BNP and D2D levels in rat serum were detected using ELISA.
TTNNI3kit, BNP, TXA2 and D2D were obtained from Youershengke
(Wuhan, China).
RT-PCR was used to detect CX3CL1, CX3CR1 and
intercellular adhesion molecule-1 (ICAM-1) expression in mRNA of
rat pulmonary tissue. A high-purity total RNA rapid extraction kit
was obtained from Generay (Shanghai, China), and a PrimeScript RT
reagent kit was purchased from Takara (Tokyo, Japan). Super Real
PreMix Plus (with SYBR-Green I) was obtained from Tiangen.
High-precision spectrophotometer (Merinton SMA4000; Merinton
Instrument, Ltd., Beijing, China) and quantitative PCR system (CFX
connect real-time PCR system; Bio-Rad, Hercules, CA, USA) were
used.
Laser confocal scanning microscopy was used to
detect the coexpression of CX3CL1/CX3CR1 and CX3CL1/NF-κB.
Fractalkine antibody (CX3CL1) (cat. no. sc-7227; batch no. B1612;
1:10; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
anti-NF-κB p65 antibody (cat. no. ab7970; batch no. GR187946-6;
1:50), anti-CX3CR1 antibody (cat. no. ab8021; batch no. GR90085-11;
1:100) (both from Abcam, Cambridge, MA, USA), anti-rabbit IgG
secondary antibody (cat. no. A21206; batch no. 1110071; 488
conjugate; Life Technologies, Carlsbad, CA, USA; excitation
wavelength/emission wavelength: 488/520 nm, 1:200), anti-goat IgG
secondary antibody (cat. no. A21432; batch no. 1620248; 555
conjugate; excitation wavelength/emission wavelength: 555/562 nm,
1:200) (Life Technologies), 4′,6-diamidino-2-phe-nylindole (DAPI)
(excitation wavelength/emission wavelength: 358/461 nm; Sigma, St.
Louis, MO, USA) were used.
Methods
Preparation of the
CX3CL1-overexpression vector and shRNA
The steps for construction of CX3CL1 overexpression
AD are as follows. AD vector plasmid was recombined and
pHBAd-MCMV-GFP vector was digested with EcoRI and
BamHI double enzymes and collected after digestion. CX3CL1
fragments were obtained, and the transformed CX3CL1 was used to
select bacterial colonies. The bacteria were incubated by shaking
for 14 h at 37°C and 250 rpm. The bacterial suspension was detected
using PCR and sequenced to obtain pHBAd-MCMV-GFP-CX3CL1 recombinant
vector, marked as AD-CX3CL1. Meanwhile, plasmid pHBAd-MCMV-GFP was
used as the control, and marked as AD-GFP. Recombinant plasmids and
recombinant AD vector package were prepared. Then, virus
harvesting, amplification, purification, and detection of the
infectious titer were performed. CX3CL1 shRNA AD construction
process was as follows. pHBAd-U6-GFP interference vector was used,
and the interference sequence was designed to construct, screen and
identify CX3CL1 shRNA (Fig.
1).
Animal model
Male SD rats (200±20 g) were randomly divided into 9
groups (n=10): normal group (group N), sham operation group (group
Sham), sham operation + aspirin group (group ASP), model group
(group M), model + ASP group (group M+A), model + shRNA (group
M+SH), sham operation + CX3CL1-overexpression vector group (group
Sham+CX3), model + ASP + shRNA group (group M+A+SH), and model +
ASP + CX3CL1-overexpression vector group (group M+A+CX3).
Autologous thrombus was injected through the jugular vein to copy a
rat PTE model. One day before surgery, 0.2 ml of blood was
withdrawn from the caudal vein, and incubated at 37°C overnight.
The concretionary thrombus was taken out to prepare 30 emboli (2×1
mm2), which were further placed into a 2-ml syringe. The
rats were anesthetized using 10% chloral hydrate (0.3 g/kg). The
right jugular vein was separated, and the puncture needle was
placed. The prepared embolus was pushed into the common vein using
the puncture needle, followed by pushing 1 ml of saline to prevent
the embolus from staying in the tube or jugular vein. Finally, the
wound was sutured after the bleeding stopped. One day before
surgery and 40 min before modeling, the drug was administrated via
gavage. The virus intervention groups (group M+SH, group Sham+CX3,
group M+A+SH, group M+A+CX3) were injected once with aspirin 300
mg/kg through the caudal vein 3 days before modeling (109 pfu/rat).
Group Sham and group M received an equal volume of saline every
day. Group N did not receive any intervention.
Detection of pulmonary artery
pressure
Six hours after modeling, the animals were
anesthetized again. A PE50 tube was inserted into the pulmonary
artery, and the other side was connected with a pressure
transducer. A waveform of pulmonary artery pressure was recorded
using the biological function experimental system (BL-420S), and
pulmonary arterial systolic pressure (PASP), pulmonary artery
diastolic pressure (PADP) and pulmonary arterial pressure (PAP)
were calculated.
Pulmonary pathology was detected using H&E
staining and CX3CL1, CX3CR1 and ICAM-1 in pulmonary tissue were
detected by RT-PCR.
TXA2, BNP, TNNI3 and D-dimer in serum were detected
by double-antibody sandwich or competitive inhibition ELISA.
CX3CL1/CX3CR1 coexpression and CX3CL1/NF-κB
coexpression in the pulmonary tissue were detected by laser
confocal scanning microscopy.
The virus intervention group was divided into two
subgroups (CX3CL1-overexpression group and shRNA group). The tissue
was cut into 8-µm slices at −20°C and observed by a
fluorescence microscope.
Statistical analysis
SPSS 21.0 (SPSS, Chicago, IL, USA) was used for data
analysis, and the results are expressed as mean ± standard
deviation. One-way analysis of variance was used, and pairwise
comparison between groups was analyzed by least significant
difference. A P-value <0.0 was considered to indicate a
statistically significant result.
Results
Detection of pulmonary artery
pressure
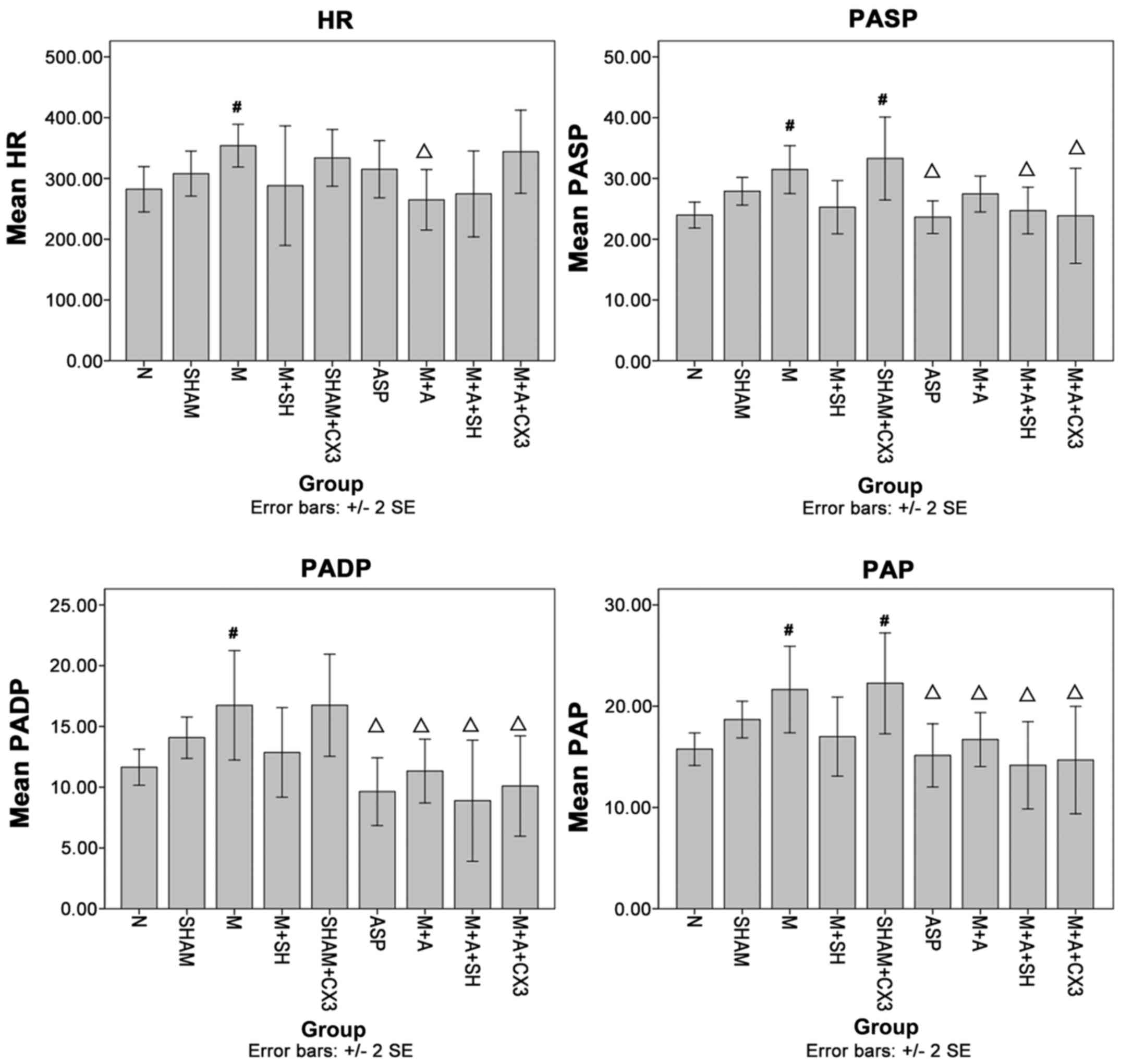
Compared with group N, hear rate (HR), PASP, PADP
and PAP in group M were significantly increased (P<0.05). PASP
and PAP in group Sham+CX3 were significantly increased (P<0.05).
Compared with group M, HR in group M+A was significantly decreased
(P<0.05), PASP in groups M+A+SH, ASP and M+A+CX3 were also
significantly decreased (P<0.05). PADP and PAP in groups ASP,
M+A, M+A+SH, and M+A+CX3 were significantly decreased (P<0.05)
(Fig. 2).
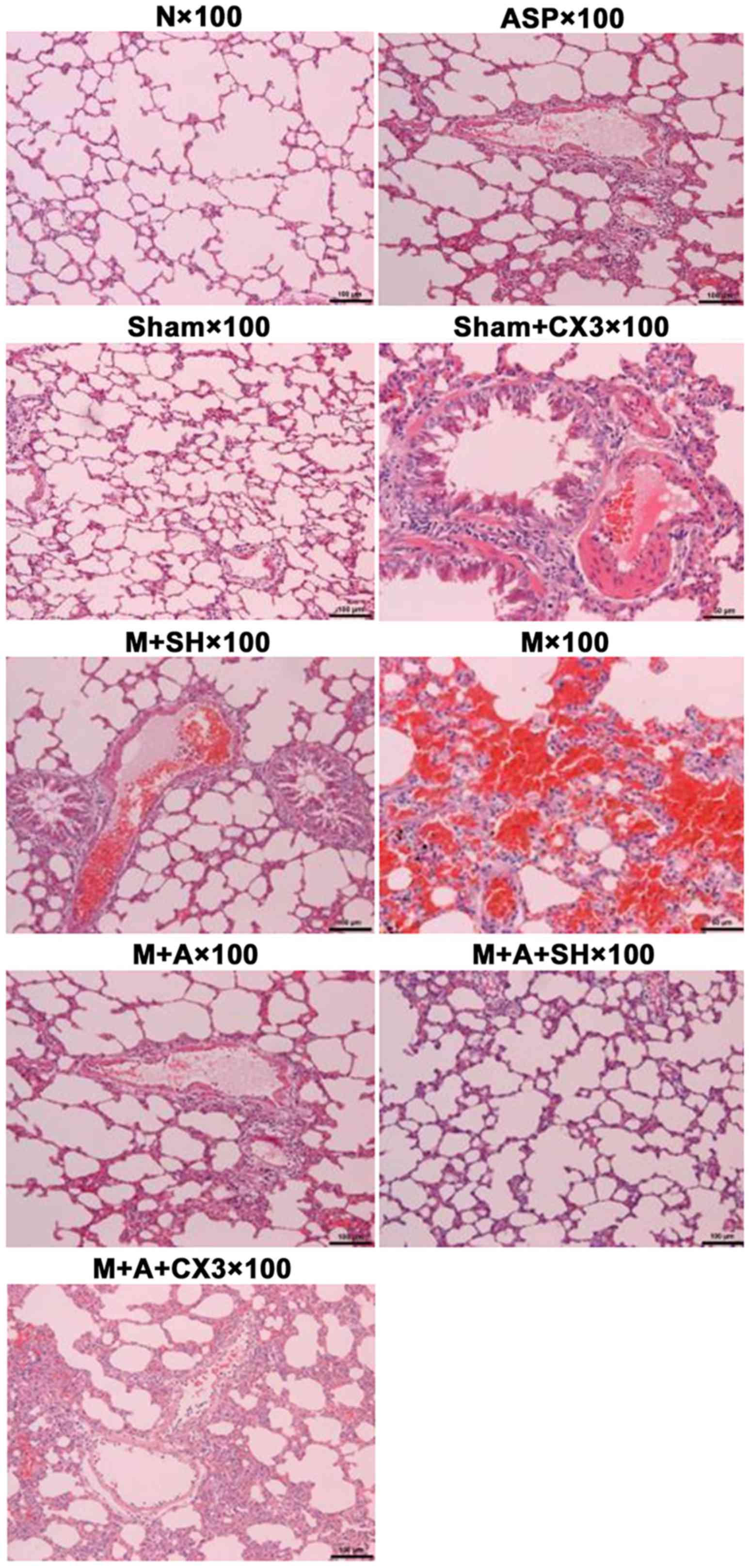
Pulmonary H&E detection
The results of lung pathology as detected by H&E
are shown in Table I and Fig. 3.
 | Table IPulmonary pathology as detected by
hematoxylin and eosin (H&E). |
Table I
Pulmonary pathology as detected by
hematoxylin and eosin (H&E).
| Group | Pathological
change |
|---|
| Group N | Clear pulmonary
structure, normal alveolar structure, no evident inflammatory cell
infiltration in pulmonary interstitial fibrosis, occasional
inflammation in airways and blood vessesl, no formation of
thromboembolism in blood vessels |
| Group Sham, ASP and
Sham+CX3 | Same as normal group,
less inflammatory cell infiltration in bronchus and blood vessels
and pulmonary interstitial fibrosis (granulocytes, lymphocytes, and
eosinophilic granulocytes), no evident formation of thromboembolism
in blood vessels |
| Group M | Mixed thrombus and
coagulation in the pulmonary artery, evident vascular endothelial
loss, alveolar septal thickening and swelling, pulmonary
hemorrhage, severe inflammatory cell infiltration in the bronchus
and blood vessels and pulmonary interstitial fibrosis, or even
pulmonary abscess |
| Group M+SH | Part of embolism in
pulmonary artery dissolved, revascularization, intravascular
subcutaneous hyperplasia with slight inflammation, less number of
thrombogenesis, good thrombolytic effect |
| Group M+ASP | Good thrombolytic
effect, evident vascular endothelial hyperplasia, severe
inflammation |
| Group M+ASP+SH | Good thrombolytic
effect, less inflammatory response |
| Group
M+ASP+CX3 | Good thrombolytic
effect, more inflammatory response |
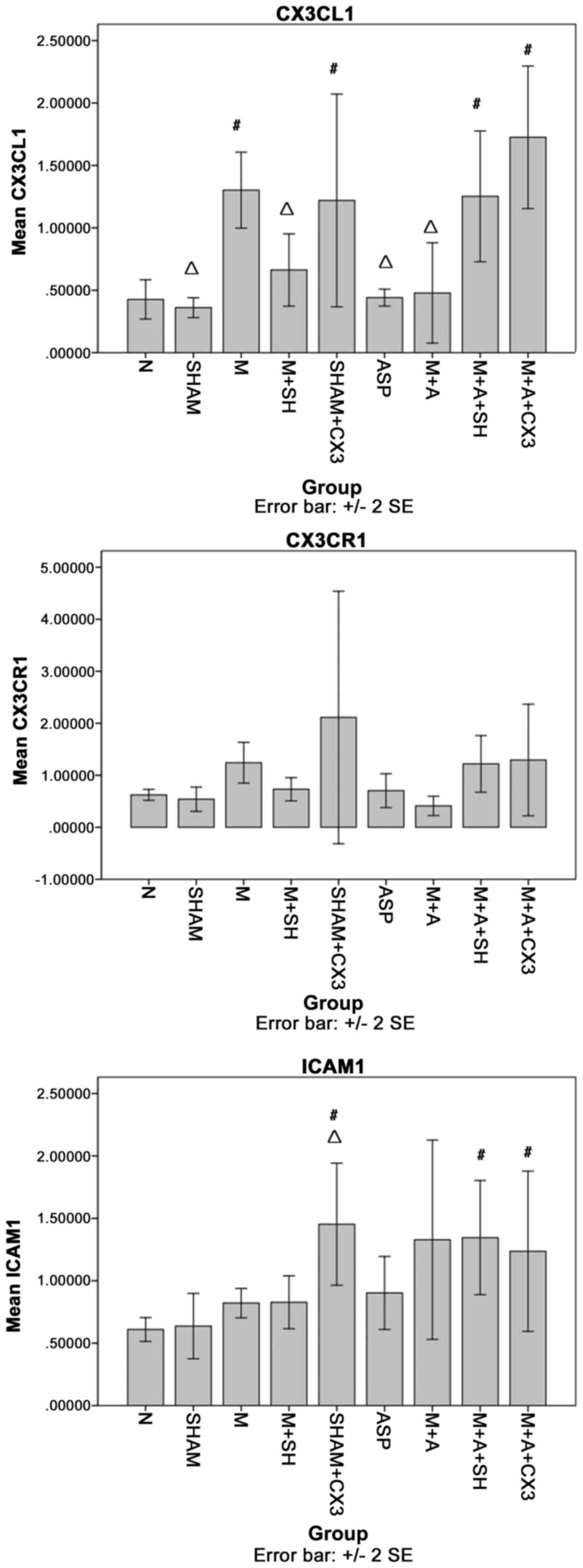
CX3CL1, CX3CR1 and ICAM-1 in the
pulmonary tissue as detected by PCR
As shown in Fig.
4, compared with group N, CX3CL1 in groups M, Sham+CX3, M+A+SH
and M+A+CX3 was significantly increased (P<0.05). Compared with
group M, CX3CL1 in groups N, Sham, M+SH, ASP and M+A was
significantly decreased (P<0.05).
The comparisons of CX3CR1 among the different groups
showed no significant differences.
Compared with group N, ICAM-1 in groups Sham+CX3,
M+A+SH and M+A+CX3 was significantly increased (P<0.05).
Compared with group M, ICAM-1 in group Sham+CX3 was significantly
decreased (P<0.05).
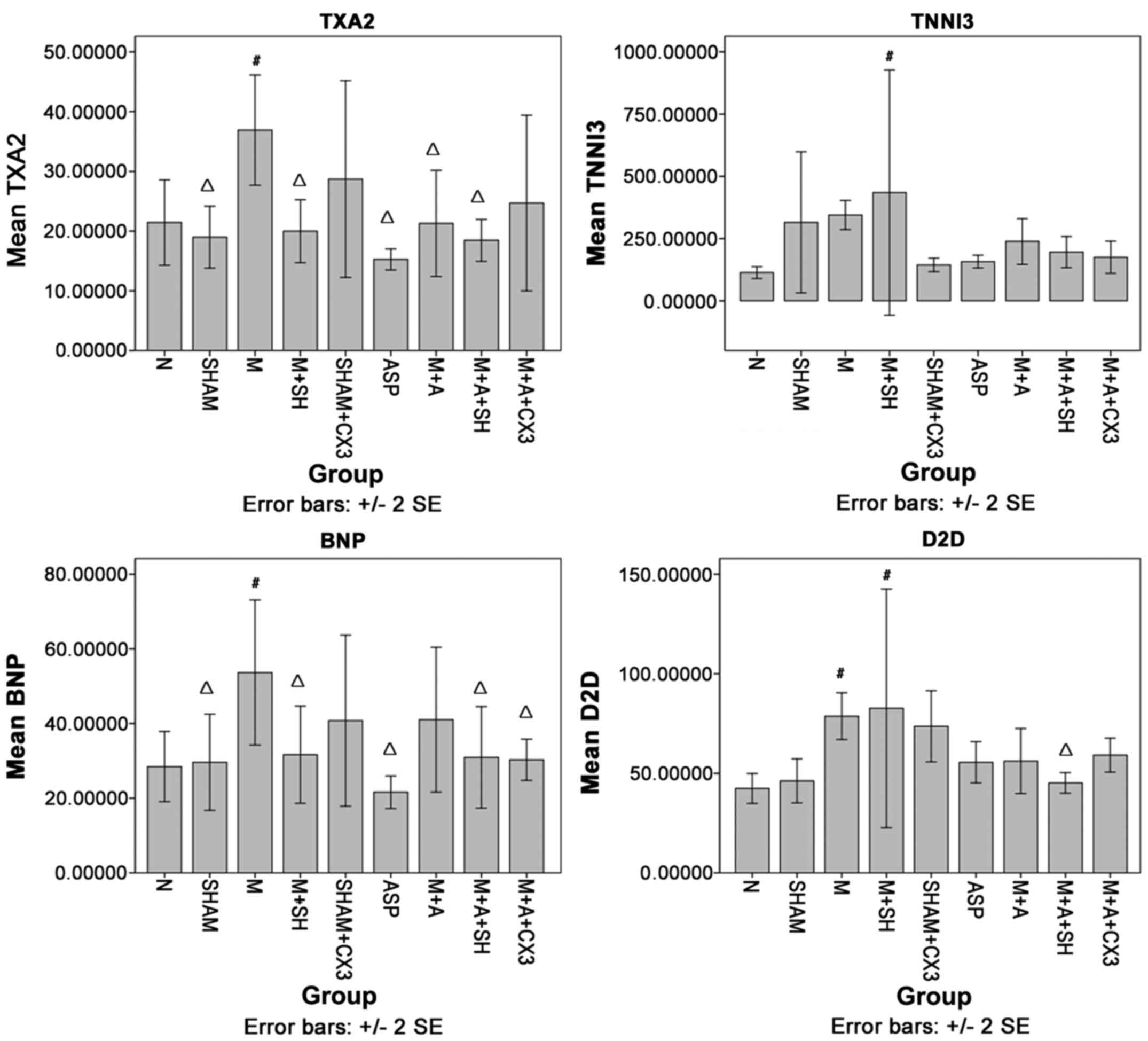
TXA2, TNNI3, BNP and D2D levels in rat
serum as detected by ELISA
As shown in Fig.
5, compared with group N, TXA2 in group M was significantly
increased (P<0.05). Compared with group M, TXA2 in groups N,
Sham, M+SH, ASP, M+A and M+A+SH was significantly decreased
(P<0.05).
Compared with group N, TNNI3 in group M+SH was
significantly increased (P<0.05). Compared with group M, no
significant difference was observed.
Compared with group N, BNP in group M was
significantly increased (P<0.05). Compared with group M, BNP in
groups N, Sham, M+SH, ASP, M+A+SH and M+A+CX3 was significantly
decreased (P<0.05).
Compared with group N, D2D in groups M and M+SH was
significantly increased (P<0.05). Compared with group M, D2D in
groups N and M+A+SH was significantly decreased (P<0.05).
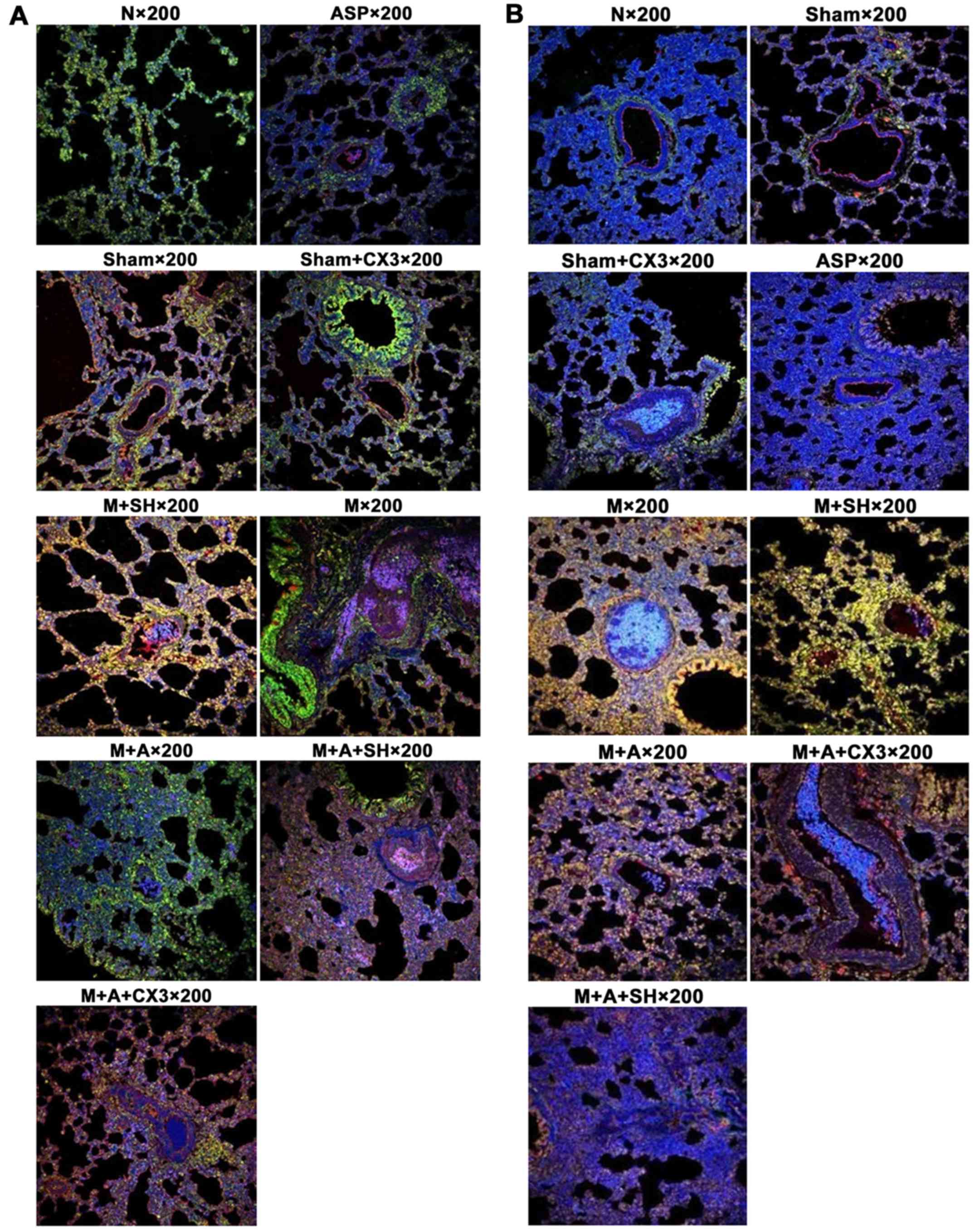
Coexpression of CX3CL1/CX3CR1 and
CX3CL1/NF-κB as detected by laser confocal scanning microscopy
The positive color of immunofluorescence indicated
that CX3CL1 (red) and CX3CR1 (green) were mainly expressed in the
cytoplasm and cytomembrane. NF-κB (green) was mainly expressed in
the cytoplasm, and rarely expressed in the cell nucleus. As shown
in Tables II and III and Fig. 6, the fluorescence strength of each
group was compared according to the following standard: slight, +;
moderate, ++; strong, +++.
 | Table IICX3CL1/CX3CR1 expression as detected
by double-labeling immunofluorescence. |
Table II
CX3CL1/CX3CR1 expression as detected
by double-labeling immunofluorescence.
| Group | Expression
strength |
|---|
| Group N and
ASP | + |
| Group Sham,
Sham+CX3 and M | ++ |
| Group M+A, M+A+SH
and M+A+CX3 | ++ |
| Group M+SH | +++ |
 | Table IIICX3CL1/NF-κB expression as detected
by double-labeling immunofluorescence. |
Table III
CX3CL1/NF-κB expression as detected
by double-labeling immunofluorescence.
| Group | Expression
strength |
|---|
| Group N, ASP, Sham,
and Sham+CX3 | + |
| Group M+A, M+A+SH
and M+A+CX3 | ++ |
| Group M and
M+SH | +++ |
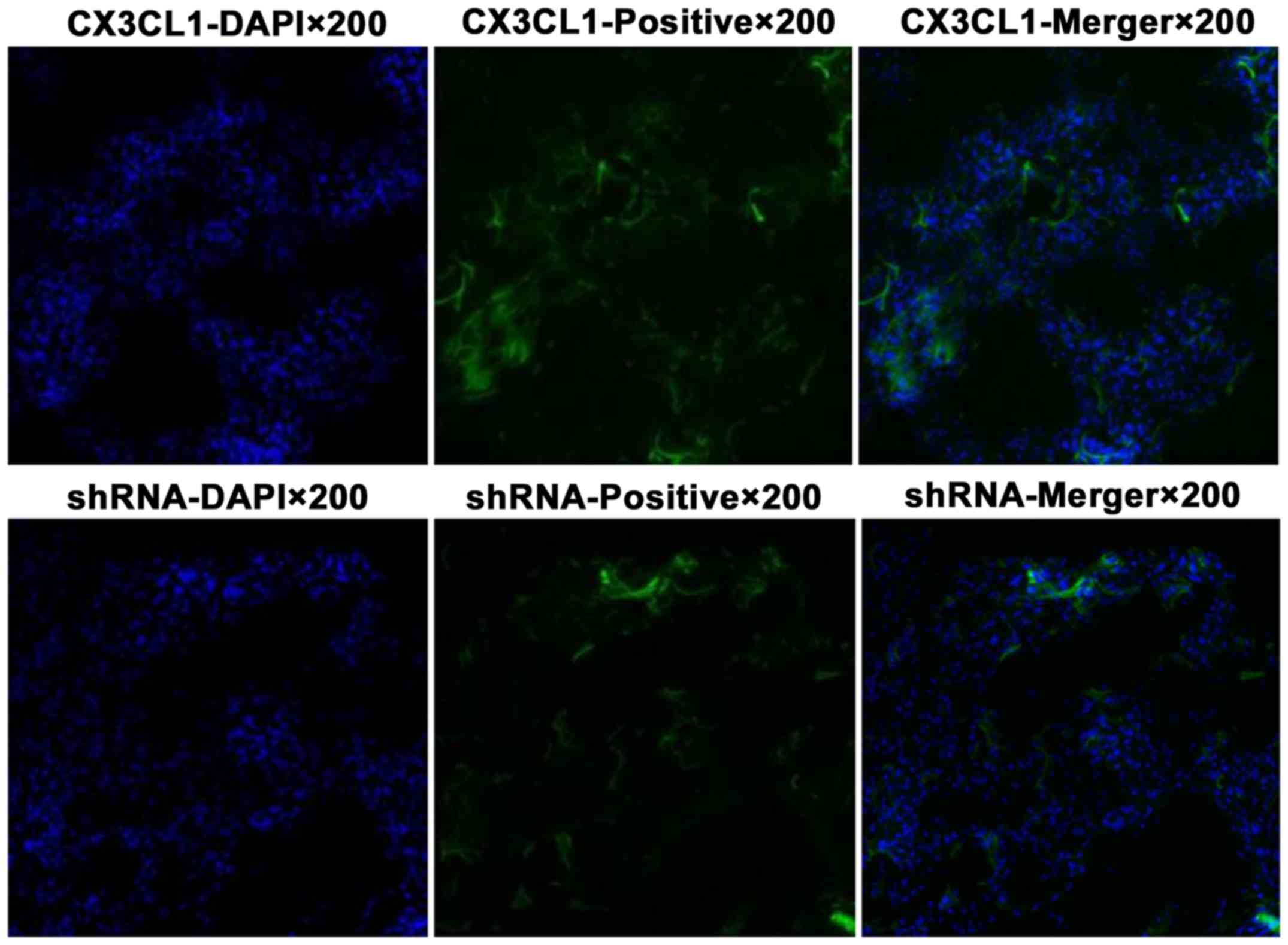
Virus infection of the rat pulmonary
tissue as observed by laser confocal scanning microscopy
The pulmonary tissue infected by the virus is shown
in green color, and the cell nucleus is shown in blue color
(Fig. 7).
Discussion
In the present study, CX3CL1-shRNA AD and
CX3CL1-overexpression vector were constructed. The study aimed to
investigate the influence of CX3CL1 on APE. Compared with a
previous study, this study detected pulmonary artery pressure.
Although the pressure was not as visual as CTPA, it still could
reflect the pressure change after APE. To directly observe the
correlation between CX3CL1 in the APE site and CX3CR1 expression
and the correlation between the change in the CX3CL1/CX3CR1
signaling pathway and NF-κB inflammatory pathway, laser confocal
scanning microscopy was used. Due to the use of many groups, the
experiment was divided into three steps: i) group N, group Sham,
group M and group M+A; ii) group M, group M+SH and group M+CX3;
iii) group M+SH, group M+CX3, group M+A+SH and group M+A+SH. The
first step focused on the drug effect and change in the signaling
pathway, the second confirmed the influence of the change in the
inflammatory pathway on APE (especially the change in proteins of
the signaling pathway), and the third investigated the relationship
between the signaling pathway and aspirin (especially the change in
the signaling pathway). It was found that aspirin significantly
decreased the pulmonary artery pressure, improved pathological
changes in the embolism, and decreased the expression of the
CX3CL1/CX3CR1 and CX3CL1/NF-κB signaling pathways. Moreover, the AD
overexpression CX3CL1 vector aggravated the inflammatory changes in
rats with APE, which were improved by aspirin. However, AD CX3CL1
intervention decreased this change, and its combination with
aspirin significantly improved the APE changes.
The presemt study explored the important role of the
CX3CL1/CX3CR1 signaling pathway in the occurrence of APE as well as
improvement in APE changes by aspirin via the signaling pathway.
Therefore, pulmonary artery pressure, pulmonary H&E and ELISA
were used to quantitatively detect TNNI3, BNP and D2D levels in rat
serum. The limitation of the study was that APE was mainly a
mechanical obstruction of the pulmonary artery; neurohumoral
(inflammatory mediator) control was not the key factor, and hence
should not be emphasized, or a disconnection between experimental
findings and clinical practice would result.
If therapy for APE is effective, it should
significantly decrease the pulmonary artery pressure. When APE
occurs, pulmonary artery pressure is increased. The present study
found that HR, PASP, PADP and PAP in group M were significantly
increased. Therefore, it was believed that the APE model was
successful from the aspect of hemodynamics. The HR in group M+A was
significantly decreased compared with group M, suggesting that
aspirin took effect on APE. The pulmonary artery pressures in group
M+A+SH and group M+A+CX3 was significantly decreased, indicating no
overexpression or inhibition of CX3CL1; aspirin decreased the
pulmonary artery pressure. It was confirmed by pulmonary H&E
detection that only APE was affected by aspirin. Irrespective of
the expression or inhibition of CX3CL1, the degree of APE and
inflammation was less. The prognosis of APE is related to TnT, BNP
and D2D (8). Therefore, these
three indicators were used in the present study. After successful
APE modeling, serum BNP and D2D were significantly increased.
Furthermore, BNP was significantly decreased in M+SH, M+A+SH and
M+A+CX3, and D2D was significantly decreased in M+A+SH, suggesting
that the inhibition of CX3CL1 could improve the pathology of
APE.
The European Society of Cardiology reported that APE
is the most severe clinical manifestation of venous thromboembolism
(VTE) (8). The basic process of
APE includes mechanical obstruction. Pulmonary artery spasm
contraction caused by neurohumoral factor (mainly inflammatory
mediator) also plays an important role, especially within a short
time. It mainly involves fibrous proteins and aggregated blood
platelets, and also infiltration of various inflammatory cells.
They continuously release a series of inflammatory mediators such
as A2 (TXA2) (9) to shrink the
pulmonary artery. More attention has been paid to the first step,
and less to the second step.
CX3CL1 (fractalkine) is a chemotactic factor
containing 373 amino acids. It possesses adhesive and chemotactic
activity. It is the only member of the CX3C family (10), and can combine with a specific
receptor CXCCR1, mediating the intimate adhesion of inflammatory
cells and vascular endothelium cells. CX3CL1 plays an important
role in the recruitment of inflammatory cells on the vascular wall
and injury of endothelial cells (11,12). It was reported that TNF-α could
influence the CX3CL1/CX3CR1 inflammatory signaling pathway
(13), which was confirmed in a
previous study (5). It was also
demonstrated that the CX3CL1/CX3CR1 signaling pathway exists in
atherosclerosis (14), and CX3CL1
plays a role in high pulmonary artery pressure combined with high
airway pressure (15). However,
still no systematic study exists on the mechanism underlying the
involvement of CX3CL1/CX3CR1 in APE.
It was also confirmed that CX3CL1 was significantly
increased in APE. Aspirin could inhibit the expression, as shown in
this study. CX3CL1 in group Sham+CX3 was significantly increased
and that in group M+SH was significantly decreased, suggesting that
the preparations of CX3CL1 AD and shRNA AD were successful.
ICAM-1 is a member of the immunoglobulin
super-family (16). It is mainly
expressed in neuronal cells, immune cells, vascular endothelium
cells, epithelial cells, and glial cells. It is one of the
important leukocyte-endothelial cell adhesion molecules, and is
involved in intracellular and cell-matrix signal exchange,
mediating adhesion, recognition, activation, proliferation,
differentiation, inflammatory reaction and damage repair. After
CX3CL1 overexpression in group Sham, the ICAM-A level was
significantly higher than that in group N and group M, indirectly
suggesting that CX3CL1 could stimulate the secretion of ICAM-1.
The TXA2 level in rats with APE was significantly
increased, and the levels in groups M+SH, M+A and M+A+SH were
significantly decreased. This indicated that aspirin could decrease
TXA2 secretion after inhibiting CX3CL1 expression.
The coexpression of CX3CL1/CX3CR1 and CX3CL1/NF-κB
as detected by double immunofluorescent staining suggested
increased expression of the aforementioned factors.
Therapy for APE includes streptokinase, urokinase,
recombinant tissue plasminogen activator thrombolysis,
low-molecular-weight heparin and new oral anticoagulants (1,8,17).
The selection of aspirin was due to the inflammatory response after
the occurrence of APE. It could irreversibly inhibit epoxidase and
further inhibit the formation of thromboxane A2 in blood platelets.
It could also inhibit the activation of NF-κB and exert
anti-inflammatory effects. As nonsteroidal anti-inflammatory drugs
are not specific, their high concentration may effectively inhibit
inflammatory factors such as NF-κB (18,19). A previous study found that aspirin
could inhibit NF-κB expression in APE (5). Aspirin inhibits CX3CL1/CX3CR1
expression (20,21). It can be safely applied in APE or
for preventing deep vein thrombosis (22,23). The daily aspirin dose was reported
to be 325 mg for 14 days. Prevention and treatment of VTE was safe,
thus the dose of aspirin was increased in the present study
(24). This study demonstrated
that aspirin improved the pathological changes in rats with APE via
the CX3CL1/CX3CR1 signaling pathway.
Future studies should investigate the effect (and
its underlying mechanism) of CX3CL1 on thrombogenesis by
influencing the damage of vascular endothelium cells and
inflammatory response.
Acknowledgments
The present study was funded by the Medical and
Health Platform Program of Zhejiang Province (key support) (grant
no. 2015ZDA022), Zhejiang Provincial Program for the Cultivation of
High-Level Innovative Health Talents (2014-108) and the Natural
Sciences Fund of Zhejiang Province (grant nos. LY17H290006 and
LY12H29005).
Glossary
Abbreviations
Abbreviations:
|
APE
|
acute pulmonary embolism
|
|
HR
|
heart rate
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL
|
interleukin
|
|
NF
|
nuclear factor
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
BNP
|
brain natriuretic peptide
|
|
TnT
|
troponin T
|
|
MMP
|
matrix metallopeptidase
|
|
shRNA
|
short hairpin RNA
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
SD
|
Sprague-Dawley
|
|
MCMV
|
murine cytomegalovirus
|
|
GFP
|
green fluorescent protein
|
|
H&E
|
hematoxylin and eosin staining
|
|
TXA2
|
thromboxane A2
|
|
TNNI3
|
troponin I type 3
|
|
D2D
|
D-dimer
|
|
ICAM
|
intercellular adhesion molecule
|
|
AD
|
adenovirus
|
|
PASP
|
pulmonary arterial systolic
pressure
|
|
PADP
|
pulmonary artery diastolic
pressure
|
|
PAP
|
pulmonary arterial pressure
|
|
VTE
|
venous thromboembolism
|
References
|
1
|
Geerts WH, Bergqvist D, Pineo GF, Heit JA,
Samama CM, Lassen MR and Colwell CW: Prevention of venous
thromboembolism: American college of chest physicians
evidence-based clinical practice guidelines (8th edition). Chest.
133(Suppl 6): 381S–453S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen AT, Agnelli G, Anderson FA, Arcelus
JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL,
Kakkar AK, et al VTE Impact Assessment Group in Europe (VITAE):
Venous thromboembolism (VTE) in Europe: The number of VTE events
and associated morbidity and mortality. Thromb Haemost. 98:756–764.
2007.PubMed/NCBI
|
|
3
|
Yang Y, Liang L, Zhai Z, He H, Xie W, Peng
X and Wang C; Investigators for National Cooperative Project for
Prevention and Treatment of PTE-DVT: Pulmonary embolism incidence
and fatality trends in chinese hospitals from 1997 to 2008: A
multicenter registration study. PLoS One. 6:e268612011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Wu J, Zhang W, Zhi Y, Wu Y, Jiang
R and Yang R: Effects of aspirin on the ERK and PI3K/Akt signaling
pathways in rats with acute pulmonary embolism. Mol Med Rep.
8:1465–1471. 2013.PubMed/NCBI
|
|
5
|
Wang LC, Jiang RL, Zhang W, Wei LL and
Yang RH: Effects of aspirin on the expression of nuclear factor-κB
in a rat model of acute pulmonary embolism. World J Emerg Med.
5:229–233. 2014. View Article : Google Scholar
|
|
6
|
Wang LC, Wu JN, Xia GL, Mao W, Ying RB,
Huang LQ and Jiang RL: Effect of aspirin on fractalkine in rats
with pulmonary embolism. Trop J Pharm Res. 13:753–760. 2014.
View Article : Google Scholar
|
|
7
|
Jiang R, Wei L, Zhu M, Wu J and Wang L:
Aspirin inhibits LPS-induced expression of PI3K/Akt, ERK, NF-κB,
CX3CL1, and MMPs in human bronchial epithelial cells. Inflammation.
39:643–650. 2016. View Article : Google Scholar
|
|
8
|
Konstantinides S, Torbicki A, Agnelli G,
Danchin N, Fitzmaurice D, Galiè N, Gibbs J, Huisman M, Humbert M,
Kucher N, et al; SEC Working Group for the ESC 2014 Guidelines on
the Diagnosis and Management of Acute Pulmonary Embolism; Expert
Reviewers for the ESC 2014 Guidelines on the Diagnosis and
Management of Acute Pulmonary Embolism. SEC Clinical Practice
Guidelines Committee: Comments on the 2014 ESC Guidelines on the
diagnosis and management of acute pulmonary embolism. Rev Esp
Cardiol (Engl Ed). 68:10–16. 2015.
|
|
9
|
Schmeck J, Koch T, Patt B, Heller A,
Neuhof H and van Ackern K: The role of endothelin-1 as a mediator
of the pressure response after air embolism in blood perfused
lungs. Intensive Care Med. 24:605–611. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bazan JF, Bacon KB, Hardiman G, Wang W,
Soo K, Rossi D, Greaves DR, Zlotnik A and Schall TJ: A new class of
membrane-bound chemokine with a CX3C motif. Nature. 385:640–644.
1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Todorova D, Sabatier F, Doria E, Lyonnet
L, Vacher Coponat H, Robert S, Despoix N, Legris T, Moal V, Loundou
A, et al: Fractalkine expression induces endothelial progenitor
cell lysis by natural killer cells. PLoS One. 6:e266632011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumiya T, Ota K, Imaizumi T, Yoshida H,
Kimura H and Satoh K: Characterization of synergistic induction of
CX3CL1/fractalkine by TNF-alpha and IFN-gamma in vascular
endothelial cells: An essential role for TNF-alpha in
post-transcriptional regulation of CX3CL1. J Immunol.
184:4205–4214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szukiewicz D, Kochanowski J, Mittal TK,
Pyzlak M, Szewczyk G and Cendrowski K: CX3CL1 (fractalkine) and
TNFα production by perfused human placental lobules under normoxic
and hypoxic conditions in vitro: The importance of CX3CR1
signaling. Inflamm Res. 63:179–189. 2014. View Article : Google Scholar
|
|
14
|
Apostolakis S and Spandidos D: Chemokines
and atherosclerosis: Focus on the CX3CL1/CX3CR1 pathway. Acta
Pharmacol Sin. 34:1251–1256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ars C, Thurion P, Delos M, Sibille Y and
Pilette C: Small airway obstruction in severe pulmonary arterial
hypertension correlates with increased airway CD8+
T-cells and fractalkine expression. Eur Respir J. 34:1494–1496.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ostrowski RP, Jadhav V, Chen W and Zhang
JH: Reduced matrix metalloproteinase-9 activity and cell death
after global ischemia in the brain preconditioned with hyperbaric
oxygen. Acta Neurochir Suppl (Wien). 106:47–49. 2010. View Article : Google Scholar
|
|
17
|
Wang C, Zhai Z, Yang Y, Cheng Z, Ying K,
Liang L, Dai H, Huang K, Lu W, et al: Inverse relationship of
bleeding risk with clot burden during pulmonary embolism treatment
with LMW heparin. Clin Respir J. 10:596–605. 2016. View Article : Google Scholar
|
|
18
|
Bhattacharyya S, Ghosh S and Sil PC:
Amelioration of aspirin induced oxidative impairment and apoptotic
cell death by a novel antioxidant protein molecule isolated from
the herb Phyllanthus niruri. PLoS One. 9:e890262014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto Y and Gaynor RB: Therapeutic
potential of inhibition of the NF-kappaB pathway in the treatment
of inflammation and cancer. J Clin Invest. 107:135–142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szukiewicz D, Wojciechowska M, Bilska A,
Stangret A, Szewczyk G, Mittal TK, Watroba M and Kochanowski J:
Aspirin action in endothelial cells: Different patterns of response
between chemokine CX3CL1/CX3CR1 and TNF-α/TNFR1 signaling pathways.
Cardiovasc Drugs Ther. 29:219–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo Y, Apostalakis S, Blann AD and Lip GY:
Plasma CX3CL1 levels and long term outcomes of patients with atrial
fibrillation: The West Birmingham Atrial Fibrillation Project.
Cerebrovasc Dis. 38:204–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogonda L, Hill J, Doran E, Dennison J,
Stevenson M and Beverland D: Aspirin for thromboprophylaxis after
primary lower limb arthroplasty: Early thromboembolic events and 90
day mortality in 11,459 patients. Bone Joint J. 98-B:341–348. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Braithwaite I, Dunbar L, Eathorne A,
Weatherall M and Beasley R: Venous thromboembolism rates in
patients with lower limb immobilization after Achilles tendon
injury are unchanged after the introduction of prophylactic
aspirin: Audit. J Thromb Haemost. 14:331–335. 2016. View Article : Google Scholar
|
|
24
|
Kaye ID, Patel DN, Strauss EJ, Alaia MJ,
Garofolo G, Martinez A and Jazrawi LM: Prevention of venous
thromboembolism after arthroscopic knee surgery in a low-risk
population with the use of aspirin. A randomized trial. Bull Hosp
Jt Dis (2013). 73:243–248. 2015.
|