Introduction
Staphylococcus aureus is a major bacterial
pathogen that can cause severe infections in both the hospital and
the community (1). This bacterium
can cause many kinds of infection, including pneumonia, sepsis,
wound sepsis, endocarditis, catheter-related infections and urinary
tract infections (2,3). Methicillin-resistant
Staphylococcus aureus (MRSA) is a bacterium that is
resistant to a variety of antibiotics, including β-lactams,
aminoglycosides, quinolones, oxazolidinone, vancomycin and
streptogramin type antibiotics (4). Infections caused by MRSA are a
worldwide healthcare problem (5).
Therefore, there is an urgent need for antibiotics to which MRSA is
susceptible to further control the spread of illness caused by
MRSA.
Antibiotic resistant MRSA is a significant challenge
for all scientists who are involved in antibiotic drug discovery.
One study suggested that combination drug treatment was an
effective method to slow down or stop the development of
drug-resistant bacteria (6). The
mechanism of antibiotic activity against infections caused by
Staphylococcus aureus (S. aureus) includes an
interference with bacterial protein and nucleic acid synthesis,
inhibition of metabolic pathways, disruption of the bacterial
membrane structure and cell wall biosynthesis (7–9).
By contrast, S. aureus strains become resistant to β-lactam
antibiotics by producing penicillin-binding protein 2a (PBP2a), a
protein with lower binding affinity to β-lactams. In normal
circumstances, S. aureus strains produce penicillin-binding
proteins (PBPs) for synthesis of bacterial cell walls (10,11). In resistant S. aureus
strains, PBP2a replaces the function of normal PBPs.
Acanthopanax henryi (A. henryi)
(Oliv.) Harms belongs to the Araliaceae family and may be used as a
traditional oriental medicine for the treatment of rheumatism and
inflammation (12,13). Some studies have also reported
that it has a strong antioxidant activity and improves the symptoms
of Alzheimer's disease (14,15). However, the antimicrobial activity
of A. henryi (Oliv.) Harms has not been evaluated.
In the present study, the antibacterial effect of
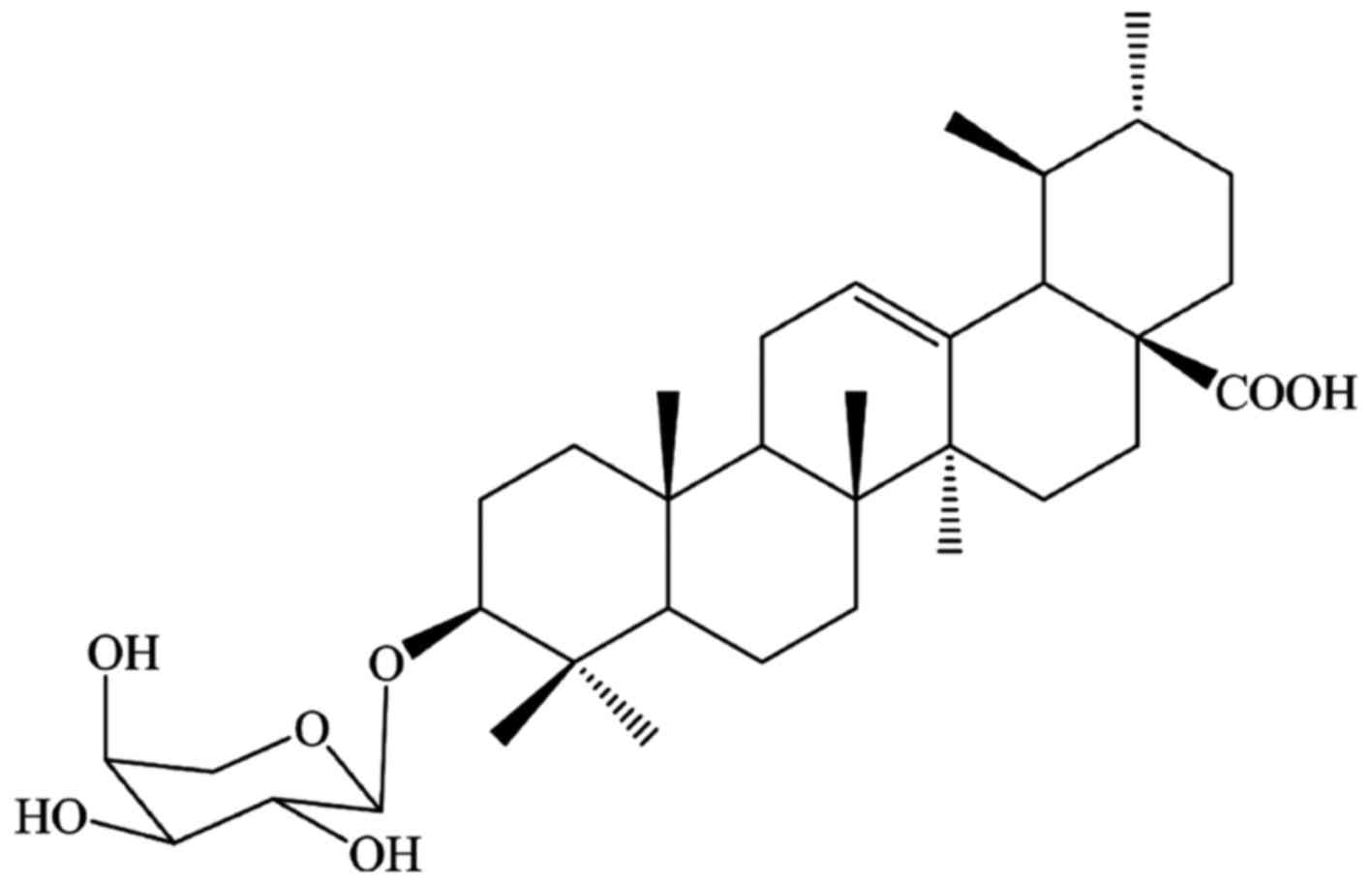
ursolic acid 3-O-α-L-arabinopyranoside (URS) (Fig. 1), isolated from the leaves of
A. henryi (Oliv.) Harms, against MRSA was investigated. To
evaluate the anti-MRSA mechanisms of URS, the synergistic effect of
URS combined with oxacillin (OXA), the anti-MRSA activity of URS
combined with a membrane permeability agent and ATPase inhibitor,
the morphological changes in bacterial cells and the levels of
PBP2a production were evaluated.
Materials and methods
Plant materials
The leaves of A. henryi (Oliv.) Harms were
collected in October 2012 in Xinhua, Changsha, China. The plant
species was confirmed by Professor Xiang-Qian Liu (Hunan Key
Laboratory of Traditional Chinese Medicine Modernization, Hunan
University of Chinese Medicine, Changsha, China) and the voucher
specimen (no. 20121125) was deposited at the School of Pharmacy,
Hunan University of Chinese Medicine.
Extraction and isolation
The dried leaves of A. henryi (Oliv.) Harms
(10 kg) were cut into small pieces, extracted three times with MeOH
(3×100 ml) at room temperature, and concentrated under reduced
vacuum to obtain a dark-green residue (0.8 kg). The residue was
then suspended in H2O and partitioned with petroleum
ether. The water fraction was fractionated using column
chromatography (CC) on macroporous resin and eluted with a gradient
of EtOH/H2O (0, 30, 50, 75 and 95%) into five fractions
(1–5). Fraction 4 (75% EtOH, 14.0 g) was
subjected to silica gel CC and eluted with
CHCl3/MeOH/H2O (25:1:0/1:1:0.2) to give
fifteen fractions (A-O). Fraction C (119 mg) was re-fractionated on
silica gel H CC and eluted with
CHCl3/MeOH/H2O (15:1:0/6:1:0.1) to give six
sub-fractions (C1-C6). Sub-fraction C3 (106.0 mg) was subjected to
ODS CC and eluted with a gradient of MeOH/H2O (70, 80,
90 and 100%) to yield 12.0 mg URS (16).
The compound structures were identified using mass
spectros copy, 1D-nuclear magnetic resonance (NMR) and 2D-NMR and
the spectral data were compared with those reported previ ously
(16). 1H NMR and
13C NMR spectra were measured on a Varian INOVA 400 M
spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA)
with chemical shifts reported as ppm (tetramethylsilane as internal
standard). Electrospray ionization mass spectra were then measured
using an Agilent 6530 Accurate-Mass Q-TOF (Agilent Technologies,
Inc.).
High performance liquid chromatography
(HPLC)
The purity of URS was >98%, as determined via
HPLC as previously described (17). Briefly, URS was dissolved in MeOH
to a concentration of 0.1 mg/ml for HPLC analysis by using a
Kinetex XB-C18 analytical column (100×4.6 mm ×2.6 µm;
Phenomenex, Inc., Torrance, CA, USA) at 30°C. Elution was conducted
using mobile phase A (water) and mobile phase B (acetonitrile) with
a gradient as follows: 0–2 min, 29–31% B; 2–13 min, 31–35% B; 13–15
min, 35–40% B; 15–23 min, 40–44% B; 23–25 min, 44–46% B; 25–31 min,
46–49% B; and 31–38 min, 49–55% B. The flow rate was constant at
1.0 ml/min and the effluents were monitored at 210 nm using an
Agilent 1200 HPLC system with variable wavelength detector (Agilent
Technologies, Inc.). The purity value was found to be >98% using
a peak area normalization method. The purity value was obtained by
calculating the percentage of the URS peak area to that of the
total peaks in the HPLC chromatogram.
Bacterial strains and culture medium
Among the eight strains of S. aureus used in
the present study, two clinical MRSA isolates, DPS-1 and DPS-2, as
the references (18,19) mentioned, were collected from two
different patients at Wonkwang University Hospital (Iksan, Korea);
two strains were MRSA ATCC 33591 and methicillin-susceptible S.
aureus (MSSA) ATCC 25923, purchased from the American Type
Culture Collection (Manassas, VA, USA); and the remaining four MRSA
strains, CCARM 3090, CCARM 3091, CCARM 3095, CCARM 3102, were
provided by the Culture Collection of Antimicrobial Resistant
Microbes (National Research Resource Bank, Seoul, Korea). All
bacteria were cultured on either Mueller-Hinton agar (MHA) or Brain
Heart Infusion agar at 37°C for 24 h. The bacterial strains were
suspended in either Mueller-Hinton broth (MHB) or brain heart
infusion broth (BHIB) and grown at 37°C for 24 h in order to
perform the experiments. The bacteria were stored in 30% glycerol
and frozen at −80°C.
Materials and reagents
Difco™ Mueller-Hinton agar, Difco™ Mueller-Hinton
broth, Difco™ brain heart infusion agar, Bacto™ Brain Heart
Infusion broth and Difco™ skim milk were obtained from Difco
Laboratories (Baltimore, MD, USA). Glycerol was obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). MTT, Triton X-100
(TX-100), N,N′-dicyclohexylcarbodiimide (DCCD), paraformaldehyde,
glutaraldehyde, sodium cacodylate buffer, osmium tetroxide, uranyl
acetate, EtOH, MeOH, propylene oxide, Spurr's resin, OXA and
solvents were purchased from Sigma-Aldrich; Merck KGaA. SMART™
bacterial protein extraction solution was purchased from Intron
Biotechnology, Inc. (Seongnam, Korea). The chemiluminescent ECL
assay kit was purchased from ATTO Corp. (Tokyo, Japan).
Determination of the minimal inhibitory
concentration (MIC)
A total of 8 bacterial strains were subjected to
antimicrobial susceptibility and MIC assays. The MIC values of URS
and the antibiotic, OXA, against MRSA and MSSA were determined via
broth microdilution assay using a 96-well microplate, according to
a previous study (19). A series
of 2-fold dilutions of URS in MHB and BHIB were prepared and the
bacteria colonies were picked with a 1 µl white sterile loop
and needle to be suspended in either MHB or BHIB. The inocula were
adjusted to the 0.5 of the McFarland standard scale
[~1.5×108 colony-forming units (CFU)/ml] and the final
inocula were adjusted to 1.5×105 CFU/spot. The
inoculated broth was incubated at 37°C for 24 h and the MIC was
determined using MTT reagent. Following the 24 h incubation, MTT (1
mg/ml) was added to the broth suspension in every well and the
plate was incubated for 30 min in a 37°C incubator. Blue color
indicated the presence of bacteria (18,20). The MIC was defined as the lowest
concentration that inhibited bacterial growth.
Checkerboard dilution test
The synergistic effect between URS and OXA was
determined using a checkerboard dilution test (21). Serial dilutions of URS with
different concentrations OXA were mixed in cation-supplemented MHB
and BHIB. Each test strain of the final inocula concentration was
adjusted to 1.5×105 CFU/ml and incubated at 37°C for 24
h. For the synergy studies, the range of concentrations used was
determined according to the previously determined MIC of OXA for
each specific isolate. The concentration of URS ranged from 6.25 to
0.19 µg/ml. Following a 24 h incubation, the MICs were
interpreted. The in vitro interaction between the drugs was
quantified by determining the fractional inhibitory concentration
(FIC). The FIC of each agent was calculated as the MIC of the
agents in combination, divided by the MIC of the agent alone. The
FIC index (FICI) was calculated using the following formula: FICI =
FICA + FICB = [A]/MICA +
[B]/MICB, where [A] and [B] were the concentrations of
drug A and B, respectively, and MICA/FICA and
MICB/FICB were the MIC/FIC of drug A and B,
respectively. The FICI was interpreted as follows: <0.5,
synergy; 0.5–0.75, partial synergy; 0.75–1, additive effect; 1–4,
no effect; and >4, antagonism. Finally, the different values of
synergy between the two agents were calculated (22).
Time-kill assay
The synergistic antimicrobial effect was determined
using a time-kill assay, as described previously (23). URS combined with OXA, OXA alone
and URS alone were compared to control (drug-free) regarding the
synergistic effect on the bacterial growth curve (24). At five different time phases (0,
4, 8, 16 and 24 h), bacterial growth curves were observed.
Bacterial cultures were diluted with fresh MHB to
~1.5×105 CFU/ml and the bacteria suspensions were
incubated at 37°C for 24 h. Aliquots (0.1 ml) of the suspension
were taken at 0, 4, 8, 16 and 24 h of incubation and serial 10-fold
dilutions were prepared in saline as needed. Following incubation
for 24 h, the number of viable cells was determined on a drug-free
MHA plate. Colony counts were performed on plates and 30–300
colonies were calculated. The lower limit of sensitivity of the
colony counts was 100 CFU/ml. The antimicrobial agents used were
considered bactericidal at the lowest concentration that reduced
the original inoculum by 3 log10 CFU/ml (99.9%) for each
of the indicated times. However, they were designated
bacteriostatic if the inoculum was reduced by 0–3 log10
CFU/ml.
Effect of URS on membrane-permeabilizing
agents and ATPase inhibitors
To explore whether the antibacterial activity of URS
was associated with the altered membrane permeability or the action
of ATPase, the authors evaluated the antibacterial activity of URS
in the presence of a detergent and an ATPase-inhibiting agent
(19). To determine the
detergent-induced permeabilization, an appropriate concentration of
URS was determined using the detergent TX-100 (25), which significantly increases
bacterial sensitivity to antibiotics (26). DCCD, a metabolic inhibitor that
can decrease ATP levels by disrupting electrochemical proton
gradients in a bacterial environment, was used as an inhibitor of
ATPase (19,27). The bacteria culture were adjusted
to 1.5×105 CFU/ml and 100 µl/well was added to
96-well microplate. A total density of 10 µl/well of URS, at
a concentration of 1/64 MIC, with the presence of 0.00001% TX-100
and 250 µg/ml DCCD were individually added to the 96-well
microplate and incubated at 37°C for 24 h. The results were read at
optical density 600 nm.
Transmission electron microscopy
(TEM)
On the basis of biological activity, morphological
changes in MRSA ATCC 33591 following the addition of URS were
evaluated using TEM, according to a previously described protocol
with some modifications (28,29). MRSA exponential phase cultures
were prepared by diluting cultures in MHB overnight and cell growth
was continued at 37°C until the cultures reached the
mid-logarithmic phase of growth. The MHB-grown exponential-phase
MRSA was treated with 31.25 µg/ml OXA alone, 1/2 MIC of URS
alone, and 31.25 µg/ml OXA + 1/2 MIC of URS for 4 h.
Following treatment, 2 ml of the culture was collected by
centrifugation at 10,000 × g for 10 min. Following the removal of
the supernatant, pellets were washed with MHB and primary fixed
with 2% paraformaldehyde and 2% glutaraldehyde at 4°C for 2 h.
Samples were washed and resuspended thrice in 0.05 M sodium
cacodylate buffer (pH 7.2) at 4°C for 10 min, then post-fixed with
1% OsO4 at 4°C for 2 h. The samples were then washed
twice with sterile-distilled water at room temperature. Thereafter,
the samples were en bloc stained with 0.5% uranyl acetate at 4°C
for 30 min and dehydrated using a graded EtOH series. Finally, 100%
propylene oxide was used for transit and infiltration with
propylene oxide and Spurr's resin reagents in a specific ratio. The
specimens were examined using an energy-filtering transmission
electron microscope (LIBRA 120; Carl Zeiss GmbH, Oberkochen,
Germany) operated at an accelerating voltage of 120 kV. Transmitted
electron signals were recorded using a 4×4 k slow-scan
charge-coupled device camera (Ultrascan 4000 SP; Gatan, Pleasanton,
CA, USA) attached to an electron microscope.
Western blotting
The western blot assay was performed according to
the standard procedures to measure the translated protein level
(30,31). The MRSA culture (ATCC 33591) was
grown at an OD600 of 0.4 in MHB and treated with various
concentrations and combinations of OXA and URS for 4 h for western
blot analysis (32). Briefly,
cells were harvested and suspended in SMART™ bacterial protein
extraction solution containing Tris-HCl (pH 7.5). The extraction
was performed according to the manufacturer's protocol. Protein
concentrations were measured using the Bio-Rad protein assay
reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and cell
lysates were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The
electrophoresed gels were transferred to Amersham™
Hybond™-P-membranes (GE Healthcare Life Sciences, Chalfont, UK).
The membranes were blocked with 5% skim milk for 1 h and hybridized
with monoclonal mouse anti-PBP2a primary antibody (1:500, cat. no.
70PB001; DiNonA Inc., Seoul, Korea) overnight at 4°C. Loading
differences were normalized with monoclonal anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH) antibody (1:500, cat. no.
sc-166574, Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Following incubation with anti-mouse IgG secondary antibody
(1:1,000, cat. no. G-21040; Enzo Life Sciences, Ann Arbor, MI, USA)
at room temperature for 1 h, immunoreactive proteins were detected
using a chemiluminescent ECL assay kit (ATTO Corp.) according to
the manufacturer's instructions. Western blot bands were visualized
using ImageQuant LAS 4000 Mini Luminescent image analyzer (GE
Healthcare Life Sciences) and the quantitative measurement of band
intensity was performed using ImageJ software (version 1.45S;
National Institutes of Health, Bethesda, MA, USA)
Statistical analysis
Analyses were performed in triplicate and data were
presented as the mean ± standard deviation. Results were
statistically analyzed using an independent Scheffe's t-test (SPSS
software version 22.0; IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Antimicrobial activity of URS and
antibiotics
Antimicrobial susceptibility studies were performed
using a broth microdilution method. The MIC values of URS and OXA
against eight strains of S. aureus are presented in Table I. The data indicated that URS,
which was isolated from the leaves of A. henryi (Oliv.)
Harms, had high antimicrobial activity against MRSA and MSSA. The
MIC values of URS against MSSA and MRSA were 3.125 and 6.25
µg/ml, respectively. The MIC of OXA against MSSA was
<0.97 µg/ml, whereas the MIC against MRSA ranged from 3.9
to 2,000 µg/ml. The high MIC values of OXA against MRSA
confirmed that the studied strains were resistant to OXA, whereas
the MIC value of OXA against MSSA indicated the susceptibility of
this strain to the antibiotic (11).
 | Table IMIC values of URS and OXA against
MSSA and MRSA. |
Table I
MIC values of URS and OXA against
MSSA and MRSA.
| S. aureus
strains | MIC (µg/ml)
|
|---|
| URS | OXA |
|---|
| ATCC 25923 | 3.125 | <0.97 |
| ATCC 33591 | 6.25 | 62.5 |
| CCARM 3090 | 6.25 | 500 |
| CCARM 3091 | 6.25 | 2000 |
| CCARM 3095 | 6.25 | 500 |
| CCARM 3102 | 6.25 | 500 |
| DPS-1 | 6.25 | 500 |
| DPS-2 | 6.25 | 3.9 |
Synergistic effects of URS and OXA based
on FICI
Evaluation of the synergistic effect of URS and OXA
in combination against MRSA was performed using a checkerboard
dilution method. The results are presented in Table II and suggested that in the
presence of URS, the susceptibility of MRSA to OXA increased.
Treatment with 1/2 MIC URS in combination with OXA reduced the MIC
of OXA by 2–32-fold.
 | Table IIResults of the combination of URS and
OXA against MRSA strains. |
Table II
Results of the combination of URS and
OXA against MRSA strains.
| Strains | Agent | MIC (µg/ml)
| FICI | Outcome |
|---|
| Alone | Combination |
|---|
| ATCC 33591 | URS | 6.25 | 1.56 | 0.75 | Partial S. |
| OXA | 62.5 | 31.25 | | |
| CCARM 3090 | URS | 6.25 | 3.125 | 0.53 | Partial S. |
| OXA | 500 | 15.6 | | |
| CCARM 3095 | URS | 6.25 | 3.125 | 0.56 | Partial S. |
| OXA | 500 | 31.25 | | |
| CCARM 3102 | URS | 6.25 | 3.125 | 0.56 | Partial S. |
| OXA | 500 | 31.25 | | |
| DPS-1 | URS | 6.25 | 0.19 | 0.53 | Partial S. |
| OXA | 500 | 250 | | |
| DPS-2 | URS | 6.25 | 1.56 | 0.75 | Partial S. |
| OXA | 3.9 | 1.95 | | |
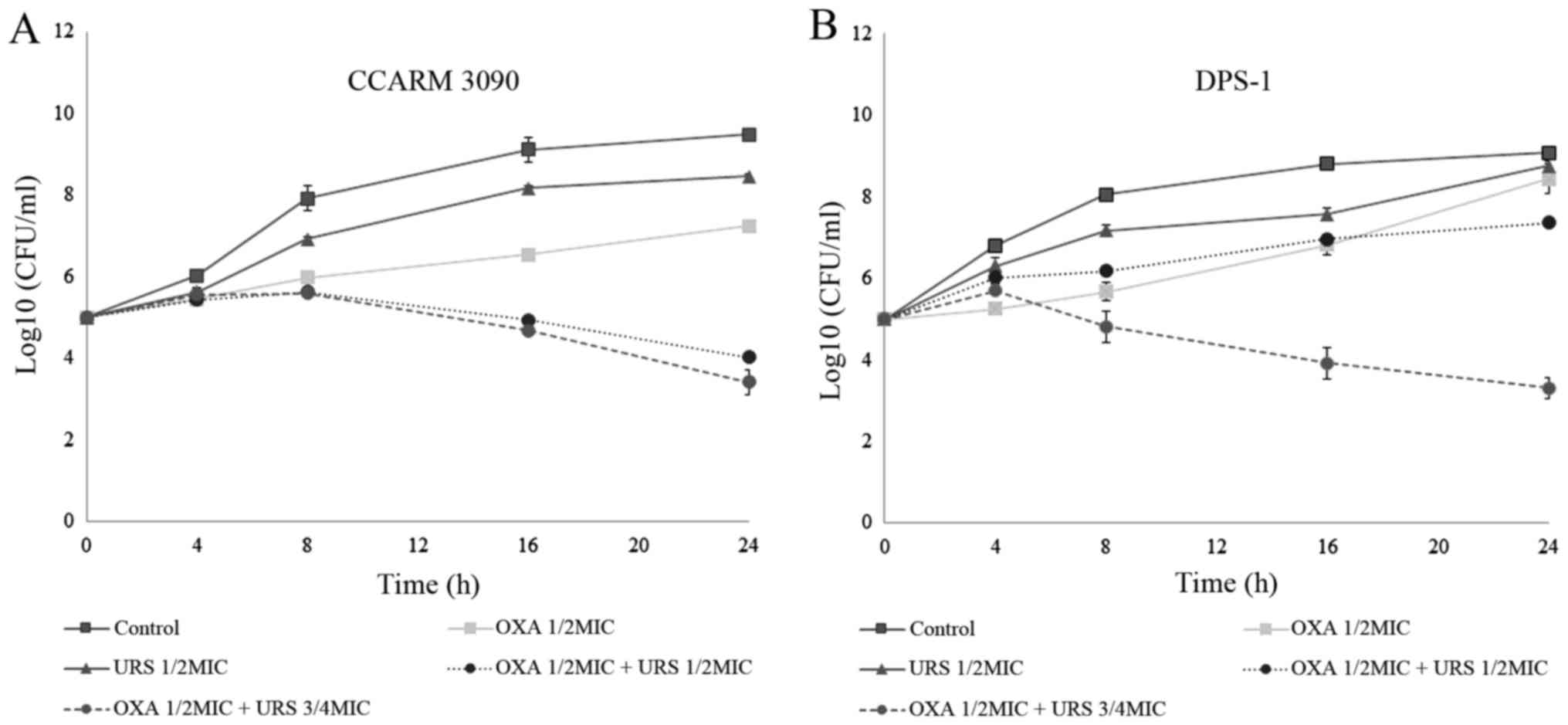
Time-kill curve assay
On the basis of FIC indices, the synergism of URS
and OXA against MRSA was confirmed using a time-kill assay. In the
present study, two strains, CCARM 3090 and DPS-1, were used to
perform the analysis. The results are reported in Fig. 2. The time-kill curves were
constructed with control, 1/2 MIC OXA alone, 1/2 MIC URS alone, 1/2
MIC OXA and 1/2 MIC URS in combination, and 1/2 MIC OXA and 3/4 MIC
URS in combination at time-points 0, 4, 8, 16 and 24 h. The results
are presented as the log value of the number of surviving bacteria
in the antimicrobial test at the different time intervals. As the
figures indicated, at 24 h, treatment with 1/2 MIC OXA and 3/4 MIC
URS in combination resulted in combined group bacteria counts that
decreased to 3 log10. However, the original
antibacterial-free control count was 1.5×105 CFU/ml,
which increased to almost 1010 CFU/ml after 24 h
(33). In addition, the time-kill
curves indicated a concentration-dependent bactericidal effect
against MRSA strains.
Antimicrobial activity with detergents
and ATPase inhibitors
MRSA CCARM 3090 was used to investigate the effects
of enhanced membrane permeability by using detergents and the
diversification of susceptibility by using ATPase inhibitors on the
activity of URS. The results are presented in Fig. 3. The membrane-permeabilizing
agent, TX-100, can increase the permeability of the outer membrane
in gram-negative bacteria (34).
Compared to the OD600 value of URS alone (1/64 MIC), the OD600
value of the suspension in the presence of 1/64 MIC URS and
0.00001% TX-100 was reduced 56.6%. From the results of the OD600
values, in the presence of either URS alone (1/64 MIC) or 250
µg/ml DCCD alone, MRSA maintained its viability. However,
the OD600 value of the suspension significantly decreased by URS in
combination with DCCD. Bacterial viability in the presence of 1/64
MIC URS and 250 µg/ml DCCD reduced to 11.7%.
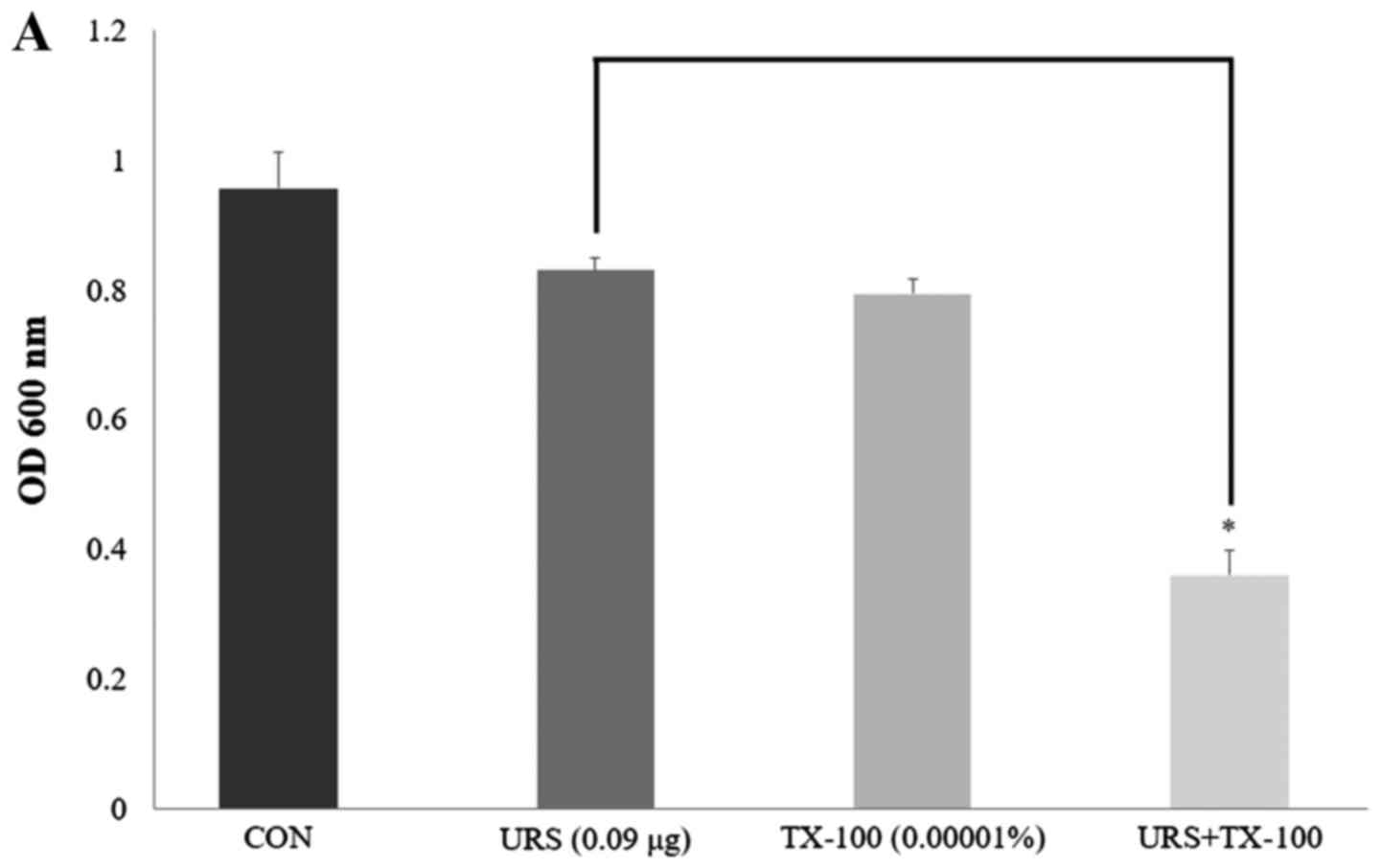 | Figure 3(A) The effect of the
membrane-permeabilizing agent, TX-100, on the susceptibility of
methicillin-resistant Staphylococcus aureus CCARM 3090 to
URS treatment. (B) The effect of the ATPase-inhibitor, DCCD, on the
susceptibility of MRSA CCARM 3090 to URS treatment. The viability
of bacteria was determined via spectrophotometry (optical density
at 600 nm, OD600) following incubation for 24 h with 0.09
µg/ml URS and 0.00001% TX-100 and 0.09 µg/ml URS and
250 µg/ml DCCD. These data are represented as the mean ±
standard deviation of three independent experiments.
*P<0.05 as indicated. CON, control S. aureus
strain, which was not treated; TX-100, Triton X-100; URS, ursolic
acid 3-O-α-L-arabinopyranoside; DCCD,
N,N′-dicyclohexylcarbodiimide; MRSA, methicillin-resistant
Staphylococcus aureus. |
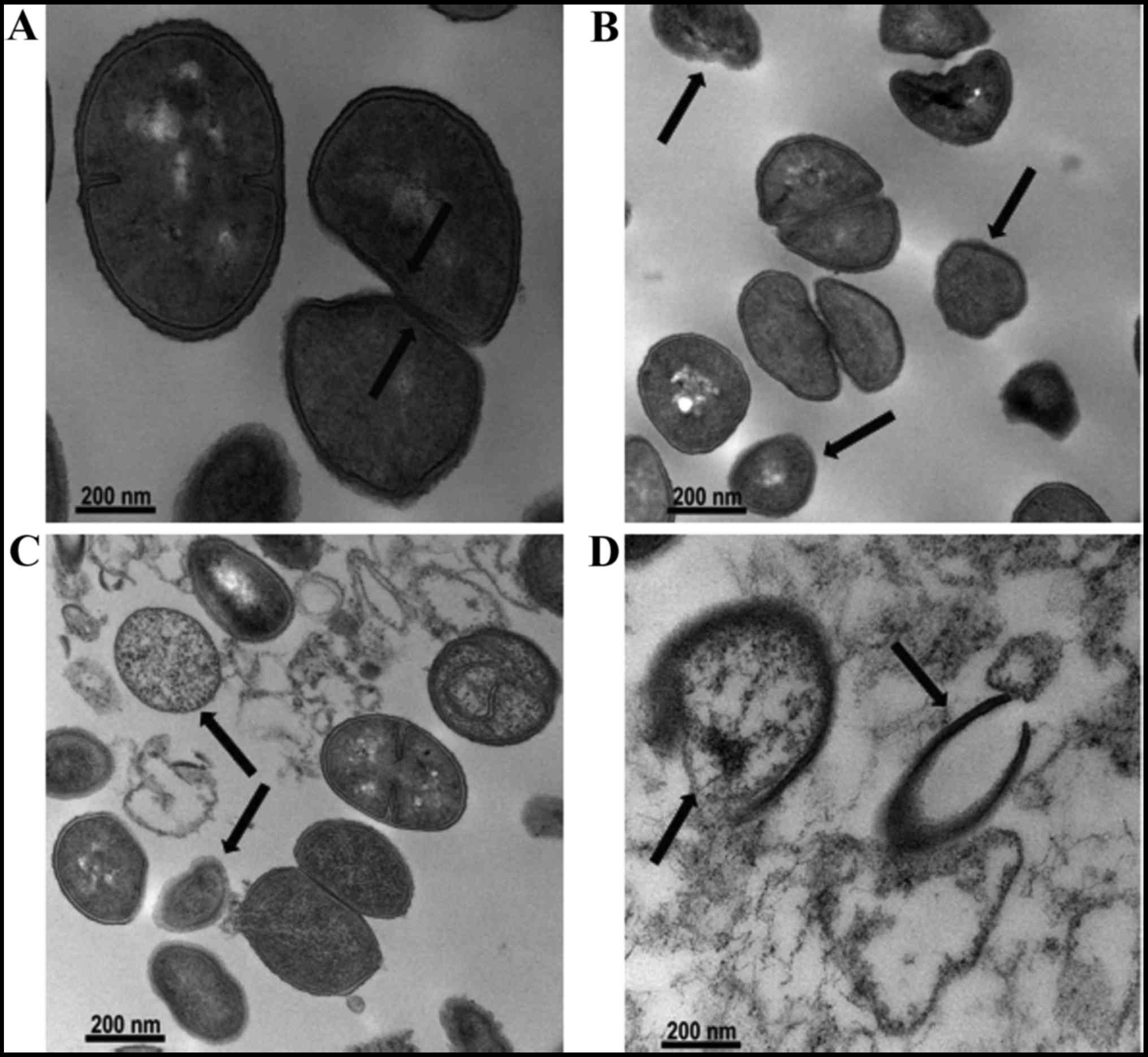
Effect on bacterial cell morphology
To determine morphological changes, MRSA ATCC 33591
treated with OXA (31.25 µg/ml) alone, URS (3.125
µg/ml) alone, and OXA and URS in combination was examined by
TEM analysis. The results are indicated in Fig. 4. The images indicated
characteristic morphological changes in the cells of MRSA ATCC
33591 after treatment with OXA and URS. The untreated bacterial
cells had normal morphology with distinct septa (Fig. 4A). In the presence of OXA and URS
individually, the cytoplasmic membranes of the bacterial cells were
damaged and had rougher surfaces (Fig. 4B and C) compared to those of
control cells. Following exposing MRSA to the combination of OXA
and URS, deformation of bacterial cells was observed compared to
groups treated with OXA alone and URS alone (Fig. 4D). This caused cell membrane
disintegration, cell lysis and release of cytoplasmic contents. In
addition, the cells appeared almost absent. This suggested a strong
bactericidal activity against MRSA. The notable changes in
bacterial cell morphology indicated that bacterial cell membrane
viscosity and permeability were compromised by treatment with the
combination of URS and OXA (24).
Expression of PBP2a protein in MRSA
To detect the protein level of PBP2a in MRSA,
western blotting was performed. PBP2a expression levels following
the tested treatments are summarized in Fig. 5. GAPDH, which served as an
internal control, was detected after all treatments (results not
shown). The experimental samples consisted of control, OXA (31.25
µg/ml), URS (6.25 µg/ml) and the combination of OXA
(31.25 µg/ml) and URS (6.25 µg/ml). As the figure
demonstrated, PBP2a was not completely inhibited; however, the
PBP2a protein level decreased non-significantly on the addition of
URS and OXA alone. Compared to control, the expression of PBP2a of
the combination group was reduced 36.98%. The decrease in the PBP2a
level may indicate that URS interrupted the process of protein
synthesis by damaging RNA.
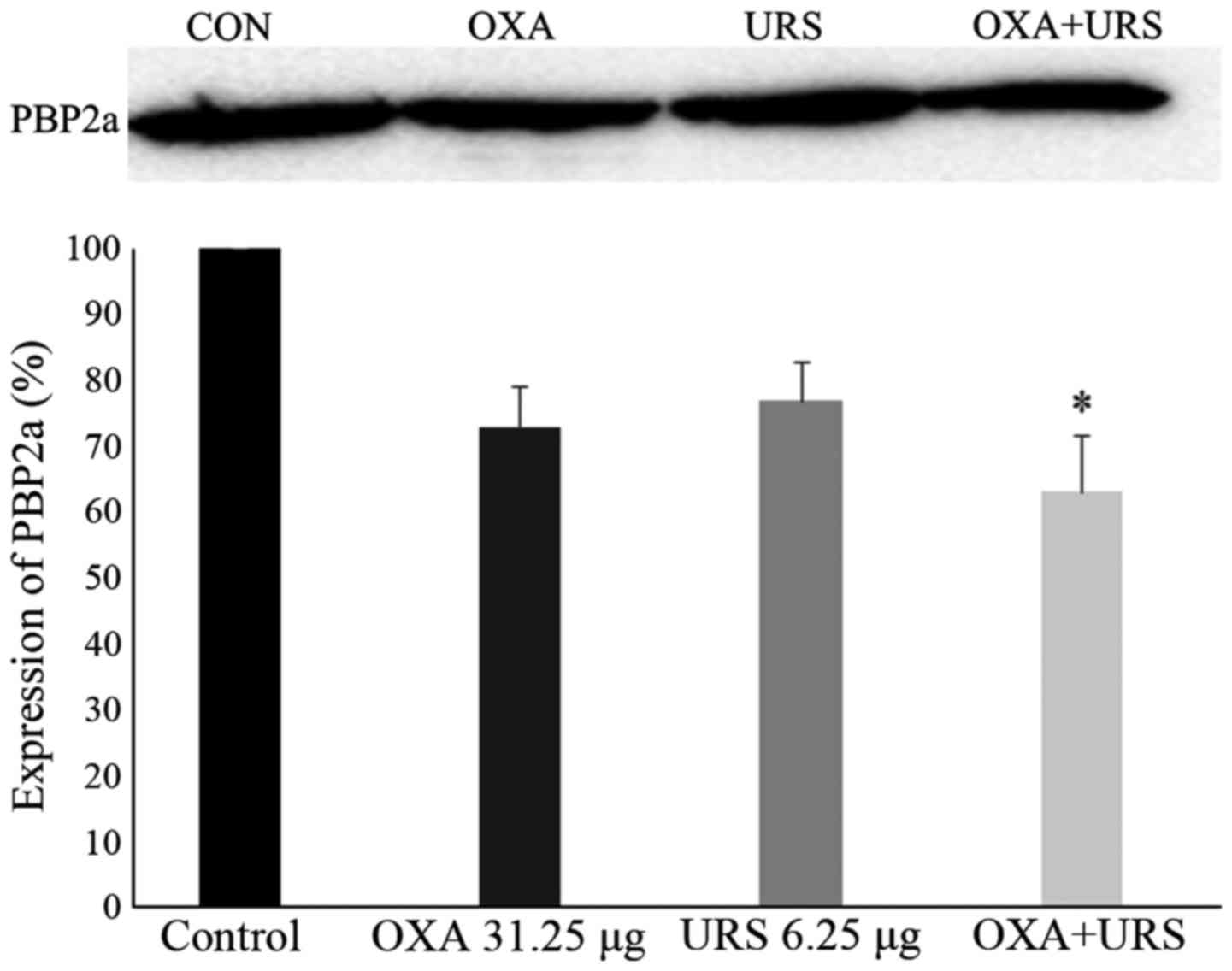 | Figure 5Expression of PBP2a in MRSA cultures
grown in the presence of sub-inhibitory concentrations of URS and
OXA. Western blotting image, lane 1, control MRSA; lane 2, OXA
31.25 µg/ml; lane 3, URS 6.25 µg/ml, lane 4, the
combination of URS and OXA. Quantitative densitometric analysis of
PBP2a expression in MRSA cultures grown in the presence of OXA
alone, URS alone, and OXA and URS in combination, normalized to
GAPDH loading control. These data are represented as the means ±
standard deviation of 3 independent experiments.
*P<0.05 vs. control. PBP2a, penicillin-binding
protein 2a; MRSA, methicillin-resistant Staphylococcus
aureus; OXA, oxacillin; URS, ursolic acid
3-O-α-L-arabinopyranoside. |
Discussion
MRSA, a gram-positive bacterial pathogen, can cause
infections in a wide range of human tissues. Because MRSA acquires
resistance to most antibiotics, few new drugs are available to
treat it (35). The increasing
emergence of multidrug-resistant bacteria is a worldwide healthcare
problem. Therefore, new effective antimicrobial agents or novel
therapeutic approaches for the treatment of infectious diseases
caused by drug-resistant bacteria, including MRSA, are clearly
needed. Previously, to control pathogenic microorganisms, there has
been considerable interest in traditional Chinese medicine natural
products isolated from herbal medicines for use as alternative
treatments (7,23). Some researchers reported that an
effective strategy to conquer resistance mechanisms was the use of
drug combinations, such as β-lactams together with β-lactamase
inhibitors (36). In the present
study, the authors demonstrated the synergism and mechanism of
action of URS, obtained from the leaves of A. henryi (Oliv.)
Harms, combined with OXA against MRSA.
In the present study, the MIC values of URS and OXA
were determined using broth microdilution assay. The MICs of URS
against MRSA and MSSA were 6.25 and 3.125 µg/ml,
respectively (Table I). These
results indicated strong antibacterial activity against MRSA and
MSSA. The low MIC exhibited by URS is very rare in natural
products. The synergistic effects of URS and OXA were analyzed
using a checkerboard dilution method, which indicated a partial
synergistic effect between URS and OXA in various MRSA strains
(Table II). When in the presence
of URS at sub-inhibitory concentrations, the MIC of OXA was reduced
by as much as 32-fold, from 500 to 15.6 µg/ml. This also
indicated that URS restored the susceptibility of MRSA to OXA
(11). Combination therapy is an
urgently recommended empirical treatment for bacterial infections
and for preventing the emergence of resistant mutant strains of
bacteria (37,38). The time-kill growth curves further
confirmed the synergism between URS and OXA at sub-inhibitory
concentrations (Fig. 2).
Treatment with the combination of 3/4 MIC URS and 1/2 MIC OXA
inhibited the growth of MRSA compared to MRSA strains that were
treated with either URS or OXA alone. In addition, bacterial growth
was suppressed after 8 h with this combination, but a total kill
was not achieved. At the beginning of the exponential phase, the
control with a rapidly growing and the combination group had same
tendency. But the combination group with a slight bacterial growth
compared to the control at exponential phase. An extended lag phase
was observed compared to control. The lag phase is a special stage
when bacteria equilibrate to adapt to a new environment by
undergoing macromolecular repair and synthesis of cellular growth
through DNA replication. Therefore, we inferred that the lengthy
lag phase observed in our study was due to the inhibition of DNA
replication, which delayed the cellular growth process (11,39).
The reagents, TX-100 and DCCD, were used in
combination with URS to detect the effects on bacterial cell
viability. The results indicated that the OD600 values of the
suspension were reduced by the combination of 0.09 µg/ml URS
with 0.00001% TX-100 or 250 µg/ml DCCD (Fig. 3). TX-100 has been reported to
enhance cell membrane permeability, decrease methicillin
resistance, and stimulate cell autolysis (28). DCCD, an inhibitor of ATPase,
inhibited the H+ translocation activity of the
F0 domain of F0F1-ATPase (7,19).
In the presence of detergent or an ATPase inhibitor, the
susceptibility of MRSA to URS was increased. Consequently, the
authors inferred that the antibacterial activity of URS was
associated with cytoplasmic membrane permeability and inhibition of
ATPase function, indicating the potential for using URS in
combination with detergents or ATPase inhibitors to treat MRSA
infections.
Understanding the fine ultrastructure of the
bacterial cell wall is important to gain insight into bacterial
physiology and the mechanism of action of antibiotics against
bacteria (40). Using TEM to
observe morphological changes in bacterial cells provides useful
insights into the mechanism underlying the activity of
antibacterial agents (41). When
bacteria cells were treated with URS and OXA, cell membrane
disintegration, cell lysis, and release of cytoplasmic contents
were observed (Fig. 4D) and the
ultrastructure impact on bacteria cells indicated URS had
antibacterial effects and was synergistic with OXA.
OXA is a β-lactam antibiotic that inhibits cell wall
peptidoglycans through binding and competitive inhibition with PBPs
(42). S. aureus
antibiotic resistance was caused by PBP2a production, which is a
protein that binds to β-lactam antibiotics with lower affinity
(10). PBP2a blocked the effects
and replaced the function of normal PBPs. Therefore, the inhibition
of PBP2a expression is an effective approach to restore the
susceptibility of MRSA to antibiotics. In the present study, the
protein expression of PBP2a was suppressed compared to the control
when samples were treated with the combination of URS and OXA, but
the presence of the band indicated that PBP2a expression was not
completely inhibited (Fig. 5).
Resistance to the β-lactam antibiotics, including OXA, is primarily
mediated by PBP2a production encoded by the mecA gene
(43). The results indicated an
antimicrobial effect of URS owing to an effect on PBP2a protein
levels and the further study of RNA levels is needed.
In this study, URS, isolated from the leaves of
A. henryi (Oliv.) Harms, is a plant-based antimicrobial
agent that was found to be effective against MRSA and MSSA. In
addition, the authors demonstrated the synergistic effect and
mechanism of action of URS combined with OXA in the treatment
against MRSA. Combination treatment indicated that URS had
potential as a novel antibacterial agent for antimicrobial therapy
of infections caused by MRSA. Notwithstanding the results obtained
in the present study proved the antimicrobial activity of URS in
vitro, a limitation of this study is the fact that we need
further confirm the antibacterial activity of URS in vivo,
and more experiments will be carried out in subsequent studies.
S. aureus secretes a wide range of virulence factors and
α-hemolysin plays an important role in the induction of lung injury
infected by S. aureus pneumonia (44). Staphylococcal enterotoxins are the
virulence factors result in gastroenteritis, which also cause the
food poisoning in human (45).
Further studies will include the influence of URS on staphylococcal
α-hemolysin and enterotoxin productions.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation (NRF) of
Korea funded by the Ministry of Education
(NRF-2016R1D1A1B03934552). Following are results of a study on the
'Leaders in Industry-University Cooperation' Project, supported by
the Ministry of Education and National Research Foundation of
Korea.
References
|
1
|
Jiang JH and Peleg AY:
Daptomycin-nonsusceptible Staphylococcus aureus: The role of
combination therapy with daptomycin and gentamicin. Genes (Basel).
6:1256–1267. 2015. View Article : Google Scholar
|
|
2
|
Mahdiyoun SM, Kazemian H, Ahanjan M, Houri
H and Goudarzi M: Frequency of aminoglycoside-resistance genes in
methicillin-resistant Staphylococcus aureus (MRSA) isolates from
hospitalized patients. Jundishapur J Microbiol. 9:e350522016.
View Article : Google Scholar :
|
|
3
|
Hu Y, Liu A, Vaudrey J, Vaiciunaite B,
Moigboi C, McTavish SM, Kearns A and Coates A: Combinations of
β-lactam or amino-glycoside antibiotics with plectasin are
synergistic against methicillin-sensitive and methicillin-resistant
Staphylococcus aureus. PLoS One. 10:e01176642015. View Article : Google Scholar
|
|
4
|
Ekambaram SP, Perumal SS, Balakrishnan A,
Marappan N, Gajendran SS and Viswanathan V: Antibacterial synergy
between rosmarinic acid and antibiotics against
methicillin-resistant Staphylococcus aureus. J Intercult
Ethnopharmacol. 5:358–363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Poulsen MØ, Jacobsen K, Thorsing M,
Kristensen NR, Clasen J, Lillebæk EM, Skov MN, Kallipolitis BH,
Kolmos HJ and Klitgaard JK: Thioridazine potentiates the effect of
a beta-lactam antibiotic against Staphylococcus aureus
independently of mecA expression. Res Microbiol. 164:181–188. 2013.
View Article : Google Scholar
|
|
6
|
McConeghy KW, Bleasdale SC and Rodvold KA:
The empirical combination of vancomycin and a β-lactam for
Staphylococcal bacteremia. Clin Infect Dis. 57:1760–1765. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee YS, Lee DY, Kim YB, Lee SW, Cha SW,
Park HW, Kim GS, Kwon DY, Lee MH and Han SH: The mechanism
underlying the antibacterial activity of Shikonin against
methicillin-resistant Staphylococcus aureus. Evid Based Complement
Alternat Med. 2015:5205782015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lowy FD: Antimicrobial resistance: The
example of Staphylococcus aureus. J Clin Invest. 111:1265–1273.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tenover FC: Mechanisms of antimicrobial
resistance in bacteria. Am J Med. 119(Suppl 1): S3–S70. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ike B, Ugwu MC, Ikegbunam MN, Nwobodo D,
Ejikeugwu C, Gugu T and Esimone CO: Prevalence, antibiogram and
molecular characterization of comunity-acquired
methicillin-resistant Staphylococcus Aureus in AWKA, Anambra
Nigeria. Open Microbiol J. 10:211–221. 2016. View Article : Google Scholar
|
|
11
|
Santiago C, Pang EL, Lim KH, Loh HS and
Ting KN: Reversal of ampicillin resistance in MRSA via inhibition
of penicillin-binding protein 2a by Acalypha wilkesiana. BioMed Res
Int. 2014:9653482014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SY, Yook CS, Nohara T, Mizutani T and
Tanaka T: Random amplified polymorphic DNA analysis of genetic
relationships among Acanthopanax species. Arch Pharm Res.
27:1270–1274. 2004. View Article : Google Scholar
|
|
13
|
Park SY: Studies on RAPD analysis and
triterpenoidal constituents of Acanthopanax species. Kumamoto
University Press. 3:1–3. 2002.
|
|
14
|
Zhang XD, Liu XQ, Kim YH and Whang WK:
Chemical constituents and their acetyl cholinesterase inhibitory
and antioxidant activities from leaves of Acanthopanax henryi:
Potential complementary source against Alzheimer's disease. Arch
Pharm Res. 37:606–616. 2014. View Article : Google Scholar
|
|
15
|
Kim JH, Liu XQ, Dai L, Yook CS and Lee KT:
Cytotoxicity and anti-inflammatory effects of root bark extracts of
Acanthopanax henryi. Chin J Nat Med. 12:121–125. 2014.PubMed/NCBI
|
|
16
|
Grace G, Paulo SE and Seligmann O: A new
saponin from mate, Ilex paraguariensis. J Nat Prod. 52:1367–1370.
1989. View Article : Google Scholar
|
|
17
|
Li Z: Simultaneous determination of
fifteen triterpenoid saponins in different medicinal parts of
Acanthopanax henryi by HPLC CAD ESI MS. Study on chemical
constituents of Acanthopanax henryi (Oliv.) Harms. Hunan University
of Traditional Chinese Medicine; pp. 45–66. 2015
|
|
18
|
Joung DK, Kang OH, Seo YS, Zhou T, Lee YS,
Han SH, Mun SH, Kong R, Song HJ, Shin DW, et al: Luteolin
potentiates the effects of aminoglycoside and β-lactam antibiotics
against methicillin-resistant Staphylococcus aureus in vitro. Exp
Ther Med. 11:2597–2601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joung DK, Mun SH, Choi SH, Kang OH, Kim
SB, Lee YS, Zhou T, Kong R, Choi JG, Shin DW, et al: Antibacterial
activity of oxyresveratrol against methicillin-resistant
Staphylococcus aureus and its mechanism. Exp Ther Med.
12:1579–1584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi YJ, Chen J and Xu M: A new method for
antimicrobial susceptibility testing of in vitro-cultured bacteria
by means of resonance light scattering technique. J Microbiol
Biotechnol. 18:118–123. 2008.PubMed/NCBI
|
|
21
|
Timurkaynak F, Can F, Azap ÖK, Demirbilek
M, Arslan H and Karaman SÖ: In vitro activities of non-traditional
antimicrobials alone or in combination against multidrug-resistant
strains of Pseudomonas aeruginosa and Acinetobacter baumannii
isolated from intensive care units. Int J Antimicrob Agents.
27:224–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mun SH, Kang OH, Joung DK, Kim SB, Seo YS,
Choi JG, Lee YS, Cha SW, Ahn YS, Han SH, et al: Combination therapy
of Sophoraflavanone B against MRSA: In vitro synergy testing. Evid
Based Complement Altern Med. 2013:8237942013. View Article : Google Scholar
|
|
23
|
Choi JG, Kang OH, Brice OO, Lee YS, Chae
HS, Oh YC, Sohn DH, Park H, Choi HG, Kim SG, et al: Antibacterial
activity of Ecklonia cava against methicillin-resistant
Staphylococcus aureus and Salmonella spp. Foodborne Pathog Dis.
7:435–441. 2010. View Article : Google Scholar
|
|
24
|
Farooqui A, Khan A, Borghetto I, Kazmi SU,
Rubino S and Paglietti B: Synergistic antimicrobial activity of
Camellia sinensis and Juglans regia against multidrug-resistant
bacteria. PLoS One. 10:e01184312015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cordwell SJ, Larsen MR, Cole RT and Walsh
BJ: Comparative proteomics of Staphylococcus aureus and the
response of methicillin-resistant and methicillin-sensitive strains
to Triton X-100. Microbiology. 148:2765–2781. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibata H, Saito H, Yomota C, Kawanishi T
and Okuda H: Alterations in the detergent-induced membrane
permeability and solubilization of saturated
phosphatidylcholine/cholesterol liposomes: Effects of poly(ethylene
glycol)-conjugated lipid. Chem Pharm Bull (Tokyo). 60:1105–1111.
2012. View Article : Google Scholar
|
|
27
|
Linnett PE and Beechey RB: Inhibitors of
the ATP synthethase system. Methods Enzymol. 55:472–518. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mun SH, Kim SB, Kong R, Choi JG, Kim YC,
Shin DW, Kang OH and Kwon DY: Curcumin reverse methicillin
resistance in Staphylococcus aureus. Molecules. 19:18283–18295.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hartmann M, Berditsch M, Hawecker J,
Ardakani MF, Gerthsen D and Ulrich AS: Damage of the bacterial cell
envelope by antimicrobial peptides gramicidin S and PGLa as
revealed by transmission and scanning electron microscopy.
Antimicrob Agents Chemother. 54:3132–3142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sambrook J and Russell DW: Molecular
Cloning: A Laboratory Manual. 2nd edition. Cold Spring Harbor
Laboratory Press; New York, NY: 1989
|
|
31
|
Eom SH, Kang SK, Lee DS, Myeong JI, Lee J,
Kim HW, Kim KH, Je JY, Jung WK and Kim YM: Synergistic
antibacterial effect and antibacterial action mode of
chitosan-ferulic acid conjugate against methicillin-resistant
Staphylococcus aureus. J Microbiol Biotechnol. 26:784–789. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klitgaard JK, Skov MN, Kallipolitis BH and
Kolmos HJ: Reversal of methicillin resistance in Staphylococcus
aureus by thioridazine. J Antimicrob Chemother. 62:1215–1221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eom SH, Lee DS, Jung YJ, Park JH, Choi JI,
Yim MJ, Jeon JM, Kim HW, Son KT, Je JY, et al: The mechanism of
antibacterial activity of phlorofucofuroeckol-A against
methicillin-resistant Staphylococcus aureus. Appl Microbiol
Biotechnol. 98:9795–9804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Irvin RT, MacAlister TJ and Costerton JW:
Tris(hydroxymethyl) aminomethane buffer modification of Escherichia
coli outer membrane permeability. J Bacteriol. 145:1397–1403.
1981.PubMed/NCBI
|
|
35
|
Joung DK, Joung H, Yang DW, Kwon DY, Choi
JG, Woo S, Shin DY, Kweon OH, Kweon KT and Shin DW: Synergistic
effect of rhein in combination with ampicillin or oxacillin against
methicillin-resistant Staphylococcus aureus. Exp Ther Med.
3:608–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ba X, Harrison EM, Lovering AL, Gleadall
N, Zadoks R, Parkhill J, Peacock SJ, Holden MT, Paterson GK and
Holmes MA: Old drugs to treat resistant bugs: Methicillin-resistant
Staphylococcus aureus isolates with mecC are susceptible to a
combination of penicillin and clavulanic acid. Antimicrob Agents
Chemother. 59:7396–7404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi JG, Choi JY, Mun SH, Kang OH, Bharaj
P, Shin DW, Chong MS and Kwon DY: Antimicrobial activity and
synergism of Sami-Hyanglyun-Hwan with ciprofloxacin against
methicillin-resistant Staphylococcus aureus. Asian Pac J Trop Med.
8:538–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mot YY, Othman I and Sharifah SH:
Synergistic antibacterial effect of co-administering
adipose-derived mesenchymal stromal cells and Ophiophagus hannah
L-amino acid oxidase in a mouse model of methicillin-resistant
Staphylococcus aureus-infected wounds. Stem Cell Res Ther. 8:52017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rolfe MD, Rice CJ, Lucchini S, Pin C,
Thompson A, Cameron AD, Alston M, Stringer MF, Betts RP, Baranyi J,
et al: Lag phase is a distinct growth phase that prepares bacteria
for exponential growth and involves transient metal accumulation. J
Bacteriol. 194:686–701. 2012. View Article : Google Scholar :
|
|
40
|
Alharbi NS, Khaled JM, Alzaharni KE,
Mothana RA, Alsaid MS, Alhoshan M, Dass LA, Kadaikunnan S and
Alobaidi AS: Effects of Piper cubeba L. essential oil on
methicillin-resistant Staphylococcus aureus: An AFM and TEM study.
J Mol Recognit. 30:1–8. 2017. View Article : Google Scholar
|
|
41
|
Joung DK, Mun SH, Lee KS, Kang OH, Choi
JG, Kim SB, Gong R, Chong MS, Kim YC, Lee DS, et al: The
antibacterial assay of tectorigenin with detergents or ATPase
inhibitors against methicillin-resistant Staphylococcus aureus.
Evid Based Complement Alternat Med. 2014:7165092014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carvalho JF, Azevedo ÍM, Rocha KB,
Medeiros AC and Carriço AD: Oxacillin magnetically targeted for the
treatment of methicillin-resistant S. aureus infection in rats.
Acta Cir Bras. 32:46–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hong SB, Rhee MH, Yun BS, Lim YH, Song HG
and Shin KS: Synergistic anti-bacterial effects of Phellinus baumii
ethyl acetate extracts and β-lactam antimicrobial agents against
methicillin-resistant Staphylococcus aureus. Ann Lab Med.
36:111–116. 2016. View Article : Google Scholar
|
|
44
|
Dong J, Qiu J, Wang J, Li H, Dai X, Zhang
Y, Wang X, Tan W, Niu X, Deng X, et al: Apigenin alleviates the
symptoms of Staphylococcus aureus pneumonia by inhibiting the
production of alpha-hemolysin. FEMS Microbiol Lett. 338:124–131.
2013. View Article : Google Scholar
|
|
45
|
Mun SH, Kong R, Seo YS, Zhou T, Kang OH,
Shin DW and Kwon DY: Subinhibitory concentrations of punicalagin
reduces expression of virulence-related exoproteins by
Staphylococcus aureus. FEMS Microbiol Lett. 363:1–6. 2016.
View Article : Google Scholar
|