Introduction
Sepsis and septic shock are associated with systemic
inflammatory response, and eventually lead to multiple organ
dysfunctions and mortality (1).
The most common cause of sepsis is exposure to bacterial structural
pathogens resulting in the excessive production of pro-inflammatory
mediators, such as nitric oxide (NO) and cyclooxygenase-2 (COX-2),
and cytokines, such as tumor necrosis factor-α (TNF-α) and
interleukin-1β (IL-1β) by aberrant activation of multiple immune
cells (1). Renal mesangial cells
(RMCs) within the glomerulus are significant in the pathogenesis of
various types of renal disease (2). Previous experimental studies have
demonstrated that endotoxins activate RMCs (3). Furthermore, RMCs produce
sepsis-associated mediators, such as TNF-α and monocyte
chemoattractant protein-1 (MCP-1) in response to lipopolysaccharide
(LPS) (4). MCP-1, a type of
chemokine, contributes to the migration and infiltration of
monocytes/macrophages in glomeruli under pathophysiological
conditions (5). Renal damage,
such as glomerulonephritis, a systemic response to infection,
occurs in critically ill sepsis and septic shock patients (6). The increased expression level of
MCP-1 was observed during the pathogenesis of renal disease models,
such as LPS-induced septic kidney and LPS-challenged macrophage
cells (7,8). Recently, aberrant production of
pro-inflammatory mediators and cytokines has been demonstrated to
be involved in the aggravation of kidney damage under septic
conditions (9). Our previous
finding indicates that natural compounds possessing
anti-inflammatory potential inhibit the expression levels of
inducible NO synthase (iNOS) and COX-2, and the secretion of TNF-α
and IL-1β in LPS-challenged macrophages (10,11). In addition, it has been reported
that mesangial cells protect themselves from the LPS-induced
overproduction of COX-2 by enhancing heme oxygenase-1 (HO-1)
expression under septic conditions (7). It is important to clearly understand
the role of mesangial cells in sepsis given that they perform
multiple roles during its progression. Therefore, suppression of
these pro-inflammatory mediators and cytokines is useful for the
treatment of inflammation-associated renal pathogenic
conditions.
Nuclear factor (erythroid-derived 2)-like-2 (Nrf-2)
is the key transcription factor required for cellular defense
against oxidative stress. It has been reported that Nrf-2 exerts
cytoprotective effects in septic mice models (12). Caffeic acid phenethyl ester (CAPE)
has been reported to contribute to the regulation of peripheral
immune functions, expressing HO-1 via the regulation of Nrf-2, a
major transcription factor of HO-1 (13). HO-1 is induced by a number of
oxidative stresses and exhibits a key cytoprotective role against
oxidative cellular injuries. Recent studies have indicated that
LPS-challenged macrophages protect themselves from overproduction
of inflammatory mediators, such as COX-2 by enhancing HO-1
expression levels (14). In
addition, it has been reported that HO-1 inhibits the excessive
production of cytokines, such as TNF-α in LPS-challenged RAW264.7
cells (15). Furthermore, HO-1
induction improved animal survival in lethal endotoxemia (12). The evidence indicated that
upregulation of HO-1 is associated with significant activation of
mitogen-activated protein kinases (MAPKs) and Nrf-2 in animal cells
(16).
Apocynin (4-hydroxy-3-methoxyacetophenone),
originally isolated from the root extract of Himalayan herb,
Picrorhiza kurroa Royle (Scrophulariaceae), inhibits the
production of superoxide by generating NADPH oxidase enzyme
(17). The aim of the present
study was to gain novel insight into the anti-inflammatory
properties of apocynin in RMCs given that previous studies
demonstrated that apocynin exhibits various pharmacological
properties, such as in the pathogenesis of inflammatory skin
diseases (18), ulcerative
colitis (19),
hyperoxaluria-induced nephrolithiasis (20) and acute lung injury (21), and is beneficial for the treatment
of osteoarthritis and rheumatoid arthritis (22). The anti-inflammatory mechanisms of
apocynin in RMCs are yet to be identified. Therefore, in the
present study, the possible protective effect of apocynin and its
underlying mechanisms were investigated in LPS-challenged RMCs.
Materials and methods
Reagents and cell culture
Bacterial LPS from Escherichia coli serotype
055: B5 and apocynin (4′-hydroxy-3′-methoxyacetophenone) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Apocynin was dissolved in dimethyl sulfoxide and the desired
concentration was added to the culture medium of rat mesangial
cells, mentioned below. Rat kidney mesangial cells were obtained
from the American Type Culture Collection (cat. no. CRL-2573;
Manassas, VA, USA) and maintained in HyClone Dulbecco's modified
Eagle's medium (GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 15% fetal bovine serum, 100 U/ml penicillin, 100
mg/ml streptomycin (both from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 0.4 mg/ml G418 disulphate (Duchefa
Biochemie, Haarlem, Netherlands) at 37°C in 5% CO2. At
~75% confluence, mesangial cells were incubated with serum free
media and treated with various concentrations of apocynin (62.5,
125, 250 and 500 µM).
Cell viability assay
The mesangial cells were seeded at
5×104/well in 96-well plates and incubated at 37°C under
5% CO2 and 95% humidified air until reaching >80%
cell confluence. The cells were treated with apocynin for 1 h prior
to the addition of LPS for 24 h. Cell viability was determined
using cell counting kit-8 (CCK-8) assay (cat no. CK04; Dojindo
Molecular Technologies, Inc, Rockville, MD, USA), which produces a
water-soluble formazan dye in living cells. The quantity of
formazan dye generated by dehydrogenases in cells is directly
proportional to the number of living cells. CCK-8 solution (10
µM) was added to each well and the plate was incubated 1–4 h
at 37°C incubator. Absorbance was measured at a wavelength of 450
nm using a microplate reader. The results were expressed as the
percentage of surviving cells over control cells.
Nitrite quantification assay
Accurate quantitative measurement of NO production
in the biological samples was estimated by measuring the quantity
of nitrite, a stable metabolite of NO, using the Griess assays.
Subsequent to apocynin-pretreated RMCs being stimulated with LPS in
at 5×105/well in 12-well plates for 24 h, 100 µl
cell supernatant was combined with an equal volume of Griess
reagent (Sigma-Aldrich, St. Louis, MO, USA). The absorbance was
measured at a wavelength of 550 nm using a microplate reader and
sodium nitrite served as a standard. The results were expressed as
the percentage of released NO from LPS-stimulated RMCs, and a
standard curve was established.
Western blot analysis
The cells were seeded at 2.5×106/well in
60-mm cell culture plates and pretreated with apocynin for 1 h.
Following treatment, the cells were stimulated with LPS for various
durations. Then the cells were washed with 1X phosphate-buffered
saline and lysed in PRO-PREP lysis buffer (Intron Biotechnology,
Inc., Seongnam, Korea). Samples from the cell lysates were
denatured with sonication on ice and equal amounts of protein were
separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel.
Proteins were transferred to polyvinylidene fluoride membrane
(Merck KGaA) and blocked in 5% skimmed milk or 3% bovine serum
albumin (BSA) in Tris-buffered saline and Tween-20 (TBST) for 1 h
at room temperature. The membranes were probed with specific
antibodies against MCP-1 (1:1,000; cat. no. ab7202; Abcam,
Cambridge, MA, USA), iNOS (1:1,000; cat. no. 610333, BD
Transduction Laboratories, San Jose, CA, USA), COX-2 (1:1,000; cat.
no. 48425; Cell Signaling Technology, Inc., Danvers, MA, USA),
phosphorylated-Nrf2 (1:1,000; cat. no. bs-2013R; Bioss, Beijing,
China), HO-1 (1:500; cat. no. ab13248; Abcam), Akt (cat. no. 9272),
phosphorylated-Akt (cat. no. 9271), extracellular signal-regulated
kinase 1/2 (Erk1/2) (cat. no. 4695), phosphorylated-Erk1/2 (cat.
no. 9101), p38 (cat. no. 9212), phosphorylated-p38 (cat. no. 9211),
phosphorylated-c-Jun N-terminal kinase (cat. no. 9251), JNK
(1:1,000; cat. no. 9252) (all from Cell Signaling Technology,
Inc.), and β-actin (1:2,500; cat. no. A5441, Sigma-Aldrich; Merck
KGaA) were diluted either in 5% skimmed milk or 3% BSA. After
thoroughly washing with TBST, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
(1:2,500, cat. no. 111-035-144) for polyclonal antibodies, or with
HRP-conjugated goat anti-mouse IgG secondary antibodies (1:2,500,
cat. no. 715-035-150; Jackson ImmunoResearch Laboratories, West
Groove, PA, USA). Immunoreactive bands were visualized using an
enhanced X-ray film and intensity was measured by ImageQuant 5.2
(GE Healthcare, Waukesha, WI, USA). All values shown in the figures
are expressed as the means ± standard deviation (SD) obtained from
at least three independent experiments.
Statistical analysis
The experimental results are expressed as means ± SD
obtained from at least three independent experiments. Statistical
significance was analyzed by one-way ANOVA. P<0.05 was
considered to indicate a statistically significant difference.
Results
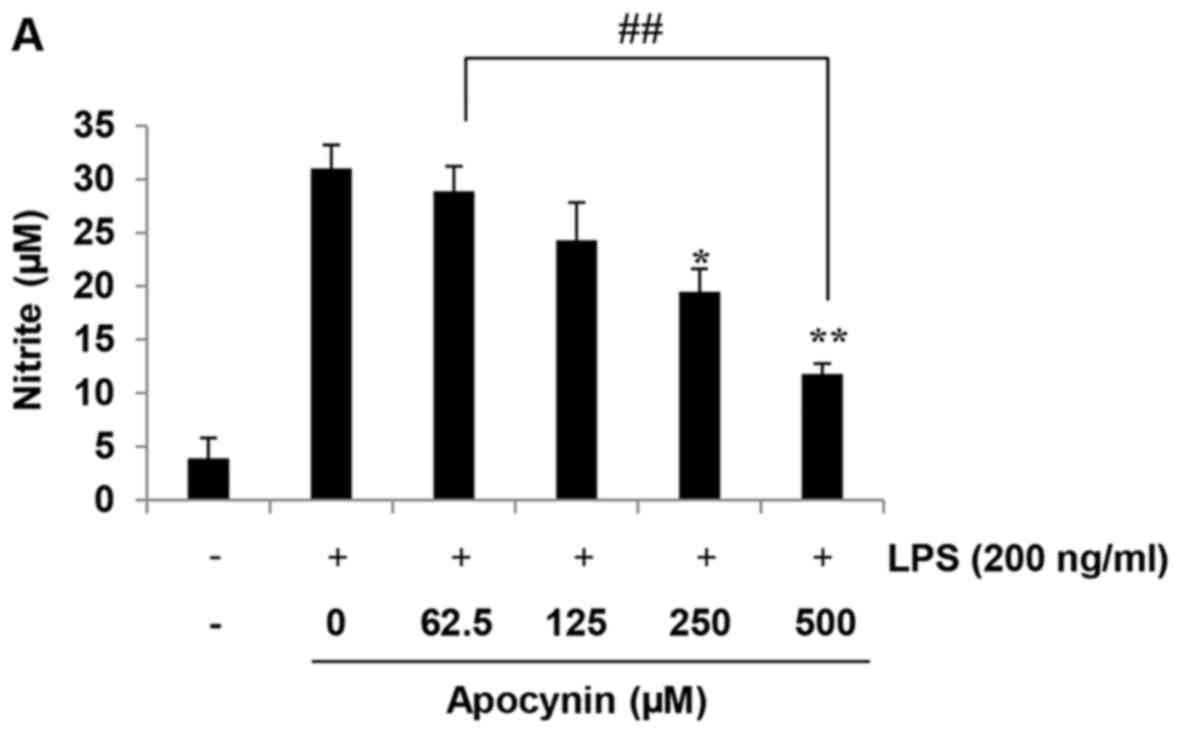
Apocynin suppresses NO production in
LPS-stimulated rat mesangial cells
The anti-inflammatory effects of apocynin were
evaluated according to the quantity of NO production in
LPS-stimulated RMCs by measuring the quantity of nitrite, a stable
metabolite of NO, using Griess reagent. Cells were incubated with
the indicated concentrations of apocynin (62.5, 125, 250 and 500
µM) for 1 h prior to LPS-treatment. The production of NO in
RMCs was increased following LPS incubation for 24 h. However,
apocynin significantly inhibited the production of NO in a
concentration-dependent manner in LPS-stimulated RMCs (Fig. 1A). Evaluation of the cytotoxicity
using CCK-8 assay demonstrated that apocynin-induced cytotoxicity
was negligible at concentrations up to 500 µM apocynin
(Fig. 1B).
Apocynin attenuates LPS-induced iNOS,
COX-2 and MCP-1 expression levels in rat mesangial cells
To investigate whether apocynin exhibits inhibitory
activity against NO via inhibition of iNOS and COX-2, western blot
analysis was performed. LPS treatment resulted in markedly
increased expression levels of iNOS and COX-2 in response to LPS.
However, pretreatment of apocynin significantly attenuated
LPS-induced overproduction of iNOS and COX-2 protein in RMCs
(Fig. 2A–C). These findings are
consistent with the inhibitory effect of the fractions on NO
release (Fig. 1A). As MCP-1 is
key in the migration of activated microphages, whether apocynin
suppresses the LPS-attenuated expression level of MCP-1 was
examined using western blot analysis. To examine the effect of
apocynin on LPS-induced MCP-1, mesangial cells were pretreated with
apocynin for ~1 h prior to LPS-treatment for 24 h. LPS-induced
MCP-1 expression levels were significantly reduced by apocynin
treatment in a concentration-dependent manner (Fig. 2A and D).
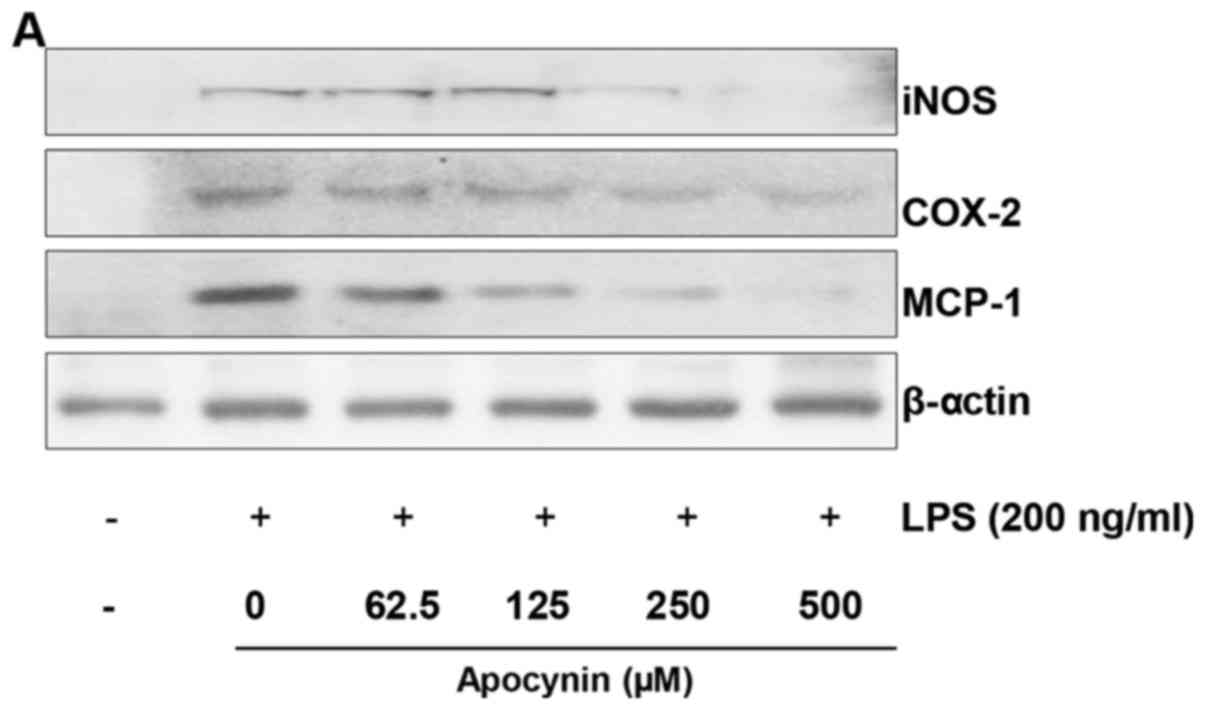 | Figure 2Apocynin inhibits LPS-induced
expression levels of iNOS, COX-2 and MCP-1 proteins in RMCs. (A)
Cell lysates were subjected to 10% SDS-PAGE, and the protein
expression levels of iNOS, COX-2 and MCP-1 were determined by
western blot analysis. The expression levels of iNOS, COX-2 and
MCP-1 were markedly increased in response to LPS, and apocynin
significantly inhibited the LPS-induced expression of these
proteins in a concentration-dependent manner. Quantitative analyses
of (B) iNOS, (C) COX-2 and (D) MCP-1 immunoblots. Apocynin
significantly suppressed the LPS-induced expression of these
proteins. Immunoblots of each protein were obtained from three
independent experiments. Data are expressed as means ± standard
deviation. *P<0.05 and **P<0.01 vs. LPS
alone; ##P<0.01. LPS, lipopolysaccharide; iNOS,
inducible nitric oxide synthase; COX-2, cyclooxygenase-2; MCP-1,
monocyte chemoattractant protein-1; RMC, renal mesangial cells. |
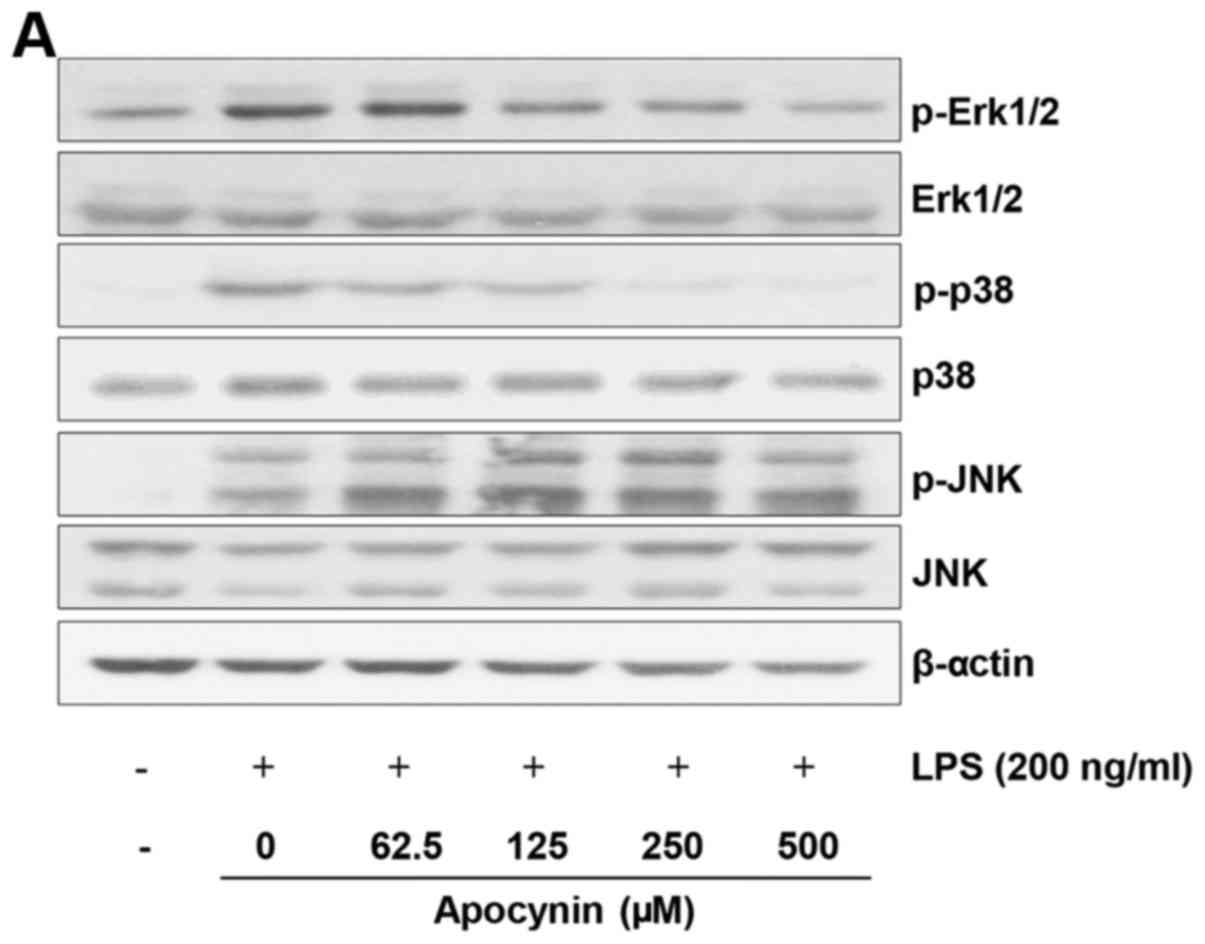
Effect of apocynin on LPS-induced
phosphorylation of MAPKs in rat mesangial cells
To examine the underlying mechanism by which
apocynin exerts its anti-inflammatory effects, the MAPK signaling
pathway, which is known to be significantly involved in
pro-inflammatory responses, was examined. Cells were pretreated
with apocynin at different concentrations for 1 h and subsequently
treated with LPS (200 ng/ml) for 30 min. LPS treatment exhibited a
robust activation of all three MAPKs (Fig. 3). Apocynin treatment significantly
attenuated the phosphorylation of Erk1/2 and p38 in a
concentration-dependent manner (Fig.
3A–C). By contrast, there was no noticeable inhibitory effect
of apocynin on phosphorylation of JNK in LPS-induced RMCs (Fig. 3A and D). The suppressive effect of
apocynin on the LPS-induced activation of Erk1/2 and p38 signaling
pathways strongly indicates that apocynin exerts significant
anti-inflammatory effects on mesangial cells. However, further
studies are required to clarify the exact mechanism by which
apocynin exerts its anti-inflammatory effects via the differential
suppression of MAPKs in RMCs.
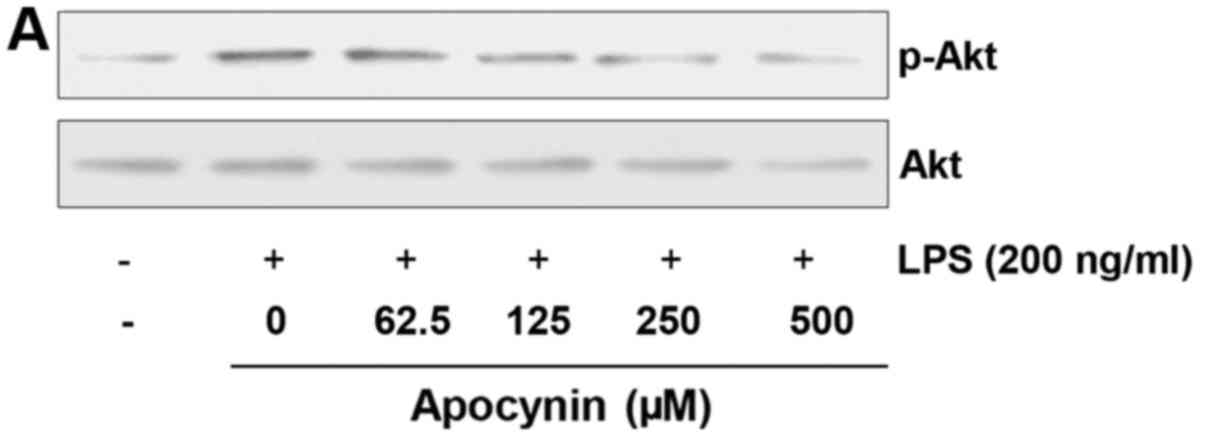
Apocynin induces phosphorylation of Akt
in LPS-stimulated rat mesangial cells
Given that the Akt signaling pathway is significant
in cytoprotection against stresses, the effect of apocynin on Akt
phosphorylation was examined. Cells were pretreated with apocynin
at different concentrations for 1 h and subsequently treated with
LPS (200 ng/ml) for 30 min. LPS treatment demonstrated an increased
phosphorylation of Akt, which is considered to be a compensatory
response to cellular damage (Fig.
4). Apocynin treatment significantly suppressed LPS-induced
phosphorylation of Akt in a concentration-dependent manner
(Fig. 4). However, apocynin,
although significant, could not reverse the LPS-induced Akt
phosphorylation back to the basal level. Further studies are
required to clearly elucidate the exact role of Akt activation in
LPS-challenged RMCs.
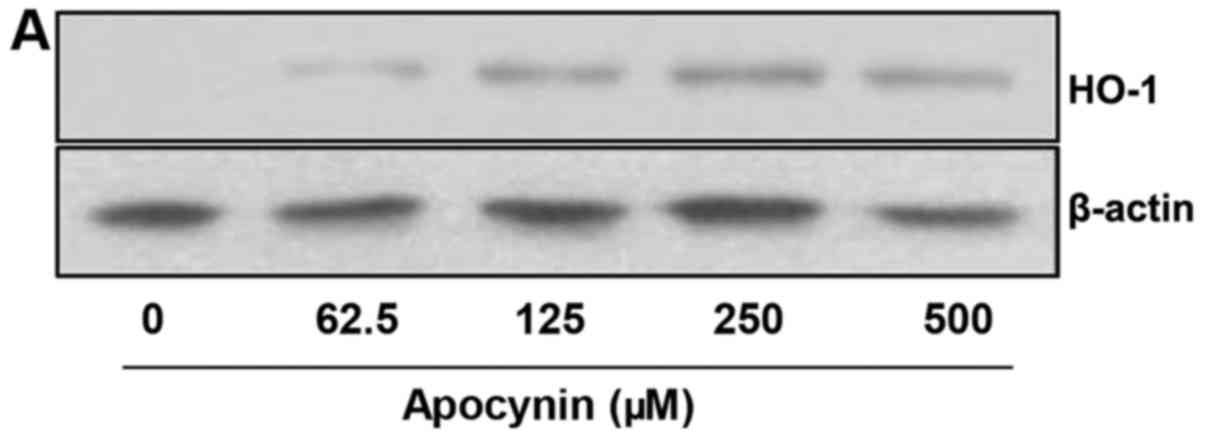
Apocynin induces HO-1 protein expression
via Nrf2 activation in rat mesangial cells
As HO-1 modulates inflammation and inhibits
LPS-induced production of pro-inflammatory mediators, and as Nrf2,
a transcription factor for HO-1, mediates major cytoprotective
responses, the role of apocynin on the HO-1/Nrf2 signaling pathway
was examined. RMCs were pretreated with apocynin at different
concentrations for 24 h. The level of HO-1 expression was
significantly increased in apocynin-treated RMCs in a
concentration-dependent manner (Fig.
5A and B). Furthermore, apocynin exhibited increased Nrf2
phosphorylation in accordance with HO-1 expression (Fig. 5C and D). The data strongly
indicate that apocynin exerts its anti-inflammatory activity via
activation of a cellular cytoprotective pathway.
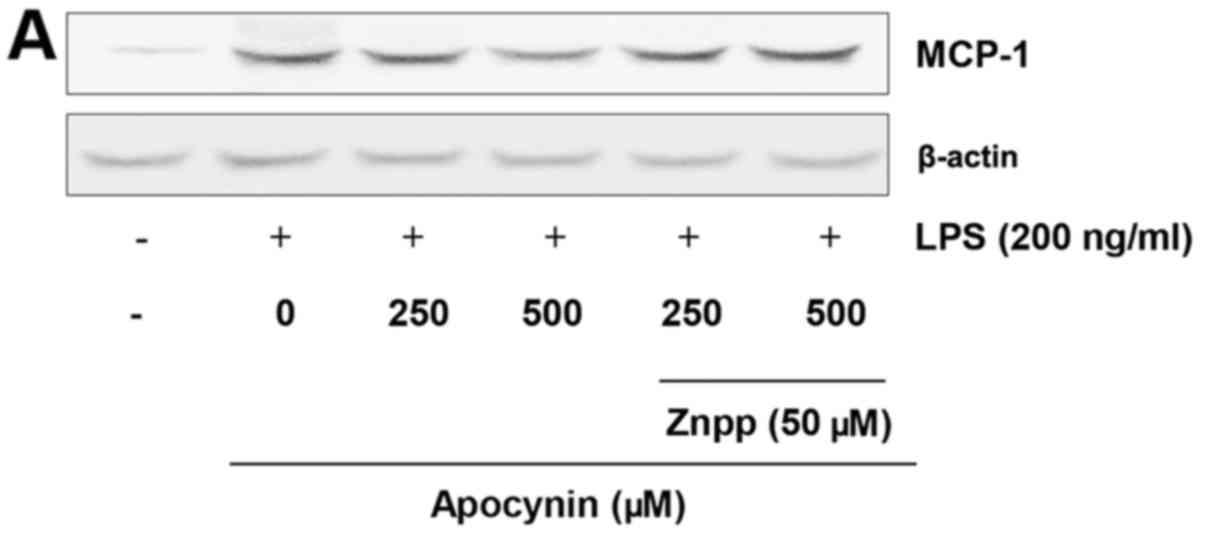
Effects of HO-1 in LPS-induced MCP-1
expression in rat mesangial cells
To verify the cytoprotective role of
apocynin-induced upregulation of HO-1 on the LPS-challenged RMCs,
the level of MCP-1 expression was examined in the absence and
presence of HO-1 activity. RMCs were pretreated with zinc
protoporphyrin (Znpp), a HO-1 inhibitor, and subsequently treated
with apocynin. Following 24-h incubation with LPS, the expression
level of MCP-1 was determined. In the presence of HO-1 activity,
apocynin significantly attenuated LPS-induced MCP-1 expression
levels in a concentration-dependent manner (Fig. 6). However, apocynin-induced
suppression of MCP-1 expression was significantly abolished in the
absence of HO-1 activity with Znpp. The data strongly indicates
that HO-1 mediates the apocynin-induced suppression of MCP-1 in
LPS-challenged RMCs.
Discussion
In the present study, apocynin was demonstrated to
significantly attenuate LPS-induced inflammatory responses via the
suppression of MAPK signaling pathways, such as Erk and p38, and
the activation of cytoprotective pathways, such as HO-1/Nrf2 in
LPS-challenged RMCs. Apocynin suppressed LPS-induced expression of
pro-inflammatory mediators, such as iNOS and COX-2. In addition,
apocynin significantly attenuated LPS-induced expression of MCP-1,
which was abolished with the suppression of HO-1, indicating that
HO-1 mediates the suppressive effect of apocynin on MCP-1
expression in LPS-challenged RMCs.
RMCs have been reported to be involved in the
autoimmune glomerulonephritis (2), generation of oxygen radicals
(23), and glomerular coagulation
and fibrinolysis (24). A
previous study indicated that the suppression of COX-2, iNOS and NO
contributes to the anti-inflammatory responses in LPS-treated human
renal mesangial cells (25).
Consistently, the present study demonstrated that apocynin exerted
an inhibitory effect on LPS-induced iNOS and COX-2 expression, and
NO production in a concentration-dependent manner.
MCP-1-induced migration and infiltration of
macrophages contributes to the aggravation of sepsis and septic
shock. MCP-1, produced by mesangial cells, has been reported to
contribute to the pathogenesis of glomerulonephritis (26). The involvement of MCP-1 is not
limited to glomerular inflammation, but may be observed in other
inflammatory diseases. Various studies have demonstrated that MCP-1
is elevated and has a detrimental role in the development of
atherosclerosis (27),
inflammatory bowel diseases (28), rheumatoid arthritis (29) and allergic encephalitis (30). Recently, it was reported that
trihydroxycinnamic acid suppressed LPS-induced MCP-1 protein
expression, resulting in the significant attenuation of LPS-induced
septic renal damage (9). In the
present study, apocynin significantly inhibited LPS-induced MCP-1
expression levels in RMCs.
The present study demonstrated that among MAPKs, the
phosphorylation of Erk1/2 and p38, but not JNK, was attenuated by
apocynin with concurrent suppression of inflammatory responses in
LPS-challenged RMCs. Previously, a similar study has demonstrated
that activation of p38, Erk1/2 and Akt, in response to hydrogen
peroxide, was suppressed by apocynin treatment (31). Another consistent finding revealed
that only Erk and p38 signaling pathways were involved in the
induction of HO-1 (7). The Erk1/2
signaling pathway has been implicated in the development of
inflammatory cellular responses in kidney injury (8), as well as in macrophage cells
(32). The present study also
indicated that Akt phosphorylation was attenuated by apocynin
treatment in LPS-challenged RMCs. In the present study, activation
of the Akt signaling pathway with LPS treatment was considered a
compensatory response to cellular damage. Apocynin attenuated the
LPS-induced Akt phosphorylation. However, further studies are
required to clearly elucidate the exact role of differential
suppression on MAPK and Akt signaling pathways in LPS-challenged
RMCs.
Nrf-2 has been demonstrated to be key in maintaining
cellular homeostasis and regulating inflammatory conditions via
cytoprotective enzymes and stress-responsive proteins (33,34). Sulforaphane-treated mice
demonstrated Nrf-2 activation and increased HO-1 expression
(35), and loss of Nrf-2
signaling increased the susceptibility to inflammation (36). Furthermore, induction of HO-1
resulted in the reduction of oxidative stress by removing free
heme, as well as an increase in the level of anti-inflammatory
substances (37). In the present
study, apocynin, resulting from upregulation of the Nrf-2 signaling
pathway, exhibited increased expression levels of HO-1 in RMCs. It
has been reported that activation of the HO-1/Nrf-2 signaling
pathway may be achieved via the various signaling pathways
(10,12,38). Considering the cytoprotective role
of HO-1, the induction of HO-1 expression by pharmacological
modulation may be a valuable therapeutic target against the
progression of sepsis and inflammatory disorders. The present study
demonstrated that apocynin resulted in an increase of HO-1
expression levels with subsequent attenuation of inflammation in
RMCs. In addition, inhibition of HO-1 with an inhibitor, Znpp,
significantly abolished apocynin-induced MCP-1 suppression in RMCs,
strongly indicating that HO-1 is critically involved in the
suppression of MCP-1 expression levels in RMCs. The data was
consistent with previous studies demonstrating that HO-1 inducers
were reported to reduce MCP-1 expression levels in sepsis and
inflammatory disorders (39,40).
Apocynin, isolated from the root extract of
Picrorhiza kurroa, has been reported to perform a range of
biological activities, including radical scavenger (41) and antioxidant in vascular cells
(31), via the suppression of
NADPH oxidase, which is responsible for the generation of
endogenous reactive oxygen species (ROS) (42). Apocynin has been reported to
inhibit the formation of functional NADPH oxidase complex (41). Aberrant production of
NADPH-induced ROS, such as superoxide anion and hydrogen peroxide,
mediates injury in various types of inflammatory disorder (41). Furthermore, apocynin was
demonstrated to provide blood pressure-lowering effects in
hypertensive animals (43,44),
as well as exerting renal protective action in rats (45). However, the pharmacological
properties of apocynin on RMCs have not been extensively elucidated
in LPS-challenged RMCs. The present study is the first, to the best
of our knowledge, to demonstrate that apocynin is an effective
inducer of HO-1 expression in RMCs. Recently, it was reported that
apocynin prevents quinolinic acid-induced neurological damage by
increasing glutathione synthesis and Nrf2 mRNA expression levels
(46) and that apocynin
attenuates diabetes-induced adverse remodeling of left ventricular
myocardium (47), indicating the
diverse therapeutic properties of apocynin.
In conclusion, the present study clearly
demonstrates that apocynin may attenuate kidney dysfunction by
suppressing the MCP-1-induced renal infiltration of macrophages via
upregulation of the HO-1/Nrf2 signaling pathway in RMCs. In
addition, apocynin significantly suppresses LPS-induced
overproduction of pro-inflammatory mediators via the inhibition of
aberrantly activated MAPK and Akt signaling pathways. These results
demonstrate that apocynin may present as a promising candidate for
in vivo evaluation of a therapeutic agent for
inflammation-associated renal disorders.
Acknowledgments
The present study was supported by the 2016 Research
Grant from Kangwon National University (grant no. 520160299).
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radeke HH and Resch K: The inflammatory
function of renal glomerular mesangial cells and their interaction
with the cellular immune system. Clin Investig. 70:825–842. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Almeida WS, Maciel TT, Di Marco GS,
Casarini DE, Campos AH and Schor N: Escherichia coli
lipopolysaccharide inhibits renin activity in human mesangial
cells. Kidney Int. 69:974–980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsing CH, Chou W, Wang JJ, Chen HW and Yeh
CH: Propofol increases bone morphogenetic protein-7 and decreases
oxidative stress in sepsis-induced acute kidney injury. Nephrol
Dial Transplant. 26:1162–1172. 2011. View Article : Google Scholar
|
|
5
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poukkanen M, Vaara ST, Pettilä V, Kaukonen
KM, Korhonen AM, Hovilehto S, Inkinen O, Laru-Sompa R, Kaminski T,
Reinikainen M, et al FINNAKI study group: Acute kidney injury in
patients with severe sepsis in Finnish Intensive Care Units. Acta
Anaesthesiol Scand. 57:863–872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YC, Chen CH, Ko WS, Cheng CY, Sue YM
and Chen TH: Dipyridamole inhibits lipopolysaccharide-induced
cyclooxygenase-2 and monocyte chemoattractant protein-1 via heme
oxygenase-1-mediated reactive oxygen species reduction in rat
mesangial cells. Eur J Pharmacol. 650:445–450. 2011. View Article : Google Scholar
|
|
8
|
Nagai T, Urushihara M, Kinoshita Y, Jamba
A, Kondo S and Kagami S: Differential regulation of angiotensin
II-induced extracellular signal regulated kinase-1/2 and -5 in
progressive glomerulonephritis. Nephrology (Carlton). 21:950–958.
2016. View Article : Google Scholar
|
|
9
|
Lee JW, Kwon JH, Lim MS, Lee HJ, Kim SS,
Lim SY and Chun W: 3,4,5-Trihydroxycinnamic acid increases
heme-oxygenase-1 (HO-1) and decreases macrophage infiltration in
LPS-induced septic kidney. Mol Cell Biochem. 397:109–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JW, Bae CJ, Choi Y-J, Kim SI, Kim NH,
Lee HJ, Kim SS, Kwon YS and Chun W: 3, 4, 5-Trihydroxycinnamic acid
inhibits LPS-induced iNOS expression by suppressing NF-κB
activation in BV2 microglial cells. Korean J Physiol Pharmacol.
16:107–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vo VA, Lee JW, Park JH, Kwon JH, Lee HJ,
Kim SS, Kwon YS and Chun W: N-(p-Coumaryol)-tryptamine suppresses
the activation of JNK/c-Jun signaling pathway in LPS-challenged
RAW264. 7 cells Biomol Ther (Seoul). 22:200–206. 2014. View Article : Google Scholar
|
|
12
|
Ha YM, Ham SA, Kim YM, Lee YS, Kim HJ, Seo
HG, Lee JH, Park MK and Chang KC: β1-adrenergic receptor-mediated
HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW
264.7 cells leads to inhibition of HMGB1 release in LPS-activated
RAW 264.7 cells and increases in survival rate of CLP-induced
septic mice. Biochem Pharmacol. 82:769–777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fidan H, Sahin O, Yavuz Y, Kilbas A,
Cetinkaya Z, Ela Y, Ozen OA and Altuntas I: Caffeic acid phenethyl
ester reduces mortality and sepsis-induced lung injury in rats.
Crit Care Med. 35:2822–2829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park SY, Seetharaman R, Ko MJ, Kim DY, Kim
TH, Yoon MK, Kwak JH, Lee SJ, Bae YS and Choi YW: Ethyl linoleate
from garlic attenuates lipopolysaccharide-induced pro-inflammatory
cytokine production by inducing heme oxygenase-1 in RAW264.7 cells.
Int Immunopharmacol. 19:253–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG,
Lee JH and Chang KC: Heme-oxygenase-1 induction and carbon
monoxide-releasing molecule inhibit lipopolysaccharide
(LPS)-induced high-mobility group box 1 release in vitro and
improve survival of mice in LPS- and cecal ligation and
puncture-induced sepsis model in vivo. Mol Pharmacol. 76:173–182.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park EJ, Lim JH, Nam SI, Park JW and Kwon
TK: Rottlerin induces heme oxygenase-1 (HO-1) up-regulation through
reactive oxygen species (ROS) dependent and PKC delta-independent
pathway in human colon cancer HT29 cells. Biochimie. 92:110–115.
2010. View Article : Google Scholar
|
|
17
|
Paracatu LC, Zeraik ML, Bertozo LC,
Bartolomeu AA, Filho LC, Fonseca LM and Ximenes VF: Synthesis,
antioxidant and anti-inflammatory properties of an apocynin-derived
dihydrocoumarin. Med Chem. 13:93–100. 2017. View Article : Google Scholar
|
|
18
|
Nam YJ, Kim A, Sohn DS and Lee CS:
Apocynin inhibits Toll-like receptor-4-mediated activation of NF-κB
by suppressing the Akt and mTOR pathways. Naunyn Schmiedebergs Arch
Pharmacol. 389:1267–1277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang YJ, Lee SJ, Park JY, Chun W, Nam SJ,
Park JM, Park SC, Choi DH and Kang CD: Apocynin suppresses
lipopolysaccharide-induced inflammatory responses through the
inhibition of MAP kinase signaling pathway in RAW264.7 cells. Drug
Dev Res. 77:271–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma M, Kaur T and Singla SK: Role of
mitochondria and NADPH oxidase derived reactive oxygen species in
hyperoxaluria induced nephrolithiasis: Therapeutic intervention
with combinatorial therapy of N-acetyl cysteine and Apocynin.
Mitochondrion. 27:15–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdelmageed ME, El-Awady MS and Suddek GM:
Apocynin ameliorates endotoxin-induced acute lung injury in rats.
Int Immunopharmacol. 30:163–170. 2016. View Article : Google Scholar
|
|
22
|
Lafeber FP, Beukelman CJ, van den Worm E,
van Roy JL, Vianen ME, van Roon JA, van Dijk H and Bijlsma JW:
Apocynin, a plant-derived, cartilage-saving drug, might be useful
in the treatment of rheumatoid arthritis. Rheumatology (Oxford).
38:1088–1093. 1999. View Article : Google Scholar
|
|
23
|
Radeke HH, Meier B, Topley N, Flöge J,
Habermehl GG and Resch K: Interleukin 1-α and tumor necrosis
factor-α induce oxygen radical production in mesangial cells.
Kidney Int. 37:767–775. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Migliorini A, Ebid R, Scherbaum CR and
Anders HJ: The danger control concept in kidney disease: Mesangial
cells. J Nephrol. 26:437–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu F, Zhang W, Li L, Zheng F, Shao X, Zhou
J and Li H: Inhibitory effects of honokiol on
lipopolysaccharide-induced cellular responses and signaling events
in human renal mesangial cells. Eur J Pharmacol. 654:117–121. 2011.
View Article : Google Scholar
|
|
26
|
Brown Z, Strieter RM, Neild GH, Thompson
RC, Kunkel SL and Westwick J: IL-1 receptor antagonist inhibits
monocyte chemotactic peptide 1 generation by human mesangial cells.
Kidney Int. 42:95–101. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Namiki M, Kawashima S, Yamashita T, Ozaki
M, Hirase T, Ishida T, Inoue N, Hirata K, Matsukawa A, Morishita R,
et al: Local overexpression of monocyte chemoattractant protein-1
at vessel wall induces infiltration of macrophages and formation of
atherosclerotic lesion: Synergism with hypercholesterolemia.
Arterioscler Thromb Vasc Biol. 22:115–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spoettl T, Hausmann M, Herlyn M, Gunckel
M, Dirmeier A, Falk W, Herfarth H, Schoelmerich J and Rogler G:
Monocyte chemoattractant protein-1 (MCP-1) inhibits the
intestinal-like differentiation of monocytes. Clin Exp Immunol.
145:190–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rantapää-Dahlqvist S, Boman K, Tarkowski A
and Hallmans G: Up regulation of monocyte chemoattractant protein-1
expression in anti-citrulline antibody and immunoglobulin M
rheumatoid factor positive subjects precedes onset of inflammatory
response and development of overt rheumatoid arthritis. Ann Rheum
Dis. 66:121–123. 2007. View Article : Google Scholar
|
|
30
|
Izikson L, Klein RS, Charo IF, Weiner HL
and Luster AD: Resistance to experimental autoimmune
encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2.
J Exp Med. 192:1075–1080. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heumüller S1, Wind S, Barbosa-Sicard E,
Schmidt HH, Busse R, Schröder K and Brandes RP: Apocynin is not an
inhibitor of vascular NADPH oxidases but an antioxidant.
Hypertension. 51:211–217. 2008. View Article : Google Scholar
|
|
32
|
Vo VA, Lee JW, Chang JE, Kim JY, Kim NH,
Lee HJ, Kim SS, Chun W and Kwon YS: Avicularin inhibits
lipopolysaccharide-induced inflammatory response by suppressing ERK
phosphorylation in RAW 264.7 macrophages. Biomol Ther (Seoul).
20:532–537. 2012. View Article : Google Scholar
|
|
33
|
Lee I-S, Lim J, Gal J, Kang JC, Kim HJ,
Kang BY and Choi HJ: Anti-inflammatory activity of xanthohumol
involves heme oxygenase-1 induction via NRF2-ARE signaling in
microglial BV2 cells. Neurochem Int. 58:153–160. 2011. View Article : Google Scholar
|
|
34
|
Surh YJ, Kundu JK and Na HK: Nrf2 as a
master redox switch in turning on the cellular signaling involved
in the induction of cytoprotective genes by some chemopreventive
phytochemicals. Planta Med. 74:1526–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin W, Wu RT, Wu T, Khor TO, Wang H and
Kong AN: Sulforaphane suppressed LPS-induced inflammation in mouse
peritoneal macrophages through Nrf2 dependent pathway. Biochem
Pharmacol. 76:967–973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yates MS, Tran QT, Dolan PM, Osburn WO,
Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams
CR, et al: Genetic versus chemoprotective activation of Nrf2
signaling: Overlapping yet distinct gene expression profiles
between Keap1 knockout and triterpenoid-treated mice.
Carcinogenesis. 30:1024–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawakami T, Takahashi T, Shimizu H,
Nakahira K, Takeuchi M, Katayama H, Yokoyama M, Morita K, Akagi R
and Sassa S: Highly liver-specific heme oxygenase-1 induction by
interleukin-11 prevents carbon tetrachloride-induced
hepatotoxicity. Int J Mol Med. 18:537–546. 2006.PubMed/NCBI
|
|
38
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-trihydroxycinnamic acid inhibits
lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in
vitro and improves survival of mice in LPS-induced endotoxemia
model in vivo. Mol Cell Biochem. 390:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boonloh K, Lee ES, Kim HM, Kwon MH, Kim
YM, Pannangpetch P, Kongyingyoes B, Kukongviriyapan U,
Thawornchinsombut S, Lee EY, et al: Rice bran protein hydrolysates
attenuate diabetic nephropathy in diabetic animal model. Eur J
Nutr. Dec 21–2016.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SE, Lee EO, Yang JH, Kang JHL, Suh YH
and Chong YH: 15-deoxy-Δ12,14-prostaglandin
J2 inhibits human immunodeficiency virus-1 tat-induced
monocyte chemoattractant protein-1/CCL2 production by blocking the
extracellular signal-regulated kinase-1/2 signaling pathway
independently of peroxisome proliferator-activated receptor-γ and
heme oxygenase-1 in rat hippocampal slices. J Neurosci Res.
90:1732–1742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mouzaoui S, Djerdjouri B, Makhezer N,
Kroviarski Y, El-Benna J and Dang PM: Tumor necrosis
factor-α-induced colitis increases NADPH oxidase 1 expression,
oxidative stress, and neutrophil recruitment in the colon:
Preventive effect of apocynin. Mediators Inflamm. 2014:3124842014.
View Article : Google Scholar
|
|
42
|
Maldonado PD, Molina-Jijón E,
Villeda-Hernández J, Galván-Arzate S, Santamaría A and
Pedraza-Chaverrí J: NAD(P)H oxidase contributes to neurotoxicity in
an excitotoxic/prooxidant model of Huntington's disease in rats:
Protective role of apocynin. J Neurosci Res. 88:620–629. 2010.
|
|
43
|
Hamilton CA, Brosnan MJ, Al-Benna S, Berg
G and Dominiczak AF: NAD(P)H oxidase inhibition improves
endothelial function in rat and human blood vessels. Hypertension.
40:755–762. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park YM, Park MY, Suh Y-L and Park JB:
NAD(P)H oxidase inhibitor prevents blood pressure elevation and
cardiovascular hypertrophy in aldosterone-infused rats. Biochem
Biophys Res Commun. 313:812–817. 2004. View Article : Google Scholar
|
|
45
|
Adler S and Huang H: Oxidant stress in
kidneys of spontaneously hypertensive rats involves both oxidase
overexpression and loss of extracellular superoxide dismutase. Am J
Physiol Renal Physiol. 287:F907–F913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cruz-Álvarez S, Santana-Martínez R,
Avila-Chávez E, Barrera-Oviedo D, Hernández-Pando R,
Pedraza-Chaverri J and Maldonado PD: Apocynin protects against
neurological damage induced by quinolinic acid by an increase in
glutathione synthesis and Nrf2 levels. Neuroscience. 350:65–74.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qiu J, Zhao J, Li J, Liang X, Yang Y,
Zhang Z, Zhang X, Fu H, Korantzopoulos P, Tse G, et al: Apocynin
attenuates left ventricular remodeling in diabetic rabbits.
Oncotarget. 8:38482–38490. 2017.PubMed/NCBI
|