Introduction
Rapid industrialization in China had certain
drawbacks, and it is now facing a great challenge in heavy metal
pollution. Processes of mining, smelting, industrial production,
pesticide application as well as oil and other fuel combustion will
inevitably result in widespread heavy metal pollution. China's main
streams are suffering from varying degrees of heavy metal pollution
(1–6). The various heavy metal elements in
contaminated water may be accumulated in aquatic weeds, plankton
and aquatic animals and finally enter the human body through
various branches of the food chain (7).
Ningbo, an eastern coastal city in China, has
>7.8 million inhabitants. In this area, the preferred daily diet
mostly comprises aquatic products, including fish, shrimp, crab and
shellfish. It is noteworthy that in recent years, the coastal
waters and aqua products in the Ningbo area have been suffering
from multi-heavy metal pollution, which mainly includes pollution
with zinc, manganese, lead, copper, cadmium, mercury, chromium and
nickel (8–10). Long-term consumption of aqua
products may result in the accumulation of heavy metals and further
human health hazards.
Multiple heavy metal elements may enter the human
body simultaneously and the combined toxicity is complex due to
their interaction. While the toxic molecular mechanisms of
different heavy metals are not identical, they have certain effects
in common. First, most heavy metal ions freely pass through the
cell membrane and directly react with intracellular organelles and
biological macromolecules, interfering with the intracellular
calcium homeostasis and transport system (11). Furthermore, most heavy metals
cause excessive generation of reactive oxygen species (ROS) and
lead to organelle and DNA damage (12–15). These common points may be useful
in searching for an effective antidote to antagonize the combined
toxicity induced by multiple heavy metals.
Luteolin, a common flavonoid, occurs in broccoli,
carrots, celery, cauliflower and peppers. Studies have confirmed
that luteolin possesses important pharmacological properties,
including antimicrobial, antiviral, anti-allergic, antioxidant,
anti-inflammatory and anticancer effects (16–21). Other studies demonstrated that
luteolin has an inhibitory effect on the toxicity induced by heavy
metals. Zhou et al (22)
suggested that luteolin protected SH-SY5Y cells against
zinc-induced, ROS-mediated apoptosis. Liu et al (23) reported that luteolin regulated the
redox imbalance, preserved mitochondrial function and depressed the
caspase family-associated apoptosis induced by copper. Excessive
ROS generation is associated with mitochondrial damage, while
luteolin was reported to protect mitochondrial function through
depression of ROS generation (24). In addition to zinc and copper,
most other heavy metals also induce excessive ROS generation
(25–30). Therefore, luteolin may be a
potential effective antidote to prevent the combined toxicity
induced by multiple heavy metals.
Therefore, the inhibitory effects of luteolin on the
combined toxicity induced in HL7702 cells by a multi-heavy metal
mixture, including eight common contamination metals identified in
aqua products in the Ningbo area, were assessed and the underlying
molecular mechanisms were investigated.
Materials and methods
Materials and reagents
(CH3COO)2Pb·3H2O,
CdCl2·2.5H2O,
NiCl2·6H2O, MnCl2·4H2O,
ZnSO4·7H2O, CuSO4·5H2O
and K2Cr2O7 (analytical grades,
99.0–99.8%) were all purchased from Sinopharm Chemical Reagent Co.,
Ltd. (Beijing, China). CH3ClHg (analytical grade,
≥99.0%) was purchased from Dr Ehrenstorfer GmbH (Augsburg,
Germany). MTT was supplied by Amresco® (Solon, OH, USA).
Luteolin (analytical grades, ≥99.0%) was obtained from
Sigma-Aldrich (St. Louis, MO, USA). Bovine serum albumin (BSA) was
from Sangon Biotech (Shanghai, China). The assay kits for the
detection of lipid peroxidation (cat. no. S0131), ROS (cat. no.
S0033), adenosine triphosphate (ATP; cat. no. S0026) and total
protein were obtained from Beyotime Institute of Biotechnology
(Shanghai, China). The mitochondrial membrane potential assay kit
with JC-1 (cat. no. M8650) and the cell mitochondria isolation kit
(cat. no. SM0020) were obtained from Solarbio Life Sciences
(Beijing, China). The Alexa Fluor® 488 Annexin V/Dead
Cell Apoptosis kit (cat. no. V13241) was from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Antibodies B-cell
lymphoma 2 (Bcl-2; cat. no. 2870), Bcl-2-associated X protein (Bax;
cat. no. 2772), apoptotic protease activating factor 1 (Apaf1; cat.
no. 8969), cleaved caspase-9 (cat. no. 7237), caspase-3 (cat. no.
9665), cleaved caspase-3 (Asp175; cat. no. 9661), cleaved PARP-1
(Asp214; cat. no. 9544), used for western blot analysis in the
present study were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Poly(adenosine diphosphate-ribose) polymerase-1
(PARP-1; cat. no. sc-1562), GAPDH (cat. no. sc-25778), cytochrome
c (cat. no. sc-13561), pro-caspase-9 (cat. no. sc-7885) were
all purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). Goat anti-mouse IgG (cat. no. BA1050) and goat anti-rabbit
IgG (cat. no. BA1054) were provided by the Boster Biological
Technology (Wuhan, China). The HL7702 cell line was received from
the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China).
Preparation of heavy metal mixture
The multi-heavy metal mixture, which included
copper, mercury, cadmium, zinc, lead, manganese, nickel and
chromium was prepared according to the proportions of daily intake
of each metal through aqua product consumption by an adult in the
Ningbo area (Table I) (10,31). The sum of the concentrations of
the eight heavy metal elements was used as the final concentration
of the multi-heavy metal mixture.
 | Table IStock solution of the multi-heavy
metal mixture prepared according to the proportions of daily
consumption of each metal element through aqua products by an adult
in the Ningbo area. |
Table I
Stock solution of the multi-heavy
metal mixture prepared according to the proportions of daily
consumption of each metal element through aqua products by an adult
in the Ningbo area.
| Heavy metal
element | Concentration in
stock solution (mg/l) | Consumption through
aqua products by a 70 kg adult (mg/day) |
|---|
| Pb | 1.042 | 0.012 |
| Cd | 2.085 | 0.024 |
| Hg | 0.174 | 0.002 |
| Cu | 48.820 | 0.562 |
| Zn | 167.047 | 1.923 |
| Mn | 27.016 | 0.311 |
| Cr | 6.428 | 0.074 |
| Ni | 4.691 | 0.054 |
| Total | 257.303 | 2.962 |
Cell culture
HL7702 hepatocyte cells were maintained in RPMI-1640
medium (cat. no. SH30809.01; GE Healthcare Life Sciences, Little
Chalfont, UK) containing 1% penicillin-streptomycin and 10% fetal
bovine serum from Tianhang Biological Technology (Huzhou, China)
under standard culture conditions (37°C; 95% humidified air and 5%
CO2).
MTT assay
HL7702 cells were seeded into a 96-well plate at a
density of 10,000 cells/well in 200 μl medium and cultured
for 48 h. The cells were then treated with multi-heavy metal
mixture (concentrations of 0, 16.73, 19.30, 21.87, 24.44 or 27.01
mg/l) with or without luteolin (20 μM). After 12 h of
incubation, 10 μl MTT solution (3.5 mg/ml) mixed with 90
μl phenol red-free culture medium was added into each well,
followed by further incubation in the dark for 4 h. After the
culture medium was discarded, 150 μl dimethyl sulfoxide was
added to each well. The plate was incubated on an incubator shaker
at room temperature for 15 min. The optical density was measured at
a wavelength of 492 nm using a microplate reader.
Lipid peroxidation detection
HL7702 cells were seeded into a 6-well plate at a
density of 250,000 cells/well in 2.5 ml medium and cultured for 48
h. The cells were then treated with multi-heavy metal mixture (0,
16.73, 19.30, 21.87, 24.44 or 27.01 mg/l) with or without luteolin
(20 μM). After 12 h of incubation, the cells were harvested
and then lysed by adding 120 μl Nonidet P-40 lysis buffer to
each well. The lysate was centrifuged at 1,200 × g for 15 min at
4°C. The protein concentration was determined using the
bicinchoninic acid method. Supernatant (100 μl) was mixed
with 200 μl malondialdehyde (MDA) detection buffer and then
boiled for 15 min. After cooling to room temperature, the mixture
was centrifuged at 1,000 × g for 15 min. The supernatant was then
transferred into a 96-well plate (200 μl/well) and the
amount of lipid peroxidation was detected at a wavelength of 532 nm
by a microplate reader. The MDA levels were normalized to the
protein concentration.
ROS detection
Intracellular ROS levels were determined using a ROS
detection kit. HL7702 cells were treated with multi-heavy metal
mixture with or without luteolin as for the MTT assay in a black
96-well plate. The cells were then gently washed 2 times with
serum-free medium. The cells were then incubated with 200 μl
serum-free medium containing 10 μM H2DCFDA at
37°C for 35 min. The fluorescence distribution of each well was
detected by a fluorospectrophotometer (excitation wavelength, 488
nm; emission wavelength, 535 nm).
ATP detection
HL7702 cells were treated with multi-heavy metal
mixture and luteolin as for the lipid peroxidation assay.
Subsequently, they were gently washed 2 times with 4°C sterile PBS
and lysed by adding 200 μl Nonidet P-40 lysis buffer. The
lysate was centrifuged at 12,000 × g for 5 min at 4°C. A
20-μl aliquot of the supernatant was transferred into a
dedicated centrifuge tube containing 100 μl ATP detection
buffer at room temperature. ATP standard solutions (0, 0.5, 1, 5,
10, 50 and 100 μM) were used to obtain a standard curve. ATP
levels were detected by measurement of the relative light unit
value with a luminometer and were normalized to the protein
concentration.
JC-1 staining assay
HL7702 cells were treated with multi-heavy metal
mixture with or without luteolin as for the MTT assay in a black
96-well plate. Subsequently, 100 μl phenol red-free culture
medium mixed with 100 μl JC-1 staining solution was added
into each well and further incubated in the dark at 37°C for 20
min. After gently washing the cells 2 times with 200 μl JC-1
staining wash buffer (4°C), 200 μl phenol red-free culture
medium was added into each well. The fluorescence of each well was
observed under a fluorescence microscope and images were
captured.
Apoptosis detection
Apoptosis was detected using the Alexa
Fluor® 488 Annexin V/Dead Cell Apoptosis kit. HL7702
cells were treated with multi-heavy metal mixture and luteolin as
for the lipid peroxidation assay. The cells were then harvested by
EDTA-free trypsinization provided by Thermo Fisher Scientific, Inc.
Following centrifugation at 67 × g for 5 min at room temperature,
the cells were resuspended in 500 μl Annexin binding buffer
containing 1 μl propidium iodide dye and 5 μl Alexa
Fluor® 488-conjugated Annexin V. The single-cell
suspension was further incubated in the dark for 30 min and
apoptosis was then monitored by flow cytometry.
Western blot analysis
HL7702 cells were seeded into 20×100 mm culture
dishes at a density of 1,200,000 cells/dish in 10 ml medium and
cultured for 48 h. The cells were then treated as described above
and then gently washed 2 times with PBS at 4°C
For whole-cell protein preparation, 500 μl
Nonidet P-40 lysis buffer containing 10 μM phenylmethane
sulfonyl fluoride was added into each dish to lyse cells on ice for
10 min. The lysate was filled into Eppendorf tubes and centrifuged
at 15,000 × g for 3 min at 4°C. Of the supernatant, 350 μl
was used for whole-cell protein detection.
To prepare cytosolic protein, a cell mitochondria
isolation kit was used according to manufacturer's instructions and
80 μl supernatant was used for the protein detection.
Protein concentrations were determined by the BCA
method. Supernatant was mixed with 5X loading buffer (4:1 in
volume) and boiled for 5 min.
Polyacrylamide stacking (6%) gels and resolving
(10%) gels were used to separate proteins of different molecular
weights. Then the proteins were transferred onto PVDF membranes,
blocked in 5% skimmed milk for 3 h at room temperature and
incubated for 12 h at 4°C with primary antibodies. After that,
membranes were probed with secondary antibodies at room temperature
for 1.5 h. Immunoblotting was performed for Bcl-2, Bax, Apaf1,
cytochrome c, pro-caspase-9, cleaved caspase-9, caspase-3,
cleaved caspase-3 (Asp175), PARP-1, cleaved PARP-1 (Asp214) and
GAPDH at 1:1,000 dilution. The Tanon imaging processing system
(Tanon Science and Technology Co., Ltd., Shanghai, China) was used
for processing and evaluation of the western blots by ECL
solution.
Immunofluorescence staining for cleaved
caspase-3
HL7702 cells (n=50,000) were seeded onto a glass
coverslip and maintained under standard culture conditions for 48
h. The cells were treated with multi-heavy metal mixture and
luteolin as described above. The glass coverslips were successively
dipped in PBS (5 min, 2 times), 4% paraformaldehyde (10 min), PBS
containing Tween-20 (PBST; 5 min, 2 times), 0.1% Triton X-100 (10
min), PBST (5 min, 2 times), 1% BSA-TBST blocking buffer (2 h), 1%
BSA-TBST solution containing cleaved caspase-3 rabbit antibody (2
h; 1:1,000 dilution at room temperature), PBST (10 min, 2 times),
1% BSA-TBST solution containing goat-anti-rabbit antibody marked
with Alexa Fluor® 488 (2 h; 1:2,000 dilution at room
temperature) and PBST (10 min, 2 times). The cell nuclei were
stained with 10 μM DAPI. The immunofluorescence staining of
cleaved caspase-3 was captured by a fluorescence microscope.
Statistical analysis
Each experiment was performed three or more times.
Differences within groups were analyzed using one-way analysis of
variance and a post hoc least significant difference test with SPSS
13.0 (SPSS, Inc., Chicago, IL, USA). P≤0.05 was considered to
indicate a statistically significant difference.
Results
Cell viability and morphological
changes
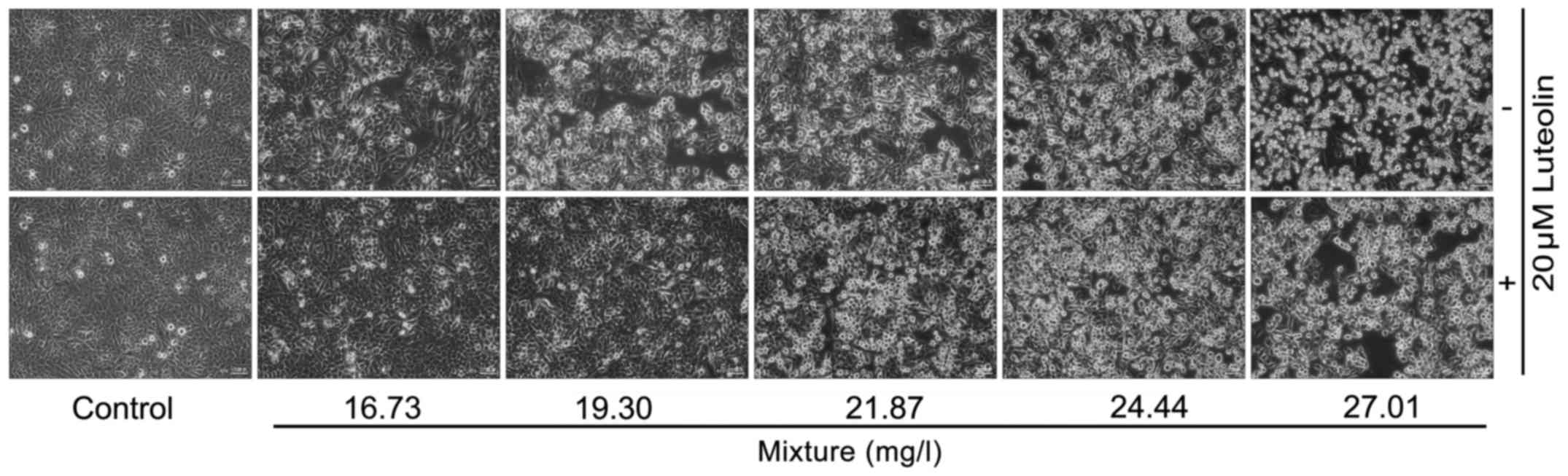
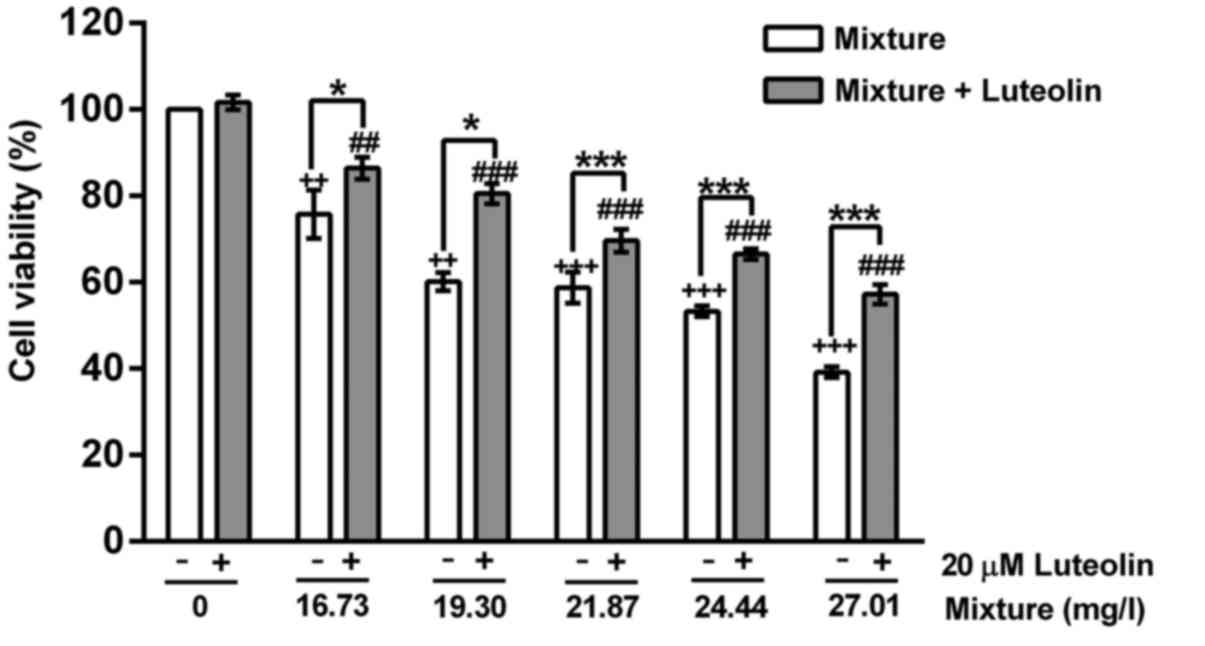
Treatment with multi-heavy metal mixture alone
significantly reduced the cell viability and induced cell
morphological changes in a dose-dependent manner, while addition of
20 μM luteolin significantly inhibited these toxic effects
induced by the multi-heavy metal mixture (Figs. 1 and 2).
ROS, lipid peroxidation and ATP level
changes
The multi-heavy metal mixture induced intracellular
ROS generation (Fig. 3A and B)
and lipid peroxidation (Fig. 3C)
in a dose-dependent manner, and decreased the intracellular ATP
content (Fig. 3D). Addition of 20
μM luteolin significantly inhibited the multi-heavy metal
mixture-induced ROS generation and lipid peroxidation as well as
the decrease of intracellular ATP.
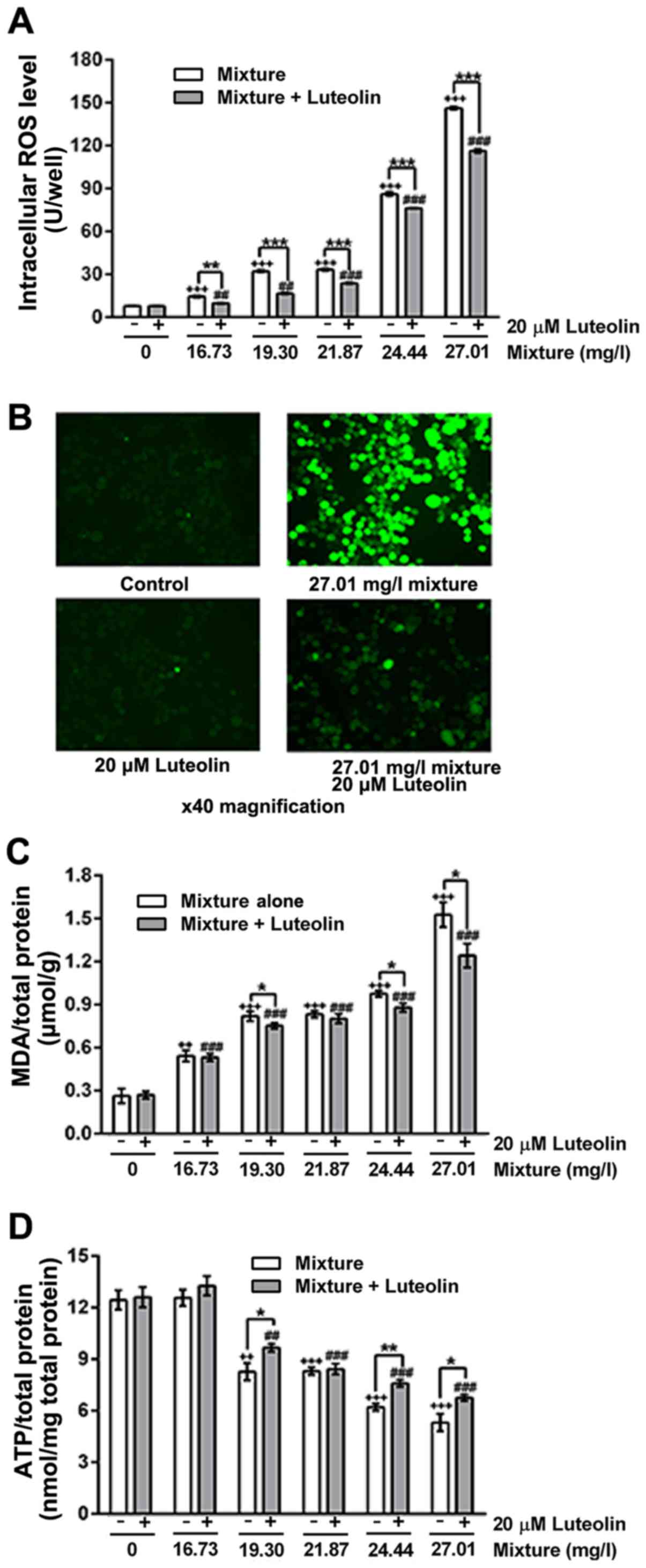 | Figure 3(A) ROS levels, (B)
immunofluorescence images of ROS (magnification, ×40), (C) MDA
content and (D) ATP levels of cells treated with multi-heavy metal
mixture alone or in combination with luteolin.
*P<0.05, **P<0.01, ***P<0.001,
mixture alone compared with mixture + luteolin;
++P<0.01, +++P<0.001, compared with
control - (without any treatment); ##P<0.01,
###P<0.001, compared with control + 20 μM
luteolin. Mixture, multi-heavy metal mixture; ROS, reactive oxygen
species; MDA malondialdehyde; ATP, adenosine triphosphate. |
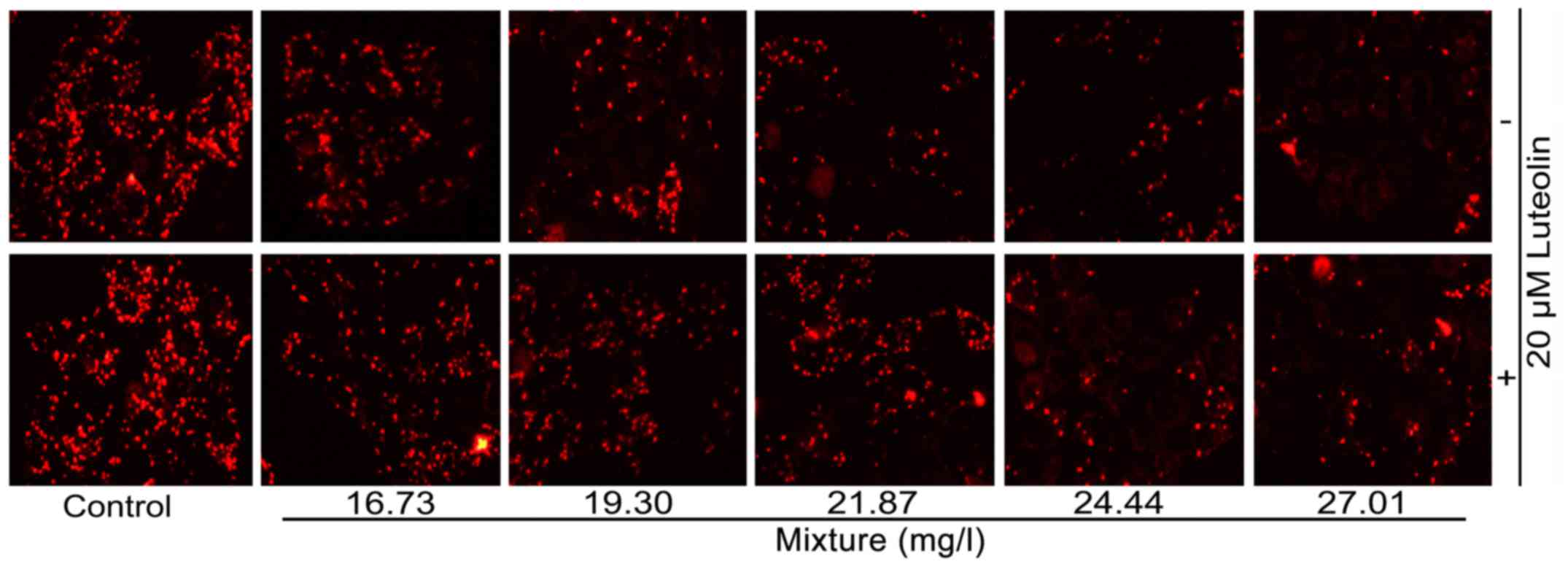
Mitochondrial membrane potential
changes
With the increase in the multi-heavy metal mixture
concentration, the mitochondrial membrane potential decreased
gradually, while 20 μM luteolin attenuated these changes
(Fig. 4).
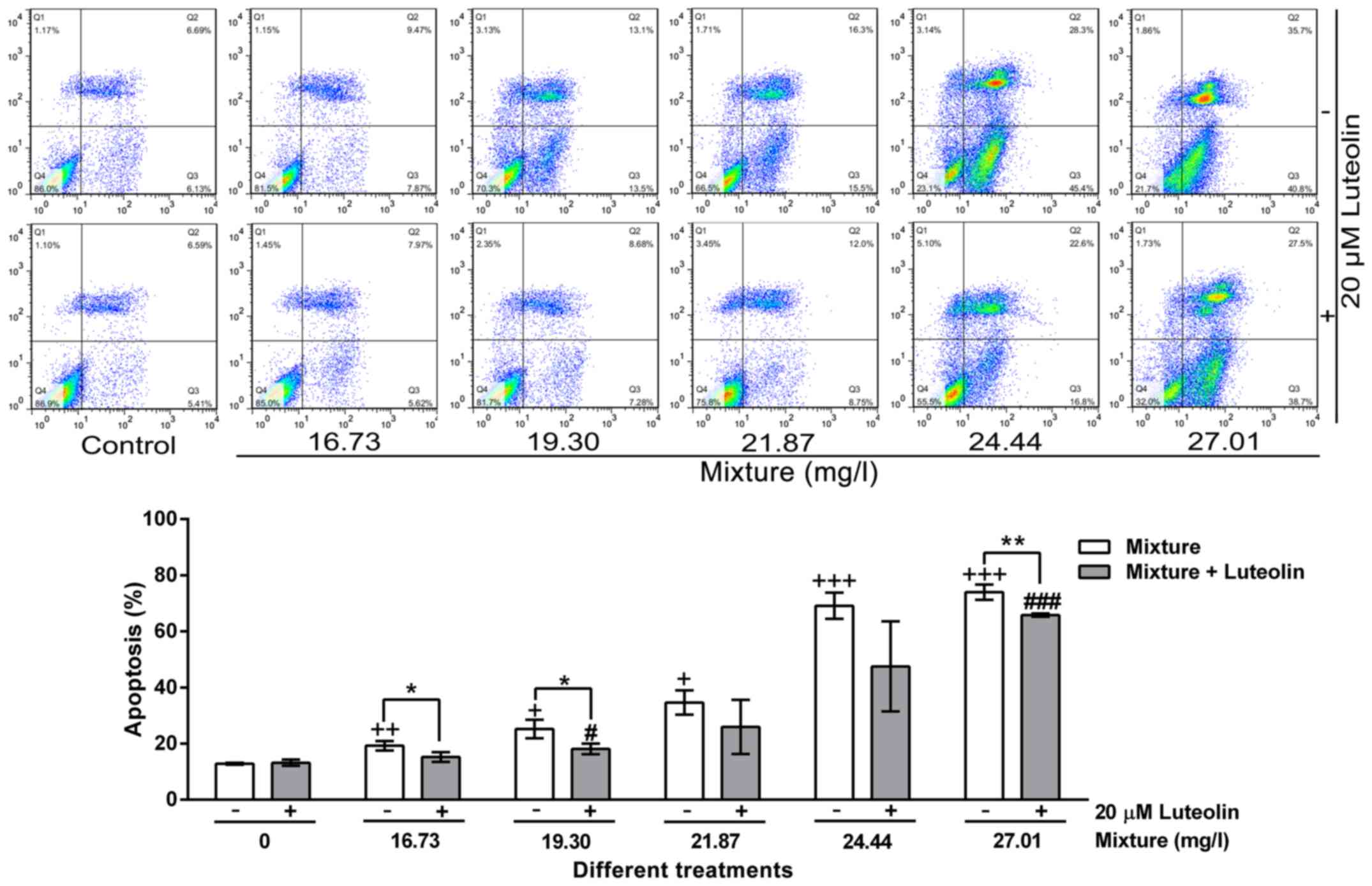
Apoptotic analysis
Multi-heavy metal mixture induced HL7702 cell
apoptosis in a dose-dependent manner, while 20 μM luteolin
inhibited this effect (Fig.
5).
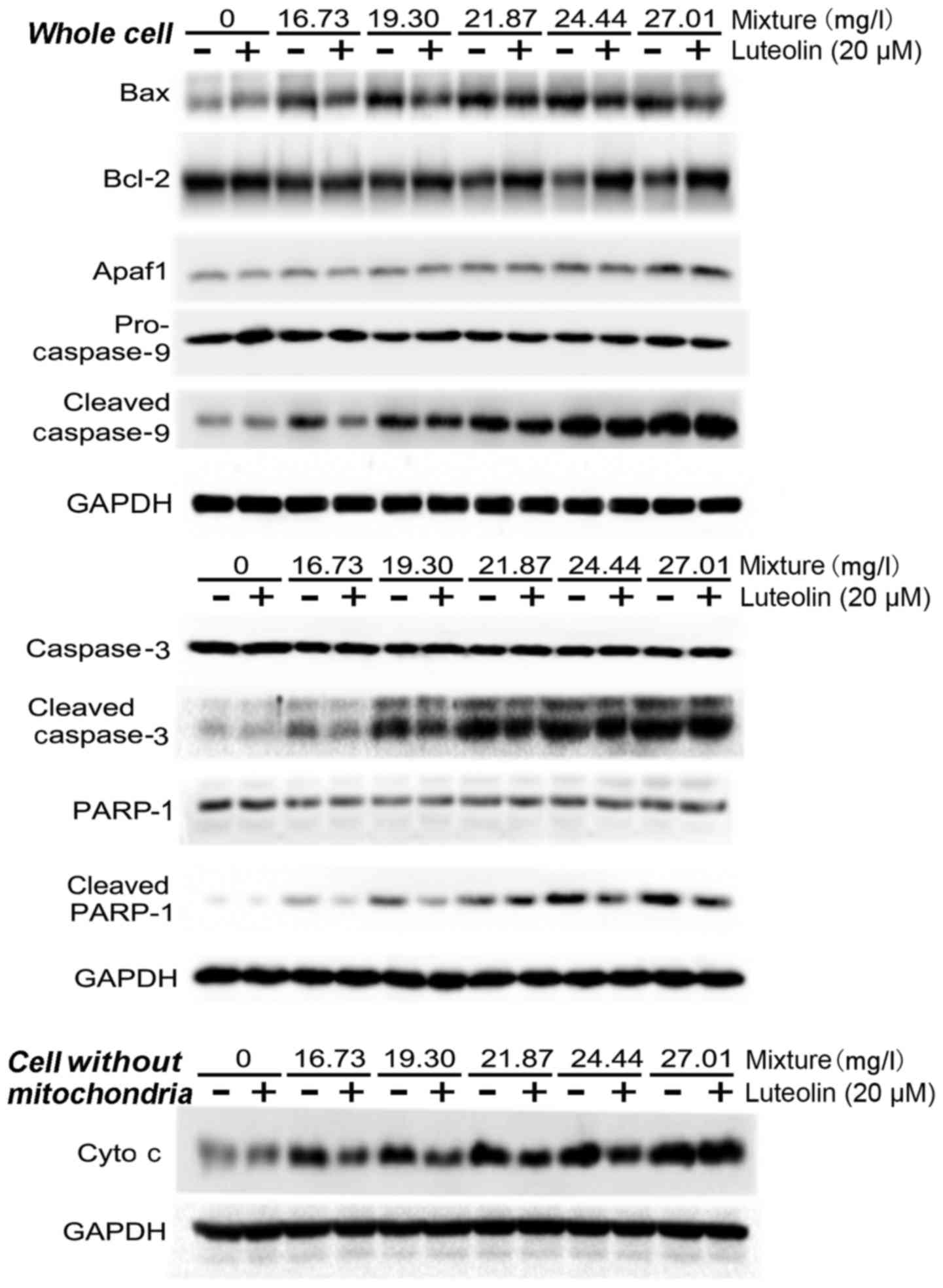
Effects on mitochondrial
apoptosis-associated signaling proteins
Addition of 20 μM luteolin inhibited the
multi-heavy metal mixture-induced increase of the Bax/Bcl-2 ratio
in the cells. Furthermore, 20 μM luteolin significantly
inhibited the multi-heavy metal mixture-induced increases of
cleaved caspase-9, cleaved caspase-3 and cleaved PARP-1 protein. In
addition, 20 μM luteolin significantly alleviated the
multi-heavy metal mixture-induced cytochrome c release from
the mitochondria into the cytosol (Fig. 6).
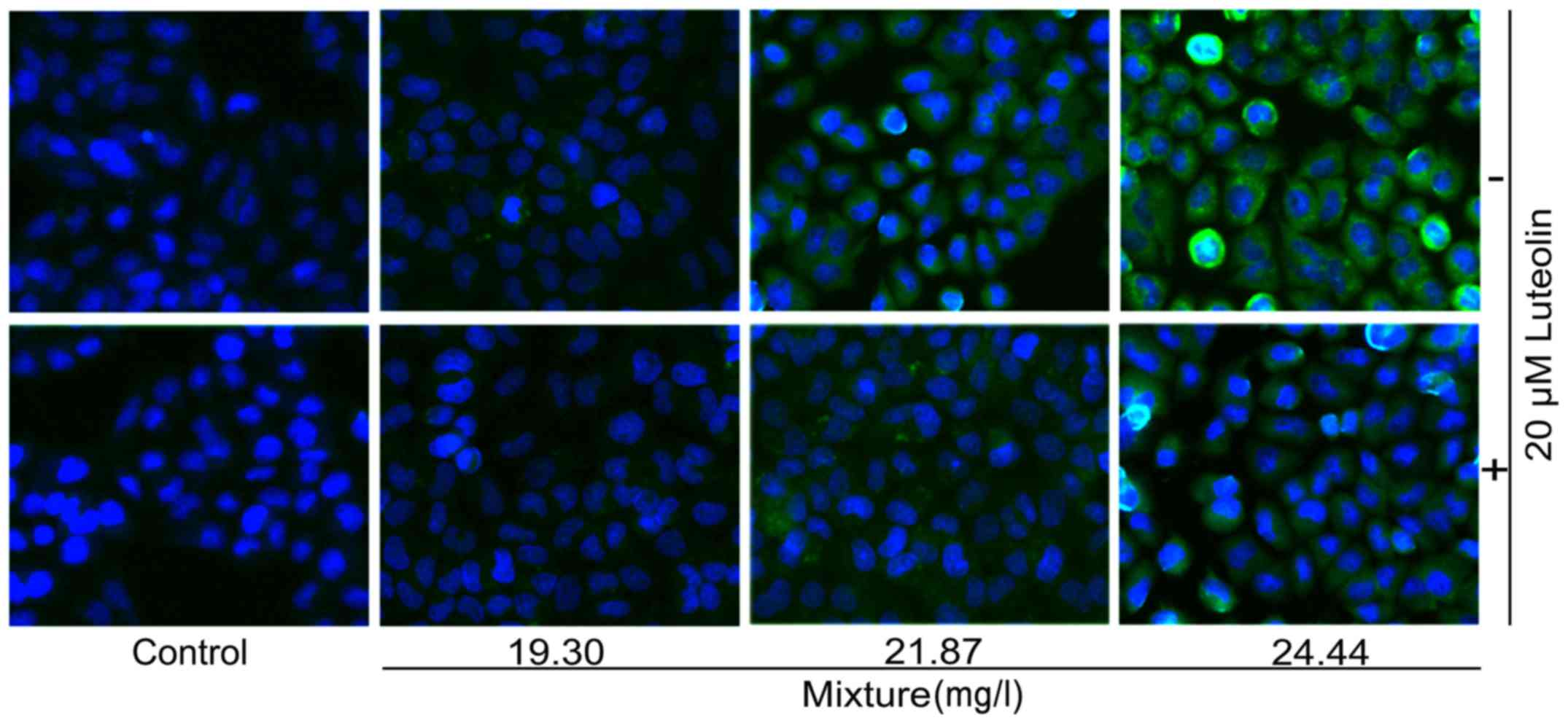
Immunofluorescence staining results in
cleaved caspase-3
Immunofluorescence staining revealed that at higher
doses, multi-heavy metal mixture treatment alone induced
significant caspase-3 cleavage (green color) in HL7702 cells, and
addition of 20 μM luteolin inhibited this effect (Fig. 7).
Discussion
The liver is an important multifunctional organ
performing detoxification and metabolism of xenobiotics (32), substance synthesis and metabolic
balancing of nutrients. In a previous study by our group, a MTT
assay was used to screen various antioxidant chemicals for their
protective effects and identified that 20 μM luteolin
significantly inhibited the cytotoxic effects if multi-heavy metal
mixture in HL7702 cells. Therefore, HL7702 hepatocytes were used in
the present study to investigate the combined toxicity of
multi-heavy metal mixture and the inhibitory effects of luteolin as
well as the underlying molecular mechanisms.
Normally, ROS generation and quenching are in a
dynamic balance state due to the intracellular antioxidant system.
Certain harmful extracellular factors may break this balance,
resulting in excessive ROS generation beyond the cell scavenging
ability to then induce organelle damage, abnormal expression of
proteins or eventually cell death (33–35). Studies have demonstrated that most
heavy metal ions cause excessive intracellular ROS generation
(12,22,36,37). The present results indicated that
the multi-heavy metal mixture induced intracellular ROS generation
in a dose-dependent manner, while the antioxidant luteolin had a
significant quenching effect on this ROS generation. Lipid
peroxidation is another indicator of cell damage from oxidative
stress. Excessive ROS released from the mitochondria into the
cytosol may induce cellular lipid peroxidation (38). The cellular MDA content is widely
used as an index of lipid peroxidation levels. In the present
study, luteolin was demonstrated to significantly prevent
multi-heavy metal mixture-induced lipid peroxidation.
As an important energy molecule, ATP participates in
most intracellular biogenic activities. The intracellular ATP
levels decline once cells undergo apoptosis, necrosis or encounter
adverse factors. The ATP detection results of the present study
suggested that 20 μM luteolin significantly inhibited the
multi-heavy metal mixture-induced effect on decreasing ATP levels.
Excessive ROS and decreased ATP levels indicate impaired
mitochondrial function (39–42). Mitochondrial JC-1 staining may be
used to detect changes in the mitochondrial membrane potential.
Under normal conditions, the JC-1 monomer aggregates in the
mitochondrial matrix to form polymer J-aggregates (red color). Once
the mitochondrial membrane potential decreases, JC-1 monomer cannot
aggregate in the mitochondrial matrix, which results in less or no
polymer J-aggregate formation (less or no red color). With
increasing multi-heavy metal mixture concentration, the
mitochondrial membrane potential gradually decreased, while 20
μM luteolin significantly attenuated this change.
The simultaneous decrease of intracellular ATP
levels and mitochondrial membrane potential often accompanies cell
apoptosis. Apoptosis is an initiative action to implement
programmed cell death (43),
which is strictly controlled by multiple genes, including the
protein families of Bcl-2 and caspases, as well as the c-myc
oncogene and P53 tumor suppressor gene (44–46). The death receptor pathways,
including membrane receptor, cytochrome c and caspase
pathways, may be activated by a series of physiological and
pathological signals (47,48).
In the present study, apoptosis was monitored by flow cytometry.
The results indicated that the multi-heavy metal mixture induced
HL7702 cell apoptosis in a dose-dependent manner, which was
significantly inhibited by 20 μM luteolin.
Bcl-2 and Bax are proteins belonging to the Bcl-2
family and control the mitochondrial membrane permeability to
regulate the release of cytochrome c (45). Bax upregulates the permeability of
the mitochondrial membrane accompanied with the release of
cytochrome c from the mitochondria into the cytosol, while
Bcl-2 has the opposite role (45,49). Cytochrome c in the cytosol
activates caspase family proteins and forms the cytochrome
c/Apaf1/caspase-9 apoptosome, which then leads to apoptosis
(50). In the present study,
mitochondrial apoptosis pathway-associated signal protein
expression was detected by western blot analysis. The results
suggest that treatment with the multi-heavy metal mixture led to a
significant upregulation of the Bax/Bcl-2 ratio, as well as the
levels of Apaf1, and cleavage of caspase-9, caspase-3 and PARP-1.
The immunofluorescence staining results in the intact HL7702 cells
confirmed that the mitochondrial apoptosis pathway was activated
(positive staining for cleaved caspase-3). Furthermore, these
results also suggested that 20 μM luteolin attenuated
multi-heavy metal mixture-induced changes in signaling proteins of
mitochondrial apoptosis pathways.
Therefore, the potential underlying molecular
mechanisms of multi-heavy metal mixture-induced cytotoxicity may be
summarized as follows: At first, the heavy metal ions enter the
cells and induce intracellular ROS generation and mitochondrial
damage. Subsequently, the permeability of the mitochondrial
membrane is upregulated by the Bax protein, which leads to
mitochondrial cytochrome c release from the mitochondria
into the cytosol and subsequent formation of the apoptosome. The
apoptosome initiates the cascades of caspase-3 and PARP-1 cleavage,
and eventually cell apoptosis. Luteolin inhibited multi-heavy metal
mixture-induced apoptosis by quenching the excessive ROS and
further by blocking the oxidative stress-mediated mitochondrial
apoptosis pathway.
In conclusion, the present study demonstrated that
the multi-heavy metal mixture containing eight common metals
prepared according to the proportions in which daily intake of each
metal occurs through aqua product consumption by an adult in the
Ningbo area induced oxidative stress injury and mitochondrial
damage in HL7702 cells. Luteolin protected HL7702 cells from
multi-heavy metal mixture-induced toxicity through downregulation
of the ROS-mediated mitochondrial apoptosis pathway. Luteolin may
be beneficial to prevent the multi-heavy metal pollution-induced
health hazards arising from long-term aqua product consumption.
However, the inhibitory effect of luteolin on the combined toxicity
of multi-heavy metals was only evaluated by in vitro
experiments in the present study. A further in vivo study
will be required to verify the above in vitro experimental
results.
Acknowledgments
This study was partly supported by the National
Nature Science Foundation of China (grant no. 81273111), Scientific
Projects of Zhejiang Province (grant nos. 2015C33148 and
2015C37117), the Ningbo Scientific Innovation Team for
Environmental Hazardous Factor Control and Prevention (grant no.
2016C51001), Zhejiang Key Laboratory of Pathophysiology (201703),
and the KC Wong Magna Fund of Ningbo University.
References
|
1
|
Liu Z, Zhang Q, Han T, Ding Y, Sun J, Wang
F and Zhu C: Heavy metal pollution in a soil-rice system in the
Yangtze River Region of China. Int J Environ Res Public Health.
13:632015. View Article : Google Scholar
|
|
2
|
Niu Y, Niu Y, Pang Y and Yu H: Assessment
of heavy metal pollution in sediments of inflow rivers to Lake
Taihu, China. Bull Environ Contam Toxicol. 95:618–623. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan Y and Li H: Investigating heavy metal
pollution in mining brownfield and its policy implications: a case
study of the Bayan Obo Rare Earth Mine, Inner Mongolia, China.
Environ Manage. 57:879–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu R, Zhang W, Hu G, Lin C and Yang Q:
Heavy metal pollution and Pb isotopic tracing in the intertidal
surface sediments of Quanzhou Bay, southeast coast of China. Mar
Pollut Bull. 105:416–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Liao Q, Shao S, Zhang N, Shen Q
and Liu C: Heavy metal pollution, fractionation, and potential
ecological risks in sediments from Lake Chaohu (Eastern China) and
the surrounding rivers. Int J Environ Res Public Health.
12:14115–14131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong S, Geng H, Zhang F, Liu Z, Wang T
and Song B: Risk assessment and prediction of heavy metal pollution
in groundwater and river sediment: a case study of a typical
agricultural irrigation area in Northeast China. Int J Anal Chem.
2015:9215392015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uluturhan E and Kucuksezgin F: Heavy metal
contaminants in Red Pandora (Pagellus erythrinus) tissues from the
Eastern Aegean Sea, Turkey. Water Res. 41:1185–1192. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao J, Bowman L, Magaye R, Leonard SS,
Castranova V and Ding M: Apoptosis induced by tungsten
carbide-cobalt nanoparticles in JB6 cells involves ROS generation
through both extrinsic and intrinsic apoptosis pathways. Int J
Oncol. 42:1349–1359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhijie Gao LW, Zheng H and Yao X: Analysis
on concentration of heavy metals lead, mercury, cadmium, chromium
in seafood in Ningbo in 2012. Zhongguo Shipin Weisheng Zazhi.
26:76–78. 2014.
|
|
10
|
Lin X, Gu Y, Zhou Q, Mao G, Zou B and Zhao
J: Combined toxicity of heavy metal mixtures in liver cells. J Appl
Toxicol. 36:1163–1172. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verma R, Xu X, Jaiswal MK, Olsen C, Mears
D, Caretti G and Galdzicki Z: In vitro profiling of epigenetic
modifications underlying heavy metal toxicity of tungsten-alloy and
its components. Toxicol Appl Pharmacol. 253:178–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou J, Jin L, Wu N, Tan Y, Song Y, Gao M,
Liu K, Zhang X and He J: DNA damage and oxidative stress in human B
lymphoblastoid cells after combined exposure to hexavalent chromium
and nickel compounds. Food Chem Toxicol. 55:533–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stephenson AP, Schneider JA, Nelson BC,
Atha DH, Jain A, Soliman KF, Aschner M, Mazzio E and Renee Reams R:
Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells:
attenuation of thymine base lesions by glutathione and
N-acetylcysteine. Toxicol Lett. 218:299–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lankoff A, Banasik A, Duma A, Ochniak E,
Lisowska H, Kuszewski T, Góźdź S and Wojcik A: A comet assay study
reveals that aluminium induces DNA damage and inhibits the repair
of radiation-induced lesions in human peripheral blood lymphocytes.
Toxicol Lett. 161:27–36. 2006. View Article : Google Scholar
|
|
15
|
Bal W and Kasprzak KS: Induction of
oxidative DNA damage by carcinogenic metals. Toxicol Lett.
127:55–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JK, Kang KA, Ryu YS, Piao MJ, Han X,
Oh MC, Boo SJ, Jeong SU, Jeong YJ, Chae S, et al: Induction of
endoplasmic reticulum stress via reactive oxygen species mediated
by luteolin in melanoma cells. Anticancer Res. 36:2281–2289.
2016.PubMed/NCBI
|
|
17
|
Kanai K, Nagata S, Hatta T, Sugiura Y,
Sato K, Yamashita Y, Kimura Y and Itoh N: Therapeutic
anti-inflammatory effects of luteolin on endotoxin-induced uveitis
in Lewis rats. J Vet Med Sc. 78:1381–1384. 2016. View Article : Google Scholar
|
|
18
|
Fan W, Qian S, Qian P and Li X: Antiviral
activity of luteolin against Japanese encephalitis virus. Virus
Res. 220:112–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Majumdar D, Jung KH, Zhang H, Nannapaneni
S, Wang X, Amin AR, Chen Z, Chen ZG and Shin DM: Luteolin
nanoparticle in chemoprevention: in vitro and in vivo anticancer
activity. Cancer Prev Res (Phila). 7:65–73. 2014. View Article : Google Scholar
|
|
20
|
Kure A, Nakagawa K, Kondo M, Kato S,
Kimura F, Watanabe A, Shoji N, Hatanaka S, Tsushida T and Miyazawa
T: Metabolic fate of luteolin in rats: its relationship to
anti-inflammatory effect. J Agric Food Chem. 64:4246–4254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Francisco V, Figueirinha A, Costa G,
Liberal J, Ferreira I, Lopes MC, García-Rodríguez C, Cruz MT and
Batista MT: The flavone luteolin inhibits liver X receptor
activation. J Nat Prod. 79:1423–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou F, Qu L, Lv K, Chen H, Liu J, Liu X,
Li Y and Sun X: Luteolin protects against reactive oxygen
species-mediated cell death induced by zinc toxicity via the
PI3K-Akt-NF-κB-ERK-dependent pathway. J Neurosci Res. 89:1859–1868.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu R, Meng F, Zhang L, Liu A, Qin H, Lan
X, Li L and Du G: Luteolin isolated from the medicinal plant
Elsholtzia rugulosa (Labiatae) prevents copper-mediated toxicity in
β-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y
cells. Molecules. 16:2084–2096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi EM: Luteolin protects osteoblastic
MC3T3-E1 cells from antimycin A-induced cytotoxicity through the
improved mitochondrial function and activation of PI3K/Akt/CREB.
Toxicol In Vitro. 25:1671–1679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hossain S, Bhowmick S, Jahan S, Rozario L,
Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK and Shahjalal
H: Maternal lead exposure decreases the levels of brain development
and cognition-related proteins with concomitant upsurges of
oxidative stress, inflammatory response and apoptosis in the
offspring rats. Neurotoxicology. 56:150–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karimi R, Vacchi-Suzzi C and Meliker JR:
Mercury exposure and a shift toward oxidative stress in avid
seafood consumers. Environ Res. 146:100–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bagchi D, Bagchi M and Stohs SJ: Chromium
(VI)-induced oxidative stress, apoptotic cell death and modulation
of p53 tumor suppressor gene. Mol Cell Biochem. 222:149–158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iakimova ET, Woltering EJ, Kapchina-Toteva
VM, Harren FJ and Cristescu SM: Cadmium toxicity in cultured tomato
cells - role of ethylene, proteases and oxidative stress in cell
death signaling. Cell Biol Int. 32:1521–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roth JA and Eichhorn M: Down-regulation of
LRRK2 in control and DAT transfected HEK cells increases
manganese-induced oxidative stress and cell toxicity.
Neurotoxicology. 37:100–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmid M, Zimmermann S, Krug HF and Sures
B: Influence of platinum, palladium and rhodium as compared with
cadmium, nickel and chromium on cell viability and oxidative stress
in human bronchial epithelial cells. Environ Int. 33:385–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin XL, Gu YL, Zhou Q, Mao GC, Ma DJ, Zhao
JS and Zou BB: Study: Joint toxicity of heavy metal mixtures
related to proportions of fish consumption. J Ningbo Univ (NSEE).
29:22–27. 2016.In Chinese.
|
|
32
|
Guillouzo A, Corlu A, Aninat C, Glaise D,
Morel F and Guguen-Guillouzo C: The human hepatoma HepaRG cells: a
highly differentiated model for studies of liver metabolism and
toxicity of xenobiotics. Chem Biol Interact. 168:66–73. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu Y, Wang Y, Zhou Q, Bowman L, Mao G, Zou
B, Xu J, Liu Y, Liu K, Zhao J, et al: Inhibition of nickel
nanoparticles-induced toxicity by epigallocatechin-3-gallate in JB6
cells may be through down-regulation of the MAPK signaling
pathways. PLoS One. 11:e01509542016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Asif M, Shafaei A, Jafari SF, Mohamed SK,
Ezzat MO, Abdul Majid AS, Oon CE, Petersen SH, Kono K and Abdul
Majid AM: Isoledene from Mesua ferrea oleo-gum resin induces
apoptosis in HCT 116 cells through ROS-mediated modulation of
multiple proteins in the apoptotic pathways: a mechanistic study.
Toxicol Lett. 257:84–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Wang H, Xu J, Zhu J and Ding K:
Inhibition of cathepsin S induces autophagy and apoptosis in human
glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K
and JNK signaling pathways. Toxicol Lett. 228:248–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hosseini A, Sharifi AM, Abdollahi M,
Najafi R, Baeeri M, Rayegan S, Cheshmehnour J, Hassani S, Bayrami Z
and Safa M: Cerium and yttrium oxide nanoparticles against
lead-induced oxidative stress and apoptosis in rat hippocampus.
Biol Trace Elem Res. 164:80–89. 2015. View Article : Google Scholar
|
|
37
|
Verma K, Mehta SK and Shekhawat GS: Nitric
oxide (NO) counteracts cadmium induced cytotoxic processes mediated
by reactive oxygen species (ROS) in Brassica juncea: cross-talk
between ROS, NO and antioxidant responses. Biometals. 26:255–269.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dutta RK, Nenavathu BP, Gangishetty MK and
Reddy AV: Studies on antibacterial activity of ZnO nanoparticles by
ROS induced lipid peroxidation. Colloids Surf B Biointerfaces.
94:143–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mathy-Hartert M, Hogge L, Sanchez C,
Deby-Dupont G, Crielaard JM and Henrotin Y: Interleukin-1beta and
interleukin-6 disturb the antioxidant enzyme system in bovine
chondrocytes: a possible explanation for oxidative stress
generation. Osteoarthritis Cartilage. 16:756–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akkerman JW, Rijksen G, Gorter G and Staal
GE: Platelet functions and energy metabolism in a patient with
hexokinase deficiency. Blood. 63:147–153. 1984.PubMed/NCBI
|
|
41
|
Chinopoulos C, Tretter L and Adam-Vizi V:
Depolarization of in situ mitochondria due to hydrogen
peroxide-induced oxidative stress in nerve terminals: inhibition of
alpha-ketoglutarate dehydrogenase. J Neurochem. 73:220–228. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tretter L, Chinopoulos C and Adam-Vizi V:
Enhanced depolarization-evoked calcium signal and reduced
[ATP]/[ADP] ratio are unrelated events induced by oxidative stress
in synaptosomes. J Neurochem. 69:2529–2537. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bennett M, Macdonald K, Chan SW, Luzio JP,
Simari R and Weissberg P: Cell surface trafficking of Fas: a rapid
mechanism of p53-mediated apoptosis. Science. 282:290–293. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis, and its inhibitor ICAD. Nature. 391:43–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Egger L, Madden DT, Rhême C, Rao RV and
Bredesen DE: Endoplasmic reticulum stress-induced cell death
mediated by the proteasome. Cell Death Differ. 14:1172–1180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eskes R, Desagher S, Antonsson B and
Martinou JC: Bid induces the oligomerization and insertion of Bax
into the outer mitochondrial membrane. Mol Cell Biol. 20:929–935.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Meichner K, Fogle JE, English L and Suter
SE: Expression of apoptosis-regulating proteins Bcl-2 and Bax in
lymph node aspirates from dogs with lymphoma. J Vet Intern Med.
30:819–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Marsden VS, O'Connor L, O'Reilly LA, Silke
J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ,
et al: Apoptosis initiated by Bcl-2-regulated caspase activation
independently of the cytochrome c/Apaf-1/caspase-9 apoptosome.
Nature. 419:634–637. 2002. View Article : Google Scholar : PubMed/NCBI
|