Introduction
Kidney transplantation is an efficacious treatment
for patients with end-stage renal disease (1,2).
The in situ regional cooling technique with static cold
storage was developed to maximize the benefit of the donated kidney
for the recipient (3), and has
achieved excellent kidney graft function and good graft survival
(4). However, renal
ischemia/reperfusion injury (IRI), which is a major cause of acute
renal injury (formerly referred to as acute renal failure) and a
risk factor for the quality of a kidney graft, rejection and renal
fibrosis, is directly correlated with the survival of the recipient
(5,6). Furthermore, the complex association
of pathophysiological processes with inflammation makes the
ischemic kidney injury an important risk factor for progression of
chronic kidney disease (7). It
was documented that inflammation, apoptosis and necrosis, hypoxic
injury and production of reactive oxygen species are involved in
the pathogenesis of IRI (8). The
modulation of inflammatory response, inhibition of apoptosis and
amelioration of oxidative stress confer an advantage to the
prevention and treatment of IRI (9,10).
Erythropoietin (EPO) has been found to confer such an advantage
(11,12), but the underlying molecular
mechanism remains unclear.
Inflammation is invariably found to be an important
initiating and aggravating factor in both acute and chronic kidney
injury (13). Nuclear factor-κB
(NF-κB), a pivotal mediator of the inflammatory response, modulates
the expression levels of adhesion molecules, chemokines and other
pro-inflammatory molecules in the kidney (14,15). It was reported that interleukin
(IL)-4 promoted the activation of signal transducer and activator
of transcription 6 (STAT6), suppressing the transcriptional
activation of NF-κB-dependent proinflammatory mediators following
liver IRI (16,17). Apoptosis is a principal cause of
cell death in the kidney following IRI (18,19). Apoptosis-related proteins, such as
caspase-3, play important roles in renal IRI (20,21). Therefore, regulating inflammation
and cell death is a promising therapeutic strategy for reversing
IRI and protecting renal allografts.
EPO is a hematopoietic hormone produced by the
kidney and fetal liver in response to hypoxia, inflammation and
cell death (22). EPO exerts
numerous protective effects. Importantly, EPO may exert
antioxidant, anti-inflammatory and anti-apoptotic effects against
IRI in the brain (23) and kidney
(24). It was demonstrated that,
under conditions of renal IRI, the expression levels of EPO in the
kidney were decreased (25). A
variety of signal transduction pathways, including
mitogen-activated protein kinase (MAPK) and NF-κB, were involved in
the EPO-mediated cytoprotective effects (26,27). The cell-protective effect of EPO
was significantly attenuated by pretreatment with the specific
p-p38 inhibitor, suggesting that MAPK pathways may be responsible
for cell survival under cytotoxic conditions (26). EPO treatment significantly
decreased the lipopolysaccharide-induced elevation of creatinine
(Cr) and NF-κB, indicating that EPO may play a protective role
against IRI by reducing the inflammatory response and tissue
degeneration, possibly via the NF-κB signaling pathway (27). Therefore, the mechanisms
underlying the protective effect of EPO against renal IRI remain to
be fully elucidated.
The aim of the present study was to investigate the
effect of EPO on the levels of chemokines, including interferon
(IFN)-γ, IL-4, IL-10 and Cr, by the siRNA technique, and determine
whether the renoprotective effect of EPO against IRI is exerted
through the STAT6/MAPK/NF-κB pathway by using specific inhibitors,
including the STAT6 inhibitor AS1517499, the JNK inhibitor
SP600125, the p38 MAPK inhibitor SB203580, and the NF-κB inhibitor
lactacystin. This detailed protective mechanism of EPO may improve
reperfusion tolerance in ischemic kidneys and benefit transplant
recipients.
Materials and methods
Animals
Specific pathogen-free (SPF) adult male Lewis rats
(n=64) and Brown Norway rats (n=64), 12–16 weeks old, weighing
250–350 g were purchased from Charles River Laboratories (Beijing,
China). All animal experiments were performed in accordance with
the Experimental Animal Regulations established by The Ministry of
Science and Technology of the People's Republic of China, and the
Guidelines for the Care and Use of Laboratory Animals published by
the National Institutes of Health (Bethesda, MD, USA). The study
received ethical approval from the Ethics Committee of Sun Yat-sen
University. Prior to performing the experiments, all the animals
were subjected to an overnight fast with unlimited access to
water.
Establishment of the animal model
The SPF rats were randomly allocated to three groups
(control, EPO and EPO-siRNA; n=8). The Lewis rats were used as the
recipients, and the Brown Norway rats were used as donors in the
renal transplants. Kidney transplantation was performed as
previously described (28). In
brief, the rats were anesthetized with inhalation of 10% chloral
hydrate 0.3 ml/100 g body weight. The left kidney was prepared by
freeing the ureter from the attachments. The donor kidney and
ureter were removed en bloc. Donor kidney grafts were washed with
4°C saline solution and stored in 4°C HCA solution. The right
kidney was removed from the recipient, and the donor kidney was
transplanted. An anastomosis was created between the donor and
recipient renal artery, renal vein and ureters with end-to-end
anastomosis, to establish the unilateral orthotopic renal
transplantation rat model. Rat rectal temperature was maintained at
35–37°C during surgery. After surgery, the rats were placed on a
heat pad until they recovered.
Experimental design
After establishment of the unilateral orthotopic
renal transplantation rat model, continuous administration of
saline solution, recombinant human EPO (500 U/kg), or
lentivirus-mediated EPO siRNA (1×107 infectious units)
(sc-37220-V; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
was performed, once a day for 7 days. After the last
administration, the rats were sacrificed and the kidneys were
removed and processed for immunohistochemistry (IHC) and
immunofluorescence (IFC) assessment of EPO levels, hematoxylin and
eosin (H&E), periodic acid-Schiff (PAS), PAS-methenamine (PASM)
and Masson's trichrome staining, for detection of the levels of
creatinine (Cr), EPO, IL-4, IL-10 and IFN-γ, and for western blot
analysis of thymic stromal lymphopoietin (TSLP), STAT6, NF-κB, JNK,
p38, and caspase-1 and -3 in renal tissues.
To further investigate the role of the
STAT6/MAPK/NF-κB pathway in EPO-mediated renoprotection. The other
40 rats were divided into 5 groups (control, and 4 groups of the
different inhibitors; n=8). The animals received the STAT6
inhibitor AS1517499 (10 mg/kg), the JNK inhibitor SP600125 (30
mg/kg), the p38 MAPK inhibitor SB203580 (15 mg/kg), or the NF-κB
inhibitor lactacystin (10 mg/kg) in the presence of EPO and using
the same route of administration of EPO following renal
transplantation. The rats were then sacrificed and the kidneys were
removed and processed for H&E, PAS, PASM, and Masson's
trichrome staining, for detection of the levels of Cr, EPO, IL-4,
IL-10 and IFN-γ, and for western blot analysis of TSLP, STAT6,
NF-κB, JNK, p38, and caspase-1 and -3 in renal tissues.
IHC and IFC
The kidneys were processed for IHC and IFC according
to standard protocols. For IHC, 3-μm paraffin sections using
rabbit anti-mouse EPO monoclonal antibody (1:100; sc-5290) and goat
anti-rabbit IgG poly-HRP (1:100; sc-2004) (both from Santa Cruz
Biotechnology, Inc.). For IFC, the sections were fixed in 4%
paraformaldehyde and incubated with rabbit anti-human EPO
monoclonal antibody (1:50; sc-80995) and FITC-labeled goat
anti-rabbit secondary antibody (1:150; sc-2004) (both from Santa
Cruz Biotechnology, Inc.). The fluorescence intensity of EPO was
calculated by using ImageJ software (National Institutes of
Health). For nuclear staining, DAPI was used and confocal images
were acquired as previously described (29,30) using the LSM 510 microscope (Carl
Zeiss AG, Oberkochen, Germany). Formalin-fixed paraffin-embedded
tissues were also stained with H&E and PAS, PASM and Masson's
trichrome staining.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Carlsbad, CA, USA) from
homogenated whole kidneys. RNA was reverse transcribed to cDNA
using SuperScript II Reverse Transcriptase (Invitrogen; Thermo
Fisher Scientific). RT-qPCR was performed on an ABI Prism 5700
Sequence Detection system (Applied Biosystems, Foster City, CA,
USA) with SYBR-Green PCR Core reagents. The primers are listed in
Table I. GAPDH served as internal
control. The relative mRNA expression levels of each target gene
were normalized to those of the controls using the
2−ΔΔCq method.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Genes | Primer sequences
(5′-3′) |
|---|
| GAPDH | F:
GGTGAAGGTCGGTGTGAAC |
| GAPDH | R:
CCTTGACTGTGCCGTTGAA |
| EPO | F:
AGAATGAAGGTGGAAGAACAGG |
| EPO | R:
CCCGAAGCAGTGAAGTGAGG |
| IFN-γ | F:
GAACTGGCAAAAGGACGGTAA |
| IFN-γ | R:
AACTTGGCGATGCTCATGAAT |
| IL-4 | F:
CCACCTTGCTGTCACCCTGT |
| IL-4 | R:
CCGTGGTGTTCCTTGTTGC |
| IL-10 | F:
CTATGTTGCCTGCTCTTACTGG |
| IL-10 | R:
ATGTGGGTCTGGCTGACTGG |
Enzyme-linked immunosorbent assay
(ELISA)
Serum Cr, EPO, IL-4, IL-10 and IFN-γ levels were
detected in a 96-well microtiter plate by using a commercial ELISA
kit (Biosource, Camarillo, CA, USA) according to the manufacturer's
instructions. All samples were tested in duplicate. The plate was
read at 450 nm on a microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). The levels were calculated from a standard
curve and expressed in pg/ml.
Western blot analysis
The protein lysates were prepared with RIPA and
subjected to three freeze/thaw cycles using liquid nitrogen. The
lysates were then electrophoresed in 10% SDS-PAGE (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), transferred to
nitrocellulose membrane, blocked with 0.5% skimmed milk and
incubated with TSLP, STAT6, NF-κB, JNK, p38 and caspase-1 and -3
primary antibodies (all from Santa Cruz Biotechnology, Inc.) for 1
h. Polyclonal anti-GAPDH was used as an internal control. After
three washes, the membrane was incubated with horseradish
peroxidase-conjugated IgG and developed under chemiluminescence
condition (Amersham Pharmacia Biotech, Piscataway, NJ, USA) using
ChemiDoc XRS (Bio-Rad Laboratories, Inc.). The quantification and
analysis for the blots were performed with ImageJ software.
Statistical analysis
All data are presented as means ± standard
deviation. Data between groups were compared using two-tailed
unpaired t-test or analysis of variance. P<0.05 was considered
to indicate statistically significant differences. All analyses
were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL,
USA).
Results
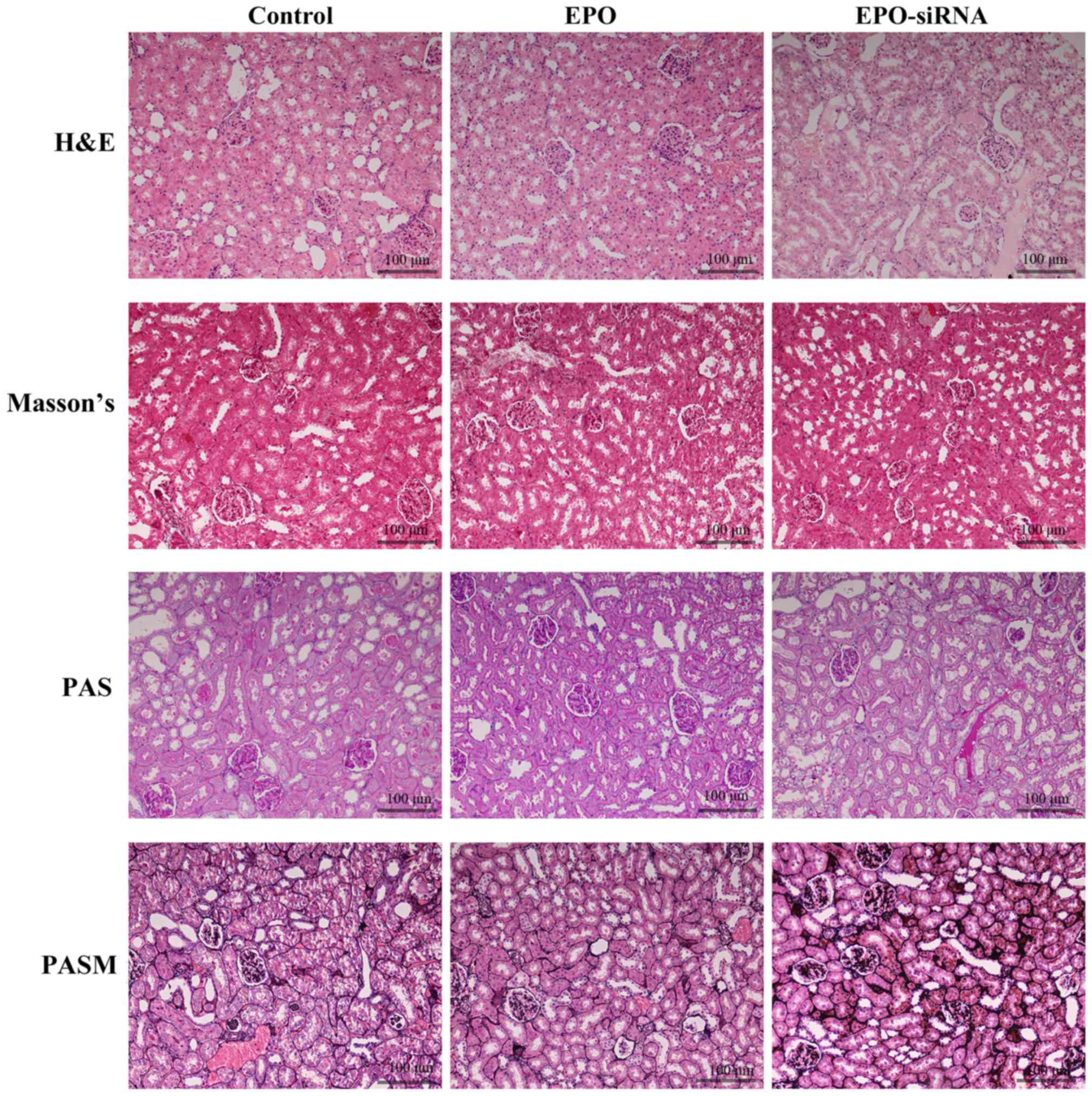
Pathological changes of the kidneys
Following continuous administration of saline
solution, recombinant human EPO (500 U/kg), or lentivirus-mediated
EPO-siRNA for 7 days, histological analysis of kidneys was
performed (Fig. 1). The images of
H&E, PAS, PASM and Masson's staining revealed that IRI occurred
after renal transplantation. Infiltration of inflammatory cells,
with a large number of lymphocytes around the blood vesselst was
observed. Furthermore, EPO protected the kidney from IRI, through
decreasing the extent of tissue congestion and inflammatory cell
infiltration; however, EPO-siRNA did not exert the same protective
effect.
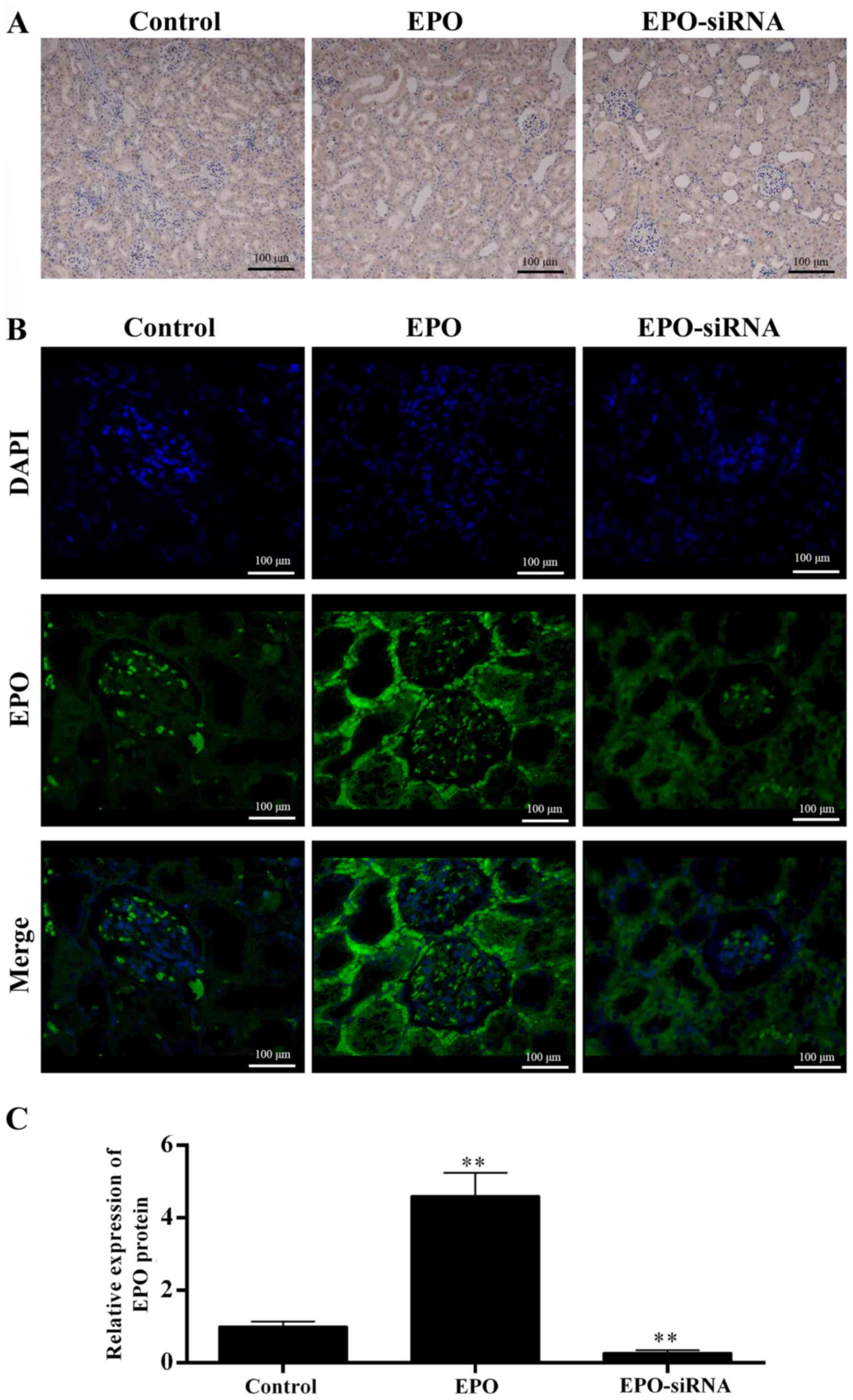
The level of EPO was detected in the kidneys
(Fig. 2). Both IHC (Fig. 2A) and IFC images (Fig. 2B), and data qualification
(Fig. 2C) revealed that the EPO
levels in EPO-treated rats were higher compared with those in the
saline solution and EPO-siRNA groups. Thus, the EPO level appears
to be inversely correlated to the renal IRI.
EPO downregulates the expression of
IFN-γ, IL-4 and Cr and upregulates the expression of IL-10, and
this effect is reversed by EPO-siRNA
The expression of EPO, IFN-γ, IL-4 and IL-10 in
tissues and serum were detected by RT-qPCR and ELISA, respectively
(Fig. 3A–G). As expected, EPO
tissue and serum levels were significantly upregulated in rats with
EPO administration, and was significantly downregulated by
EPO-siRNA (Fig. 3A and E). EPO
treatment reduced the expression of IFN-γ (Fig. 3B and F) and IL-4 (Fig. 3C and G), as well as the serum
levels, whereas it increased the expression of IL-10 at the mRNA
level and in the serum (Fig. 3D and
H). All these effects induced by EPO were reversed by
EPO-siRNA.
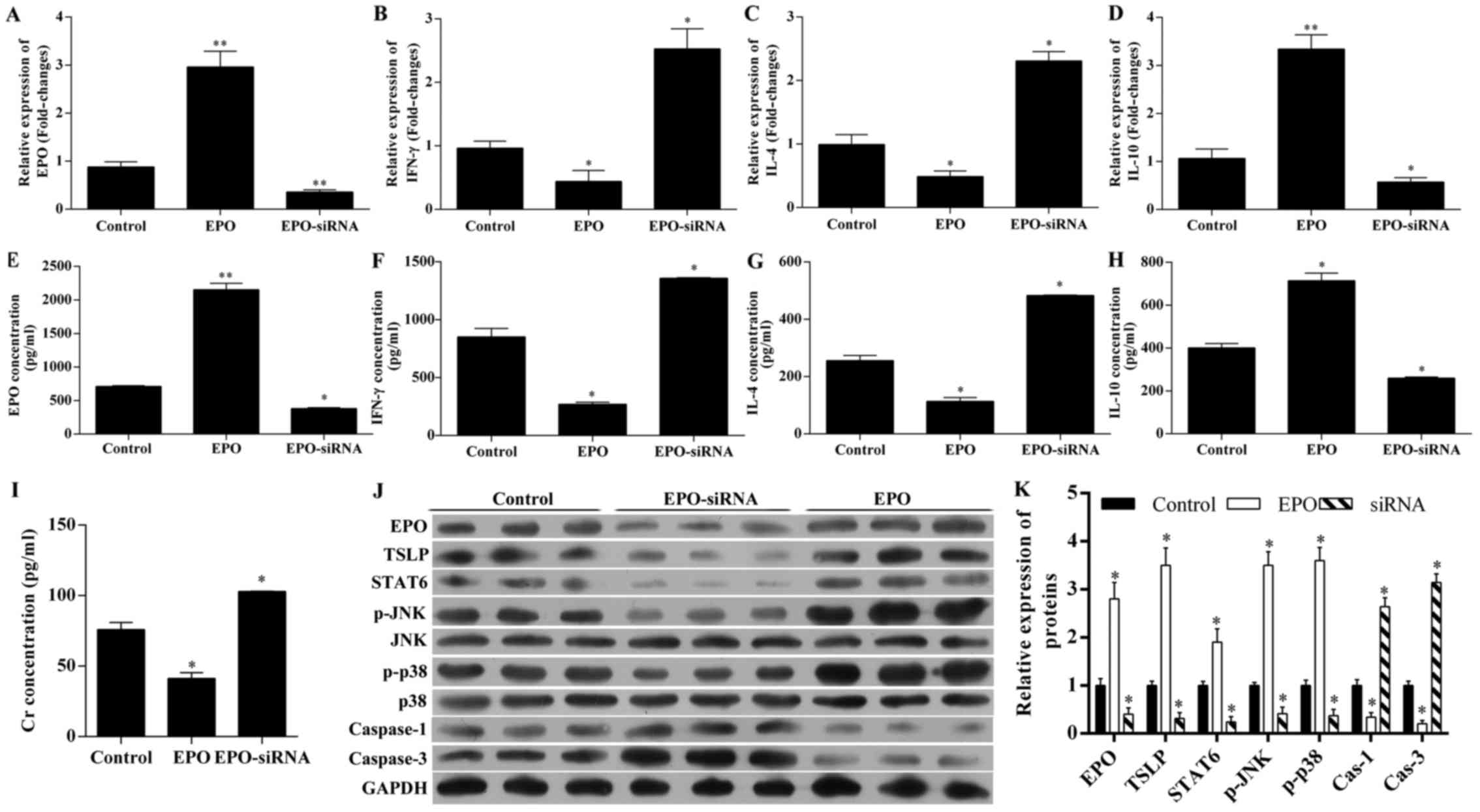 | Figure 3Changes in the levels of
erythropoietin (EPO), interferon (IFN)-γ, interleukin (IL)-4,
IL-10, creatinine (Cr), thymic stromal lymphopoietin (TSLP), signal
transducer and activator of transcription 6 (STAT6), p-JNK, p-p38,
and caspase-1 and -3. The unilateral orthotopic renal
transplantation rat model was established by administration of
saline solution (control), recombinant human EPO (500 U/kg), or
lentivirus-mediated EPO-siRNA. Using RT-qPCR, the expression of (A)
EPO, (B) IFN-γ, (C) IL-4 and (D) IL-10 in renal tissues were
detected. Using ELISA, the serum level of (E) EPO, (F) IFN-γ, (G)
IL-4, (H) IL-10 and (I) Cr were detected. (J) Using western blot
analysis, the expression of EPO, TSLP, STAT6, p-JNK, p-p38 and
caspase-1 and -3 were also detected and (K) quantified.
*P<0.05; **P<0.01 vs. control. RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
The Cr level in the serum was also investigated
(Fig. 3I). The results revealed
that, compared with controls, EPO administration decreased the
serum level of Cr, and EPO-siRNA increased the serum level of
Cr.
EPO upregulated the expression of TSLP,
STAT6, p-JNK and p-p38, and downregulated the expression of
caspase-3, while EPO-siRNA exerted the opposite effects
To analyze the underlying molecular mechanisms for
EPO-mediated renoprotection, the changes in TSLP and
STAT6/MAPK/NF-κB pathway were detected by western blot analysis
(Fig. 3J and K). EPO
administration upregulated the expression of TSLP, STAT6, p-JNK,
p-p38, but downregulated the expression of caspase-3. By contrast,
EPO-siRNA decreased the expression of EPO, TSLP, STAT6, p-JNK,
p-p38 and caspase-1, but increased the expression of caspase-3,
indicating that EPO protects the kidney from IRI via the
STAT6/MAPK/NF-κB signaling pathway.
Assessment of the role of the
STAT6/MAPK/NF-κB pathway in EPO-mediated renoprotection from
IRI
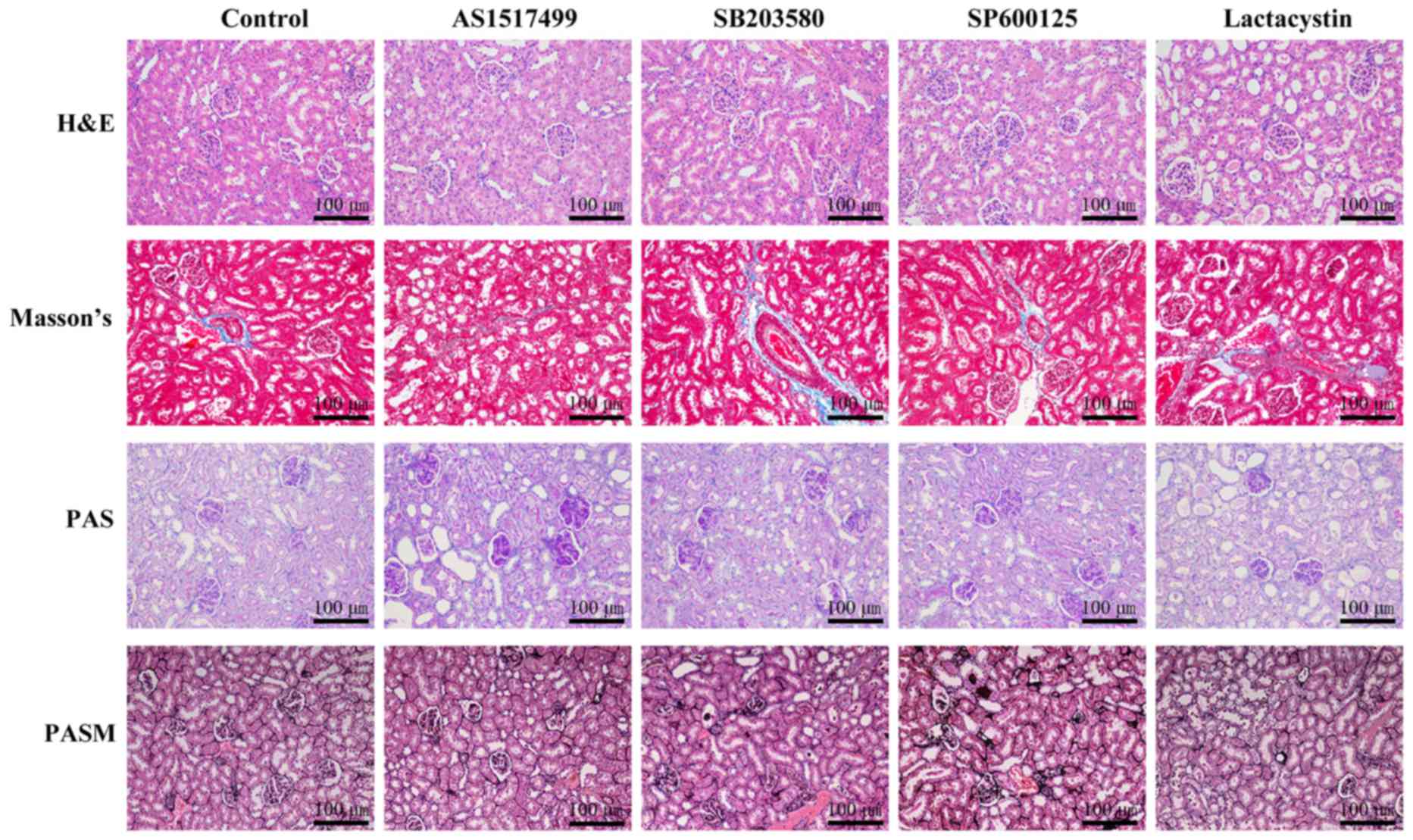
We further investigated whether the STAT6 inhibitor
AS1517499, the JNK inhibitor SP600125, the p38 MAPK inhibitor
SB203580, or the NF-κB inhibitor lactacystin suppressed the effects
of EPO in inhibiting renal IRI (Figs.
4 and 5). The histological
analysis revealed that EPO-mediated renoprotection was obviously
inhibited by AS1517499, SP600125, SB203580 and lactacystin
(Fig. 4).
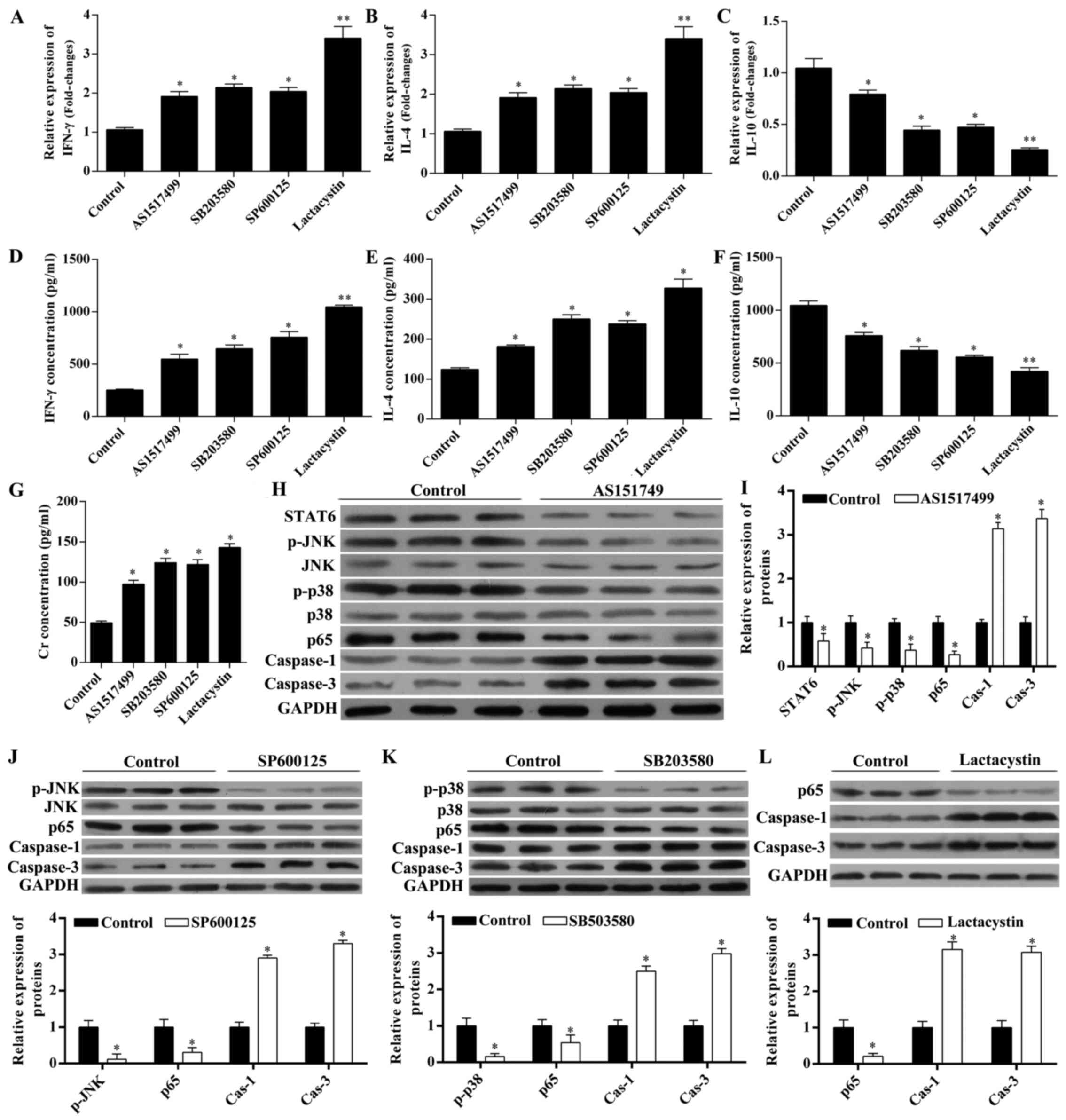 | Figure 5Erythropoietin (EPO) modulates the
levels of interferon (IFN)-γ, interleukin (IL)-4, IL-10, creatinine
(Cr) and caspase-1 and -3 via the signal transducer and activator
of transcription 6 (STAT6)/mitogen-activated protein kinase
(MAPK)/nuclear factor-κB (NF-κB) pathway. Rats with unilateral
orthotopic renal transplantation received the STAT6 inhibitor
AS1517499, the JNK inhibitor SP600125, the p38 MAPK inhibitor
SB203580, or the NF-κB inhibitor lactacystin, in the presence of
EPO. Rats administered EPO were used as control. Using RT-qPCR, the
expression of (A) IFN-γ, (B) IL-4 and (C) IL-10 in renal tissues
was detected. Using ELISA, the serum level of (D) IFN-γ, (E) IL-4,
(F) IL-10 and (G) Cr was detected. (H) Using western blot analysis,
the expression of STAT6, p-JNK, p-p38, p65 and caspase-1 and -3
with concurrent administration of EPO and AS1517499 were detected
and (I) quantified. The (J) expression of p-JNK, p65 and caspase-1
and -3 with administration of EPO and SP600125; (K) expression of
p-p38, p65 and caspase-1 and -3 with administration of EPO and
SB203580; and the (L) expression of p65 and caspase-1 and -3 with
administration of EPO and lactacystin were detected and quantified.
*P<0.05 and **P<0.01 vs. control. |
The chemokine levels were also measured (Fig. 5A–G). IFN-γ (Fig. 5A and C) and IL-4 (Fig. 5B and D) at the mRNA level and in
the serum levels were significantly increased by these inhibitors
in the presence of EPO, while IL-10 levels (Fig. 5C and F) were significantly
decreased in the presence of EPO. The serum level of Cr in the
presence of EPO was also increased by each of these inhibitors
(Fig. 5G).
The STAT6 inhibitor AS1517499 inhibited the
expression of STAT6, p-JNK, p-p38 and p65, while it increased the
expression of caspase-1 and -3 in the presence of EPO (Fig. 5H and I), suggesting that STAT6
acted upstream of MAPK (p-JNK and p-p38) and NF-κB. The JNK
inhibitor SP600125 and the p38 MAPK inhibitor SB203580 inhibited
the expression of p-JNK and p65, while they increased the
expression of caspase-1 and -3 in the presence of EPO (Fig. 5J and K), suggesting that MAPK
(p-JNK and p-p38) acted upstream of NF-κB. The NF-κB inhibitor
lactacystin upregulated the expression of caspase-1 and -3 in the
presence of EPO (Fig. 5L).
Therefore, all the inhibitors of STAT6, JNK, p38
MAPK and NF-κB reversed the changes in the chemokine levels, Cr
levels, and caspase-1 and -3 expression. These results suggest that
the STAT6/MAPK/NF-κB signaling pathway was involved in EPO-mediated
renoprotection from IRI.
Discussion
Recent studies suggested that the MAPK/NF-κB
signaling pathway is involved in the EPO-mediated protection from
IRI following renal transplantation (26,27), and that the STAT6̸NF-κB signaling
pathway is involved in the inflammatory responses (16,17). Moreover, apoptosis is known to
play a key role in renal IRI (18,19). Apoptosis-related proteins, such as
caspase-3, may be involved in renal IRI (20,21). However, the role of the
STAT6/MAPK/NF-κB signaling pathway in EPO-induced renoprotection
remains poorly understood. In the present study, a sham-operation
group was not constructed; all experiments were performed following
establishment of the unilateral orthotopic renal transplantation
rat model. We demonstrated that recombinant human EPO protected
against IRI following renal transplantation by reducing the
inflammatory responses and promoting cell survival under cytotoxic
conditions. Specific inhibitors, including the STAT6 inhibitor
AS1517499, the JNK inhibitor SP600125, the p38 MAPK inhibitor
SB203580, and the NF-κB inhibitor lactacystin, reversed the effects
of EPO, further suggesting the importance of the STAT6̸MAPK̸NF-κB
signaling pathway in EPO-mediated renoprotection from IRI.
EPO is produced by the kidney and fetal liver and
has long been considered as merely a factor modulating
erythropoiesis. However, recent studies demonstrated that EPO may
also exert protective effects against hypoxia, inflammation and
cell death following IRI in the kidney (27), brain (31), and myocardium (32–34). Our results demonstrated that EPO
protected the kidney from IRI, through decreasing the extent of
tissue congestion and inflammatory cell infiltration; however,
EPO-siRNA did not exert such a protective effect. IHC (Fig. 2A) and IFC (Fig. 2B) images revealed that the EPO
levels in EPO-treated rats were higher compared with those the the
saline solution and EPO-siRNA groups, suggesting that the EPO level
is inversely correlated with renal IRI.
EPO increased the levels of anti-inflammatory
cytokines, such as IL-10, while it reduced the levels of
pro-inflammatory cytokines, such as IFN-γ and IL-4, and Cr. This is
consistent with previous studies demonstrating that treatment with
anti-inflammatory cytokine IL-10 reduced renal and systemic
inflammation (35), and reduced
the serum levels of proinflammatory cytokine IFN-γ, protecting the
kidney from injury (36). These
data suggest that EPO not only suppressed the expression of
pro-inflammatory cytokines, but also increased the level of
anti-inflammatory cytokines. TLSP is a critical cytokine that
exacerbates allergic and fibrotic reactions (37). TLSP is mainly produced by
epithelial cells and its production is induced by inflammatory
cytokines (38). NF-κB also
regulates TSLP mRNA expression (38). TSLP was highly expressed in the
kidney in the presence of EPO, and this was inhibited by EPO-siRNA,
indicating that TSLP may be involved in EPO-mediated protection
from IRI.
It has been reported that MAPK and NF-κB played an
important role in EPO-mediated cytoprotective effects (26,27). EPO significantly decreased the
lipopolysaccharide-induced elevation of Cr and NF-κB levels,
indicating that EPO may play a protective role against IRI by
reducing the inflammatory response and tissue degeneration,
possibly via the NF-κB signaling pathway (27). Furthermore, STAT6 was elevated by
inflammatory cytokines, such as IL-4, and then suppressed the
transcriptional activation of NF-κB-dependent proinflammatory
mediators following liver IRI (16,17). In the present study, the specific
inhibitors of STAT6, JNK, p38 MAPK and NF-κB reversed the
EPO-induced changes in the levels of IFN-γ, IL-4, IL-10, Cr and
TSLP. In addition, MAPK (p-JNK and p-p38) acted upstream of NF-κB,
and NF-κB signaling regulated the expression of caspase-1 and -3,
which may responsible for the cytotoxicity observed under IRI
conditions.
In conclusion, the present study demonstrated that
EPO exerted a protective effect in renal IRI via the
STAT6/MAPK/NF-κB pathway.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81470977 and
81270835), the Science and Technology Planning Project of Guangdong
Province, China (grant no. 2014A020212121), and The Basic Service
Charge Young Teachers Cultivation Project of Sun Yat-sen University
(grant no. 13ykpy35).
References
|
1
|
Naylor KL, Zou G, Leslie WD, Hodsman AB,
Lam NN, McArthur E, Fraser LA, Knoll GA, Adachi JD, Kim SJ, et al:
Risk factors for fracture in adult kidney transplant recipients.
World J Transplant. 6:370–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfe RA, Ashby VB, Milford EL, Ojo AO,
Ettenger RE, Agodoa LY, Held PJ and Port FK: Comparison of
mortality in all patients on dialysis, patients on dialysis
awaiting transplantation, and recipients of a first cadaveric
transplant. N Engl J Med. 341:1725–1730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kosieradzki M and Rowiński W:
Ischemia/reperfusion injury in kidney transplantation: mechanisms
and prevention. Transplant Proc. 40:3279–3288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoshinaga K, Shiroki R, Fujita T, Kanno T
and Naide Y: The fate of 359 renal allografts harvested from
non-heart beating cadaver donors at a single center. Clin Transpl.
213–220. 1998.
|
|
5
|
Perico N, Cattaneo D, Sayegh MH and
Remuzzi G: Delayed graft function in kidney transplantation.
Lancet. 364:1814–1827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gueler F, Gwinner W, Schwarz A and Haller
H: Long-term effects of acute ischemia and reperfusion injury.
Kidney Int. 66:523–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Devarajan P: Update on mechanisms of
ischemic acute kidney injury. J Am Soc Nephrol. 17:1503–1520. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bajwa A, Kinsey GR and Okusa MD: Immune
mechanisms and novel pharmacological therapies of acute kidney
injury. Curr Drug Targets. 10:1196–1204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silver SA, Cardinal H, Colwell K, Burger D
and Dickhout JG: Acute kidney injury: preclinical innovations,
challenges, and opportunities for translation. Can J Kidney Health
Dis. 2:302015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu QS, Cheng ZW, Xiong JG, Cheng S, He XF
and Li XC: Erythropoietin pretreatment exerts anti-inflammatory
effects in hepatic ischemia/reperfusion-injured rats via
suppression of the TLR2/NF-κB pathway. Transplant Proc. 47:283–289.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McCook O, Georgieff M, Scheuerle A, Möller
P, Thiemermann C and Radermacher P: Erythropoietin in the
critically ill: do we ask the right questions. Crit Care.
16:3192012. View
Article : Google Scholar
|
|
13
|
Akcay A, Nguyen Q and Edelstein CL:
Mediators of inflammation in acute kidney injury. Mediators
Inflamm. 137072:2009. View Article : Google Scholar
|
|
14
|
Kono H, Nakagawa K, Morita S, Shinoda K,
Mizuno R, Kikuchi E, Miyajima A, Umezawa K and Oya M: Effect of a
novel nuclear factor-κB activation inhibitor on renal
ischemia-reperfusion injury. Transplantation. 96:863–870. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Latanich CA and Toledo-Pereyra LH:
Searching for NF-kappaB-based treatments of ischemia reperfusion
injury. J Invest Surg. 22:301–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bennett BL, Cruz R, Lacson RG and Manning
AM: Interleukin-4 suppression of tumor necrosis factor
alpha-stimulated E-selectin gene transcription is mediated by STAT6
antagonism of NF-kappaB. J Biol Chem. 272:10212–10219. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato A, Yoshidome H, Edwards MJ and
Lentsch AB: Reduced hepatic ischemia/reperfusion injury by IL-4:
potential anti-inflammatory role of STAT6. Inflamm Res. 49:275–279.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Z, Zhong Z, Li M, Xiong Y, Wang Y,
Peng G and Ye Q: Hypothermic machine perfusion increases A20
expression which protects renal cells against ischemia/reperfusion
injury by suppressing inflammation, apoptosis and necroptosis. Int
J Mol Med. 38:161–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toronyi E: Role of apoptosis in the kidney
after reperfusion. Orv Hetil. 149:305–315. 2008.In Hungarian.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu H, Jiang W, Xi X, Zou C and Ye Z:
MicroRNA-21 attenuates renal ischemia reperfusion injury via
targeting caspase signaling in mice. Am J Nephrol. 40:215–223.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haylor JL, Harris KP, Nicholson ML, Waller
HL, Huang Q and Yang B: Atorvastatin improving renal ischemia
reperfusion injury via direct inhibition of active caspase-3 in
rats. Exp Biol Med (Maywood). 236:755–763. 2011. View Article : Google Scholar
|
|
22
|
Fisher JW: Erythropoietin: physiology and
pharmacology update. Exp Biol Med (Maywood). 228:1–14. 2003.
View Article : Google Scholar
|
|
23
|
Pellegrini L, Bennis Y, Velly L,
Grandvuillemin I, Pisano P, Bruder N and Guillet B: Erythropoietin
protects newborn rat against sevoflurane-induced neurotoxicity.
Paediatr Anaesth. 24:749–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Liu C, Hou L, Lv J, Wu F, Yang X,
Ren S, Ji W, Wang M and Chen L: Protection against
ischemia/reperfusion-induced renal injury by co treatment with
erythropoietin and sodium selenite. Mol Med Rep. 12:7933–7940.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Zou YR, Zhong X, Deng HD, Pu L,
Peng K and Wang L: Erythropoietin pretreatment ameliorates renal
ischaemia-reperfusion injury by activating I3K/Akt signalling.
Nephrology (Carlton). 20:266–272. 2015. View Article : Google Scholar
|
|
26
|
Kwon MS, Kim MH, Kim SH, Park KD, Yoo SH,
Oh IU, Pak S and Seo YJ: Erythropoietin exerts cell protective
effect by activating I3K/Akt and MAPK pathways in C6 cells. Neurol
Res. 36:215–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li XJ, Zhang GX, Sun N, Sun Y, Yang LZ and
Du YJ: Protective effects of erythropoietin on endotoxin-related
organ injury in rats. J Huazhong Univ Sci Technolog Med Sci.
33:680–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cugini D, Azzollini N, Gagliardini E,
Cassis P, Bertini R, Colotta F, Noris M, Remuzzi G and Benigni A:
Inhibition of the chemokine receptor CXCR2 prevents kidney graft
function deterioration due to ischemia/reperfusion. Kidney Int.
67:1753–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng Y, Adamson RH, Curry FR and Tarbell
JM: Sphin-gosine-1-phosphate protects endothelial glycocalyx by
inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol.
306:H363–H372. 2014. View Article : Google Scholar
|
|
30
|
Zeng Y, Liu XH, Tarbell J and Fu B:
Sphingosine 1-phosphate induced synthesis of glycocalyx on
endothelial cells. Exp Cell Res. 339:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ratilal BO, Arroja MM, Rocha JP, Fernandes
AM, Barateiro AP, Brites DM, Pinto RM, Sepodes BM and Mota-Filipe
HD: Neuroprotective effects of erythropoietin pretreatment in a
rodent model of transient middle cerebral artery occlusion. J
Neurosurg. 121:55–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jun JH, Jun NH, Shim JK, Shin EJ and Kwak
YL: Erythropoietin protects myocardium against ischemia-reperfusion
injury under moderate hyperglycemia. Eur J Pharmacol. 745:1–9.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu MJ, Chen YS, Huang HS and Ma MC:
Erythropoietin alleviates post-ischemic injury of rat hearts by
attenuating nitrosative stress. Life Sci. 90:776–784. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu X, Cao Z, Cao B, Li J, Guo L, Que L, Ha
T, Chen Q, Li C and Li Y: Carbamylated erythropoietin protects the
myocardium from acute ischemia/reperfusion injury through a
I3K/Akt-dependent mechanism. Surgery. 146:506–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soranno DE, Rodell CB, Altmann C,
Duplantis J, Andres-Hernando A, Burdick JA and Faubel S: Delivery
of interleukin-10 via injectable hydrogels improves renal outcomes
and reduces systemic inflammation following ischemic acute kidney
injury in mice. Am J Physiol Renal Physiol. 311:F362–F372. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun P, Liu J, Li W, Xu X, Gu X, Li H, Han
H, Du C and Wang H: Human endometrial regenerative cells attenuate
renal ischemia reperfusion injury in mice. J Transl Med. 14:282016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Segawa R, Mizuno N, Hatayama T, Jiangxu D,
Hiratsuka M, Endo Y and Hirasawa N: Lipopolysaccharide-activated
leukocytes enhance thymic stromal lymphopoietin production in a
mouse air-pouch-type inflammation model. Inflammation.
39:1527–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takai T: TSLP expression: cellular
sources, triggers, and regulatory mechanisms. Allergol Int.
61:3–17. 2012. View Article : Google Scholar : PubMed/NCBI
|