Introduction
Endometriosis is an estrogen-dependent chronic
gynecological disease that is difficult to cure. The main clinical
characteristics include pelvic masses, chronic pelvic pain and
infertility. Although it is a benign disorder, endometriosis
exhibits invasive growth potential, which is similar to that of
malignant tumors (1). The
incidence of endometriosis is increasing year by year, but its
etiology and pathogenesis remain unclear (2). Although the classical theory of
endometriosis is Sampson's 'retrograde menstruation theory', which
suggests that endometrial fragments undergo retrograde menstruation
through the fallopian tubes and implant in the peritoneal cavity,
this does not explain why the prevalence of endometriosis is only
~10% in fertile women, the majority of whom experience retrograde
menstruation (3). Investigations
have shown that cell adhesion, invasion, angiogenesis (4) and apoptosis (5) in the eutopic endometrium in
endometriosis are different from that of the normal endometrium,
particularly for the secretory endometrium. There is also evidence
to suggest that abnormal molecular aberrations in the eutopic
endometrium promote the development of endometriosis (6). Thus, an evaluation of specific genes
and their associated molecular mechanisms in the eutopic
endometrium may provide a new theoretical basis for the
pathogenesis of endometriosis. The present study team previously
identified 10 upregulated genes in the eutopic endometrium using
cDNA representational difference analysis (7). Among them, the abnormal expression
of the cofilin 1, methionine adenosyltransferase 2A and LIM domain
kinase 1 genes in the eutopic endometrium has been reported
(8). Additionally, the present
study team also detected that B-Raf proto-oncogene,
serine/threonine kinase (BRAF) was overexpressed in the
eutopic endometrium of endometriosis (7).
BRAF is a component of the RAS-rapidly accelerated
fibrosarcoma (RAF)-mitogen-activated protein kinase kinase
(MEK)-extracellular signal-regulated kinase (ERK) signaling
pathway. Mutations in BRAF are associated with the
development of malignant tumors (9). BRAF mutations have been
detected in exons 11 and 15 in numerous tumors, and the most common
mutation is the V600EBRAF mutation
(10). BRAF mutations may
activate the mitogen-activated protein kinase (MAPK) signal pathway
constitutively, leading to abnormal cell proliferation,
differentiation and tumorigenicity (11). Other studies have shown that the
overexpression of wild-type BRAF (wtBRAF)
promotes the activation of the RAS-BRAF-MAPK signaling pathway
(12,26). Notably, BRAF has been
reported to promote cell proliferation through regulation of the
Ras/Raf/MAPK signaling pathway in the eutopic endometrial stromal
cells of patients with endometriosis (13). Previous studies by the present
research team have demonstrated that the mRNA and protein levels of
BRAF are markedly overexpressed in the eutopic endometrium tissues
of endometriosis compared with normal endometrial tissues (7,14).
This suggests that BRAF may regulate the occurrence and development
of endometriosis. However, BRAF mutations and the mechanism
associated with the upregulation of wtBRAF
expression in endometriosis remain unclear.
In the present study, BRAF mutations were
detected and the potential transcription factors binding to the
region upstream of the wtBRAF transcription start
site (TSS) were predicted. The correlation of mRNA and protein
expression between wtBRAF and the predicted
transcription factor was then analyzed. The results may provide a
novel insight into the molecular mechanisms of BRAF in the
regulation of the occurrence and development of endometriosis.
Materials and methods
Tissue collection
Ectopic endometrium and paired eutopic endometrium
samples were collected from 30 patients (37.30±6.83 years old) with
endometriosis who were undergoing total hysterectomy at the
Department of Gynecology, Cancer Hospital of China Medical
University (Shenyang, China) from January 2015 to June 2016 as the
experimental group. Normal endometrium samples from 25 patients
(40.46±5.26 years old) without estrogen-dependent disease were also
collected as the control group. All patients had regular menstrual
cycles and none had malignant diseases, autoimmune disease,
surgical diseases or inflammatory diseases. They also did not
receive gonadotrophin-releasing hormone analogs, other hormonal
medications or antibiotic therapy in the 6 months prior to the
surgery. The tissue samples were all collected in the secretory
phase of the menstrual cycle, which was confirmed by pathology. The
present study was approved by the China Medical University Research
Ethics Committee in accord with the Declaration of Helsinki.
Written informed consent was obtained from each patient prior to
the surgical procedures. All samples were divided into two groups:
One was immersed in 10% formalin solution for immunohistochemistry
(IHC), and another was frozen in liquid nitrogen for polymerase
chain reaction (PCR) and direct sequencing, quantitative PCR (qPCR)
or western blot analysis.
Genomic DNA isolation, PCR and direct
sequencing
Genomic DNA was extracted from freshly frozen tissue
(100 mg) using the TIANamp Genomic DNA kit (Tiangen Biotech Co.,
Ltd., Beijing, China) according to the manufacturer's protocol.
Approximately 200 ng genomic DNA was used for PCR in a 20-µl
reaction system. The DNA polymerase used for PCR is TaKaRa Ex
Taq® (Cat. no. R001A) purchased from Takara Bio, Inc.,
Otsu, Japan. The primer sequences are shown in Table I. The reaction conditions were as
follows: 94°C for 5 min, 1 cycle; 94°C for 30 sec, 56°C (for exon
11)/59°C (for exon 15) for 30 sec, 72°C for 30 sec, 35 cycles, and
a final extension at 72°C for 10 min. The integrity of all PCR
products was observed by 2% agarose gel electrophoresis. PCR
products were analyzed using an ABI 3730xl DNA sequencer following
purification (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The positive control,
V600EBRAF mutation, was detected using BCPAP
thyroid carcinoma cells (provided by the Central Laboratory of
Shengjing Hospital of China Medical University).
 | Table IPrimer sequences of exons used in the
present study. |
Table I
Primer sequences of exons used in the
present study.
| Exon | Primer sequences
(5′→3′) | Product
length
(bp) |
|---|
| Exon 11 | F:
ATAAGGTAATGTACTTAGGGTGAAACATAA | 356 |
| R:
TTTTGTTAGAAACTTTTGGAGGAGTC | |
| Exon 15 | F:
GCTTGCTCTGATAGGAAAATGAGA | 249 |
| R:
AATGACTTTCTAGTAACTCAGCAGCA | |
Prediction of transcription factor
binding sites upstream of the wtBRAF TSS
A region of ~2,000 bp located upstream of the
wtBRAF TSS was screened using National Center for
Biotechnology Information (NCBI: http://www.ncbi.nlm.nih.gov/) and University of
California, Santa Cruz (http://www.genome.ucsc.edu/) databases. Transcription
factors that may be able to bind to the ~2,000-bp region upstream
of the wtBRAF TSS were then predicted using
JASPAR (http://jaspar.genereg.net/) and
Transcription Factor (TRANSFAC: http://www.gene-regulation.com/pub/databases.html)
datasets.
RNA extraction, reverse transcription and
qPCR
Total RNA was extracted from freshly frozen tissues
(100 mg) using RNAiso Plus (Takara Bio, Inc.) according to the
manufacturer's protocol. RNA purity and concentration were detected
by spectrophotometry, and RNA integrity was observed by 1% agarose
gel electrophoresis. Total RNA was synthesized into cDNA using the
PrimeScript RT Reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocol. Primers were designed using PrimerPremier
5.0 (Premier Biosoft International, Palo Alto, CA, Canada), and the
sequences used in this study are shown in Table II. qPCR analysis was performed
using SYBR Premix Ex Taq (Takara Bio, Inc.) according to the
manufacturer's protocol using a LightCycler 480 detection system
(Roche Diagnostics International AG, Rotkreuz, Switzerland). The
conditions were as follows: One cycle at 95°C for 5 min, followed
by 45 cycles at 95°C for 10 sec and 60°C for 30 sec. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as an internal
control. All experiments were performed in triplicate. The relative
expression of cAMP responsive element binding protein 1
(CREB1) and BRAF was calculated using the
2−ΔΔCq method (15).
 | Table IIPrimer sequences used for
quantitative polymerase chain reaction in the present study. |
Table II
Primer sequences used for
quantitative polymerase chain reaction in the present study.
| Gene | Primer sequences
(5′→3′) | Product length
(bp) |
|---|
| CREB1 | F:
GGAGTGCCAAGGATTGAAGAAGA | 333 |
| R:
TGCTGTGCGAATCTGGTATGTT | |
|
wtBRAF | F:
GGCAGAGTGCCTCAAAAAGAA | 134 |
| R:
AACCAGCCCGATTCAAGGA | |
| GAPDH | F:
GCACCGTCAAGGCTGAGAAC | 138 |
| R:
TGGTGAAGACGCCAGTGGA | |
IHC staining
The IHC SP kit (ZSGB-BIO, Beijing, China) was used
to detect the protein expression levels of CREB1 and
wtBRAF. Paraffin-embedded specimens were cut into
4-µm sections. The sections were dewaxed with xylene and
dehydrated with graded alcohol. Following washing with
phosphate-buffered saline (PBS), the sections were incubated in 3%
H2O2 for 15 min at room temperature followed
by microwave antigen retrieval (oven fire to 100%, for 7 min). The
sections were blocked with serum (contained in The IHC SP kit) for
30 min at 37°C and incubated with mouse anti-human BRAF monoclonal
antibody (cat. no. sc-5284; 1:50; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and rabbit anti-human CREB1 polyclonal antibody
(cat. no. 12208-1-AP; 1:200; ProteinTech Group, Inc., Chicago, IL,
USA) overnight at 4°C. The sections were washed with PBS and then
incubated with secondary antibody (contained in the IHC SP kit) for
30 min at 37°C. Staining was performed using a diaminobenzidine kit
(Beyotime Institute of Biotechnology, Nanjing, China) according to
the manufacturer's protocol. Immunostaining results were scored
using a light microscope (E100; Nikon, Tokyo, Japan) according to
the positive cell percentage and positive staining intensity.
First, the extent of the staining was scored according to the
positive cell percentage: <5%, 0 points; 5–10%, 1 point; 10–50%,
2 points; >50%, 3 points. Additionally, the intensity of
staining was scored as follows: no staining, 0 points; light
yellow, 1 point, moderate yellow, 2 points; strong yellow, 3
points. The extent of the staining multiplied by the intensity was
the final score; scores of 0–3 and 4–9 were considered negative and
positive expression, respectively.
Western blot detection of protein
expression levels
To 100 mg frozen endometrial tissue was added lysis
buffer (quantity to volume ratio in mg/µl; 1:5) with 1%
protease inhibitor, lysed in ice and centrifuged at 12,000 × g and
4°C for 15 min. A bicinchoninic acid reagent kit (Beyotime
Institute of Biotechnology) was used to detect the protein
concentration of the supernatant. The protein sample (80 µg)
was separated by 8% SDS-PAGE, followed by transfer to a
polyvinylidene fluoride (EMD Millipore, Billerica, MA, USA)
membrane. After blocking with 5% non-fat milk for 2 h at room
temperature, the membranes were incubated with mouse anti-human
BRAF monoclonal antibody (cat. no. sc-5284; 1:100; Santa Cruz
Biotechnology, Inc.), rabbit anti-human CREB1 polyclonal antibody
(cat. no. 12208-1-AP; 1:500) and mouse anti-human GAPDH monoclonal
antibody (cat. no. 0004-1-Ig; 1:10,000) (both from ProteinTech
Group, Inc.) overnight at 4°C. GAPDH was used as a loading control.
The membrane was washed with TBS with 0.1% Tween-20 buffer three
times, and then the matched secondary antibodies (goat anti-mouse;
cat. no. A00001-1; 1:2,000; goat anti-rabbit; cat. no. A00001-2;
both ProteinTech Group, Inc.) were added for 2 h at room
temperature. The binding was detected using a BeyoECL Plus kit
(Beyotime Institute of Biotechnology), and the integrated density
was analyzed using ImageJ 1.48v software (National Institutes of
Health, Bethesda, MD, USA). The ratio of the integrated density
between the target band and GAPDH was considered the relative
expression level of each protein.
Statistical analysis
All data are presented as mean ± standard deviation.
ANOVA and Chi-squared test were used to analyze differences in the
mRNA and protein expression of BRAF and CREB1 among
eutopic and ectopic endometrium tissues from patients with
endometriosis and normal endometrium. Pearson's coefficient
correlation was applied to analyze the correlation between the
expression of CREB1 protein and the mRNA and protein expression of
wtBRAF. Data analysis was performed with the
statistical software SPSS 20.0 (IBM Corp., Armonk, NY, USA), and
P<0.05 was considered to indicate a statistically significant
result.
Results
BRAF mutation at exons 11 and 15
All DNA specimens were amplified using specific
primers and detected using gel electrophoresis. The band size of
exon 11 was 356 bp and that of exon 15 was 249 bp (Fig. 1A). No BRAF mutations were
detected among the 30 cases of ectopic and matched eutopic
endometrium samples from patients with endometriosis and the 25
cases of normal endometrium (Fig.
1B), compared with the positive control in which the
BRAF mutation (T1799A) was detected at exon 15 in the BCPAP
papillary thyroid cancer cell line (Fig. 1C).
Prediction of the transcription factor
binding sites of the BRAF TSS
A region of ~2,000 bp upstream of the BRAF
TSS was screened, and transcription factors binding to the
~2,000-bp region were predicted using the JASPAR core transcription
factor database. In total, 323 transcription factors (profile score
threshold 80%) were identified. Among them, 5 were filtered
(relative score >1.000; Table
III), including CREB1 (NCBI Gene ID 1385), Spi-B
transcription factor (SPIB; NCBI Gene ID 6689), nuclear
factor of activated T-cells 2 (NFATC2; NCBI Gene ID 4773),
zinc finger protein 354C (ZNF354C; NCBI Gene ID 30832) and
SRY-box 10 (SOX10; NCBI Gene ID 6663). Combined analysis
with the JASPAR and TRANSFAC databases predicted a cAMP-responsive
element (CRE) binding site (TGACGTCA) at −266 to −259 bp upstream
of the wtBRAF TSS (Fig. 2). Additionally, in a previous
study, it was detected that CREB1 mRNA expression was
significantly higher in the eutopic endometrium of patients with
endometriosis compared with that in normal endometrium (16). These findings suggest that
CREB1 may activate BRAF gene transcription by
directly binding to the CRE sequence of the BRAF promoter
region.
 | Table IIITranscription factor binding sites
predicted by the JASPAR database. |
Table III
Transcription factor binding sites
predicted by the JASPAR database.
| Model ID | Model name | Score | Relative score | Start | End | Strand | Predicted site
sequence |
|---|
| MA0018.2 | CREB1 | 11.569 | 1.0000161 | 1735 | 1742 | −1 | TGACGTCA |
| MA0018.2 | CREB1 | 11.569 | 1.0000161 | 1735 | 1742 | 1 | TGACGTCA |
| MA0081.1 | SPIB | 10.470 | 1.0000147 | 1599 | 1605 | 1 | AGAGGAA |
| MA0152.1 | NFATC2 | 11.360 | 1.0000115 | 938 | 944 | 1 | TTTTCCA |
| MA0152.1 | NFATC2 | 11.360 | 1.0000115 | 1243 | 1249 | −1 | TTTTCCA |
| MA0130.1 | ZNF354C | 8.916 | 1.0000095 | 823 | 828 | 1 | ATCCAC |
| MA0442.1 | SOX10 | 8.910 | 1.0000092 | 252 | 257 | −1 | CTTTGT |
| MA0442.1 | SOX10 | 8.910 | 1.0000092 | 1098 | 1103 | −1 | CTTTGT |
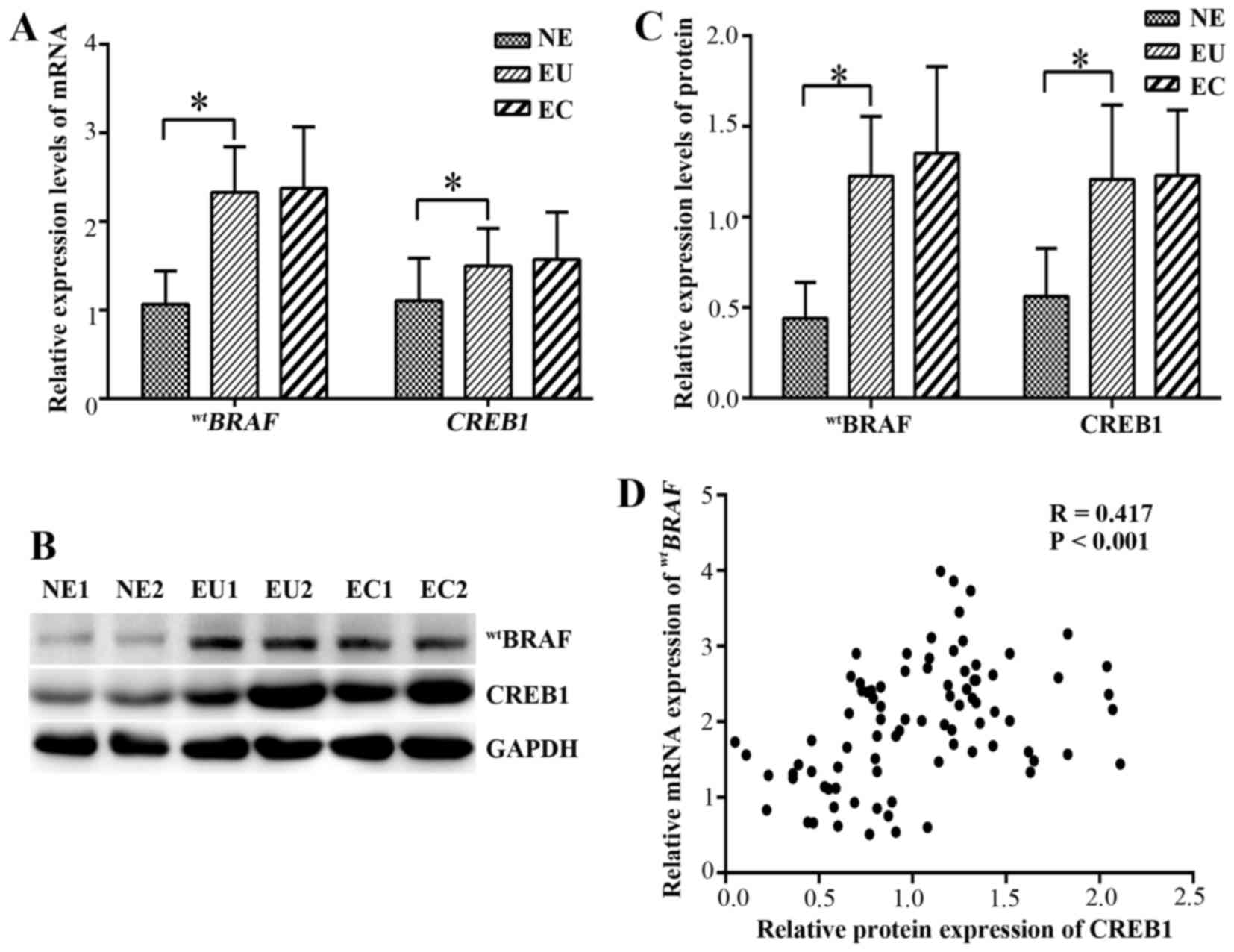
mRNA expression of wtBRAF and
CREB1 in endometrial tissues
qPCR was used to evaluate the mRNA expression levels
of wtBRAF and CREB1 in the ectopic and
eutopic endometrium of endometriosis patients. The mRNA and protein
levels are shown in (Fig. 3). In
the eutopic endometrial tissues, the relative mRNA expression
levels of wtBRAF and CREB1 were
significantly higher than those in the normal endometrial tissues
(P<0.001). However, no significant difference in
wtBRAF and CREB1 mRNAs was detected
between the ectopic and eutopic endometrium (P=0.989 and P=0.548,
respectively; Fig. 3A).
Protein expression of wtBRAF
and CREB1 in endometrial tissues by IHC
The location and expression of wtBRAF and
CREB1 in the ectopic and eutopic endometrial tissues of patients
with endometriosis and the normal endometrial tissues of the
control group were detected using IHC (Fig. 4). The results revealed that the
wtBRAF protein was located in the cytoplasm of the
epithelial and stromal cells, while CREB1 was located in the nuclei
of these cells. In the normal, eutopic and ectopic endometrial
tissues, the positive expression rates of wtBRAF were
24, 70 and 63.3% and those of CREB1 were 36, 80 and 76%,
respectively. The positive rates of wtBRAF and CREB1 in
the eutopic endometrial tissue were significantly higher than those
in normal tissues (P<0.05), while no significant difference was
detected between the eutopic and ectopic endometrial tissues
(P=0.584 and P=0.766, respectively; Table IV).
 | Table IVProtein expression of
wtBRAF and CREB1 in different endometrial tissues
determined by immunohistochemistry. |
Table IV
Protein expression of
wtBRAF and CREB1 in different endometrial tissues
determined by immunohistochemistry.
| Protein | Tissue samples, n
(%)
| P-value |
|---|
| EC (n=30) | EU (n=30) | NE (n=25) |
|---|
|
wtBRAF | | | | |
| Positive | 19 (63.3) | 21 (70) | 6 (24) | 0.584a |
| Negative | 11 (36.7) | 9 (30) | 19 (76) | <0.001b |
| CREB1 | | | | |
| Positive | 22 (76) | 23 (80) | 9 (36) | 0.766a |
| Negative | 8 (24) | 7 (20) | 16 (64) | 0.002b |
Protein expression of wtBRAF
and CREB1 in endometrial tissues by western blot analysis
The protein expression of wtBRAF and
CREB1 in the endometriosal tissues was further detected using
western blot analysis (Fig. 3B and
C). In the eutopic endometrium tissues, the wtBRAF
and CREB1 expression levels were significantly upregulated compared
with those in the normal endometrial tissue (P<0.001), while no
significant differences in expression were observed between the
eutopic and ectopic endometrium (P=0.566 and P=0.811,
respectively).
CREB1 positively correlates with
wtBRAF expression
The correlation between wtBRAF and CREB1
protein expression as determined by IHC was evaluated (Table V). The results indicated that
wtBRAF immunostaining was positively correlated with
that of CREB1 (correlation coefficient R=0.529, P<0.001).
Furthermore, correlation of the western blotting and qPCR data
suggested that there was a positive correlation between CREB1
protein and the wtBRAF transcript level in all
tissues (correlation coefficient R=0.417, P<0.001; Fig. 3D).
 | Table VCorrelation between wtBRAF
and CREB1 protein expression in endometriosis. |
Table V
Correlation between wtBRAF
and CREB1 protein expression in endometriosis.
| CREB1 (cases) | wtBRAF
(cases)
| P-value |
|---|
| Positive | Negative | R |
|---|
| Positive | 40 | 14 | 0.529 | P<0.001 |
| Negative | 6 | 25 | | |
Discussion
In the present study, no BRAF mutations were
detected in exons 11 or 15 in the ectopic and eutopic endometrium
samples of patients with endometriosis. However, significant
overexpression of wtBRAF and CREB1 mRNA
and protein was detected in the eutopic endometrium of these
patients compared with normal endometrium. However, no significant
difference was identified between the ectopic and eutopic
endometrium. In addition, analysis of the protein expression of
CREB1 indicated that it was positively correlated with the
transcript level and protein expression of
wtBRAF.
BRAF is a proto-oncogene that is also known
as v-raf murine sarcoma viral homolog B1. It belongs to the Raf
kinase family and was first discovered in 1988 (17). BRAF mutations are
associated with numerous types of malignant tumor, where they
activates the MAPK signaling pathway constitutively, resulting in
uncontrolled cellular proliferation and survival (18). The most common BRAF point
mutation, V600EBRAF, accounts for ~90% of all BRAF
mutations, and derives from a point mutation that results in an
amino acid change from valine to glutamic acid (19). However, other malignant tumors,
including primary uveal melanoma and uveal melanoma demonstrate a
lack of BRAF mutations (20,21). Zannoni et al (22) found no mutations in the hotspot
regions of BRAF (i.e. exon 15) in primary clear cell ovarian
carcinoma. However, several studies have shown the overexpression
of the wtBRAF gene in other cancer types. For
example, one study found that the overexpression of BRAF
activated the RAS-BRAF-MAPK signaling pathway (12). Furthermore, BRAF has been
demonstrated to be overexpressed in advanced hepatocellular
carcinoma (23). The
overexpression of BRAF may participate in the molecular
mechanisms of thyroid papillary carcinoma, and detection of the
expression of the BRAF gene has been shown to predict the
cell invasion ability of papillary thyroid carcinoma (24). In previous studies, the high
expression of BRAF increased the activity of ERK in the
Rat-1 cell line (25), and also
stimulated the growth of malignant melanoma cells (26). It has been suggested that BRAF
serves an important role in tumor development by binding to
specific molecular signaling molecules. Previous studies by the
present research team demonstrated that BRAF is a candidate
gene in the development of endometriosis (7), and preliminarily verified that
BRAF mRNA and protein levels are significantly upregulated
in the eutopic endometrium of patients with endometriosis compared
with normal endometrium (14). In
the present study, no BRAF mutation was detected in exons 11
or 15 among the 30 pairs of ectopic and matched eutopic endometrium
samples from patients with endometriosis. This is consistent with
another study that screened for BRAF mutations in ectopic
endometrial tissue (27).
Additionally, it was observed in the present study that the mRNA
and protein expression levels of wtBRAF in the
eutopic endometrial tissues from patients with endometriosis were
significantly higher than those in normal endometrium, which
further suggests a role for BRAF in endometriosis. However,
no significant difference was detected between the eutopic and
matched ectopic endometrial tissues from patients with
endometriosis. There are two possible reasons for this observation:
i) The BRAF gene promotes the occurrence of endometriosis,
but does not serve a role in its development; ii) heterogeneity
exists in the ectopic endometrium of endometriosis cases, and the
quantity of ectopic endometrium tissues obtained from ectopic focus
is too little to influence the research results. Further study is
required to investigate these hypotheses.
To explore the mechanism of wtBRAF
overexpression, a region of ~2,000 bp upstream of the
wtBRAF TSS was selected. Using the JASPAR and
TRANSFAC databases, a CRE binding site (TGACGTCA) at −266 to −259
bp upstream of the wtBRAF TSS was predicted. In
addition, a previous study by the present research team found that
1,216 mRNAs were expressed differentially between eutopic and
normal endometrium by long non-coding RNA microarray, among which
the function of cyclin-dependent kinase 6 in endometriosis has been
preliminarily validated, and CREB1 was an overexpressed mRNA
(16). These findings suggest
that CREB1 may function as a transcription factor of
wtBRAF, and is involved in the regulation of the
development of endometriosis.
CREB1 is a proto-oncogenic transcription
factor that is involved in oncogenesis in numerous organs.
CREB1 increases the expression of its target genes, which
are involved in various cell functions, including metabolism, the
cell cycle, cell survival and DNA repair (28). As a potent oncogene, CREB1
promotes tumorigenesis by significantly impacting the growth,
proliferation, survival, metastasis and invasion of tumor cells.
The overexpression of CREB1 promotes these functions, and is
also closely associated with the recurrence and poor prognosis of
diseases (23). For example, it
has been reported that CREB1 is overexpressed in gastric
cancer and increases gastric adenocarcinoma cell growth (29). In addition, Yang et al
(30) demonstrated that the
expression of CREB1 was associated with the migration of
hepatocellular carcinoma cells. Furthermore, the overexpression of
CREB1 has been reported to be associated with poor prognosis
in non-smokers with non-small cell lung cancer and in patients with
breast cancer (31,32). In the present study, the
expression of CREB1 mRNA and protein in the eutopic
endometrium of patients with endometriosis was significantly higher
than that in normal endometrium. In addition, it has been suggested
that the CREB protein may act as a transcription regulator of
aromatase in breast cancer cells to increase the synthesis of
estrogen (31). Breast cancer and
endometriosis are typical estrogen-dependent diseases. The present
study found that the expression of CREB1 was significantly
higher in eutopic endometrium than in normal endometrium, which
suggests that CREB1 may be a candidate gene for
endometriosis intervention and treatment. However, no significant
difference in CREB1 expression was detected between the
ectopic and eutopic endometrium; this difference remains to be
confirmed by microdissection or cell sorting. The correlation
analysis conducted in the present study revealed that the protein
expression of CREB1 was positively correlated with the transcript
level and protein expression of wtBRAF. It is
reasonable to speculate that CREB1 may activate the transcription
of wtBRAF through directly binding to its
promoter, increasing BRAF expression and regulating the cell
proliferation, migration and invasion of endometriosis. However,
additional studies are required to further verify the exact
molecular mechanism by which CREB1 regulates the expression
of wtBRAF.
Acknowledgments
The present study was supported as a project of the
National Natural Science Foundation of China (grant no.
81270675).
References
|
1
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang Y, Li Y, Liu K, Chen P and Wang D:
Expression and significance of WNT4 in ectopic and eutopic
endometrium of human endometriosis. Reprod Sci. 23:379–385. 2016.
View Article : Google Scholar
|
|
3
|
Gupta D, Hull ML, Fraser I, Miller L,
Bossuyt PM, Johnson N and Nisenblat V: Endometrial biomarkers for
the non-invasive diagnosis of endometriosis. Cochrane Database Syst
Rev. 4:CD0121652016.PubMed/NCBI
|
|
4
|
Zhang Z, Chen P, Guo C, Meng X and Wang D:
Effect of LIM kinase 1 overexpression on behaviour of
endometriosis-derived stromal cells. Cell Tissue Res. 359:885–893.
2015. View Article : Google Scholar
|
|
5
|
Harada T, Kaponis A, Iwabe T, Taniguchi F,
Makrydimas G, Sofikitis N, Paschopoulos M, Paraskevaidis E and
Terakawa N: Apoptosis in human endometrium and endometriosis. Hum
Reprod Update. 10:29–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ulukus M, Cakmak H and Arici A: The role
of endometrium in endometriosis. J Soc Gynecol Investig.
13:467–476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Q, Zhang C, Chen Y, Lou J and Wang D:
Identification of endometriosis-related genes by representational
difference analysis of cDNA. Aust N Z J Obstet Gynaecol.
52:140–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu YL, Wang DB, Liu QF, Chen YH and Yang
Z: Silencing of cofilin-1 gene attenuates biological behaviours of
stromal cells derived from eutopic endometria of women with
endometriosis. Hum Reprod. 25:2480–2488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitchell B, Dhingra JK and Mahalingam M:
BRAF and epithelial-mesenchymal transition: Lessons from papillary
thyroid carcinoma and primary cutaneous melanoma. Adv Anat Pathol.
23:244–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahman MA, Salajegheh A, Smith RA and Lam
AK: B-Raf mutation: a key player in molecular biology of cancer.
Exp Mol Pathol. 95:336–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lopes JP and Fonseca E: BRAF gene mutation
in the natural history of papillary thyroid carcinoma: diagnostic
andprognostic implications. Acta Med Port. 24(Suppl 4): 855–868.
2011.
|
|
12
|
Ewing I, Pedder-Smith S, Franchi G,
Ruscica M, Emery M, Vax V, Garcia E, Czirják S, Hanzély Z, Kola B,
et al: A mutation and expression analysis of the oncogene BRAF in
pituitary adenomas. Clin Endocrinol (Oxf). 66:348–352. 2007.
View Article : Google Scholar
|
|
13
|
Yotova IY, Quan P, Leditznig N, Beer U,
Wenzl R and Tschugguel W: Abnormal activation of Ras/Raf/MAPK and
RhoA/ROCKII signalling pathways in eutopic endometrial stromal
cells of patients with endometriosis. Hum Reprod. 26:885–897. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chu DM, Wang DB, Li Y and Liu KR:
Expression and significance of BRAF gene in ectopic endometrium of
women with endometriosis. Zhongguo Yike Daxue Xuebao. 43:790–793.
2014.In Chinese.
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Wang Y, Li Y, Yang Z, Liu K and Wang D:
Genome-wide microarray analysis of long non-coding RNAs in eutopic
secretory endometrium with endometriosis. Cell Physiol Biochem.
37:2231–2245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikawa S, Fukui M, Ueyama Y, Tamaoki N,
Yamamoto T and Toyoshima K: B-raf, a new member of the raf family,
is activated by DNA rearrangement. Mol Cell Biol. 8:2651–2654.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sclafani F, Gullo G, Sheahan K and Crown
J: BRAF mutations in melanoma and colorectal cancer: a single
oncogenic mutation with different tumour phenotypes and clinical
implications. Crit Rev Oncol Hematol. 87:55–68. 2013. View Article : Google Scholar
|
|
19
|
Roskoski R Jr: RAF
protein-serine/threonine kinases: Structure and regulation. Biochem
Biophys Res Commun. 399:313–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen Y, Goldenberg-Cohen N, Parrella P,
Chowers I, Merbs SL, Pe'er J and Sidransky D: Lack of BRAF mutation
in primary uveal melanoma. Invest Ophthalmol Vis Sci. 44:2876–2878.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rimoldi D, Salvi S, Liénard D, Lejeune FJ,
Speiser D, Zografos L and Cerottini JC: Lack of BRAF mutations in
uveal melanoma. Cancer Res. 63:5712–5715. 2003.PubMed/NCBI
|
|
22
|
Zannoni GF, Improta G, Chiarello G,
Pettinato A, Petrillo M, Scollo P, Scambia G and Fraggetta F:
Mutational status of KRAS, NRAS, and BRAF in primary clear cell
ovarian carcinoma. Virchows Arch. 465:193–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Newell P, Toffanin S, Villanueva A, Chiang
DY, Minguez B, Cabellos L, Savic R, Hoshida Y, Lim KH,
Melgar-Lesmes P, et al: Ras pathway activation in hepatocellular
carcinoma and anti-tumoral effect of combined sorafenib and
rapamycin in vivo. J Hepatol. 51:725–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng L, Li M, Zhang QP, Piao ZA, Wang ZH
and Lv S: Utility of BRAF protein overexpression in predicting the
metastasis potential of papillary thyroid carcinoma. Oncol Lett.
2:59–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erhardt P, Schremser EJ and Cooper GM:
B-Raf inhibits programmed cell death downstream of cytochrome c
release from mitochondria by activating the MEK/Erk pathway. Mol
Cell Biol. 19:5308–5315. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanami H, Imoto I, Hirasawa A, Yuki Y,
Sonoda I, Inoue J, Yasui K, Misawa-Furihata A, Kawakami Y and
Inazawa J: Involvement of overexpressed wild-type BRAF in the
growth of malignant melanoma cell lines. Oncogene. 23:8796–8804.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vestergaard AL, Thorup K, Knudsen UB, Munk
T, Rosbach H, Poulsen JB, Guldberg P and Martensen PM: Oncogenic
events associated with endometrial and ovarian cancers are rare in
endometriosis. Mol Hum Reprod. 17:758–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan X, Wang S, Zhu L, Wu C, Yin B, Zhao J,
Yuan J, Qiang B and Peng X: cAMP response element-binding protein
promotes gliomagenesis by modulating the expression of oncogenic
microRNA-23a. Proc Natl Acad Sci USA. 109:15805–15810. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong WQ, Bai R, Liu T, Cai CL, Liu M, Li X
and Tang H: MicroRNA-182 targets cAMP-responsive element-binding
protein 1 and suppresses cell growth in human gastric
adenocarcinoma. FEBS J. 279:1252–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z, Tsuchiya H, Zhang Y, Hartnett ME
and Wang L: MicroRNA-433 inhibits liver cancer cell migration by
repressing the protein expression and function of cAMP response
element-binding protein. J Biol Chem. 288:28893–28899. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chhabra A, Fernando H, Watkins G, Mansel
RE and Jiang WG: Expression of transcription factor CREB1 in human
breast cancer and its correlation with prognosis. Oncol Rep.
18:953–958. 2007.PubMed/NCBI
|
|
32
|
Seo HS, Liu DD, Bekele BN, Kim MK, Pisters
K, Lippman SM, Wistuba II and Koo JS: Cyclic AMP response
element-binding protein overexpression: A feature associated with
negative prognosis in never smokers with non-small cell lung
cancer. Cancer Res. 68:6065–6073. 2008. View Article : Google Scholar : PubMed/NCBI
|