1. Introduction
Sphingosine kinases (SphKs) are biological lipid
kinases that regulate the sphingolipid metabolic pathway. SphKs
control multiple important cell processes and play an important
role in numerous hyperproliferative and inflammatory diseases.
Briefly, the de novo way of sphingolipid
synthesis is a conduction from serine and palmitate to the
formation of 3-ketodihydrosphingosine through serine
palmitoyltransferase (SPT) enzyme. In turn,
3-ketodihydrosphingosine is reduced to dihydrosphingosine, followed
the acylation by a (dihydro)-ceramide synthase (also known as lass
or CerS) (1). After desaturation
of dihydroceramide, ceramide is produced. Ceramide is a central hub
of sphingolipid metabolism which can be phosphorylated by ceramide
kinase to form ceramide-1-phosphate; glycosylated by
glucosylceramide synthase or galactosylceramide synthase; converted
to sphingomyelin by sphingomyelin synthase; or finally broken down
by a ceramidase to form sphingosine (2). Sphingosine can be recycled into
sphingolipid pathways by ceramide synthase or it can be
phosphorylated by one of two SphKs (SphK1 and SphK2) to form
sphingosine-1-phosphate (S1P). Also, the product S1P can be
dephosphorylated to regenerate sphingosine by S1P phosphatases. The
only exit pathway of sphingolipid pathways is metabolism of S1P
mediated by S1P lyase (3)
(Fig. 1). These interconnected
metabolites interact with specific protein targets, such as
kinases, phosphatases and G protein coupled receptors (S1P
receptors) (4), to exert their
cell responses.
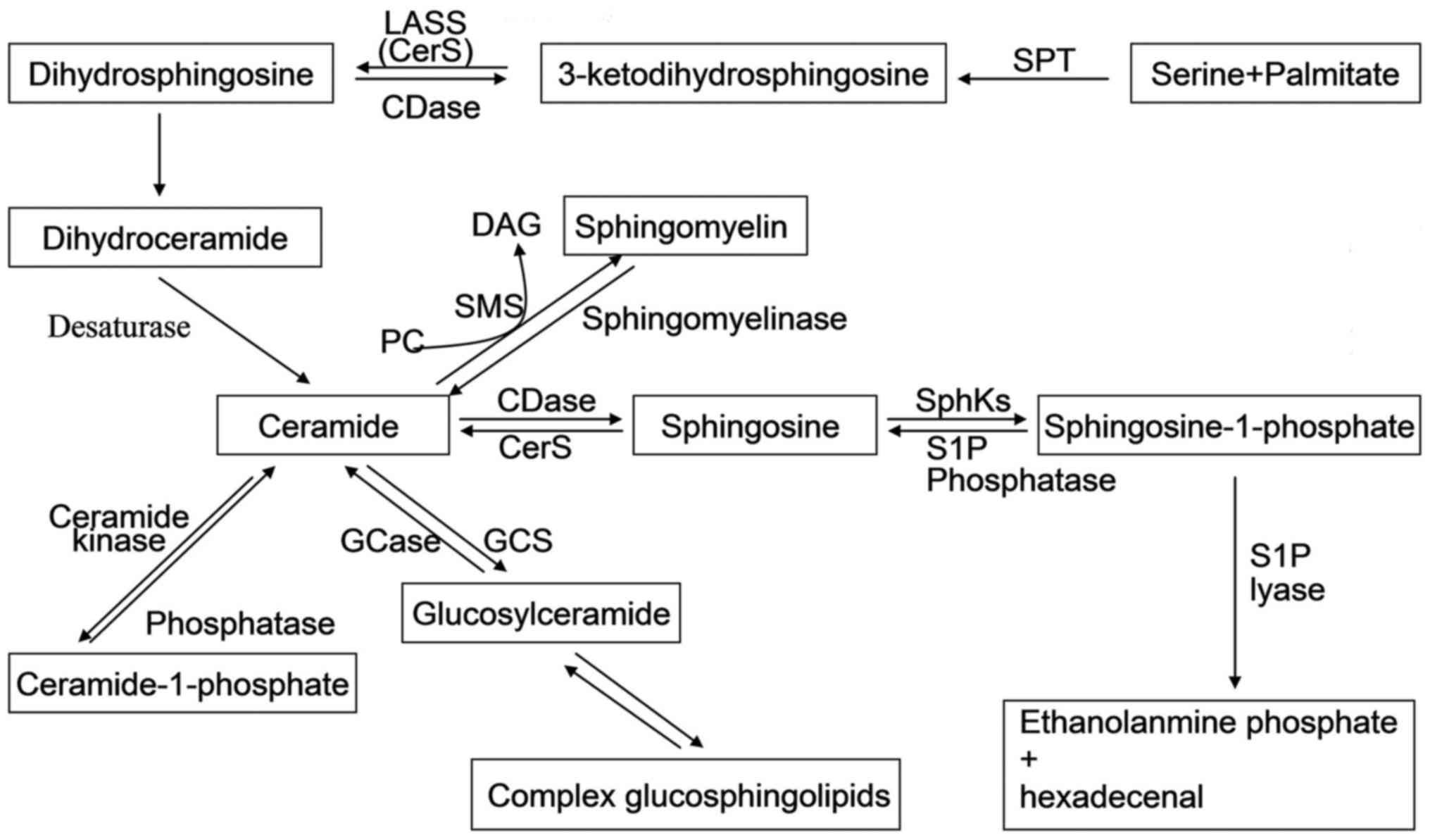 | Figure 1Sphingolipid metabolic pathway.
Ceramide is in the center of sphingolipid metabolism, generated by
de novo-sphingolipid synthesis or by sphingomyelin
degradation. The de novo synthesis of ceramide begins with
3-ketodihydrosphingosine formed by serine and palmitate through the
action of SPT; has experienced the process of the formation of
dihydrosphingosine, dihydroceramide, and is eventually generated by
the action of desaturases. Ceramide can be glycosylated by
glucosylceramide synthase or galactosylceramide synthase; converted
to sphingomyelin by sphingomyelin synthase; or finally broken down
by a ceramidase to form sphingosine. Sphingosine can be
phosphorylated by one of two SphKs (SphK1 and SphK2) to form
sphingosine-1-phosphate (S1P). The only exit pathway of
sphingolipid pathways is metabolism of S1P mediated by S1P lyase.
Most of sphingolipids can be recycled into sphingolipid pathways by
specific enzymes. SPT, serine palmitoyl transferase; CDase,
ceramidase; DAG, diacylglycerol; GCase, glucosyl ceramidase; GCS,
glucosylceramide synthase; PC, phosphatidylcholine; SphKs,
sphingosine kinases; SMS, sphingomyelin synthase. |
SphKs are the only enzymes that catalyze
ATP-dependent phosphorylation of sphingosine to S1P. The balance
between the cellular levels of S1P and ceramide/sphingosine,
so-called sphingolipid rheostat, acts as an important regulator of
cell fate (5). S1P is both an
extracellular messenger and an intracellular second messenger. As
an extracellular messenger, S1P is a specific and high-affinity
ligand for a family of G-protein-coupled receptors (GPCRs)
(6,7). S1P binding to these receptors could
trigger a wide range of cellular responses, including
proliferation, inhibition of apoptosis, formation of actin stress
fibres, stimulation of adherent junctions and enhanced
extracellular matrix assembly (8-11).
As an intracellular second messenger, S1P stimulates various
plasma-membrane receptors, such as platelet-derived growth factor
receptor (PDGFR) and Fcγ receptor 1 (FcγRI) (12,13). Inhibition of SphKs strongly
reduces cellular events triggered by these receptors. Now, SphK/S1P
signaling is considered to be involved in many physiological and
pathological processes, including cardiovascular system,
inflammation, cancer process, transplantation, virus infections and
central nervous system, which will be introduced in detail in the
application section of this review (14-19).
SphKs have five conserved domains (C1-C5). C1-C3
domains are also found in ceramide kinase (CERK) and diacylglycerol
kinases (DAGK), whereas C4 domain appears to be unique domain in
SphKs (20). The two SphK
isoforms (SphK1 and SphK2) regulate the phosphorylation metabolism.
Compared to SphK1, SphK2 has ~240 additional amino acids. Human
SphK1 localizes to chromosome 17 (17q25.2), whereas
SphK2 maps to chromosome 19 (19q13.2) (21), indicating different functions of
SphKs. SphK1 exists mostly in cytoplasm and migrates to plasma
membrane upon phosphorylation. It is involved in the formation of
cancers by enhancing cancer cell proliferation and invasion
(22,23). Moreover, SphK1 expression
is correlated with severity and poor prognosis of cancers, and
chemotherapy resistance (24-27). Also, SphK1 participates in
immunological and inflammatory responses in human body (28). SphK2 localizes in the nucleus to
inhibit DNA synthesis and regulate HDAC1/2 activity (29). Downregulation of SphK2 inhibits
the effects of inflammation, proliferation and migration in tumor
cells (30-33). Specifically, SphK2 affects the
function of mitochondria involved in the central nervous system
diseases (34). Levels of mRNA
encoding SphK1/2 are significantly higher in tumors of
rectum, stomach, kidney, colon, uterus, ovary, breast and lung
compared with normal tissues from the same patient (35). Administration of polynucleotides
encoding SphKs can induce blood vessel formation (36,37). Overall, the evidence suggests that
inhibition of SphKs may be an effective therapy for many diseases
(38).
Due to the function of SphKs and S1P, the SphK/S1P
pathway is now considered as a novel and innovative target for the
treatment of many diseases. Since the past decade, there is growing
interest in developing novel inhibitors of SphKs and their
application in clinical practice. This review consists of three
parts. The first part summarizes the history and development of
SphK inhibitors; the second part introduces SphK inhibitors by
their structures; the third part describes some applications of
SphK inhibitors.
2. The history and development of SphK
inhibitor
Sphingolipids, are a class of lipids containing a
backbone of sphingoid bases, a set of aliphatic amino alcohols that
includes sphingosine. They were discovered in brain extracts in the
1870s and named after the mythological Sphinx because of their
enigmatic nature (39,40). Sphingosine was discovered around
1880 by Thudichum, who isolated it from the hydrolysis products of
phrenosin, characterized it as a base of empirical composition
C17H35NO2, and described a number
of its salts (41). In 1968,
Stoffel et al proposed the importance of SphK is not
restricted to catabolism of sphingolipids (42). In 1989, Hannun and Bell found that
sphingosine and lysosphingolipids elicited a variety of cellular
responses, including antagonism of phorbol eater-induced responses,
blockage of platelet and neutrophil function, and inhibition of
growth factor action (43). Later
in the same year, Okazaki et al demonstrated the existence
of a 'sphingomyelin cycle' in human cells and suggested the
products of sphingolipids may play a role in cell differentiation
(44). In 1998, Olivera et
al purified SphKs from rat kidney for the first time (20), providing the basis for molecular
characterization of key enzymes in sphingolipid signaling. To date,
two mammalian SphKs have been characterized, cloned and
sequenced.
Initial SphK inhibitor development has utilized
molecules derived from sphingosine, including
L-threodihydrosphingosine (DHS:Sfingol), N,N-dimethylsphingosine
(DMS) and N,N,N-trimethylsphingosine (TMS). They block the
activities of both SphK1 and SphK2 by competing with the natural
substrate sphingosine. Safingol has been proven to show therapeutic
value in treating patients with locally advanced or metastatic
solid tumors, which has now entered phase I clinical trials
(NCT00084812). However, these sphingosine mimic compounds have low
specificity with several important off-targets, such as protein
kinase C (PKC), ceramide synthase (CerS) (45). In 2003, French et al
revealed four different types of nolipidic small molecules, SKI-I
SKI-II; SKI-III; SKI-IV, with SphK inhibitory effect at
submicromolar to micromolar concentrations (38). These four derivatives were shown
to be selective toward human SphKs compared with a panel of human
lipid and protein kinases (38).
Moreover, these compounds had antitumor activity in vivo
without obvious toxicity, providing the first evidence that SphK
inhibitor can be developed as anticancer drugs. In 21st century,
selective SphK inhibitor drugs attracted more attention. ABC294640
is the first selective SphK2 inhibitor, which has completed phase I
clinical trials recently for treatment of advanced solid tumors
(NCT01488513). The first SphK1-specific inhibitor, SK1-I, was
described by Paugh et al in 2008 (46). From then, more selective SphK
compounds were developed.
3. SphK inhibitors
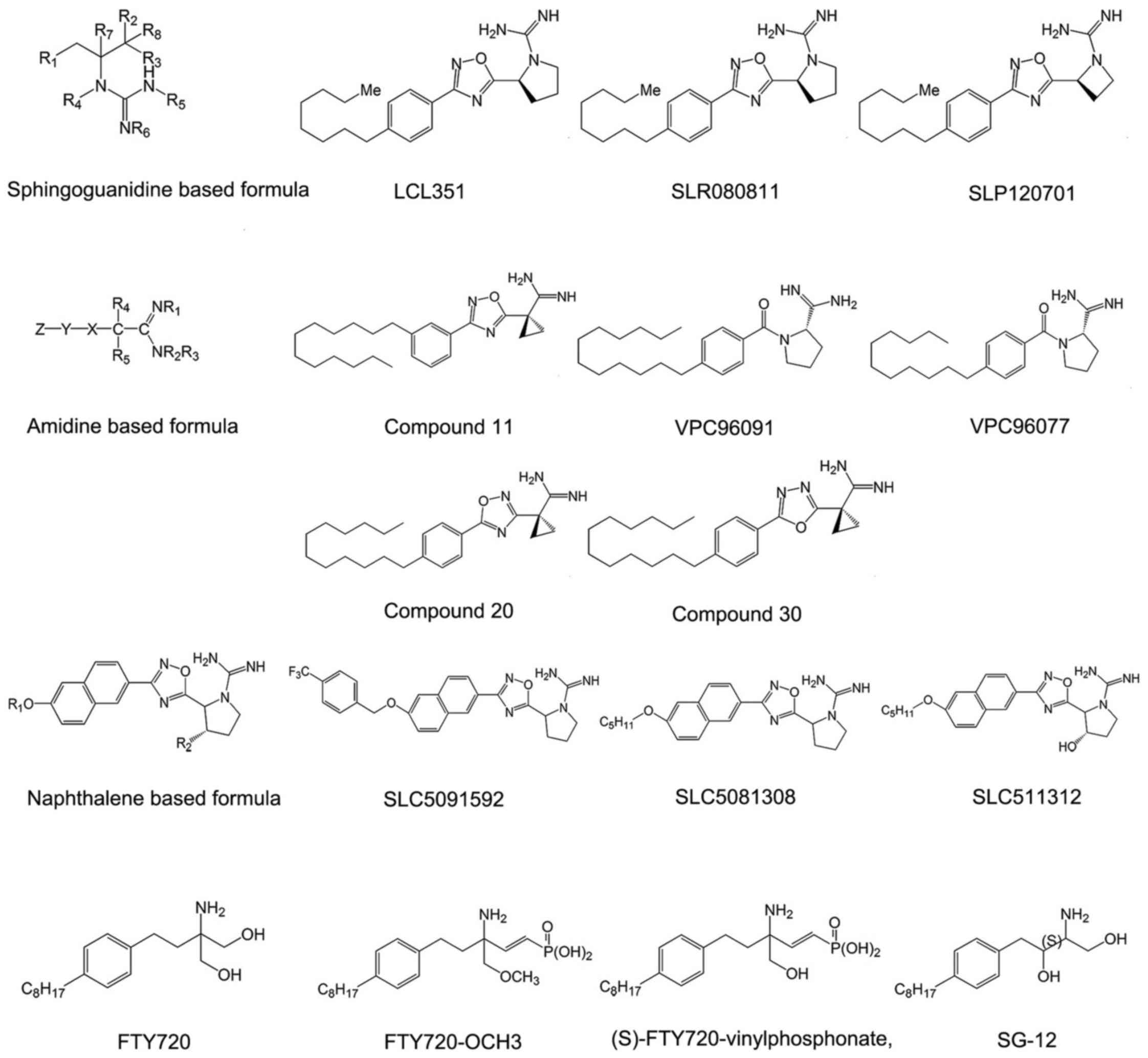
Sphingosine-based SphK inhibitors
Sphingoguanidine based SphK
inhibitors
Sphingoguanidine based SphK inhibitors are composed
of a sphingolipid backbone and a guanidine moiety at the head. The
guanidine region mimics the polar head of sphingosine. Guanidine is
believed to be able to interact with ATP directly in the catalytic
center of some enzymes and to impede the phosphorylation reaction
(47,48). Representative compounds of this
type of SphK inhibitor are SLR080811, SLP120701 and LCL351
(Fig. 2).
Among the compounds in this category, LCL351 is the
most effective agent. It exhibits half maximal inhibitory
concentration (IC50) values of 40 and 300 nmol/l for
inhibition of SphK1 and SphK2 respectively, indicating a special
selectivity for SphK1 (47,48). Except the long chain followed by
guanidine moiety, these sphingoguanidine based SphK inhibitors
usually bear an oxadizole linker ring. The structure-activity
relationship studies indicated that the removal of a hydroxyl group
on the pyrrolidine ring of sphingoguanidine based inhibitors acted
as a molecular switch to selective SphK2 inhibition (49). Furthermore, insertion of a single
methylene unit as a spacer between the oxadiazole and pyrrolidine
rings can increase the selectivity to inhibit the function of SphK1
in this category (49).
Amidine based SphK inhibitors
Amidine-based SphK inhibitors are also structural
analogues of sphingosine that prevent from the substrate binding to
the domain of the SphKs, including VPC96091, VPC96077, and compound
11 in patent WO2013/119946A1 (50-52) (Fig.
2). However, the hydroxyl group located in the polar head of
sphingosine is removed in this category. Considering that the
hydroxyl group is responsible for phosphorylation (53), these compounds, which are
difficult to phosphorylate, would be more effective in suppressing
the activity of SphKs.
The amidine-based SphK inhibitors showed high
selective activity to SphK1 with in vitro potency in the
nanomolar range. Compound 11 is the representative one with
705-fold selectivity for SphK1 and is the most selective SphK1
inhibitor reported until now (50). The unique torsional angle of the
cyclopropane ring in compound 11 provides improved presentation of
amidine in the active site. Biological evaluation of amidine based
SphK inhibitors reveals substantial differences in inhibitory
quality of molecules containing isomeric oxadiazoles. Compounds
containing 3-aryl display significantly higher potency and
selectivity for SphK1 than those containing 5-aryl 1,2,4-oxadiazole
isomers (compound 20 in patent WO2013/119946A1) (Fig. 2), while the compounds containing
1,3,4-oxadiazoles (compound 30 in patent WO2013/119946A1) (Fig. 2) display intermediate potency
(52). Moreover,
meta-substitution in the phenyl ring increases the efficacy for all
isomers (50).
Bicyclic aryl based SphK
inhibitors
Bicyclic aryl based SphK inhibitors are fused
bicyclic sphingosine analogs. The fused bicycle is naphthalene,
isoquinoline, quinoline, quinazoline or indole (54,55). Among them, Naphthalene based
compounds are studied most thoroughly. Naphthalene based SphK
inhibitors are tail region modifications of sphingosine, including
SLC5091592, SLC5081308 and SLC5111312 (Fig. 2). Removal of the lipophilic tail
completely abolishes the inhibitory activity of naphthalene based
SphK inhibitors. Also it is necessary for the tail region to bear
internal phenyl ring(s) (56).
Detailed investigations on the tail region of a scaffold that
features a lipophilic naphthalene ring holding SphK inhibition
activity were conducted by Congdon et al (57). These analogues possess a binding
mode similar to sphingosine in the ligand binding pocket and are
competitive with sphingosine, independent of ATP.
SLC5091592 is the most representative and potent
agent of naphthalene-based SphK inhibitors, which incorporates a
4-trifluomethylbenzyl 'tail' and displays high selectivity toward
SphK2 (57). The replacement of
4-trifluomethylbenzyl results in significant inhibition of SphK2 at
1 μM (μmol/l) inhibitor concentration without any
inhibition of SphK1, as the CF3 group can impact SphK2
selectivity. The corresponding meta version was also generated by
Congdon et al, but it was less potent (57). Other halogens (Br and Cl) are
equally potent in the meta and para positions. Also, the length of
the molecule is of great significance. The ideal head to tail
(positive charge to terminal methyl group) length is ~18-21 atoms
(57). Moreover, there is a
dependence on SphK2 activity and selectivity with alkyl chain
length of naphthalene based inhibitors, suggesting a larger lipid
binding pocket in SphK2 compared to SphK1 (56,58).
Amino-alcohol based SphK
inhibitors
Amino-alcohol based SphK inhibitors are sphingosine
analog inhibitors bearing an amino alcohol head group, including
FTY720, (S)-FTY720-vinylphosphonate, FTY720-OCH3 and SG-12
(Fig. 2). The amino alcohol group
is molecular target of SphK2 competing with sphingosine (53). Apart from the amino alcohol group,
the structures of these derivatives all contain a 1,4-disubstituted
phenyl ring acting as a rigid linker group and a lipophilic tail
which is important for interactions with hydrophobic binding pocket
of S1P receptors (S1PRs) (53).
FTY720 (2-amino-2-[2-(4-octylphenyl) ethyl]
propane-1, 3-diol), also known as fingolimod, is one of most
studied sphingosine analog inhibitors. It is phosphorylated by
SphK2, and the product (FTY720-P) acts as an agonist of four of the
five S1PRs (excluding S1PR2), with concentration for 50% of maximal
effect (EC50) values in the nanomolar range (59). This compound was approved by US
Food and Drug Administration (FDA) in September 21, 2010 for the
treatment of multiple sclerosis and has been used in the clinic to
quell symptoms and slow the progression of multiple sclerosis.
Moreover, it completed phase 3 clinical trials for its efficacy and
safety in de novo adult renal transplant recipients
(NCT00239876).
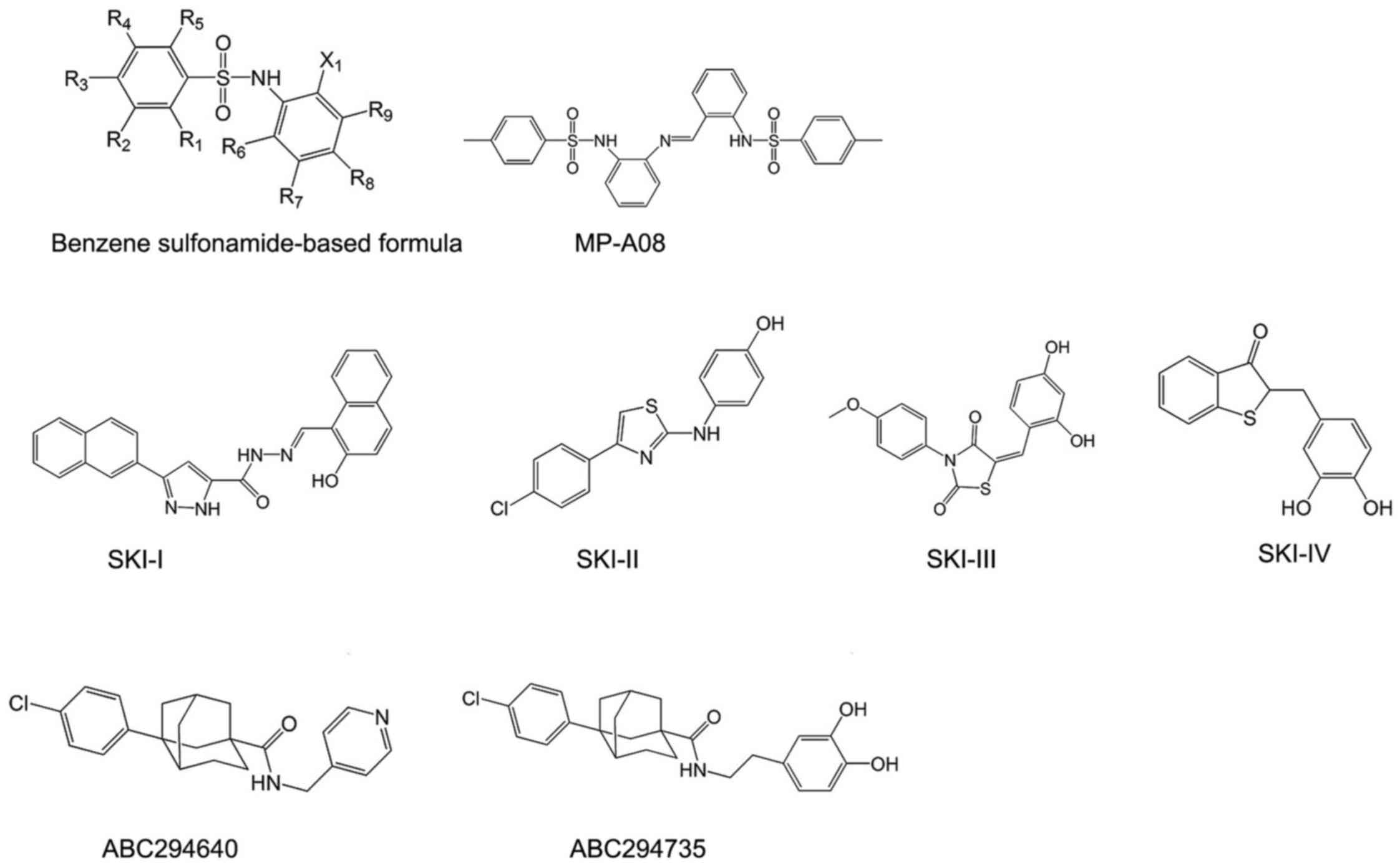
Small molecule SphK inhibitors
Lipidic small molecule SphK
inhibitors
Benzene sulfonamide-based inhibitors of SphKs were
identified through a structure-base approach to target ATP-binding
pocket of SphKs. These compounds are docked into ATP-binding pocket
of SphKs, and are predicted to form close associations with Asn22,
Arg24, Thr54, Ser79, Gly80, Asp81, Gly82, Leu83 and Ser112 of
ATP-binding pocket in SphKs (60). To confirm the orientation binding
of these compounds, its ability of inhibiting the ATP-binding
pocket mutants of SphKs was assessed in patent WO2016007993 (A1)
through alanine mutagenesis. Their inhibition was reduced by
~3-fold by the T54A, L83A, R185A and S112A mutations and ~2-fold by
the S79A, R24A and R191A mutations. These findings confirmed the
inhibitor target of ATP-binding pocket of SphKs (60).
The most representative compounds in this category
is MP-A08 (Fig. 3),
4-methyl-N-[2-[[2-[(4-ethylphenyl)sulfonylamino]phenyl]iminomethyl]phenyl]
benzenesulfonamide, containing two benzenesulfonamide groups joined
by a benzylidene-aniline group. To assess the selectivity, MP-A08
was tested against purified recombinant SphKs. MP-A08 showed no
inhibition of human DAGK and only weak influence on CERK. This
hinted that inhibitors of this type may overcome at least some of
the off-target effects of sphingosine-like molecules. MP-A08 is a
higher affinity inhibitor of SphK2 than SphK1, with inhibitory
constant (Ki) values of 6.9±0.8 and 27±3 mM, respectively (60).
Nolipidic small molecule SphK
inhibitors
SKI-I, SKI-II, SKI-III and SKI-IV were identified by
a high throughput screen by Smith and co-workers in 2003 (Fig. 3). They are the first nolipidic
small molecules of SphK inhibitors. Among them, SKI-II,
2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole, is the most
extensively studied compound, with high oral bioavailability and
limited toxicity (61). It is an
SphK1/2-dual inhibitor which favors SphK2 inhibition (62) and has been commercialized
(63). SKI-II induces lysosomal
degradation of SphK1 rather than directly inhibiting catalytic
SphK1 activity or competing the ATP-binding site (64). However, whether this is an
SphK1-specific effect or a more general effect also affecting other
proteins determined for lysosomal degradation remains unclear, thus
providing additional targets for SKI-II (64). A recent study revealed a dual
mechanism of action for SKI-II: abrogating estrogen receptor (ER)
signaling, possibly through direct binding to ER, similar to
tamoxifen (63), which is
consistent with previous speculation of its additional targets.
A series of phenyladamantane-based compounds showed
the inhibitory activity to SphKs. They are competitive inhibitors
in respect to sphingosine. The most famous compound is ABC294640
(Fig. 3). ABC294640,
3-(4-chlorophenyl)-adamantane-1-carboxylic acid
(pyridin-4-ylmethyl) amide, is the first SphK2 inhibitor with a Ki
of 9.8 μM, and it reduces the amount of S1P in intact cells
(65). It shows great therapeutic
value in cancer treatment (32)
and has completed phase I clinical trials for advanced solid tumors
recently (NCT01488513). Moreover, ABC294640 was found not only to
inhibit STAT3 phosphorylation, which is the key signaling pathways
in regulating cholangiocarcinoma cell proliferation and survival,
but also to induce autophagy of cholangiocarcinoma cells (66). Furthermore, it possesses the same
antiestrogenic activity as SKI-II, similar to tamoxifen (67). Apart from ABC294640, ABC294735 is
another potent compound in this series (Fig. 3). It is a modification of the
pyridylmethyl substituent on amide group of ABC294640 by a
dihydroxyphenyl ring. Different from ABC294640, it induced
inhibition in a dose-dependent manner of both SphK1 and SphK2
(IC50=10 μM) (126). Therefore, it is considered a
dual inhibitor of SphK1 and SphK2.
SphK inhibitors from natural
sources
Nature has provided us with basis of many medicines
in clinical use today. Many compounds from natural sources have
also been recognized as SphK inhibitors. For example, SphK
inhibitors B-5354a, b and c are from marine bacterium, SANK 71896.
They inhibit SphKs activity with IC50 values of 21, 58
and 38 μM, respectively (68). The structures of these compounds
were elucidated by a combination of spectroscopic analyses to be
new esters of 4-amino-3-hydroxybenzoic acid with long-chain
unsaturated alcohols (69). Three
functional groups including the long unsaturated aliphatic chain,
4-amino and 3-hydroxyl were identified (69). Other inhibitors were developed
from natural sources, include Jaspine B (70), F-12509A, and polyphenols. Although
these inhibitors are moderately potent, with in vitro Ki
ranging from 2 to 58 mM, their selectivity and large-scale
production capabilities remain unknown.
4. The applications of SphK inhibitors
Cancer
SphK1/2 are expressed 2-8-fold higher in various
cancer tissues (e.g., breast, lung, ovarian, stomach and colon
cancers) than in normal control tissues in both messenger (m)RNA
and protein levels (35),
indicating participation of SphKs in evolution and progression in
cancer. They take part in cancer progression through two major
different ways: one is SphKs themselves as oncogenes, the the other
is their functions of metabolites.
Firstly, SphKs may act as oncogenes, involved in
oncogenic H-Ras-mediated transformation. Xia et al proved
that SphK1 acted as an oncogene, involving in a novel signaling
pathway for Ras activation (23).
Furthermore, SphK1 itself could induce cancer cell migration and
tumor angiogenesis through its key role in epidermal growth factor
(EGF) directed motility (71,72). SphK1 and SphK2 can promote breast
cancer cell growth through mediating mitogenic action of insulin
(73). However, there is an
opposite example that specific inhibition of SphK1 had no effect on
the proliferation and survival of head and neck carcinoma cells
(74), which means that different
cancer show different tissue specificity of SphKs.
Secondly, the products of SphKs, especially S1P, can
participate in cancer progression. S1P can specifically bind to
amino-terminal ring domain of tumor necrosis factor (TNF)
receptor-associated factor 2 (TRAF2) (75,76), an E3 ubiquitin ligase that
modulates TNF-α-induced activation of nuclear factor-κB (NF-κB)
signaling. It also activates signal transducer activator of
transcription 3 (STAT3) (77-81). So, S1P can serve as a master
switch in carcinogenesis by activation of two transcriptional
regulators, TRAF2 and STAT3. S1P not only exerts function by
binding to other receptors, but also precedes its function by its
own receptors.
Many SphK inhibitors mentioned above have antitumor
activity, some of which have entered the stage of clinical trials.
For example, MP-A08 reduces the growth of human lung adenocarcinoma
in a mouse xenograft model by both inducing tumor cell apoptosis
and inhibiting tumor angiogenesis; ABC294640 was reported in
reducing the growth of prostate and colorectal cancer (32,82), and also effective in other solid
tumors, which was completed in phase I clinical trials
(NCT01488513); and SKI-II can induce growth inhibition and
apoptosis in human gastric cancer (83,84).
Allergic and inflammatory diseases
Allergic inflammatory disease is caused by
hypersensitivity of the immune system and leads to inflammation. It
was reported that level of S1P was increased in bronchoalveolar
lavage fluid of allergic asthma after allergen challenge, revealing
a potential mechanism of S1P involving in allergic inflammatory
diseases (85). The mechanisms
are varied and introduced below. Firstly, S1P mediates
intercellular adhesion molecule-1 (ICAM-1) through p38
mitogen-activated protein kinase (MAPK) and p42/p44 MAPK-dependent
Akt activation, and then causes lung injury (86). Secondly, intracellular S1P can
induce cyclooxygenase-2 (COX-2) expression via activation of S1PR2
and p42/p44 MAPK-dependent signaling in renal mesangial cells,
leading to enhanced prostaglandin E2 (PGE2)
formation and cell migration (15,87). Apart from COX-2, CD23 expression
is significantly increased in S1P-treated animal models. CD23 is a
low-affinity receptor for IgE and considered to regulate IgE
production (88,89). High expression of CD23 causes high
level of IgE, which is responsible for the activation of mast cells
(90). Thirdly, IgE mediated mast
cell activation leads to the activation of SphK, resulting in
increased formation of S1P (90),
which forms a vicious circle. Administration of S1P to nude mice
does not elicit airway smooth muscle hyper-reactivity and lung
inflammation, which indicates a Th2-like bias mechanism of S1P
(85). Fourthly, S1P signaling
plays a role in immune-cell trafficking (91,92). S1P regulates both the homing of
immune cells to lymphoid organs and their egress into blood and
lymph (93). For example, S1PR1
is decisive for T and B cell egress from lymph nodes and for the
exit of mature thymocytes, both conventional T cells and natural
killer T (NKT) cells (94).
The therapeutic value of SphK inhibitors in allergic
inflammatory diseases has been confirmed in animal models (16). SK1-I and DMS were both reported to
reduce ovalbumin-induced airway hyper-responsiveness (AHR) and
airway inflammation in mice sensitized to ovalbumin significantly
(95). These compounds decrease
the number of eosinophils and levels of the cytokines, TNF-α,
chemokines eotaxin and chemokine ligand 2 (CCL2) in bronchoalveolar
lavage fluid. SphK inhibitors also inhibit activation of NF-κB in
lungs (96), a master
transcription factor that regulates the expression of
proinflammatory cytokines. Recently, a clinical trial recruited for
evaluation the S1P as a novel biomarker in food allergy
(NCT01776489). Thus, SphKs are novel therapeutic targets for the
treatment of allergic diseases.
Cardiovascular diseases
Cardiovascular disease (CVD) is a general term for
conditions affecting the heart or blood vessels. The majority of
S1P in plasma is associated with high-density lipoproteins (HDL),
and the amount of HDL-S1P affects the quantity and quality of
HDL-dependent functions (97,98). A number of studies have linked
plasma HDL-S1P to the incidence of coronary artery disease (CAD) i)
HDL-S1P was lower in patients with stable CAD than in healthy
individuals; ii) in contrast, HDL-S1P was higher in CAD independent
of HDL-cholesterol than in healthy individuals and even higher in
acute myocardial infarction (AMI); iii) HDL-S1P correlated
negatively with the severity of coronary atherosclerosis (99,100). So the plasma S1P may act as a
biomarker for discriminating patients with myocardial infarction
(MI) and stable CAD from healthy individuals. Also, S1P takes an
important part in chronic cardiac remodeling. Firstly,
SphK1/S1P/S1PR1 signaling is a main cause of collagen deposition
and myofibroblast activation stimulated by transforming growth
factor (TGF), which contributes to cardiac fibrosis and remodeling
(101). Secondly, the NF-κB and
STAT3 in the cardiomyocyte can be activated by cardiac
SphK1/S1P/S1PR1 signaling, leading to the overproduction of
proinflammatory cytokines and pathological cardiac remodeling
(102,103). Thirdly, SphK1/S1P/S1PR1
signaling regulates β1-adrenergic receptor (β1-AR)
stimulation-induced activation of proinflammatory responses in the
cardiomyocyte, a well-recognized mechanism of chronic cardiac
inflammation following MI (104). Stimulation of β1-AR upregulates
SphK1 expression and increases S1P production in the cardiomyocyte
(104). So cardiac
SphK1/S1P/S1PR1 signaling plays a crucial role in the regulation of
cardiomyocyte pro-inflammatory responses and contributes to cardiac
remodeling. Furthermore, as we mentioned before, SphK1 and S1P are
critical for EGF-based angiogenesis. S1P signaling regulates
vascular function (105), such
as vascular tone and endothelial barrier, the regulation of blood
pressure, and anti-atherosclerotic effect. S1PR1 also regulates
cardiac function by modulating intracellular Ca2+
homeostasis and Na+/H+ exchange through its
inhibitory effect on adenylate cyclase (106). These key findings on SphK and
S1P may offer novel therapeutic approaches to CVD.
However, there is no approved clinical use or
clinical trials of SphK inhibitors in treatment of CVD. Only a few
compounds have been confirmed to show therapeutic values in CVD on
mice or rats, for example, SKI-II exacerbating atherosclerosis in
low-density lipoprotein receptor-deficient mice on high cholesterol
diet. SphK/S1P-based therapy is a completely new area in CVD
treatment.
Transplantation
Graft-versus-host disease (GVHD) and transplant
vasculopathy (TV) represent the main cause of graft failure and
reduce the ratio of long-term success after organ transplantation
(107,108). As mentioned above, SPHK1/S1P
signaling is involved in immune response and vascular function
which are both important factors of transplantation. S1PR1 is
essential for lymphocyte recirculation (109). It regulates lymphocyte egress
from both thymus and peripheral lymphoid organs, which can reduce
the chance of lymphocyte reaching the lesion site and prolong the
survival time of the transplanted organ (109,110). Also, many studies have linked
SphK1/S1P signaling to the prognostic of transplantation: i)
transplant arteriosclerosis was markedly reduced in animals with
SphK1 suppressed by fish oil (111); ii) GVHD and graft rejection was
prevented by antagonizing the function of S1P in rat small bowel
transplantation (107); iii)
SphK1/S1P pathway contributed to in vivo intimal hyperplasia
and caused the transplant vasculopathy (108).
FTY720, an SphK inhibitor, reduces the number of
lymphocytes in the peripheral circulation selectively and prolongs
the survival of transplanted organs, with no harm to the immune
response to virus and immune memory function (110,112). It has been used on patients who
receive transplantation in clinic and has showed great synergistic
effect with cyclosporin A, FK506 and other first-line
immunosuppressants (113-116).
FTY720 finished its phase 3 clinical trails for its efficacy and
safety in de novo adult renal transplant recipients in 2011
and has achieved a clear effect and slight adverse reaction in
preventing immune rejection after transplantation.
The central nervous system
SphK/S1P has different roles in different central
nervous diseases (117). SphK2
plays primary roles by regulation of mitochondria function and
modulation to dopaminergic neurons in Parkinson's disease (118,119). Mitochondrial dysfunction is a
feature of Parkinson's disease. SphK2 is located predominantly in
mitochondria. The inhibition of SphK2 decreases the expression of
peroxisome proliferator-activated receptor γ coactivator-1α
(PGC-1α) and its downstream targets nuclear respiratory factor 1
(NRF-1) and mitochondrial transcription factor A (TFAM), which are
the key transcription factors regulating mitochondrial function
(118). Also, SphK2/S1P
regulates the survival of the dopaminergic neurons (119). Dopaminergic neurons are related
to the clinical development of Parkinson's disease. In Alzheimer's
disease (AD), amyloid-β (Aβ) induces neuronal apoptosis, which is
an important part in the pathogenesis of AD. siRNA knockdown of
SphK1 expression increases the load of Aβ, altering the toxicity of
Aβ, and worsening learning and memory ability in animal models
(120,121). S1P formed by SphK2 can bind to
full-length b-secretase-1 (BACE1) and increase its proteolytic
activity (122,123). SphK2 is also involved in
nociception. SphK2 knockout mice demonstrated its facilitation of
nociceptive transmission during the late response (124), but the exact mechanism is still
unknown.
In multiple sclerosis, the immunomodulating drug
FTY720, also a sphingosine-based inhibitor of SphKs and S1PR,
completed phase 3 clinical trials for relapsing-remitting multiple
sclerosis and was approved for oral treatment of multiple sclerosis
by FDA, September 22, 2010. It can cross the blood-brain barrier
(BBB) and accumulate in the central nervous system. FTY720 acts on
brain cells directly and provides protection against the
neurodegenerative component (125). In addition, because SphK1 and
SphK2 exert different effect on the central nervous system,
development of selective SphK inhibitors becomes particularly
important.
Virus infections
Negative-strand RNA virus, including influenza virus
and measles virus, have become major health concerns and
significant economic burden throughout the world.
SphK1-overexpressing cells are more susceptible to viral infection
and have more progeny virus and viral protein expression than
control cells after being infected, which provides supporting
evidence of increased viral replication in SphK-cells. SphK2 is
co-localized with viral RNA and nonstructural proteins. Targeted
impairment of SphK2 expression or function significantly inhibits
virus infection. Furthermore, affinity purification-mass
spectrometry studies revealed that SphK2 associates with a number
of proteins involved in cellular gene expression specifically
during viral infection, suggesting its role in viral replication
(19).
DMS and SKI-II have been identified to inhibit
influenza viral protein expression in MDCK cells. These two
inhibitors also suppress the production of infectious influenza
virus particles from the cells. SKI-II displays a stronger activity
on inhibiting virus production with an EC50 of 2.5
μM, than DMS, which has an EC50 of 4.1 μM
(19). At present, there is no
SphK inhibitor entering clinical trials for virus infections and
animal experiments are also limited, so this application needs many
further studies.
5. Discussion
SphKs are key enzymes in the sphingolipid metabolic
pathway which phosphorylates sphingosine into S1P, thus providing
an essential checkpoint that regulates the relative levels of
ceramide, sphingosine and S1P. The two isoforms of SphKs (SphK1 and
SphK2) are the only enzymes responsible for the equilibrium between
the S1P and sphingosine (62).
They differ in the enzyme kinetic properties, temporal and spatial
distribution, implyiing that they have distinct physiological
functions. S1P participates in many cellular functions, including
cell proliferation, cell morphology, tumor cell invasion, platelet
aggregation, endothelial cell chemotaxis and angiogenesis. SphK/S1P
signaling pathway has become a focal point in biological research,
with therapeutic values for many diseases, including cancer,
central nervous system diseases, virus infections, rejection after
transplantation, cardiovascular diseases, and inflammatory
diseases.
With the intense interest in SphK/S1P signaling
pathway, many SphK inhibitors were developed. Three of five
conserved domains identified within SphK are found in DAGK and
CERK, whereas C4 appears to be unique in SphKs. Also SphK
inhibitors have been shown to inhibit the ERK and Akt signaling
pathways as a downstream event of SphK inhibition. These signaling
pathways and kinases are also involved in many other physiological
and pathological processes. So SphK inhibitors more or less have
some off-targets effects. Identification of these off-targets that
would work synergistically with SphKs, and develop compounds to
target the unique C4 domain should be the focus of future
studies.
Most SphK inhibitors target the substrate of SphKs
or binding pocket of sphingosine or ATP in SphKs. Sphingosine-based
SphK inhibitors are the structural modifications of sphingosine in
its polar head, lipophilic tail or the linker. Initial
sphingosine-based SphK inhibitors, including DHS, DMS and TMS, have
low specificity, with several important off-targets effects. Later
on, more selective inhibitors were identified, including amidine
based SphK inhibitors, naphthalene based SphK inhibitors,
sphingoguanidine based SphK inhibitors and many small molecules.
The modifications, increasing the selectivity to one of the two
isoforms, now focus on the alkyl length, the spacer between the
head and linker rings, and the insertion and the position of
lipidic group in tail region.
Recently, SphK1 is the most developed SphK
inhibitor. Some promising potent and selective SphK1 inhibitors are
appearing with best biochemically characterized inhibitor in
vitro and in vivo. Unfortunately, current SphK2
inhibitors are moderately potent and only ~10-fold selective. The
first SphK2 selective inhibitor described, ABC294640, has been
tested in a variety of animal models of disease with some success,
but some of the effects observed in in vivo studies may be
attributed to off-target effects of this low-potency compound.
SLR080811 is a second-generation SphK2 inhibitor that is ~10-fold
more potent than ABC294640 but no more selective. A curious aspect
of SphK2 is its absence in the results in mice in circulating S1P
levels that are three times that of normal (SphK1 ablation results
in one-half normal levels). SLR080811 treatment recapitulates this
phenomenon. If the propensity to raise S1P levels is a general
property of SphK2 inhibitors and if this effect extends beyond
rodent species, then application of selective SphK inhibitors could
be used to raise or lower circulating S1P levels.
The physiological role of S1P is complex, and SphKs
play a pivotal role in the S1P signaling axis. Numerous in
vitro and in vivo studies indicate targeting SphKs for
the potential treatment of many diseases. SphK1 and SphK2 were
reported to hold therapeutic values, however, whether the
inhibition of one of them alone is enough remains uncertain. More
kinases are targeted, more side effects will occur, so it is
necessary to find the exact function of each isoforms of SphKs.
With further investigation of S1P/SphK signal pathway, the
mechanisms of its role in different systems will be better
understood. Candidate SphK inhibitors will be optional therapeutic
choice and need to be proven by clinical trials. Only a few
compounds have entered the clinical trials. The assumption that
animal or in vitro responses would be predictive of human
in vivo reaction is not considered to be accurate. So we
consider that increasing the number of SphK inhibitors in clinical
trials is necessary.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This project was sponsored by the grants from the
National Natural Science Foundation of China (nos. 81400037,
81571568, 31340073 and 81273274); Beijing Hospital Nova Project
(no. BJ-2016-038). The Priority Academic Program Development of
Jiangsu Higher Education Institutions (PAPD); the Six Talents Peak
projects of Jiangsu Province (to JFW).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
XT and JFW conceived and designed this review. MC,
CJ, YZ, WH and WN collected materials regarding sphingosine kinase
inhibitors. MC wrote the paper. XT and JFW reviewed and edited the
manuscript. All authors read and approved the manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Orr Gandy KA and Obeid LM: Targeting the
sphingosine kinase/sphingosine 1-phosphate pathway in disease:
Review of sphingosine kinase inhibitors. Biochim Biophys Acta.
1831:157–166. 2013. View Article : Google Scholar
|
|
2
|
Kitatani K, Idkowiak-Baldys J and Hannun
YA: The sphingolipid salvage pathway in ceramide metabolism and
signaling. Cell Signal. 20:1010–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hannun YA and Obeid LM: Principles of
bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol
Cell Biol. 9:139–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herr DR, Grillet N, Schwander M, Rivera R,
Müller U and Chun J: Sphingosine 1-phosphate (S1P) signaling is
required for maintenance of hair cells mainly via activation of
S1P2. J Neurosci. 27:1474–1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pitman MR, Powell JA, Coolen C, Moretti
PA, Zebol JR, Pham DH, Finnie JW, Don AS, Ebert LM, Bonder CS, et
al: A selective ATP-competitive sphingosine kinase inhibitor
demonstrates anti-cancer properties. Oncotarget. 6:7065–7083. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Archbold JK, Martin JL and Sweet MJ:
Towards selective lysophospholipid GPCR modulators. Trends
Pharmacol Sci. 35:219–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishii I, Fukushima N, Ye X and Chun J:
Lysophospholipid receptors: Signaling and biology. Annu Rev
Biochem. 73:321–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Peyruchaud O, French KJ,
Magnusson MK and Mosher DF: Sphingosine 1-phosphate stimulates
fibronectin matrix assembly through a Rho-dependent signal pathway.
Blood. 93:2984–2990. 1999.PubMed/NCBI
|
|
9
|
Melendez AJ: Sphingosine kinase signalling
in immune cells: Potential as novel therapeutic targets. Biochim
Biophys Acta. 1784:66–75. 2008. View Article : Google Scholar
|
|
10
|
Porcelli AM, Ghelli A, Hrelia S and Rugolo
M: Phospholipase D stimulation is required for
sphingosine-1-phosphate activation of actin stress fibre assembly
in human airway epithelial cells. Cell Signal. 14:75–81. 2002.
View Article : Google Scholar
|
|
11
|
Meng Y, Xu Z, Wu F, Chen W, Xie S, Liu J,
Huang X and Zhou Y: Sphingosine-1-phosphate suppresses
cyclophosphamide induced follicle apoptosis in human fetal ovarian
xenografts in nude mice. Fertil Steril. 102:871–877.e873. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Cai KY, Li W and Huang H:
Sphingosine-1-phosphate induces the migration and angiogenesis of
Epcs through the Akt signaling pathway via Sphingosine-1-phosphate
receptor 3/platelet-derived growth factor receptor-β. Cell Mol Biol
Lett. 20:597–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Florey O and Haskard DO: Sphingosine
1-phosphate enhances Fc gamma receptor-mediated neutrophil
activation and recruitment under flow conditions. J Immunol.
183:2330–2336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stieber F and Wienke D: Inhibitors of
sphingosine kinase. Patent SG181643 A1. 2010
|
|
15
|
Hla T, Sanchez T, Paik J and Claffey KP:
Methods of inhibiting vascular permeability and apoptosis. Patent
WO/2005/002559 A2. Filed June 18, 2004; issued January 13. 2005
|
|
16
|
Lynch K and Santos W: Sphingosine kinase
inhibitors. Patent WO2016054261 A1. Filed 30 September, 2015;
issued 7 April. 2016
|
|
17
|
Smith C and French K: Methods for the
treatment and prevention of inflammatory diseases. Patent
US20060270630 A1. Filed 19 May 2006; issued 30 November. 2006
|
|
18
|
Hahm B, Seo YJ and Alexander S: Modulation
of sphingosine 1-phosphate metabolizing enzymes for the treatment
of negative-strand rna virus infections. Patent WO2012166859 A2.
Filed 31 May, 2012; issued 6 December. 2012
|
|
19
|
Sinha UK and Masood R: Compositions and
methods of sphingosine kinase inhibitors for use thereof in cancer
therapy. Patent WO2008067560 A9. Filed November 30, 2007; issued
July 17. 2008
|
|
20
|
Olivera A, Kohama T, Tu Z, Milstien S and
Spiegel S: Purification and characterization of rat kidney
sphingosine kinase. J Biol Chem. 273:12576–12583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai WQ, Wong WS and Leung BP: Sphingosine
kinase and sphingosine 1-phosphate in asthma. Biosci Rep.
31:145–150. 2011. View Article : Google Scholar
|
|
22
|
Li J, Song Z, Wang Y, Yin Y, Liu Y, Yuan R
and Nan X: Overexpression of SphK1 enhances cell proliferation and
invasion in triple-negative breast cancer via the PI3K/AKT
signaling pathway. Tumour Biol. 37:10587–10593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia P, Gamble JR, Wang L, Pitson SM,
Moretti PA, Wattenberg BW, D'Andrea RJ and Vadas MA: An oncogenic
role of sphingosine kinase. Curr Biol. 10:1527–1530. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng XD, Zhou ZS, Qiu JH, Shen WH, Wu Q
and Xiao J: Increased SPHK1 expression is associated with poor
prognosis in bladder cancer. Tumour Biol. 35:2075–2080. 2014.
View Article : Google Scholar
|
|
25
|
Matula K, Collie-Duguid E, Murray G,
Parikh K, Grabsch H, Tan P, Lalwani S, Garau R, Ong Y, Bain G, et
al: Regulation of cellular sphingosine-1-phosphate by sphingosine
kinase 1 and sphingosine-1-phopshate lyase determines chemotherapy
resistance in gastroesophageal cancer. BMC Cancer. 15:7622015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Selvam SP and Ogretmen B: Sphingosine
kinase/sphingosine 1-phosphate signaling in cancer therapeutics and
drug resistance. Handb Exp Pharmacol. 216:3–27. 2013. View Article : Google Scholar
|
|
27
|
Zhang Y, Wang Y, Wan Z, Liu S, Cao Y and
Zeng Z: Sphingosine kinase 1 and cancer: A systematic review and
meta-analysis. PLoS One. 9:e903622014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iwabuchi K, Nakayama H, Oizumi A, Suga Y,
Ogawa H and Takamori K: Role of ceramide from glycosphingolipids
and its metabolites in immunological and inflammatory responses in
humans. Mediators Inflamm. 2015:1207482015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alemany R, van Koppen CJ, Danneberg K, Ter
Braak M and Meyer Zu Heringdorf D: Regulation and functional roles
of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol.
374:413–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Q, Li J, Li G, Li Y, Xu C, Li M, Xu G
and Fu S: Prognostic significance of sphingosine kinase 2
expression in non-small cell lung cancer. Tumour Biol. 35:363–368.
2014. View Article : Google Scholar
|
|
31
|
Zhang L, Liu X, Zuo Z, Hao C and Ma Y:
Sphingosine kinase 2 promotes colorectal cancer cell proliferation
and invasion by enhancing MYC expression. Tumour Biol.
37:8455–8460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xun C, Chen MB, Qi L, Tie-Ning Z, Peng X,
Ning L, Zhi-Xiao C and Li-Wei W: Targeting sphingosine kinase 2
(SphK2) by ABC294640 inhibits colorectal cancer cell growth in
vitro and in vivo. J Exp Clin Cancer Res. 34:942015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Q, Rehman H, Shi Y, Krishnasamy Y,
Lemasters JJ, Smith CD and Zhong Z: Inhibition of sphingosine
kinase-2 suppresses inflammation and attenuates graft injury after
liver transplantation in rats. PLoS One. 7:e418342012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Strub GM, Paillard M, Liang J, Gomez L,
Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, et
al: Sphingosine-1-phosphate produced by sphingosine kinase 2 in
mitochondria interacts with prohibitin 2 to regulate complex IV
assembly and respiration. FASEB J. 25:600–612. 2011. View Article : Google Scholar :
|
|
35
|
Shida D, Takabe K, Kapitonov D, Milstien S
and Spiegel S: Targeting SphK1 as a new strategy against cancer.
Curr Drug Targets. 9:662–673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liau G, Stefansson S and Su J: Induction
of blood vessel formation through administration of polynucleotides
encoding sphingosine kinases. Patent WO2002028406 A2. Filed October
5, 2001; issued October 5. 2002
|
|
37
|
Pitson SM, Wattenberg BW, Xia P, Dandrea
RJ, Gamble JR and Vadas MA: Sphingosine kinase enzyme. Patent
WO2000070028 A1. Filed May 12, 2000; issued November 23. 2000
|
|
38
|
French KJ, Schrecengost RS, Lee BD, Zhuang
Y, Smith SN, Eberly JL, Yun JK and Smith CD: Discovery and
evaluation of inhibitors of human sphingosine kinase. Cancer Res.
63:5962–5969. 2003.PubMed/NCBI
|
|
39
|
Chun J and Hartung HP: Mechanism of action
of oral fingolimod (FTY720) in multiple sclerosis. Clin
Neuropharmacol. 33:91–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Spiegel S and Milstien S:
Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat Rev Mol
Cell Biol. 4:397–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lai WQ, Melendez AJ and Leung BP: Role of
sphingosine kinase and sphingosine-1-phosphate in inflammatory
arthritis. World J Biol Chem. 1:321–326. 2010. View Article : Google Scholar
|
|
42
|
Stoffel W, Sticht G and LeKim D: Synthesis
and degradation of spingosine bases in Hansenula ciferrii. Hoppe
Seylers Z Physiol Chem. 349:1149–1156. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hannun YA and Bell RM: Functions of
sphingolipids and sphingolipid breakdown products in cellular
regulation. Science. 243:500–507. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Okazaki T, Bell RM and Hannun YA:
Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role
in cell differentiation. J Biol Chem. 264:19076–19080.
1989.PubMed/NCBI
|
|
45
|
Pitman MR and Pitson SM: Inhibitors of the
sphingosine kinase pathway as potential therapeutics. Curr Cancer
Drug Targets. 10:354–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Paugh SW, Paugh BS, Rahmani M, Kapitonov
D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant
S, et al: A selective sphingosine kinase 1 inhibitor integrates
multiple molecular therapeutic targets in human leukemia. Blood.
112:1382–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szulc Zdzislaw M, Bielawska Alicja, Obeid
Lina M, Hannun Yusuf A, Norris James and Xiang Liu:
Sphingo-guanidines and their use as inhibitors of sphingosine
kinase. Patent WO2010078247 A1. Filed 28 December, 2009; issued 8
July. 2010
|
|
48
|
Szulc ZM, Bielawska A, Obeid LM, Hannun
YA, Norris J and Xiang L: Sphingo-guanidines and their use as
ihibitors of sphingosine kinase. Patent US2012035268 A1. Filed 28
December, 2009; issued 9 February. 2012
|
|
49
|
Patwardhan NN, Morris EA, Kharel Y, Raje
MR, Gao M, Tomsig JL, Lynch KR and Santos WL: Structure-activity
relationship studies and in vivo activity of guanidine-based
sphingosine kinase inhibitors: Discovery of SphK1- and
SphK2-selective inhibitors. J Med Chem. 58:1879–1899. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Houck JD, Dawson TK, Kennedy AJ, Kharel Y,
Naimon ND, Field SD, Lynch KR and Macdonald TL: Structural
requirements and docking analysis of amidine-based sphingosine
kinase 1 inhibitors containing oxadiazoles. ACS Med Chem Lett.
7:487–492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lynch KR, MacDonald TL and Mathews TP:
Imidamide sphingosine kinase inhibitors. Patent WO2011/020116 A1.
Filed 16 August, 2010; issued 17 February. 2011
|
|
52
|
University Of Virginia Patent Foundation;
Santos WL, Lynch KR, Macdonald TL, Kennedy A, Kharel Y, Raje MR and
Houck J: Long chain base sphingosine kinase inhibitors. Patent
WO2013/119946A1. Filed 8 February, 2013; issued 15 August. 2013
|
|
53
|
Plano D, Amin S and Sharma AK: Importance
of sphingosine kinase (SphK) as a target in developing cancer
therapeutics and recent developments in the synthesis of novel SphK
inhibitors. J Med Chem. 57:5509–5524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thomas J, Liu XG, Kumaravel G, Guckian KM,
Caldwell RD, Ma B, Lin EY, Zheng GZ and Taveras AG: Bicyclic aryl
sphingosine 1-phosphate analogs. Patent NZ597596 A. 2014
|
|
55
|
Thomas J, Liu XG, Kumaravel G, Guckian KM,
Caldwell RD, Ma B, Lin EY, Zheng GZ and Taveras AG: Bicyclic aryl
sphingosine 1-phosphate analogs. Patent US2016129023 A1. Filed 5
October, 2015; issued 12 May. 2016
|
|
56
|
Congdon MD, Childress ES, Patwardhan NN,
Gumkowski J, Morris EA, Kharel Y, Lynch KR and Santos WL:
Structure-activity relationship studies of the lipophilic tail
region of sphingosine kinase 2 inhibitors. Bioorg Med Chem Lett.
25:4956–4960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Congdon MD, Kharel Y, Brown AM, Lewis SN,
Bevan DR, Lynch KR and Santos WL: Structure-activity relationship
studies and molecular modeling of naphthalene-based sphingosine
kinase 2 inhibitors. ACS Med Chem Lett. 7:229–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Knott K, Kharel Y, Raje MR, Lynch KR and
Santos WL: Effect of alkyl chain length on sphingosine kinase 2
selectivity. Bioorg Med Chem Lett. 22:6817–6820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brinkmann V, Davis MD, Heise CE, Albert R,
Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, et
al: The immune modulator FTY720 targets sphingosine 1-phosphate
receptors. J Biol Chem. 277:21453–21457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pitman MR and Pitson SM: Benzene
sulfonamide-based inhibitors of sphingosine kinases. Patent
WO2016007993 A1. Filed 16 July, 2015; issued 21 January. 2016
|
|
61
|
French KJ, Upson JJ, Keller SN, Zhuang Y,
Yun JK and Smith CD: Antitumor activity of sphingosine kinase
inhibitors. J Pharmacol Exp Ther. 318:596–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gao P, Peterson YK, Smith RA and Smith CD:
Characterization of isoenzyme-selective inhibitors of human
sphingosine kinases. PLoS One. 7:e445432012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Antoon JW, Meacham WD, Bratton MR,
Slaughter EM, Rhodes LV, Ashe HB, Wiese TE, Burow ME and Beckman
BS: Pharmacological inhibition of sphingosine kinase isoforms
alters estrogen receptor signaling in human breast cancer. J Mol
Endocrinol. 46:205–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ren S, Xin C, Pfeilschifter J and Huwiler
A: A novel mode of action of the putative sphingosine kinase
inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (SKI
II): induction of lysosomal sphingosine kinase 1 degradation. Cell
Physiol Biochem. 26:97–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
French KJ, Zhuang Y, Maines LW, Gao P,
Wang W, Beljanski V, Upson JJ, Green CL, Keller SN and Smith CD:
Pharmacology and antitumor activity of ABC294640, a selective
inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther.
333:129–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ding X, Chaiteerakij R, Moser CD, Shaleh
H, Boakye J, Chen G, Ndzengue A, Li Y, Zhou Y, Huang S, et al:
Antitumor effect of the novel sphingosine kinase 2 inhibitor
ABC294640 is enhanced by inhibition of autophagy and by sorafenib
in human cholangiocarcinoma cells. Oncotarget. 7:20080–20092. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Antoon JW, White MD, Meacham WD, Slaughter
EM, Muir SE, Elliott S, Rhodes LV, Ashe HB, Wiese TE, Smith CD, et
al: Antiestrogenic effects of the novel sphingosine kinase-2
inhibitor ABC294640. Endocrinology. 151:5124–5135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kono K, Tanaka M, Mizuno T, Kodama K,
Ogita T and Kohama T: B-535a, b and c, new sphingosine kinase
inhibitors, produced by a marine bacterium; taxonomy, fermentation,
isolation, physico-chemical properties and structure determination.
J Antibiot (Tokyo). 53:753–758. 2000. View Article : Google Scholar
|
|
69
|
Kono K, Tanaka M, Ogita T and Kohama T:
Characterization of B-5354c, a new sphingosine kinase inhibitor,
produced by a marine bacterium. J Antibiot (Tokyo). 53:759–764.
2000. View Article : Google Scholar
|
|
70
|
Salma Y, Lafont E, Therville N, Carpentier
S, Bonnafé MJ, Levade T, Génisson Y and Andrieu-Abadie N: The
natural marine anhydrophytosphingosine, Jaspine B, induces
apoptosis in melanoma cells by interfering with ceramide
metabolism. Biochem Pharmacol. 78:477–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sarkar S, Maceyka M, Hait NC, Paugh SW,
Sankala H, Milstien S and Spiegel S: Sphingosine kinase 1 is
required for migration, proliferation and survival of MCF-7 human
breast cancer cells. FEBS Lett. 579:5313–5317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gamble J, Vadas M, Pitson S, Xia P and
Limaye V: Method of modulating epithelial cell activity by
modulating the functional levels of sphingosine kinase. Patent
US20060205688A1. Filed 14 October, 2003; issued 14 September.
2006
|
|
73
|
Dai L, Qi Y, Chen J, Kaczorowski D, Di W,
Wang W and Xia P: Sphingosine kinase (SphK) 1 and SphK2 play
equivalent roles in mediating insulin's mitogenic action. Mol
Endocrinol. 28:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Schnute ME, McReynolds MD, Kasten T, Yates
M, Jerome G, Rains JW, Hall T, Chrencik J, Kraus M, Cronin CN, et
al: Modulation of cellular S1P levels with a novel, potent and
specific inhibitor of sphingosine kinase-1. Biochem J. 444:79–88.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Park ES, Choi S, Shin B, Yu J, Yu J, Hwang
JM, Yun H, Chung YH, Choi JS, Choi Y, et al: Tumor necrosis factor
(TNF) receptor-associated factor (TRAF)-interacting protein (TRIP)
negatively regulates the TRAF2 ubiquitin-dependent pathway by
suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction. J
Biol Chem. 290:9660–9673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Alvarez SE, Harikumar KB, Hait NC,
Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T,
et al: Sphingosine-1-phosphate is a missing cofactor for the E3
ubiquitin ligase TRAF2. Nature. 465:1084–1088. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Garris CS, Wu L, Acharya S, Arac A, Blaho
VA, Huang Y, Moon BS, Axtell RC, Ho PP, Steinberg GK, et al:
Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation
exacerbates TH17-mediated autoimmune neuroinflammation. Nat
Immunol. 14:1166–1172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nguyen AV, Wu YY and Lin EY: STAT3 and
sphingosine-1-phosphate in inflammation-associated colorectal
cancer. World J Gastroenterol. 20:10279–10287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Theiss AL: Sphingosine-1-phosphate: Driver
of NFκB and STAT3 persistent activation in chronic intestinal
inflammation and colitis-associated cancer. JAKSTAT.
2:e241502013.
|
|
80
|
Liang J, Nagahashi M, Kim EY, Harikumar
KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, et
al: Sphingosine-1-phosphate links persistent STAT3 activation,
chronic intestinal inflammation, and development of
colitis-associated cancer. Cancer Cell. 23:107–120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Degagné E, Pandurangan A, Bandhuvula P,
Kumar A, Eltanawy A, Zhang M, Yoshinaga Y, Nefedov M, de Jong PJ,
Fong LG, et al: Sphingosine-1-phosphate lyase downregulation
promotes colon carcinogenesis through STAT3-activated microRNAs. J
Clin Invest. 124:5368–5384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
McNaughton M, Pitman M, Pitson SM, Pyne NJ
and Pyne S: Proteasomal degradation of sphingosine kinase 1 and
inhibition of dihydroceramide desaturase by the sphingosine kinase
inhibitors, SKi or ABC294640, induces growth arrest in
androgen-independent LNCaP-AI prostate cancer cells. Oncotarget.
7:16663–16675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li PH, Wu JX, Zheng JN and Pei DS: A
sphingosine kinase-1 inhibitor, SKI-II, induces growth inhibition
and apoptosis in human gastric cancer cells. Asian Pac J Cancer
Prev. 15:10381–10385. 2014. View Article : Google Scholar
|
|
84
|
Liu Y and Zhu Z, Cai H, Liu Q, Zhou H and
Zhu Z: SKI-II reverses the chemoresistance of SGC7901/DDP gastric
cancer cells. Oncol Lett. 8:367–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Roviezzo F, Sorrentino R, Bertolino A, De
Gruttola L, Terlizzi M, Pinto A, Napolitano M, Castello G,
D'Agostino B, Ianaro A, et al: S1P-induced airway smooth muscle
hyperresponsiveness and lung inflammation in vivo: Molecular and
cellular mechanisms. Br J Pharmacol. 172:1882–1893. 2015.
View Article : Google Scholar :
|
|
86
|
Lin CC, Lee IT, Hsu CH, Hsu CK, Chi PL,
Hsiao LD and Yang CM: Sphingosine-1-phosphate mediates
ICAM-1-dependent monocyte adhesion through p38 MAPK and p42/p44
MAPK-dependent Akt activation. PLoS One. 10:e01184732015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Völzke A, Koch A, Meyer Zu Heringdorf D,
Huwiler A and Pfeilschifter J: Sphingosine 1-phosphate (S1P)
induces COX-2 expression and PGE2 formation via S1P
receptor 2 in renal mesangial cells. Biochim Biophys Acta.
1841:11–21. 2014. View Article : Google Scholar
|
|
88
|
Selb R, Eckl-Dorna J, Twaroch TE, Lupinek
C, Teufelberger A, Hofer G, Focke-Tejkl M, Gepp B, Linhart B,
Breiteneder H, et al: Critical and direct involvement of the CD23
stalk region in IgE binding. J Allergy Clin Immunol.
139:281–289.e5. 2017. View Article : Google Scholar :
|
|
89
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Melendez AJ: Allergy therapy: The
therapeutic potential of targeting sphingosine kinase signalling in
mast cells. Eur J Immunol. 38:2969–2974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kunisawa J, Kurashima Y, Gohda M, Higuchi
M, Ishikawa I, Miura F, Ogahara I and Kiyono H: Sphingosine
1-phosphate regulates peritoneal B-cell trafficking for subsequent
intestinal IgA production. Blood. 109:3749–3756. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Garris CS, Blaho VA, Hla T and Han MH:
Sphingosine-1-phosphate receptor 1 signalling in T cells:
Trafficking and beyond. Immunology. 142:347–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Matloubian M, Lo CG, Cinamon G, Lesneski
MJ, Xu Y, Brinkmann V, Allende ML, Proia RL and Cyster JG:
Lymphocyte egress from thymus and peripheral lymphoid organs is
dependent on S1P receptor 1. Nature. 427:355–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rivera J, Proia RL and Olivera A: The
alliance of sphingosine-1-phosphate and its receptors in immunity.
Nat Rev Immunol. 8:753–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lai WQ, Goh HH, Bao Z, Wong WS, Melendez
AJ and Leung BP: The role of sphingosine kinase in a murine model
of allergic asthma. J Immunol. 180:4323–4329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Price MM, Oskeritzian CA, Falanga YT,
Harikumar KB, Allegood JC, Alvarez SE, Conrad D, Ryan JJ, Milstien
S and Spiegel S: A specific sphingosine kinase 1 inhibitor
attenuates airway hyperresponsiveness and inflammation in a mast
cell-dependent murine model of allergic asthma. J Allergy Clin
Immunol. 131:501–11.e1. 2013. View Article : Google Scholar
|
|
97
|
Levkau B: Cardiovascular effects of
sphingosine-1-phosphate (S1P). Handb Exp Pharmacol. 216:147–170.
2013. View Article : Google Scholar
|
|
98
|
Argraves KM and Argraves WS: HDL serves as
a S1P signaling platform mediating a multitude of cardiovascular
effects. J Lipid Res. 48:2325–2333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Levkau B: HDL-S1P: Cardiovascular
functions, disease-associated alterations, and therapeutic
applications. Front Pharmacol. 6:2432015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jing XD, Wei XM, Deng SB, Du JL, Liu YJ
and She Q: The relationship between the high-density lipoprotein
(HDL)-associated sphingosine-1-phosphate (S1P) and coronary
in-stent restenosis. Clin Chim Acta. 446:248–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pchejetski D, Foussal C, Alfarano C,
Lairez O, Calise D, Guilbeau-Frugier C, Schaak S, Seguelas MH,
Wanecq E, Valet P, et al: Apelin prevents cardiac fibroblast
activation and collagen production through inhibition of
sphingosine kinase 1. Eur Heart J. 33:2360–2369. 2012. View Article : Google Scholar
|
|
102
|
Frias MA, James RW, Gerber-Wicht C and
Lang U: Native and reconstituted HDL activate Stat3 in ventricular
cardiomyocytes via ERK1/2: Role of sphingosine-1-phosphate.
Cardiovasc Res. 82:313–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Whetzel AM, Bolick DT, Srinivasan S,
Macdonald TL, Morris MA, Ley K and Hedrick CC: Sphingosine-1
phosphate prevents monocyte/endothelial interactions in type 1
diabetic NOD mice through activation of the S1P1 receptor. Circ
Res. 99:731–739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang F, Xia Y, Yan W, Zhang H, Zhou F,
Zhao S, Wang W, Zhu D, Xin C, Lee Y, et al: Sphingosine 1-phosphate
signaling contributes to cardiac inflammation, dysfunction, and
remodeling following myocardial infarction. Am J Physiol Heart Circ
Physiol. 310:H250–H261. 2016. View Article : Google Scholar
|
|
105
|
Li N and Zhang F: Implication of
sphingosin-1-phosphate in cardiovascular regulation. Front Biosci
(Landmark Ed). 21:1296–1313. 2016. View
Article : Google Scholar
|
|
106
|
Keul P, van Borren MM, Ghanem A, Müller
FU, Baartscheer A, Verkerk AO, Stümpel F, Schulte JS, Hamdani N,
Linke WA, et al: Sphingosine-1-phosphate receptor 1 regulates
cardiac function by modulating Ca2+ sensitivity and
Na+/H+ exchange and mediates protection by
ischemic preconditioning. J Am Heart Assoc. 5:52016. View Article : Google Scholar
|
|
107
|
Song J, Hagiya H, Kurata H, Mizuno H and
Ito T: Prevention of GVHD and graft rejection by a new S1P receptor
agonist, W-061, in rat small bowel transplantation. Transpl
Immunol. 26:163–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Trayssac M, Galvani S, Augé N, Sabbadini
R, Calise D, Mucher E, Sallusto F, Thomsen M, Salvayre R and
Nègre-Salvayre A: Role of sphingosine-1-phosphate in transplant
vasculopathy evoked by anti-HLA antibody. Am J Transplant.
15:2050–2061. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY,
Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, et al: Sphingosine
1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively,
regulate lymphocyte recirculation and heart rate. J Biol Chem.
279:13839–13848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Brinkmann V, Cyster JG and Hla T: FTY720:
Sphingosine 1-phosphate receptor-1 in the control of lymphocyte
egress and endothelial barrier function. Am J Transplant.
4:1019–1025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li Q, Wang C, Zhang Q, Tang C, Li N and Li
J: The reduction of allograft arteriosclerosis in intestinal
transplant is associated with sphingosine kinase
1/sphingosine-1-phosphate signaling after fish oil treatment.
Transplantation. 93:989–996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chiba K1, Yanagawa Y, Masubuchi Y, Kataoka
H, Kawaguchi T, Ohtsuki M and Hoshino Y: FTY720, a novel
immunosuppressant, induces sequestration of circulating mature
lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720
selectively decreases the number of circulating mature lymphocytes
by acceleration of lymphocyte homing. J Immunol. 160:5037–5044.
1998.PubMed/NCBI
|
|
113
|
Zhang J, Zhang A, Sun Y, Cao X and Zhang
N: Treatment with immunosuppressants FTY720 and tacrolimus promotes
functional recovery after spinal cord injury in rats. Tohoku J Exp
Med. 219:295–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yuzawa K, Fukunaga K and Ohkohchi N: Back
transplantation for survival of the graft. Transplant Proc.
37:192–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sugito K, Uekusa S, Kawashima H, Masuko T,
Furuya T, Konuma N, Ohashi K, Inoue M, Ikeda T and Koshinaga T:
Effect of combined treatment with FK506, FTY720, and ex vivo graft
irradiation in rat small bowel transplantation: Expression of
mucosal addressin cell adhesion molecule-1. Pediatr Transplant.
14:614–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lopes CT, Gallo AP, Palma PV, Cury PM and
Bueno V: Skin allograft survival and analysis of renal parameters
after FTY720 + tacrolimus treatment in mice. Transplant Proc.
40:856–860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Pyne S, Adams DR and Pyne NJ: Sphingosine
1-phosphate and sphingosine kinases in health and disease: Recent
advances. Prog Lipid Res. 62:93–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sivasubramanian M, Kanagaraj N, Dheen ST
and Tay SS: Sphingosine kinase 2 and sphingosine-1-phosphate
promotes mitochondrial function in dopaminergic neurons of mouse
model of Parkinson's disease and in MPP+-treated MN9D
cells in vitro. Neuroscience. 290:636–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Pyszko J and Strosznajder JB: Sphingosine
kinase 1 and sphingosine-1-phosphate in oxidative stress evoked by
1-methyl-4-phenylpyridinium (MPP+) in human dopaminergic
neuronal cells. Mol Neurobiol. 50:38–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang Y, Yu Q, Lai TB, Yang Y, Li G and
Sun SG: Effects of small interfering RNA targeting sphingosine
kinase-1 gene on the animal model of Alzheimer's disease. J
Huazhong Univ Sci Technolog Med Sci. 33:427–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yang Y, Wang M, Lv B, Ma R, Hu J, Dun Y,
Sun S and Li G: Sphingosine kinase-1 protects differentiated N2a
cells against beta-amyloid25-35-induced neurotoxicity via the
mitochondrial pathway. Neurochem Res. 39:932–940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yi H, Lee SJ, Lee J, Myung CS, Park WK,
Lim HJ, Lee GH, Kong JY and Cho H: Sphingosylphosphorylcholine
attenuated β-amyloid production by reducing BACE1 expression and
catalysis in PC12 cells. Neurochem Res. 36:2083–2090. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Takasugi N, Sasaki T, Suzuki K, Osawa S,
Isshiki H, Hori Y, Shimada N, Higo T, Yokoshima S, Fukuyama T, et
al: BACE1 activity is modulated by cell-associated
sphingosine-1-phosphate. J Neurosci. 31:6850–6857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Canlas J, Holt P, Carroll A, Rix S, Ryan
P, Davies L, Matusica D, Pitson SM, Jessup CF, Gibbins IL, et al:
Sphingosine kinase 2-deficiency mediated changes in spinal pain
processing. Front Mol Neurosci. 8:292015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hunter SF, Bowen JD and Reder AT: The
direct effects of fingolimod in the central nervous system:
Implications for relapsing multiple sclerosis. CNS Drugs.
30:135–147. 2016. View Article : Google Scholar :
|
|
126
|
Aurelio L, Scullino CV, Pitman MR, Sexton
A, Oliver V, Davies L, Rebello RJ, Furic L, Creek DJ, Pitson SM, et
al: From sphingosine kinase to dihydroceramide desaturase: A
structure-activity relationship (SAR) study of the enzyme
inhibitory and anticancer activity of
4-((4-(4-chlorophenyl)thiazol-2-yl)amino) phenol (SKI-II). J Med
Chem. 59:965–984. 2016. View Article : Google Scholar : PubMed/NCBI
|