Introduction
Mesangial cells (MCs) are essential in physiological
functions, including the regulation of intraglomerular capillary
flow, phagocytosis and removal of foreign bodies, secretion of
cytokines, and generation of extracellular matrix (ECM) (1). However, these cells are vulnerable
to the effect of various stimuli, which can give rise to kidney
damage by releasing inflammatory factors and ECM components,
ultimately leading to glomerular sclerosis and interstitial
fibrosis. Patients suffering the effects of these diseases
experience the development of end-stage renal disease after several
years owing to renal fibrosis (2,3).
Therefore, the inhibition of MC proliferation has become an
important therapy in treating glomerular proliferative diseases.
MRL/lpr lupus nephritis (LN) in mice is characterized by the
activation of MCs in the kidney leading to the uncontrolled release
of inflammatory mediators. The renal pathology of LN in mice
includes the proliferation of MCs and thickening of the mesangial
matrix at the age of ~24 weeks (4). Therefore, MRL/lpr LN mice are well
recognized as a representative pathological model of human MC
proliferative nephropathy (5,6).
Apoptosis, a specialized form of cellular suicide,
is important in a broad range of physiological functions, including
cell growth, morphogenesis, tissue homeostasis and immunity.
Members of the B-cell lymphoma-2 (Bcl-2) family of proteins are
essential in regulating apoptosis (7-9).
The effects of Bcl-2, Bcl-extra large (xL) and Bcl-2-associated X
protein (Bax) proteins on the regulation of MC apoptosis in
vivo and in vitro have been investigated previously. It
has been suggested that expression levels of Bcl-2 and Bcl-xL in
MCs are positively correlated with the downregulation of apoptosis,
whereas the overexpression of Bax protein facilitates a process of
apoptosis (10-12). This suggests that the regulation
of intrinsic targets, including the Bcl-2 family of proteins, is a
potential strategy for the development of MC anti-proliferation
agents.
An intracellular signaling pathway implicated in the
regulation of apoptosis is the protein kinase B (AKT) cascade
(13,14). AKT is mainly activated by growth
factors, and is associated with cell proliferation, differentiation
and cell death. Gong et al (15) reported that aplysin inhibits cell
proliferation and induces apoptosis in vitro, possibly
through suppressing the phosphatidylinositol 3-kinase (PI3K)/AKT
pathway. In vivo, the tissues of AKT1-knockout mice with
increased apoptosis was smaller, compared with the tissues of
wild-type littermates (16). In
LN mice, MC proliferation has been shown to be a critical event, in
which the phosphorylation of AKT becomes overexpressed (17). These data suggest that the
abnormal proliferation of MCs may be associated with the inhibition
of apoptosis induced by the expression of overactive AKT.
Trifluoperazine (TFP) is a calmodulin inhibitor, and
a classic anxiolytic and antipsychotic drug. Previous
investigations have demonstrated that TFP can arrest cell cycle,
inhibit cell proliferation and induce apoptosis. Previous studies
have reported that TFP suppressed the proliferation of fibrosarcoma
HT1080, leukemia, breast cancer and human A549 lung adenocarcinoma
cells by regulating different signaling pathways (18-20). Yeh et al (21) reported that TFP inhibited cancer
stem cell proliferation by suppressing the apoptotic pathway.
However, the role and mechanism of TFP in MC remain to be fully
elucidated.
The present study aimed to investigate the effects
of TFP on the progression of cell proliferation and cell apoptosis
in an MC line in vivo and in vitro.
Materials and methods
Animals
A total of 10 six-week-old female C57BL/6 mice
weighing ~20±5 g were purchased from the Experimental Animal Center
of Shanxi Medical University (Taiyuan, China); 20 female MRL/lpr LN
mice (~22±5 g, 8-weeks-old) were purchased from the Model Animal
Institute of Nanjing University (induced from Jackson Laboratory,
Ben Harbor, ME, USA). The mice were housed under controlled
pathogen-free environmental conditions (temperature 22°C, 12 h
light-dark cycle). Animals were given free access to water and fed
a standard laboratory diet. The experiments were performed
according with protocols approved by the Institutional Animal Care
and Use Committee of Shanxi Medical University.
Treatment protocols
The mice were randomly divided into three groups
(n=6/group): Control group, LN group, and LN+TFP group. The animals
in the LN+TFP group were intraperitoneally injected with 20
mg/kg·day TFP (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
at 12 weeks of age. The mice in the LN group received the same
injection ratio, but of saline. After 12 weeks, the animals were
sacrificed. Peripheral blood and kidney samples were obtained, and
the renal samples were embedded in paraffin for histopathological
and immunohistochemical analysis. Additional renal tissue was
acquired and immediately frozen in liquid nitrogen until
analysis.
Immunohistochemistry
The renal samples were fixed in 10% formalin at room
temperature for >48 h and embedded in paraffin. Serial
5-μm sections were deparaffinized in xylene and rehydrated
using sequential passage with 100, 95, 80 and 70% ethanol for 10
min each followed by three washes with distilled water. Unspecific
staining was blocked with 1.5% standard goat serum (ab138478;
Abcam, Cambridge, UK) for 15 min at room temperature and then
incubated at 4°C overnight with 1:100 diluted primary Ki67
antibodies (sc-23900; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Sections maintained in PBS were used as a negative control.
The tissue was incubated with a pre-diluted horseradish
peroxidase-conjugated secondary antibody (PV-9000; ZSGB-BIO
Technology, Co., Ltd., Beijing, China) for 1 h at room temperature.
The immunohistochemical analysis was performed in a blinded manner
by two independent investigators. Mice glomeruli (10 per mouse)
were examined in a blinded manner at each time point using
high-power light microscopy (magnification, ×400). ImageJ software
6.0 (National Institutes of Health, Bethesda, MD, USA) was used to
quantitatively count the number of Ki67-positive cells and total
glomerular cells. Ki67 relative density was calculated as the ratio
of Ki67-positive cells to total glomerular cells.
H&E staining
The formalin-fixed tissue was embedded in paraffin,
and sections of 5-μm thickness were cut, deparaffinized in
xylene and rehydrated in a descending alcohol series. The sections
were conducted via sequential passage. Sections were stained with
hematoxylin for 5 min at room temperature, differentiated in 1%
acid alcohol for 30 sec, blued in 0.2% ammonia water for 30 sec and
subsequently stained with eosin for 15 sec at room temperature.
Mice glomeruli (10 per mouse) were obtained in a blinded manner at
each time point using high-power light microscopy (magnification,
×400). ImageJ software 6.0 (National Institutes of Health) was used
to count the number of mesangial cells.
Cell culture and treatments
The T-SV40 (22)
human mesangial cell line was donated by Dr Xuewang Li of Peking
Union Medical College Hospital (Beijing, China). The cells were
cultured in RMPI-1640 medium (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) containing 10% fetal bovine serum (FBS; GE
Healthcare Life Sciences) at 37°C with 5% CO2. The cells
at ~70% confluence were planted in serum-free medium for 24 h, and
treated with 10 and 20% FBS as control and model groups,
respectively. Different concentrations of TFP (0, 5, 10, 20 and 30
μM) were added to the corresponding groups. Another grouping
method in the present study comprised normal cells, which were
treated with 20 ng/ml platelet-derived growth factor (PDGF;
PeproTech, Inc. Rocky Hill, NJ, USA), and cells treated with PDGF
and 20 μM TFP (PDGF+TFP) to detect the AKT signaling
pathway.
Cell counting kit-8 (CCK-8) assay and
cell counting
The MCs were grown at a density of 5×103
cells/well in 96-well plates and cell viability was assessed using
a CCK-8 assay (Beyotime Institute of Biotechnology, Jiangsu, China)
in accordance with the manufacturer's protocols. The cells were
grown in 96-well plates at a density of 106 cells/well
and treated with 10 or 20% FBS and different concentrations of TFP
(0, 5, 10, 20 and 30 μM) for 24 h. Following the relative
treatment, 10 μl CCK-8 solution was added to each well, and
the cells in the plate were incubated at 37°C for 2 h The
absorbance was measured at a test wavelength of 450 nm and a
reference wavelength of 650 nm on an automated reader (Bio-Rad
Model 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Cell
survival was calculated according the optical density (OD) as a
percentage using the following formula: Cell survival (%) = (mean
OD of treated cells/mean OD of control cells) ×100. The results
were calculated as a percentage of the untreated control cells. For
cell counting, the MCs were seeded into 24-well plates at a density
of 1×105 cells per well. Following treatment, the cells
were harvested and counted. The living cell population was
estimated using a trypan blue dye exclusion test; cells were
counted under a low-power light microscope (magnification,
×40).
Flow cytometric analysis of
apoptosis
The effects of TFP on cell apoptosis were analyzed
using flow cytometry. The cells were treated with 10 or 20% FBS and
TFP (0, 5, 10 and 20 μM) for 24 h. The cells were then
centrifuged at 447.2 × g and 4°C for 10 min and washed twice with
PBS. The supernatant was then discarded and the cells were
processed with an Annexin V/PI staining apoptosis detection kit
(Aria II; BD Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocols. The cells were separated into three
groups: Viable, early apoptotic, and late apoptotic or dead. Viable
cells exhibit only weak Annexin V staining of the cellular
membrane, whereas early apoptotic cells exhibit a significantly
higher degree of surface labeling. Late apoptotic or dead cells
exhibit membrane staining by Annexin V and marked nuclear staining
by propidium iodide. Following staining, cell apoptosis was
analyzed by the number of early apoptotic cells using a flow
cytometer (Beckman Coulter, Inc., Palo Alto, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the MCs or mouse
tissues independently using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
The spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
measure the concentration of RNA in each sample. Total RNA (2
μg) was reverse transcribed to cDNA using a reverse
transcription kit (Takara Bio, Inc., Otsu, Japan). The qPCR
analysis was performed using SYBR Premix Ex Taq (2X) (RR820A;
Takara Bio, Inc.). A total of 2 μl cDNA (<100 ng), 10
μl buffer, 0.3 μl forward primer, 0.3 μl
reverse primer and double-distilled water (ddH2O) ≤20
μl. The qPCR thermal cycling protocol was programmed in the
CFX96™ Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.)
and consisted of an initial denaturation step at 95°C for 30 sec,
followed by 40 cycles of denaturation for 5 sec at 95°C, and
annealing and extension for 30 sec at 56°C. The β-actin (mouse
tissue) or GAPDH (MC cell) gene was used as the internal control.
The primers used for mouse tissues were as follows: Bcl-2 forward,
5′-CTTCAGGGATGGGGTGAACT-3′ and reverse, 5′-CAGCCTCCGTTATCCTGGAT-3′;
Bax forward, 5′-TCATGAAGACAGGGGCCTTT-3′ and reverse,
5′-GTCCACGTCAGCAATCATCC-3′; β-actin forward,
5′-CCTCTATGCCAACACAGTGC-3′ and reverse, 5′-CCTGCTTGCTGATCCACATC-3′.
The primes for MCs were as follows: Bax forward,
5′-AAGCTGAGCGAGTGTCTCAAG-3′ and reverse,
5′-CAAAGTAGAAAGGGCGACAAC-3′; Bcl-2 forward,
5′-TGGGAGAACAGGGTACGATAAC-3′ and reverse,
5′-GAACTCAAAGAAGGCCACAATC-3′; GAPDH forward,
5′-GGGAAACTGTGGCGTGAT-3′ and reverse, 5′-GAGTGGGTGTCGCTGTTGA-3′.
The mRNA levels were normalized to internal control and assessed
using the 2−∆∆Cq method (23).
Western blot analysis
The MCs or kidney tissue were lysed in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and centrifuged at 14,000 × g for 10 min at 4°C. The
protein was collected and concentration was determined using a BCA
assay kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
Samples containing 60 μg of protein were resolved via 12%
SDS-PAGE and transferred onto nitrocellulose membranes (Whatman
International, Ltd., Maidstone, UK). Following blocking with 5%
skimmed milk powder in Tris-buffered saline (50 mmol/l Tris-base
and NaCl) and 0.1% Tween-20 at room temperature for 70 min, the
membranes were incubated with the following primary antibodies
overnight at 4°C: Bcl-2 (cat. no. SC-492), Bax (cat. no. SC-526),
phosphorylated (p-)AKT/Thr308 (p-AKT) (cat. no. SC-16646-R), AKT
(cat. no. SC-8312) and β-actin (sc-4967S) antibodies (dilution
1:200) from Santa Cruz Biotechnology, Inc. Following being washed
three times for 15 min with TBST buffer, the membranes were
incubated with a corresponding horseradish peroxidase-conjugated
secondary antibody (1:5,000, ZB-5301; ZSGB-BIO Technology, Co.,
Ltd.) for 1 h at room temperature and analyzed using the Quantity
One analysis system, version 4.62 version (Bio-Rad Laboratories,
Inc.)
Measurement of renal and liver
function
Blood urea nitrogen (BUN) and serum creatinine (Cr),
two key renal function markers, were determined with commercially
available kits according to the manufacturer's protocols (cat. nos.
KA0201 and KA0314, respectively; Shanghai Yuanye Science and
Technology Co., Ltd., Shanghai, China). The observation absorbance
was read at 450 nm. The same ELISA procedure was used for aspartate
aminotransferase (AST), a key liver function marker (cat. no.
KA0565; Shanghai Yuanye Science and Technology Co., Ltd.) according
to the manufacturer's protocols.
Statistics analysis
The statistical analyses were performed with SPSS
19.0 (IBM SPSS, Armonk, NY, USA). The values are expressed as the
mean ± standard deviation. Data analyses were performed using
one-way analysis of variance tests followed by two-tailed t-test
post-hoc comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
TFP reduces MC proliferation in
vitro
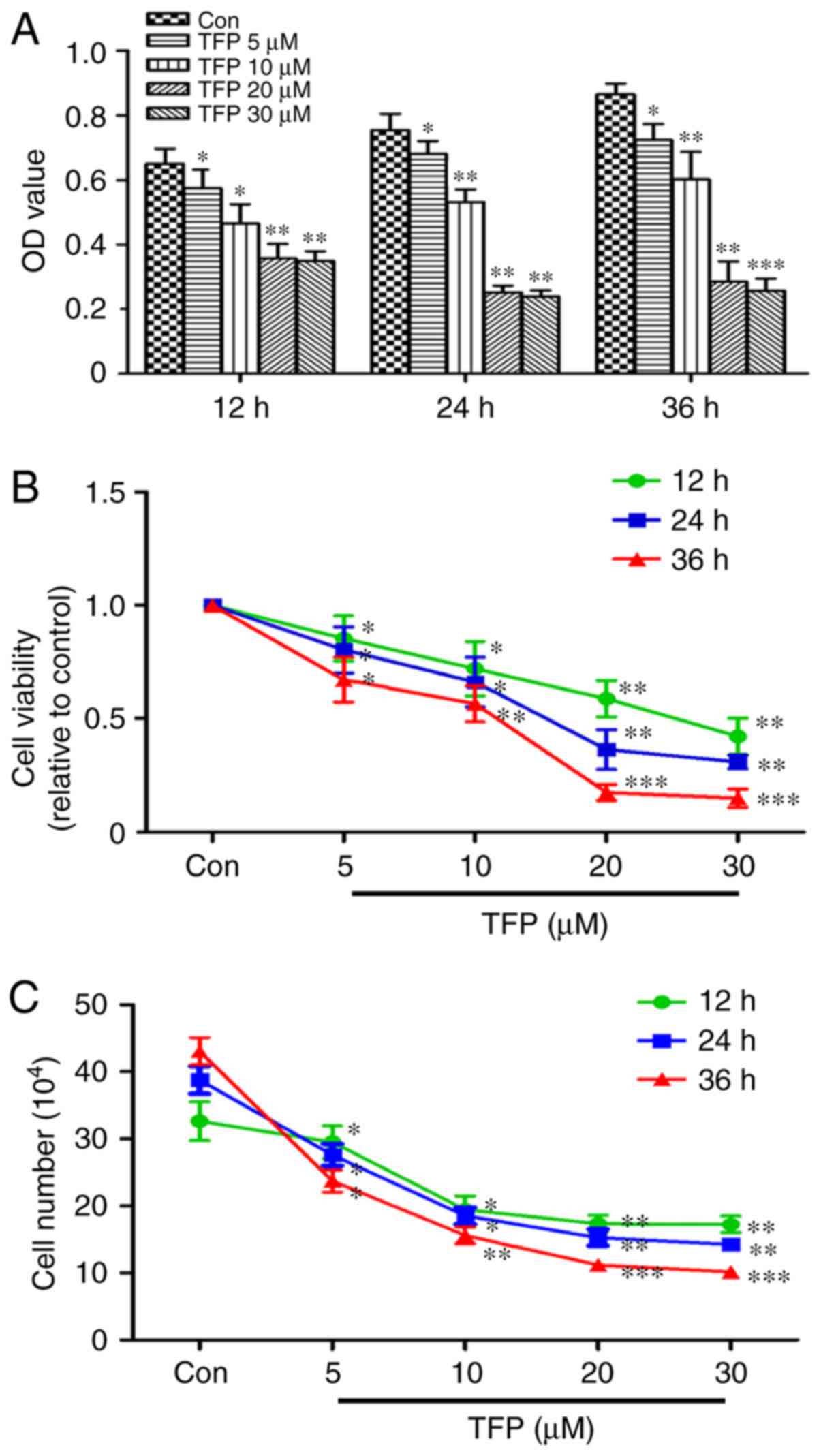
The CCK-8 assay showed time- and dose-dependent
inhibition of MC proliferation elicited by TFP. With an increased
concentration of TFP and duration of treatment, the percentage of
viable cells was significantly decreased, particularly following
treatment with 20 μM TFP (Fig.
1A and B). The results of the CCK-8 assay were reinforced using
the cell counting assay. Proliferation of the cells in response to
TFP was significantly inhibited in a time- and dose-dependent
manner in the MCs (Fig. 1C).
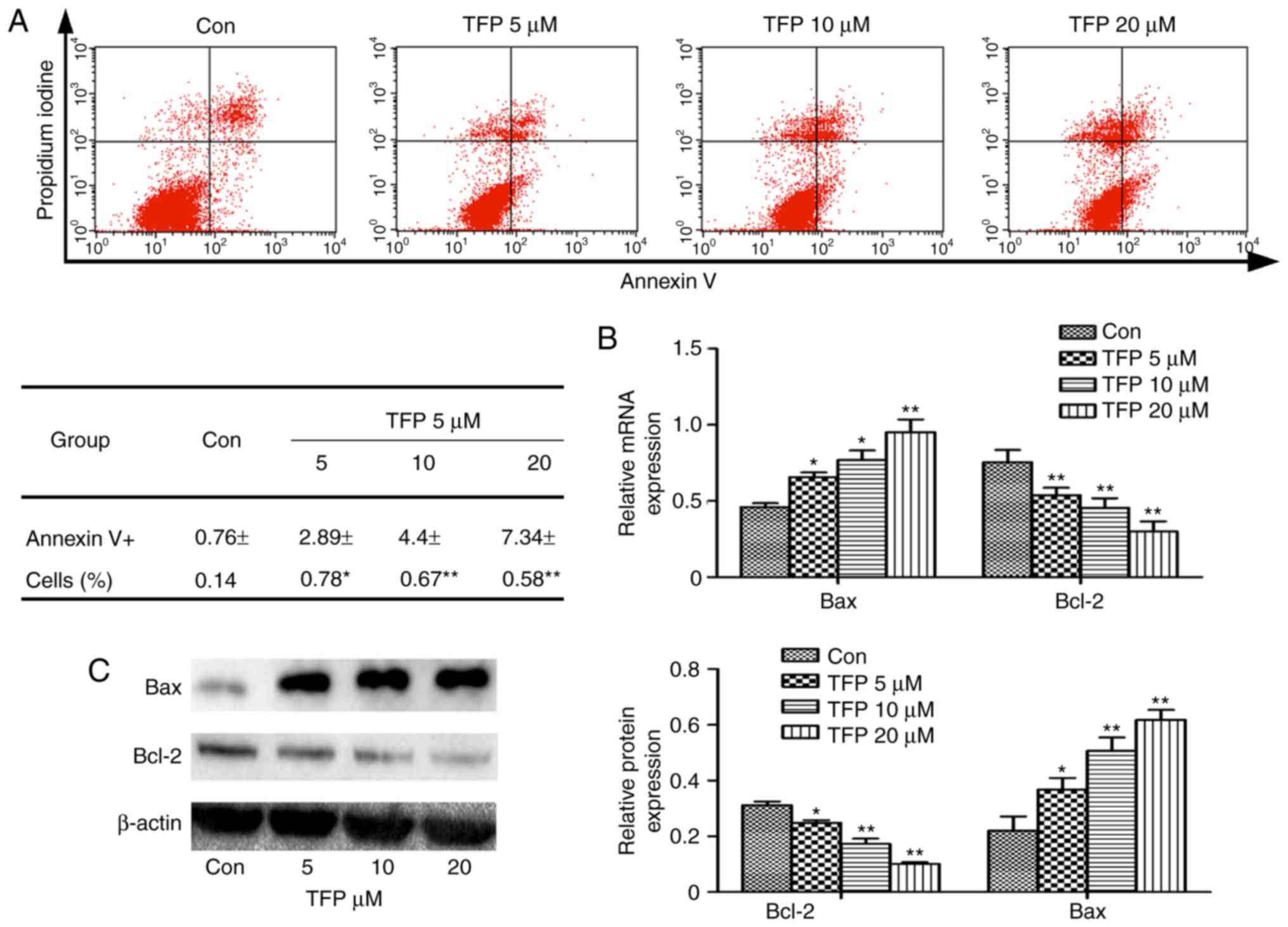
TFP promotes MC apoptosis in vitro
To examine whether TFP treatments induced apoptosis,
the MCs were treated with various concentrations of TFP for 24 h.
The results of flow cytometry showed that TFP treatments induced a
significant increase in of Annexin V-positive cell populations in a
dose-dependent manner (Fig. 2A).
In the experiment, Bcl-2 and Bax were the two important
apoptosis-related proteins regulating cellular apoptosis. It was
observed that TFP (5, 10 and 20 μM) treatment decreased the
level of Bcl-2 and increased the expression of Bax in a
dose-dependent manner. This was observed at the gene and protein
levels (Fig. 2B and C).
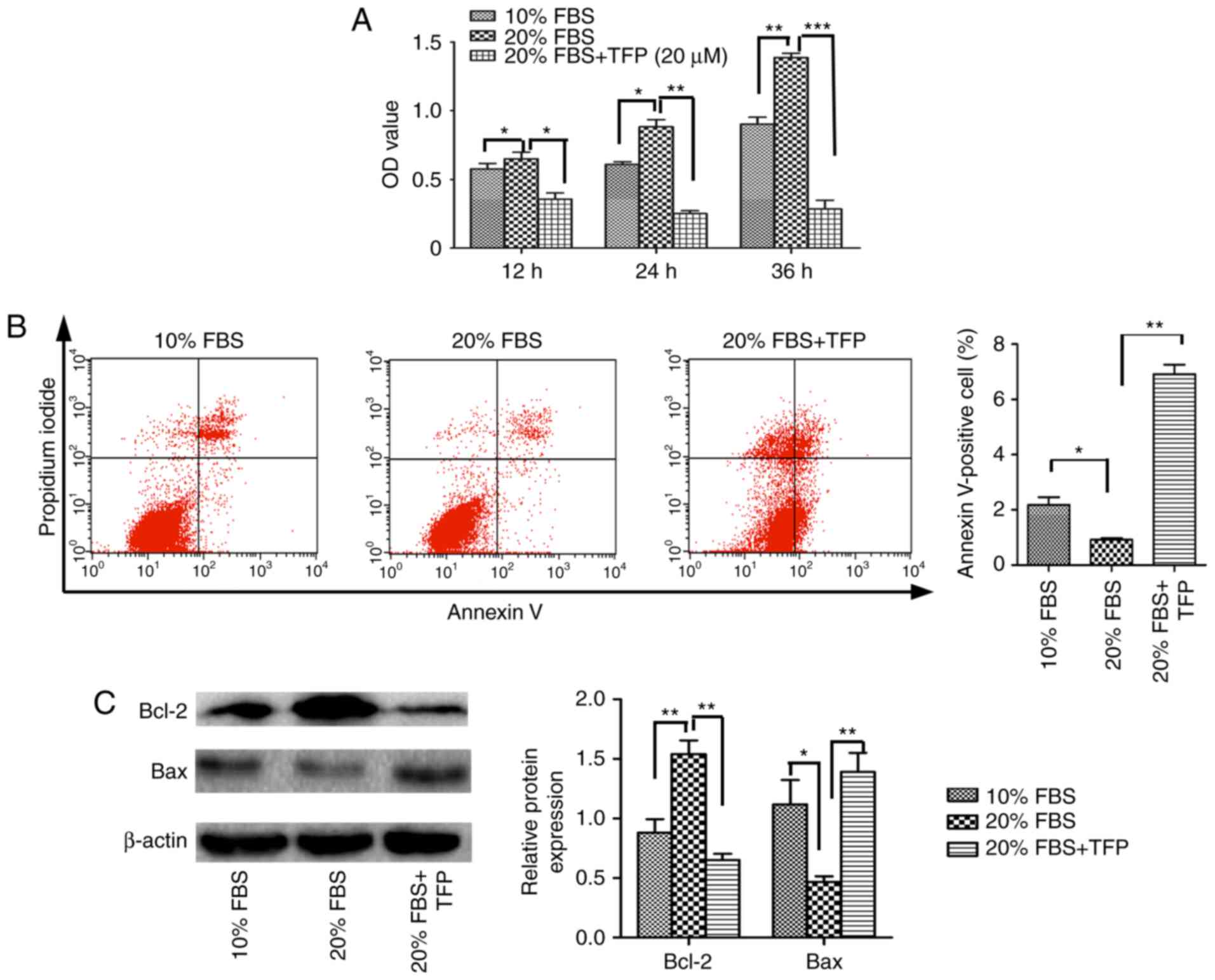
TFP suppresses 20% FBS-stimulated MC
proliferation
The present study demonstrated that TFP inhibited
the proliferation of normal MCs by promoting cellular apoptosis.
Subsequently, MC proliferation was stimulated with exogenous
factors (20% FBS), and whether TFP inhibited this effect was
examined. The results revealed that, following treatment with 20%
FBS, the proliferation rate of the cultured MCs was significantly
increased, compared with that of the 10% FBS-treated MCs
(P<0.05). Following TFP treatment, the proliferation rate of MCs
cultured with 20% FBS was significantly lower, compared with that
in the 20% FBS control group (P<0.05; Fig. 3A). Compared with the 20% FBS
group, the apoptotic rates were lower in the normal (10% FBS) group
and increased following TFP treatment (Fig. 3B). Compared with the 20% FBS
control group, the protein levels of Bcl-2 were significantly
decreased in the 20% FBS-cultured MC following TFP treatment
(P<0.05), whereas the expression of Bax was higher in the
TFP-treated cells, compared with that in the control group
(Fig. 3C).
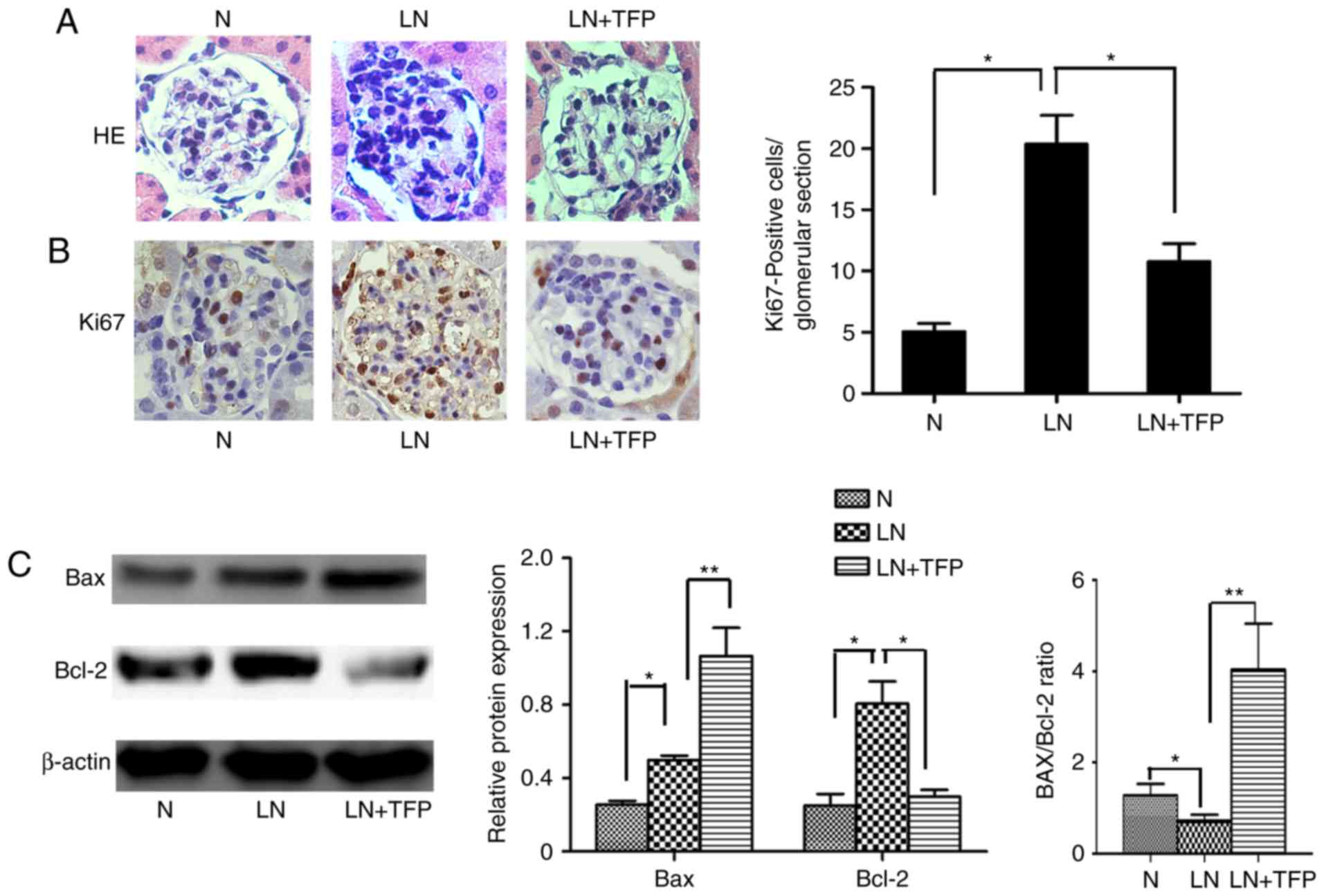
TFP promotes MC apoptosis in vivo
In order to further examine whether TFP inhibits the
proliferation of MCs by inducing MC apoptosis in vivo, the
present study measured cell proliferation rates, and levels of
apoptosis-related factors Bcl-2 and Bax in the kidney tissues of
mice. H&E staining indicated diffuse proliferation of MCs and
the glomerular matrices of LN mice, compared with the control,
which decreased following treatment with TFP for 3 months (Fig. 4A). The results of Ki-67
immunohistochemical analysis confirmed these findings (Fig. 4B). The apoptosis-related proteins
Bcl-2 and Bax in the kidney tissue were also detected using western
blot analysis. Compared with those in the normal group, the
expression levels of anti-apoptotic Bcl-2 and pro-apoptotic Bax
were increased. However, the Bax/Bcl-2 ratio was decreased in the
LN group. Compared with the LN group, the expression of Bcl-2 was
decreased whereas the expression of Bax was increased. However, the
Bax/Bcl-2 ratio was increased in the LN+TFP group (Fig. 4C).
Effect of TFP on renal function in LN
mice
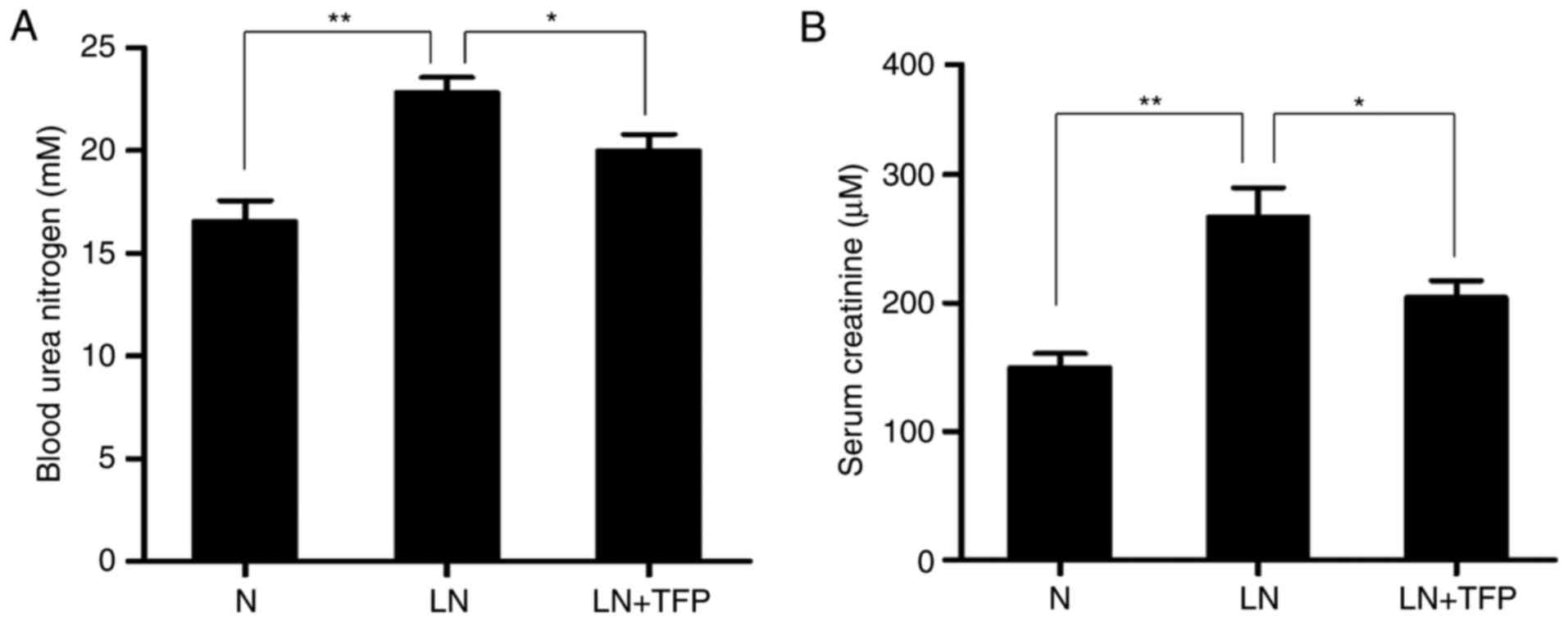
The effect of TFP on renal function in LN mice was
investigated. The level of BUN was significantly higher in the LN
group (22.84±0.56 mM), compared with that in the normal group
(16.57±3.46 mM). However, the level of BUN was significantly
decreased in the TFP-treated LN mice (19.99±0.92 mM) (Fig. 5A). The serum Cr levels exhibited
the same trend, with levels of 143.54±23.35, 237.19±13.70 and
210.56±36.26 μM in the normal, LN and LN+TNF groups,
respectively (Fig. 5B). These
data confirmed that TFP improved the renal function in LN mice.
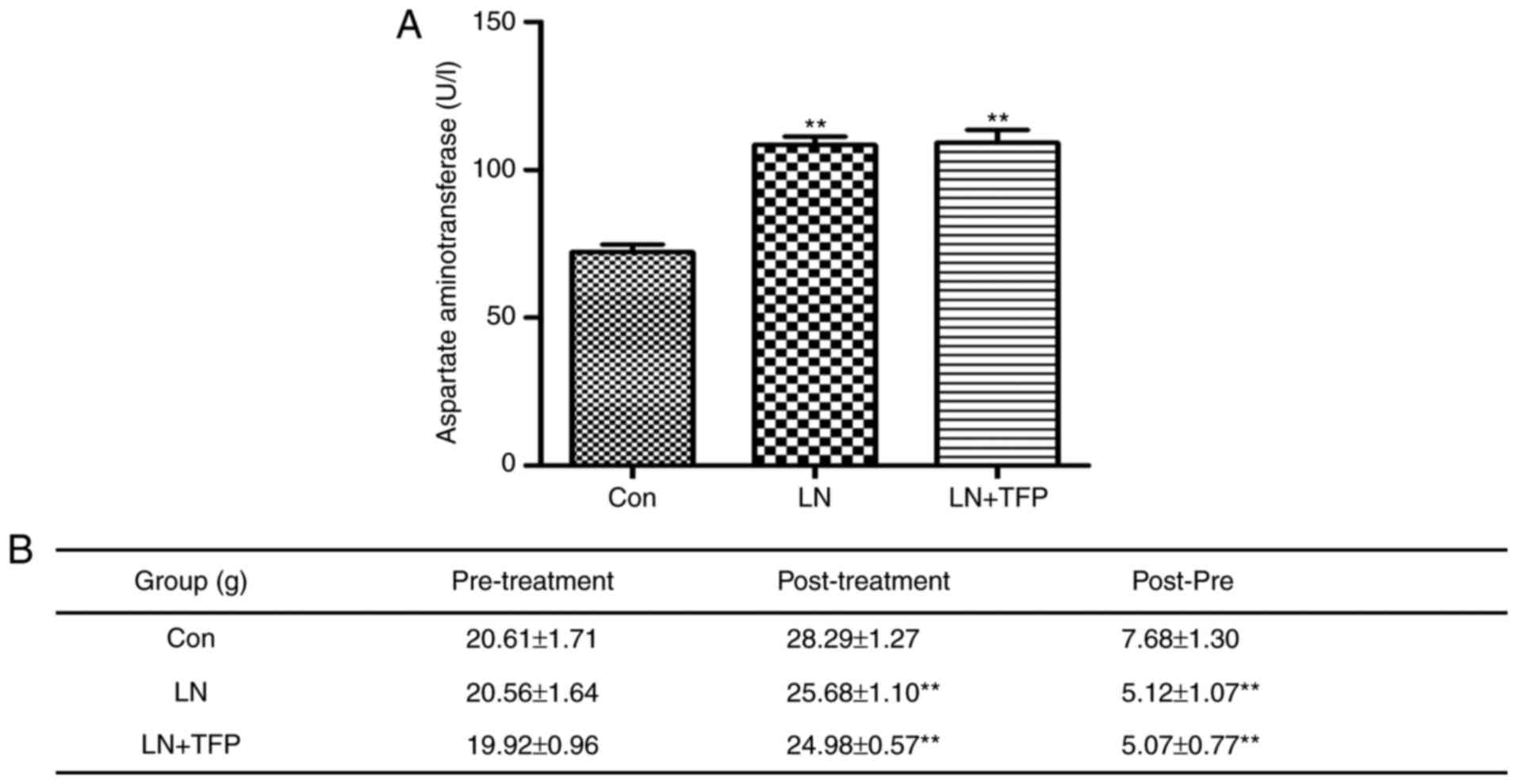
TFP toxic effects of TFP in LN mice
The present study also performed drug toxicity
experiments with TFP in vivo. The level of AST was
significantly higher in the LN group (108.40±7.17 U/l) and LN+TFP
(110.20±10.51 U/l), compared with that in the normal group
(72.18±6.13 U/l). However, no significant differences were found
between the LN group and TFP-treated LN group (Fig. 6A). No significant differences in
body weight change (post-pre) were observed between the mice in the
LN group (5.12±1.07 g) and those in the TFP-treated LN group
(5.07±0.77 g) (Fig. 6B). These
results indicated that the intraperitoneal administration of TFP
did not produce marked toxic effects in LN mice.
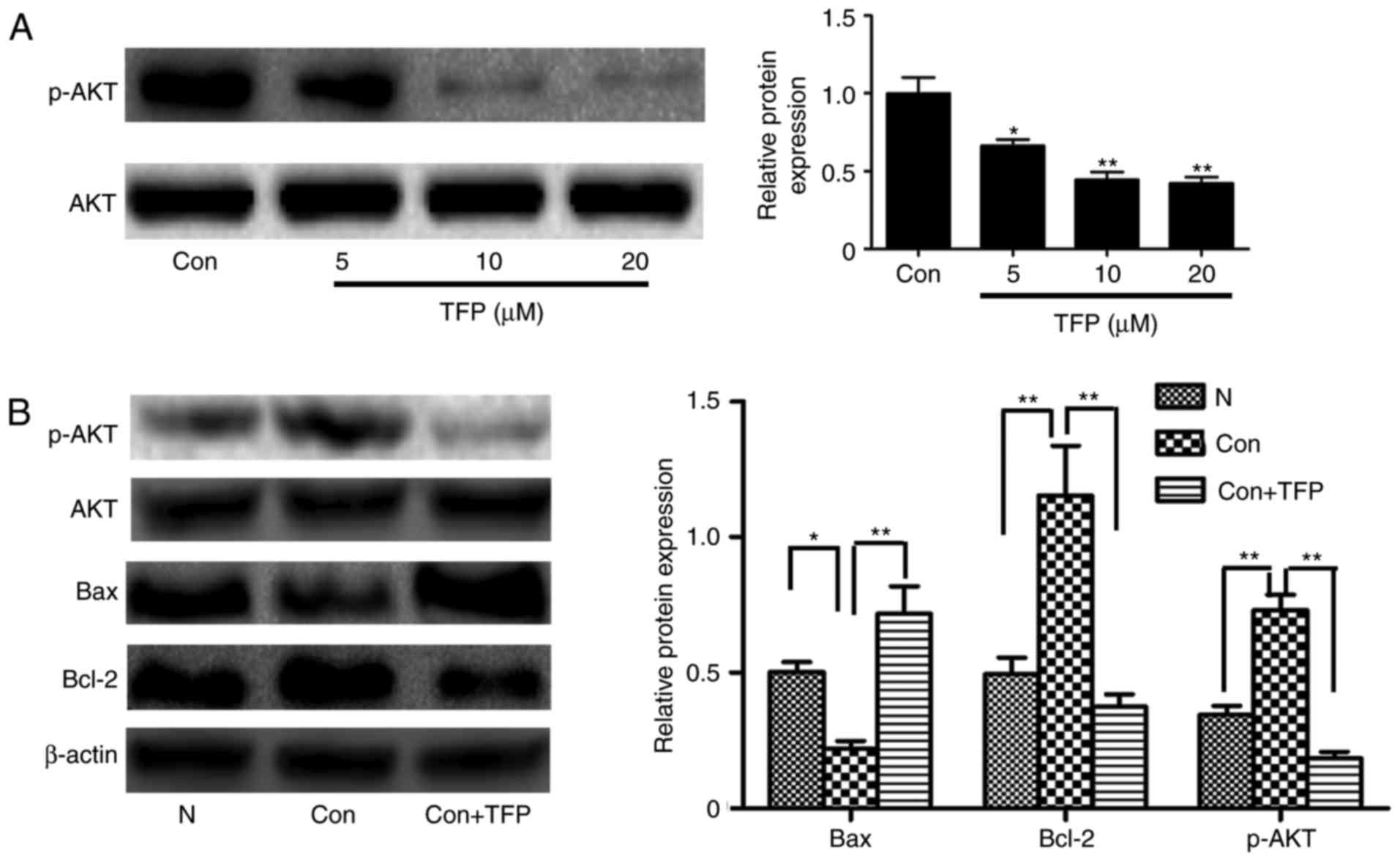
Reduced protein expression of AKT in MCs
by TFP
The results of the western blot analysis showed that
TFP significantly and dose-dependently decreased the level of p-AKT
in MCs, compared with that in the control group (Fig. 7A). To further examine whether TFP
inhibited the AKT pathways associated with apoptosis, PDGF was used
to stimulate MCs. PDGF induced the phosphorylation of AKT,
decreased the levels of Bcl-2 and increased the expression of Bax.
However, following treatment with TFP, the opposite trend was
observed (Fig. 7B). These data
demonstrated that TFP inhibited the AKT pathways and this may be
involved in the TFP-induced apoptosis of MCs.
Discussion
The present study examined whether TFP had a
potential effect on the activity of MCs. It was found that TFP
applied in vitro and in vivo significantly inhibited
the proliferation of MCs, induced apoptosis and improved renal
function in MRL/lpr LN mice without exhibiting any major adverse
effects. These analyses showed that TFP inhibited the expression of
p-AKT, indicating that TFP may inhibit MCs through promoting
apoptosis, at least in part via the AKT signaling pathway.
The proliferation of MCs induced by various factors
is detrimental as it accelerates kidney damage and promotes
glomerulosclerosis. Therefore, the inhibition of MC proliferation
has emerged as an important treatment option for proliferative
glomerular diseases. Several previous studies (18-20) have shown that TFP can inhibit the
proliferation of cancer cells. In the present study, it was found
that TFP also inhibited the MC proliferation in vitro, as
shown in Fig. 1.
Apoptosis is a genetically programmed cell death and
has a key function in the repair process evoked by proliferative
diseases. In the repair process during inflammation caused by
proliferative renal disease, apoptosis avoids the excessive
proliferation of MCs (24). Bcl-2
and Bax proteins are the two principal members of the Bcl-2
multi-gene family. Bcl-2 prevents apoptosis, whereas Bax produces a
pro-apoptotic effect (25). The
present study revealed that TFP treatment resulted in notable rises
in Annexin V-positive cell populations and augmented the Bax/Bcl-2
ratio in a dose-dependent manner, as shown in Fig. 2. These results are consistent with
those reported for TFP in other diseases and tissue types (19,21,26). The present study showed that TFP
upregulated the ratio of Bax/Bcl-2 to promote MC apoptosis in
vitro.
The present study also confirmed that TFP inhibited
normal MC proliferation by promoting the apoptosis pathways
described above. A higher concentration of serum (20% FBS) was used
to stimulate MCs and cause them to proliferate abnormally, prior to
treating the MCs with TFP to antagonize the effect. The results
showed that high concentrations of serum accelerated cell
proliferation and inhibited cell apoptosis. However, the use of TFP
together with a high concentration of serum in the cell resulted in
the cell proliferation rate being <20% of that in the serum
culture group with the additional promotion of cell apoptosis, as
shown in Fig. 3. Therefore, TFP
antagonized the abnormal proliferation of MCs induced by exogenous
factors in vitro.
For the in vivo experiment, an MRL/lpr LN
mouse model was used to examine the effect of TFP on MCs. As shown
in Fig. 4A and B, the data showed
that TFP inhibited the abnormal proliferation of MCs in the LN
mice. It was observed that the protein expression levels of Bax and
Bcl-2 were higher in the LN mice, compared with those in the normal
mice. This result was consistent with a study by Cui et al
(27). The present study aimed to
further understand the underlying factors, and it was found that
the ratio of Bax/Bcl-2 was lower in LN mice, compared with that in
normal group mice. This suggested that Bcl-2 was more important in
inhibiting the apoptosis of MCs in LN, compared with Bax. Following
TFP treatment, the ratio of Bax/Bcl-2 was upregulated, which
promoted apoptosis. The findings in vivo were consistent
with those in vitro. These results revealed that TFP
upregulated the ratio of Bax/Bcl-2 to promote MC apoptosis.
The activation of MCs can lead to damage to renal
function, therefore, the present study investigated whether TFP can
alleviate renal pathological injury and improve renal function
following inhibiting MCs. The, two blood measurements of BUN and
serum Cr are important indicators of renal function as they are
easily measured. An increase in these indicators in the blood
predicts significant damage to functioning nephrons. In the present
study, the levels of these indicators indicated severe injury in
the LN mice group; a salient decrease was observed following TFP
treatment (20 mg/kg·day for 12 weeks), as shown in Fig. 5. These results indicated that TFP
slowed the progression of LN in the model mice and was accounted
for by the inhibited proliferation of MCs. Of note, no significant
changes in behavior, mental state, diet or sleep were observed
following intraperitoneal administration of TFP in the LN mice.
Compared with the control group, no significant differences in
liver function or change in body weight were found between the LN
group and TFP-treated LN group, as shown in Fig. 6.
AKT is a serine/threonine kinase, which is important
in the regulation of cell proliferation, survival/apoptosis,
angiogenesis and protein synthesis (28,29). In previous years, several reports
have consistently observed that AKT is frequently activated among
several types of cancer, initiating a potent anti-apoptotic signal
(30). Shimamura et al
reported that the proliferation of MCs in vitro was
associated with the PI3K/AKT signaling pathway, which led to
proliferation by inhibiting apoptosis (31). Gong et al reported that
aplysin effectively inhibited the growth of a glioma and induced
apoptosis via suppressing the PI3K/AKT signaling pathway (15). Therefore, the effect of TFP on MC
apoptosis may also be associated with the PI3K/AKT signaling
pathway. The present study aimed to elucidate this interaction and
quantify the effect of TFP on the PI3K/AKT signaling pathway. The
results of the present study showed that TFP significantly and
dose-dependently decreased the levels of p-AKT, as shown in
Fig. 7A. To further establish
whether TFP can inhibit AKT pathways, PDGF was used to stimulate
MCs as a positive control. This induces the phosphorylation of AKT
and affects the AKT signaling pathway to decrease the level of
apoptosis (32). The present
study showed that, following treatment with TFP, the recorded trend
was opposite to that observed in the PDGF group without TFP; a
downregulation of p-AKT and corresponding increases to the
Bax/Bcl-2 ratio were observed, as shown in Fig. 7B. The findings revealed that TFP
upregulated the levels of apoptosis, at least in part via
suppressing AKT signaling pathways in MCs.
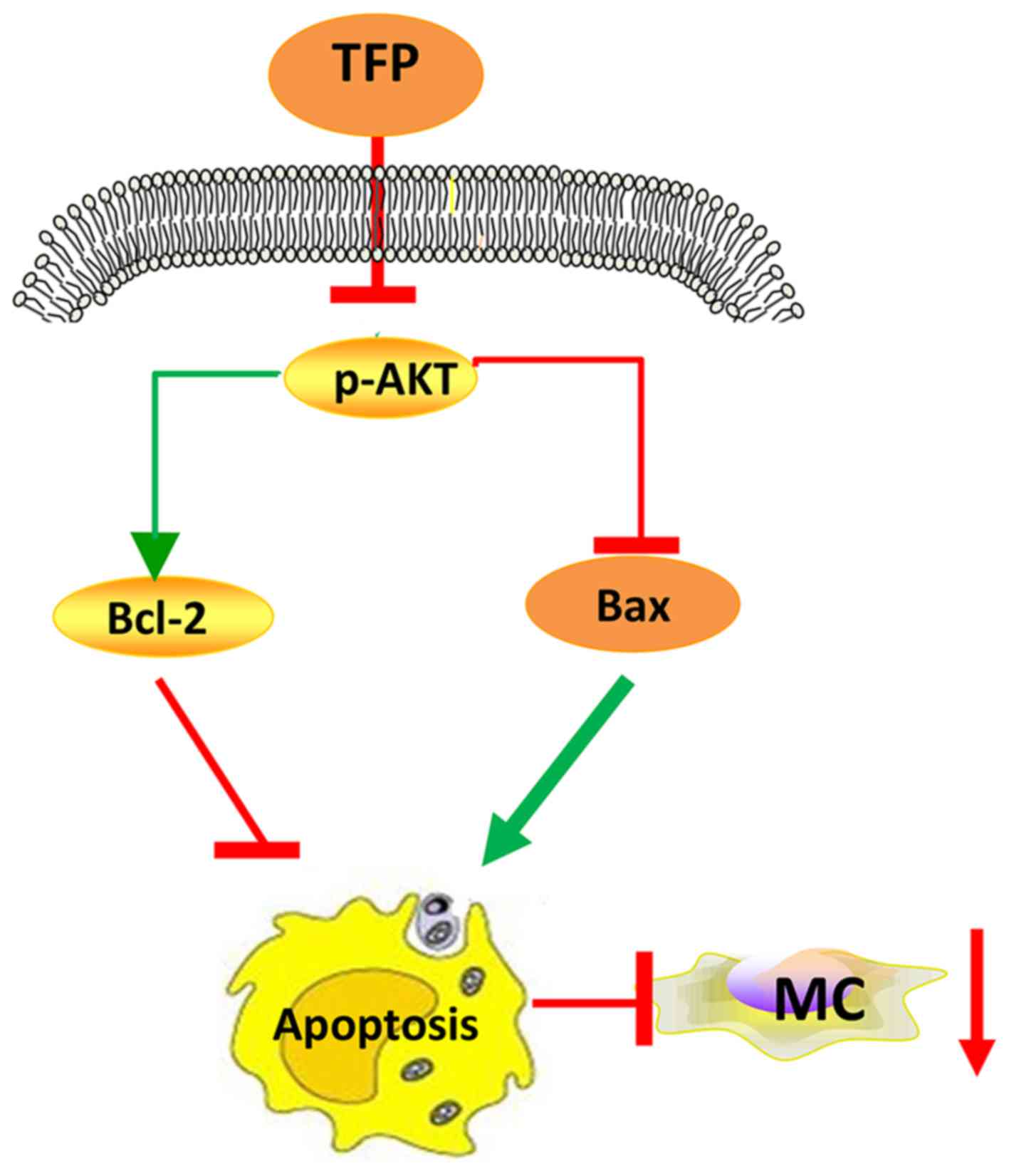
In conclusion, TFP inhibited the proliferation of
human MCs in vitro and in vivo via promoting
apoptosis (augmenting the Bax/Bcl-2 ratio), at least in part by
suppressing the AKT signaling pathway (Fig. 8). Further investigations on the
anti-proliferative efficiency of TFP and the underlying mechanisms
are required to broaden this understanding. In continuation of the
present study, future investigations aim to examine the effect of
TFP on cell cycle. The Food and Drug Administration approval
concerning TFP suggests that it is a safe drug to use in large
populations and that it has manageable or tolerable side effects
(33); therefore, these findings
may provide a novel strategy for the treatment of LN.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81570626 and 81450033). The
authors would like to thank Dr Xuewang Li of Peking Union Medical
College Hospital for providing the human mesangial cells.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Badr KF, Murray JJ, Breyer MD, Takahashi
K, Inagami T and Harris RC: Mesangial cell, glomerular and renal
vascular responses to endothelin in the rat kidney. Elucidation of
signal transduction pathways. J Clin Invest. 83:336–342. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wyatt RJ and Julian BA: IgA nephropathy. N
Engl J Med. 368:2402–2414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grassmann A, Gioberge S, Moeller S and
Brown G: ESRD patients in 2004: Global overview of patient numbers,
treatment modalities and associated trends. Nephrol Dial
Transplant. 20:2587–2593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumoto K, Yoshikai Y, Asano T, Himeno
K, Iwasaki A and Nomoto K: Defect in negative selection in lpr
donor-derived T cells differentiating in non-lpr host thymus. J Exp
Med. 173:127–136. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugiyama N, Nakashima H, Yoshimura T,
Sadanaga A, Shimizu S, Masutani K, Igawa T, Akahoshi M, Miyake K,
Takeda A, et al: Amelioration of human lupus-like phenotypes in
MRL/lpr mice by overexpression of interleukin 27 receptor alpha
(WSX-1). Ann Rheum Dis. 67:1461–1467. 2008. View Article : Google Scholar
|
|
6
|
Lamoureux JL, Watson LC, Cherrier M, Skog
P, Nemazee D and Feeney AJ: Reduced receptor editing in lupus-prone
MRL/lpr mice. J Exp Med. 204:2853–2864. 2011. View Article : Google Scholar
|
|
7
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar
|
|
10
|
Uda S, Yoshimura A, Sugenoya Y, Inui K,
Taira T and Ideura T: Mesangial proliferative nephritis in man is
associated with increased expression of the cell survival factor,
Bcl-2. Am J Nephrol. 18:291–295. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuan B, Ding Y, Zhang H and Yu Y: Effects
of Xueniaoting powder and S-9 on apoptosis and expression of bax
and bcl-2 in glomerular mesangial cells. Shenzhen J Integ Trad Chin
Western Med. 13:447–456. 2003.
|
|
12
|
Zhuan B, Ding Y and Wu L: Effects of
shenbining on apoptosis and experssion of bax and Bcl-2 in the
kidney of mesangial proliferative glomerulonephritis. Chin J Integ
Trad Western Nephrol. 4:316–318. 2003.
|
|
13
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao T, Furnari F and Newton AC: PHLPP: A
phosphatase that directly dephosphorylates Akt, promotes apoptosis,
and suppresses tumor growth. Mol Cell. 18:13–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong AJ, Gong LL, Yao WC, Ge N, Lu LX and
Liang H: Aplysin induces apoptosis in glioma cells through
HSP90/AKT pathway. Exp Biol Med (Maywood). 240:639–644. 2015.
View Article : Google Scholar
|
|
16
|
Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol
K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al: Growth
retardation and increased apoptosis in mice with homozygous
disruption of the Akt1 gene. Genes Dev. 15:2203–2208. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stylianou K, Petrakis I, Mavroeidi V,
Stratakis S, Vardaki E, Perakis K, Stratigis S, Passam A,
Papadogiorgaki E, Giannakakis K, et al: The PI3K/Akt/mTOR pathway
is activated in murine lupus nephritis and downregulated by
rapamycin. Nephrol Dialy Transplant. 26:498–508. 2011. View Article : Google Scholar
|
|
18
|
Gulino A, Barrera G, Vacca A, Farina A,
Ferretti C, Screpanti I, Dianzani MU and Frati L: Calmodulin
antagonism and growth-inhibiting activity of triphenylethylene
antiestrogens in MCF-7 human breast cancer cells. Cancer Res.
46:6274–6278. 1986.PubMed/NCBI
|
|
19
|
Chen QY, Wu LJ, Wu YQ, Lu GH, Jiang ZY,
Zhan JW, Jie Y and Zhou JY: Molecular mechanism of trifluoperazine
induces apoptosis in human A549 lung adenocarcinoma cell lines. Mol
Med Rep. 2:811–817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polischouk AG, Holgersson A, Zong D,
Stenerlöw B, Karlsson HL, Möller L, Viktorsson K and Lewensohn R:
The antipsychotic drug trifluoperazine inhibits DNA repair and
sensitizes non small cell lung carcinoma cells to DNA double-strand
break induced cell death. Mol Cancer Ther. 6:2303–2309. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN,
Yang SC, Ho CC, Chen CC, Kuo YL, Lee PY, et al: Trifluoperazine, an
antipsychotic agent, inhibits cancer stem cell growth and overcomes
drug resistance of lung cancer. Am J Respir Crit Care Med.
186:1180–1188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Delarue F, Virone A, Hagege J, Lacave R,
Peraldi MN, Adida C, Rondeau E, Feunteun J and Sraer JD: Stable
cell line of T-SV40 immortalized human glomerular visceral
epithelial cells. Kidney Int. 40:906–912. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Savill J, Mooney A and Hughes J: Apoptosis
and renal scarring. Kidney Int Suppl. 54:S14–S17. 1996.PubMed/NCBI
|
|
25
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong SH, Lee MY, Shin KS and Kang SJ:
Perphenazine and trifluoperazine induce mitochondria-mediated cell
death in SH-SY5Y cells. Animal Cells Syst. 16:20–26. 2012.
View Article : Google Scholar
|
|
27
|
Cui JH, Qiao Q, Guo Y, Zhang YQ, Cheng H,
He FR and Zhang J: Increased apoptosis and expression of FasL, Bax
and caspase-3 in human lupus nephritis class II and IV. J Nephrol.
25:255–261. 2012. View Article : Google Scholar
|
|
28
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal-regulating kinase 1. Mol Cell Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Majumder PK and Sellers WR: Akt-regulated
pathways in prostate cancer. Oncogene. 24:7465–7474. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimamura H, Terada Y, Okado T, Tanaka H,
Inoshita S and Sasaki S: The PI3-kinase-Akt pathway promotes
mesangial cell survival and inhibits apoptosis in vitro via
NF-kappa B and Bad. J Am Soc Nephrol. 14:1427–1434. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franke TF, Yang SI, Chan TO, Datta K,
Kazlauskas A, Morrison DK, Kaplan DR and Tsichlis PN: The protein
kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Howland RH: Trifluoperazine: A sprightly
old drug. J Psychosoc Nurs Ment Health Serv. 54:20–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|