Introduction
Myocardial infarction is a major form of ischemic
heart disease defined as imbalance ischemia and myocardial necrosis
(1,2). Even though prognosis has improved
substantially over the past decade, acute myocardial infarction
remains the most severe manifestation of coronary artery disease,
affecting more than 7 million individuals worldwide, accounting for
more than 4 million deaths in Europe and Northern Asia every year
(3,4). It has been well characterized that
oxidative stress and inflammation are the main pathophysiological
processes involved in myocardial infarction (5,6).
Evidence is accumulating that antioxidant therapy has a potential
to prevent ISO-induced myocardial injury (7–9)
and myocardial ischemia/reperfusion (I/R) injury (10,11). Accordingly, NOD-like receptor
superfamily, pyrin domain containing 3 (NLRP3) inflammasome is
implicated in cellular inflammation processes in response to
oxidative stress (12). This
inflammasome is protein complex containing NLRP3, ASC and
caspase-1. Once the NLRP3 inflammasome is activated, it stimulates
caspase-1 activation, which in turn promotes the processing and
secretion of pro-inflammatory cytokine interleukin-1β (IL-1β)
(13), which has been implicated
to play a role in I/R injury. Reactive oxygen species (ROS) have
been identified as an important NLRP3 inflammasome activator in
various diseases, such as hepatic (14), and renal I/R injury (15). Moreover, previous studies have
demonstrated that NLRP3 inflammasome was activated in myocardial
I/R injury in cardiac microvascular endothelial cells (16), cardiac fibroblasts (17), which indicates a role of NLRP3
inflammasome in the development of myocardial injury.
Rosuvastatin, a member of 3-hydroxy-3-methylglutaryl
coenzyme A reductase inhibitors, exerts various pharmacological
activities, such as anti-inflammatory (18,19), anti-oxidative (20,21), cardioprotective activities
(22,23). Previous studies have reported that
rosuvastatin inhibited neuronal cell apoptosis and improved
neurological deficit in transient middle cerebral artery occlusion
(tMCAO)/reperfusion injury (24),
and promoted angiogenesis in myocardial infarct rats (25). However, the mechanisms by which
rosuvastatin protects against myocardial injury are still
incompletely understood. Furthermore, rosuvastatin alleviates
diabetic cardiomyopathy by suppressing the cardiac NLRP3
inflammasome activation and IL-1β production in a type 2 diabetes
rat model (26). Importantly,
rosuvastatin treatment significantly decreases NLRP3 expression,
and its downstream cytokines in peripheral blood monocytes of acute
myocardial infarction patients and unstable angina patients
(27), suggest that rosuvastatin
may ameliorate myocardial infarction injury via NLRP3
inflammasome.
Based on the above, the present study investigated
the cardioprotective effect of rosuvastatin on ISO-induced
myocardial injury in rats, focusing on the antioxidant and
anti-inflammatory role, and elucidated whether the cardioprotective
effect of rosuvastatin in ISO-induced myocardial injury is mediated
by NLRP3 inflammasome.
Materials and methods
Materials and chemicals
Isoproterenol hydrochloride was purchased from Sigma
Chemical Co. (St. Louis, MO, USA). Rosuvastatin was kindly provided
by AstraZeneca (Shanghai, China). The aspartate transaminase (AST),
alanine transaminase (ALT), creatine kinase (CK-MB), and lactate
dehydrogenase (LDH) kits were procured from Nanjing Jiancheng
Bioengineering Institute (Jiangsu, China). Superoxide dismutase
(SOD), catalase (CAT) and glutathione peroxidase (GPX) activities
as well as reduced glutathione (GSH) and malondialdehyde (MDA)
levels in heart tissues were measured by commercially available
kits (Beyotime Institute of Biotechnology, Haimen, China).
Commercially ELISA kits for IL-1β and IL-18 were obtained from
R&D Systems (Minneapolis, MN, USA). Antibodies of NLRP3 and ASC
were obtained from Cell Signaling Technology (Danvers, MA, USA),
antibodies of caspase-1 and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). All other chemicals and reagents used
in this study were of analytical grade.
Animals
Forty-five male Wistar-Albino rats (180–200 g) were
purchased from the Experimental Animal Center of Fudan University
(Shanghai, China). The animals were maintained under standard
laboratory conditions at 25±2°C and 50±15% humidity with an
alternate 12 h cycle of light and dark. They were acclimatized to
the conditions of the animal house for 1 week before the experiment
and allowed free access to standard laboratory diet and water ad
libitum. All animal procedures were done in accordance with the
guidelines for the care and use of laboratory animals approved by
the Ethics Committee for Animal Experimentation of Fudan
University.
Experimental protocol
Rats were randomly divided into two groups, NC group
(n=9) and ISO group (n=36). A rat model of myocardial ischemia was
induced by subcutaneous injection of ISO hydrochloride for 2
consecutive days, while NC group were injected with normal saline
for 2 consecutive days. After one week, ISO group were randomly
divided into four subgroups: Model group: rats received 1 ml/kg/day
1% Tween-80 suspension in distilled water by oral gavage for 8
consecutive weeks, RSV5, RSV10 and RSV15 group: rats received
rosuvastatin (5, 10 or 15 mg/kg, respectively) in distilled water
by oral gavage for 8 consecutive weeks. The dose of ISO and RSV was
selected based upon previous studies (24,28,29). After the end of the animal
experiment, rats were anesthetized and sacrificed, blood samples
were collected and centrifuged to obtain serum for the biochemical
assays.
Histopathological studies
After blood collection, the heart tissues were
rapidly removed, then the cardiac apex was immediately fixed in 4%
paraformaldehyde, processed in ethanol and embedded in paraffin
wax. The cardiac apex were stained with hematoxylin and eosin
(H&E) and examined under a light microscope (Olympus, Tokyo,
Japan).
Determination of pro-inflammatory
cytokines in heart
Enzyme immunoassay of IL-1β and IL-18 in heart
homogenate was performed by using ELISA kits according to
manufacturer's instructions (R&D Systems). The color intensity
was read at 450 nm with a microplate reader (Tecan Ltd., Mannedorf,
Switzerland) and the cytokines levels were expressed as pg/mg of
tissue.
Measurement of MI markers in the
serum
The serum was used to assay AST, ALT, CK-MB and LDH
activities. The activities of AST, ALT, CK-MB and LDH were assayed
using commercial kits purchased from Jiancheng Bioengineering
Institute (Nanjing, China) according to the manufacturer's
instructions.
Evaluation of lipid peroxidation and
antioxidant enzyme levels
After experimental treatment, the homogenates of
heart tissues were centrifuged at 16,000 rpm for 10 min. The
supernatant was used to assay MDA levels, and SOD, CAT, GPX
activities, as well as GSH concentrations according to the
manufacturer's instructions, on a microplate reader at 560 and 532
nm. The commercially available assay kits were purchased from
Jiancheng Bioengineering Institute.
qRT-PCR
The mRNA expression levels of NLRP3, ASC and
caspase-1 were analyzed via qRT-PCR; total RNA sample from rat
heart tissues was extracted and purified using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) following the manufacturer's
instructions. Total RNA was then reverse transcribed into cDNA with
an M-MLV reverse transcriptase kits according to the manufacturer's
protocol. Following the reverse transcription, qPCR was performed
to quantify the RNA levels of NLRP3, ASC and caspase-1 using
SYBR-Green Supermix (Bio-Rad, Hercules, CA, USA) and the data were
analyzed using the 2−ΔΔCt method. GAPDH was used as a
housekeeping gene for mRNA analysis. The primer sequences are
listed in Table I.
 | Table IRT-PCR primer sequences. |
Table I
RT-PCR primer sequences.
| Gene | Forward | Reverse |
|---|
| NLRP3 |
5′-CAGCGATCAACAGGCGAGAC-3′ |
5′-AGAGATATCCCAGCAAACCTATCCA-3′ |
| ASC |
5′-TTATGGAAGAGTCTGGAGCT-3′ |
5′-CAGCTGATGGACCTGACTGA-3′ |
| Caspase-1 |
5′-CGTGGAGAGAAACAAGGAGTG-3′ |
5′-AATGAAAAGTGAGCCCCTGAC-3′ |
| GAPDH |
5′-TTCAACGGCACAGTCAAGG-3′ |
5′-CACCAGTGGATGCAGGGAT-3′ |
Western blot analysis
Total protein was loaded per well, resolution on a
10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE), and then
transferred onto a polyvinylidene difluoride membrane (Millipore,
Bedford, MA, USA). The membrane was then blocked with 5% skim milk
and subsequently incubated with primary antibodies for NLRP3
(1:1,000), ASC (1:1,000) and caspase-1 antibodies (1:500) overnight
at 4°C. Membranes were subsequently incubated with appropriate
HRP-conjugated secondary antibody at room temperature for 1 h.
Immunoreactive bands were visualized via enhanced chemiluminescence
(Millipore) and quantified via densitometry using ImageJ (National
Institutes of Health).
Statistical analysis
All data were expressed as mean ± SD, and analyzed
using one-way ANOVA followed by a post-hoc test to determine the
statistical difference between groups. Statistical analysis was
performed using the GraphPad Prism 5 (GraphPad Software, Inc., San
Diego, CA, USA). A value of P<0.05 was considered statistically
significant.
Results
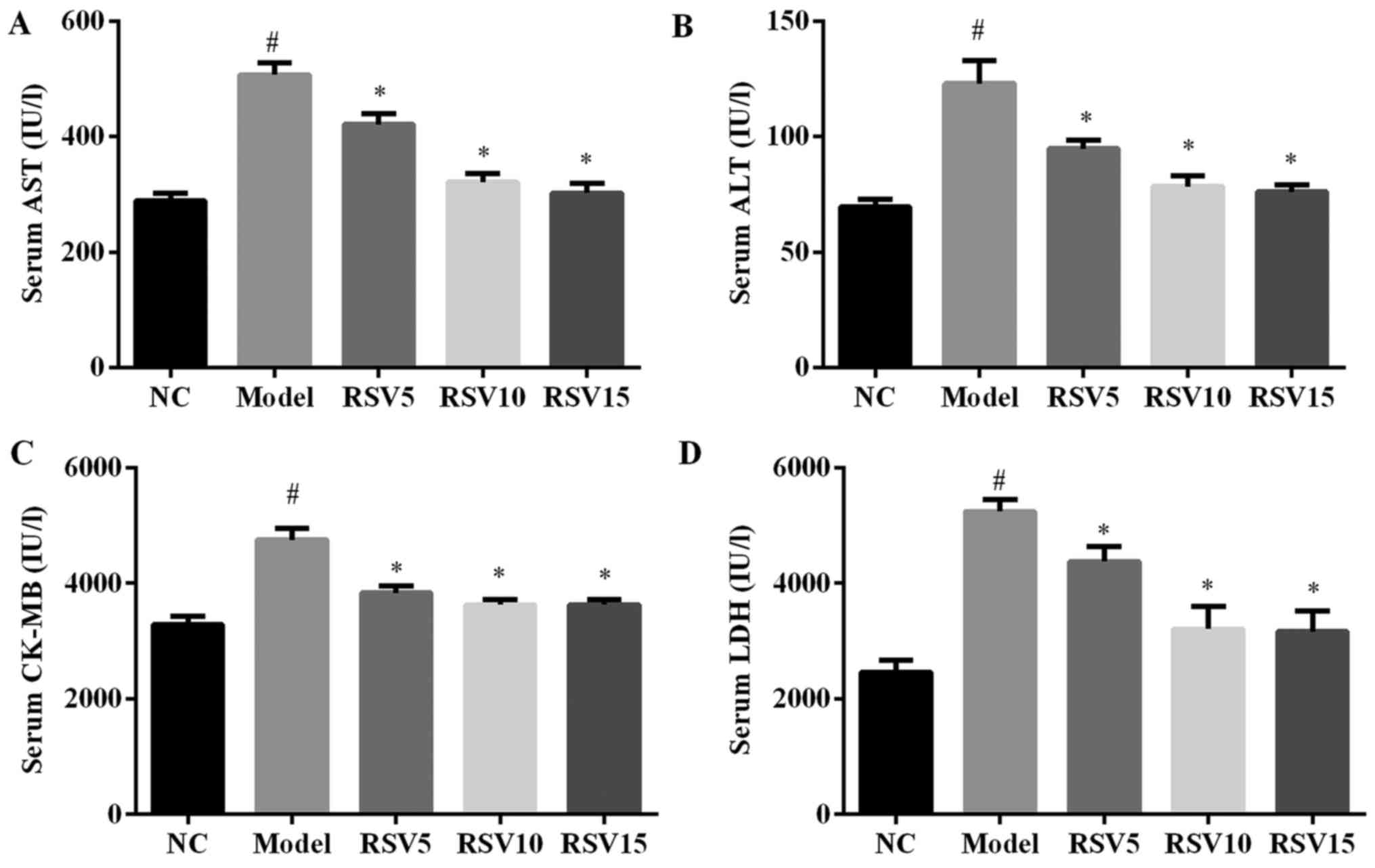
Effect of rosuvastatin on AST, ALT, CK-MB
and LDH enzyme activities in ISO induced rats
To investigate whether rosuvastatin could ameliorate
ISO-induced myocardial injury, we analyzed the myocardial injury
marker activities in heart tissue of all groups. As illustrated in
Fig. 1, two subcutaneous
injections of ISO significantly induces cardiac dysfunction as
evidenced by greatly increase serum activities of AST, ALT, CK-MB
and LDH in comparison to normal control group (P<0.05). However,
by administration of rosuvastatin, serum AST, ALT, CK-BA and LDH
activities were obviously relieved in this animal model
(P<0.05).
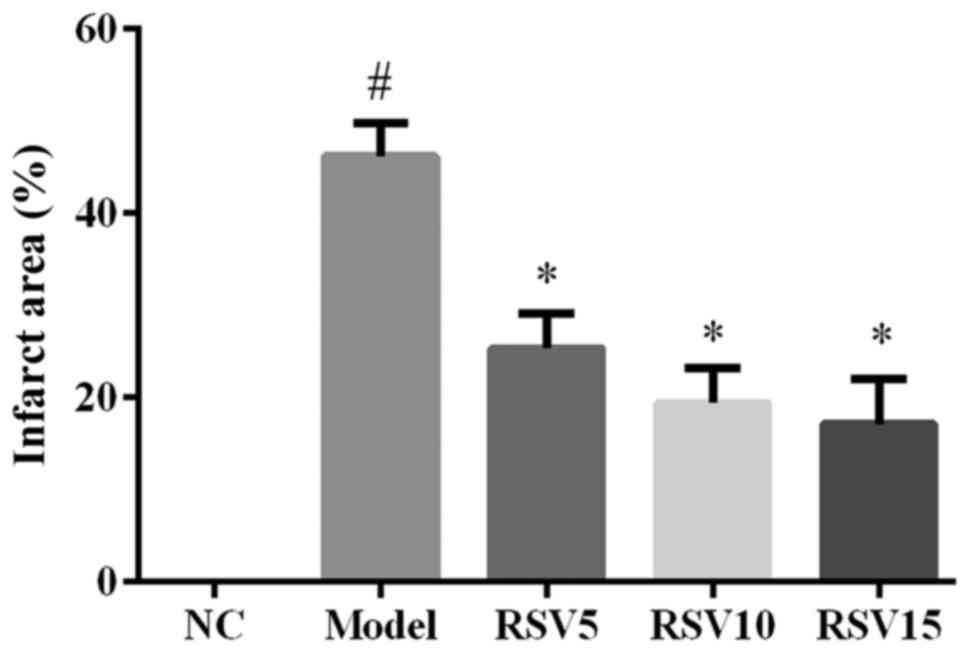
Effect of rosuvastatin on
histopathological assessments in heart in ISO induced rats
Fig. 2 shows that
ISO induced a significant infarction area in comparison to the
normal control group (P<0.05). Compared with the model group,
myocardial infarction area of rosuvastatin treated groups were
remarkably diminished (P<0.05) (Fig. 2). Furthermore, results of
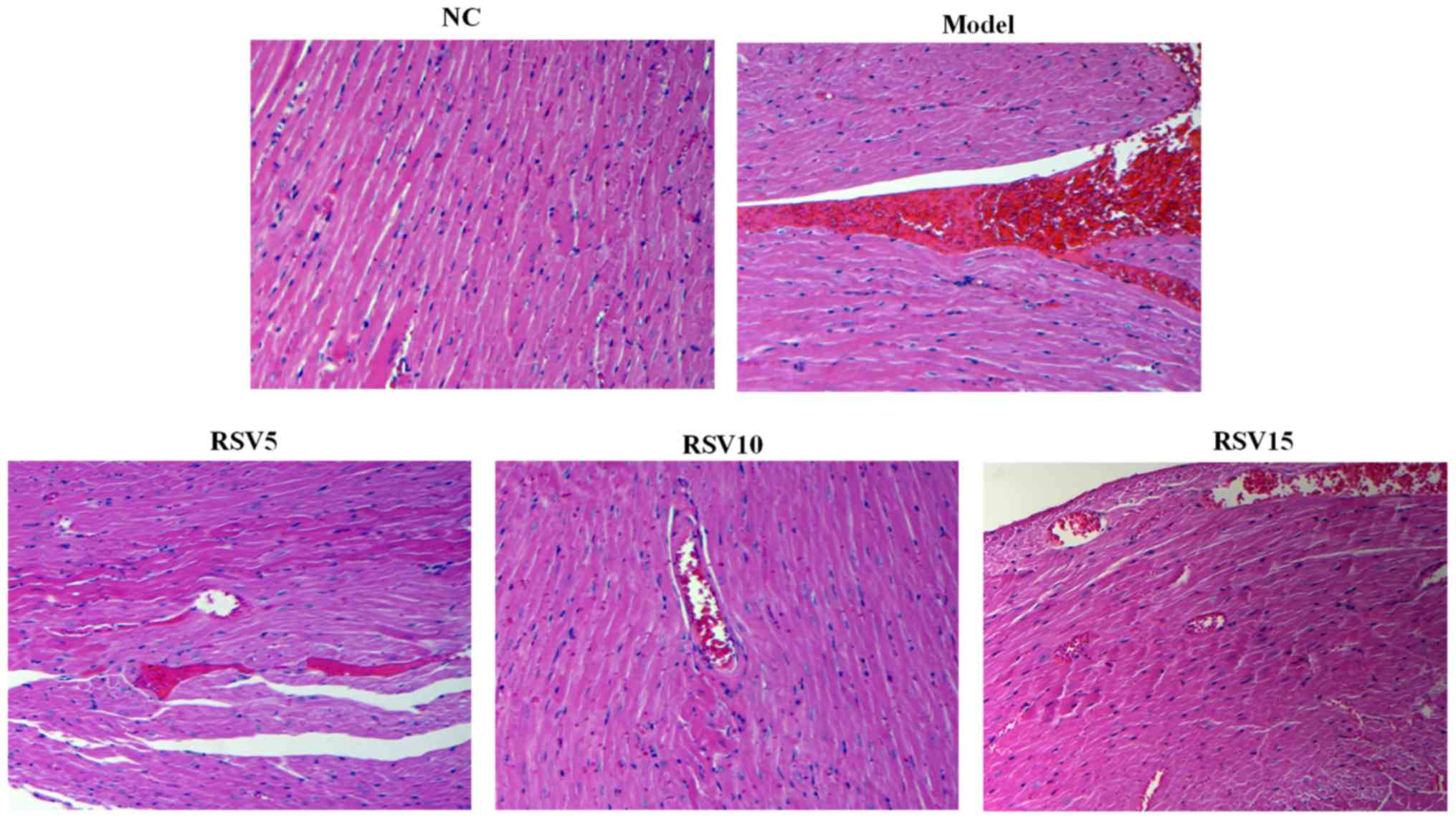
histopathological examination also confirmed the protective effect
of rosuvastatin in ISO-induced myocardial injury. As illustrated in
Fig. 3, NC group showed a normal
histoarchitecture, while obvious necrosis of myofibers with cell
infiltration, as well as extravasations of red blood cells were
observed in the heart tissue of ISO rats (Fig. 3). Importantly, rosuvastatin
significantly ameliorated these changes in ISO rats.
Effect of rosuvastatin on lipid
peroxidation and oxidative stress parameters in ISO induced
rats
Then, we investigated the effect of rosuvastatin on
ISO-induced lipid peroxidation and oxidative stress in rats. As
shown in Fig. 4, marked increase
of MDA, a lipid peroxidation byproduct, was observed in the heart
of ISO group. Moreover, rats administered ISO showed decreased
activities of antioxidants such as SOD, CAT and GPX, downregulated
non-enzymatic antioxidant (GSH) concentrations, as compared to
normal control rats. However, rosuvastatin significantly reduced
cardiac MDA levels, increased SOD, CAT and GPX activities and GSH
concentrations in ISO-treated rats (P<0.05). These results
suggest that ISO injection causes oxidative stress in heart of
rats, which is suppressed by the treatment of rosuvastatin.
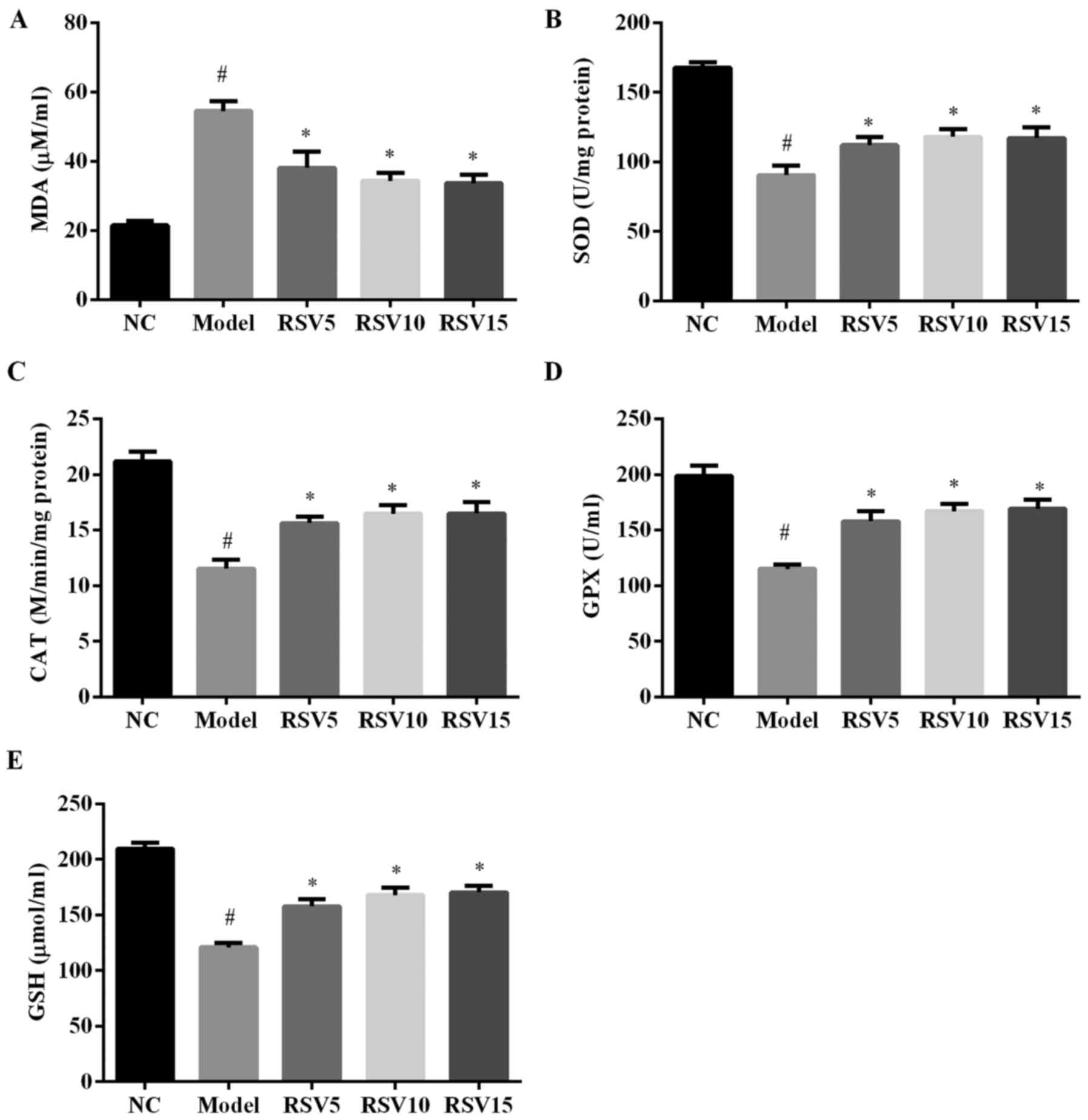 | Figure 4(A) Cardiac malondialdehyde (MDA)
levels, (B) superoxide dismutase (SOD), (C) catalase (CAT), (D)
glutathione peroxidase (GPX) activities, and (E) glutathione (GSH)
concentrations between the groups. Results are expressed as mean ±
SD, n=9. Activity is expressed as U/mg protein for SOD, µmol
of H2O2 decomposed/second/mg protein for CAT,
µmol of GSH, oxidized/min/mg of protein for GPX. NC, normal
control group; model, isoproterenol group; RSV, rosuvastatin group.
#P<0.05 compared with normal control group;
*P<0.05 compared with the model group. |
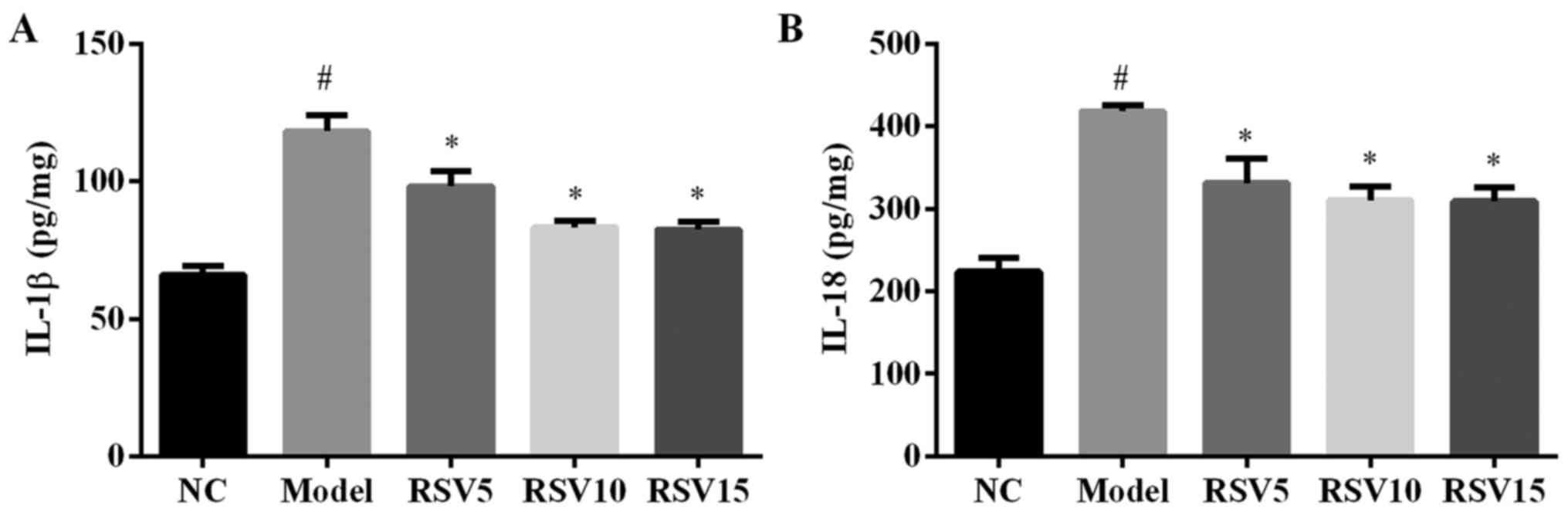
Effect of rosuvastatin on
pro-inflammatory cytokines in ISO induced rats
The production of pro-inflammatory cytokines such as
IL-1β and IL-18 in heart tissues are shown in Fig. 5. Compared to normal control group,
injection of ISO significantly increased the secretion levels of
IL-1β and IL-18 in the heart (P<0.01). Treatment with
rosuvastatin reduced ISO-induced elevation of cardiac IL-1β and
IL-18 secretion in this animal model.
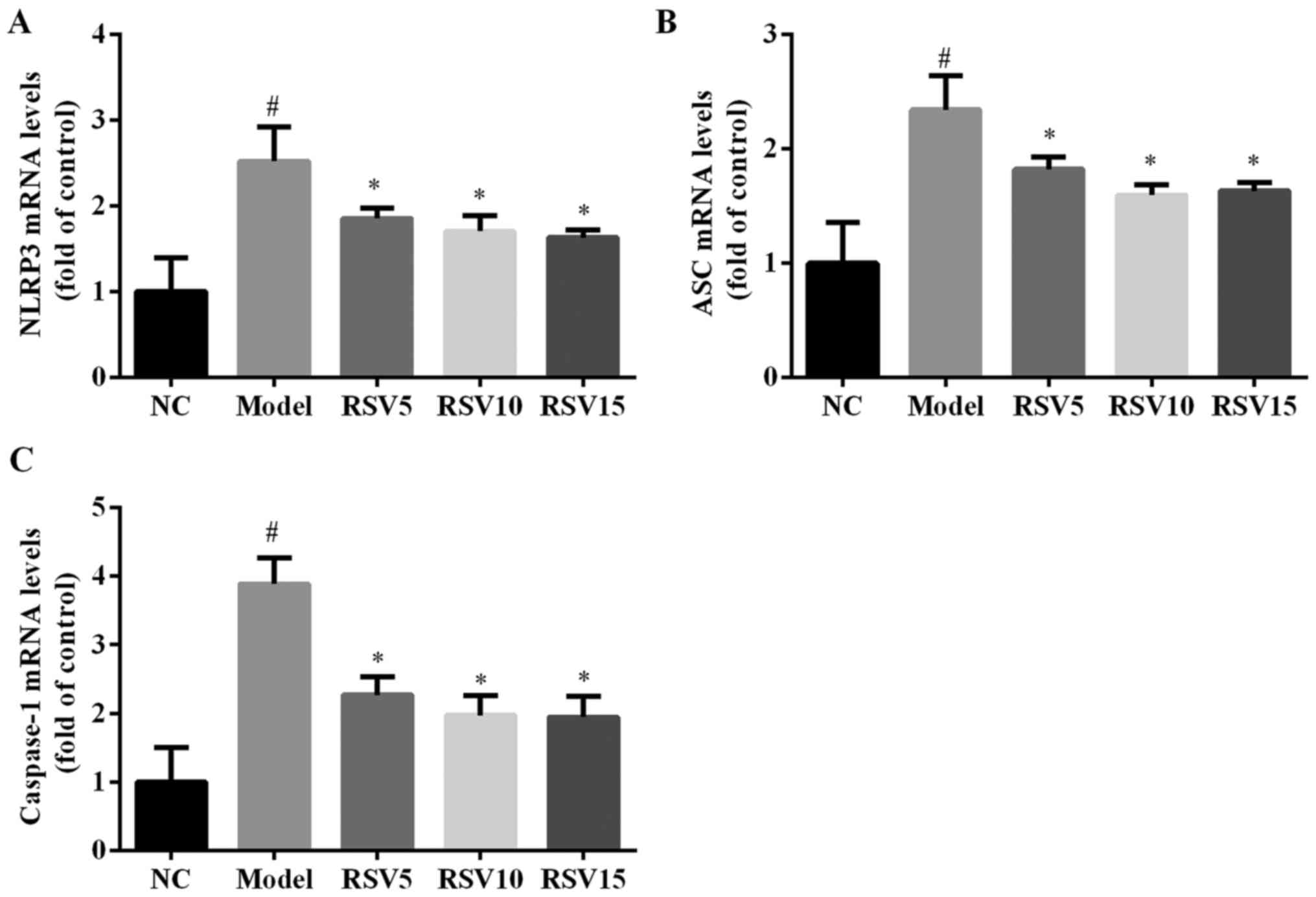
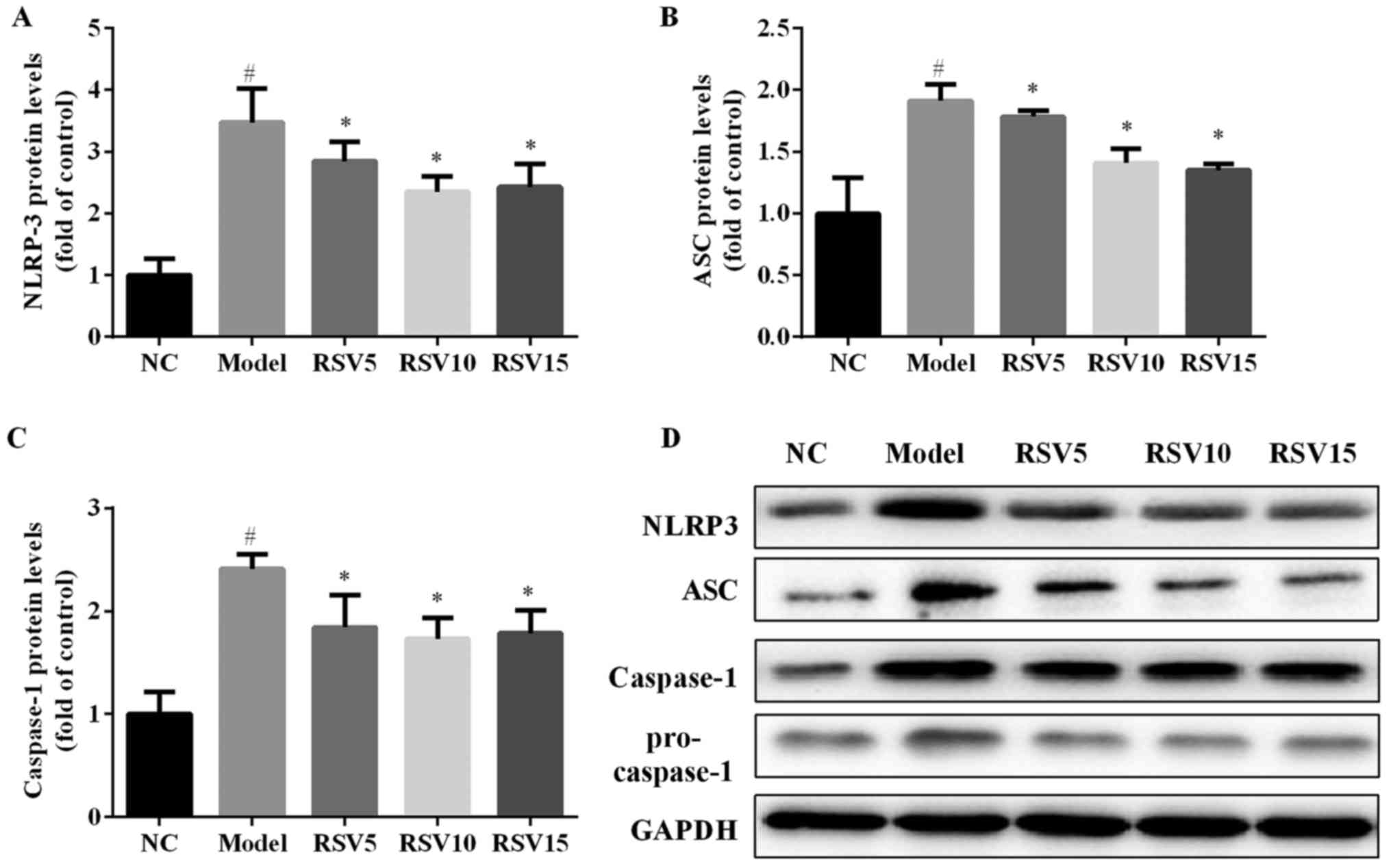
Effect of rosuvastatin on NLRP3
inflammasome activation in ISO induced rats
In order to evaluate whether rosuvastatin alleviated
ISO-induced myocardial injury via NLRP3 inflammasome, the mRNA and
protein expression levels of NLRP3, ASC and caspase-1 in the heart
of all experimental groups were detected. ISO injection obviously
induced the activation of NLRP3, characterized by significantly
increased cardiac mRNA (Fig. 6)
and protein (Fig. 7) expression
levels of NLRP3, ASC and caspase-1 (P<0.05). Rosuvastatin
markedly decreased NLRP3, ASC and caspase-1 at both mRNA and
protein levels.
Discussion
In this study, we confirmed the cardioprotective
effects of rosuvastatin against ISO-induced myocardial infarction
injury in rats. Treatment with rosuvastatin significantly reduced
myocardial infract area, improved myocardial histoarchitecture, and
decreased serum levels of myocyte marker enzymes in ISO-induced
myocardial injury in rats. In addition, rosuvastatin remarkably
restored ISO-induced elevation of antioxidants and decreased lipid
peroxidation.
ISO, a synthetic non selective β-adrenergic agonist,
ISO-induced myocardial injury has been widely used to investigate
the effect of drugs on myocardial infraction (30,31). In the present study, a rat model
of myocardial injury was successfully established, as evidenced by
dramatically increased serum levels of AST, ALT, CK-MB and LDH, and
abnormal cardiac microstructure observed on histopathological
examination. These results are in line with previous in vivo
studies (9,32). Furthermore, the present study
further confirmed that rosuvastatin can ameliorate ISO-induced
myocardial infarction injury, evidenced by dramatically decreasing
serum levels of myocardial injury markers and obviously diminished
histopathological alterations.
There is a relationship between oxidative stress and
myocardial infraction injury (33). Disturbance in oxidants and
antioxidant metabolism has been noted in patients with acute
myocardial infraction (34,35). Free radical scavenging enzymes,
such as CAT, SOD and GPX, GSH are first line cellular defense
against oxidative damage (36).
This study observed increased MDA and decreased antioxidants such
as SOD, CAT, GPX and GSH in ISO-treated rat heart tissues. Thus, it
suggests that oxidative stress may be involved in ISO-induced
myocardial infraction injury of rats. However, the treatment of
rosuvastatin remarkably restored ISO-induced oxidative stress by
increasing antioxidants activities and decreasing lipid
peroxidation.
NLRP3 inflammasome is implicated in cellular
inflammation processes in response to oxidative stress (12). Activated NLRP3 inflammasome has
been observed in the peripheral blood monocytes of patients with
acute myocardial infarction (27,37). Moreover, overproduction of
pro-inflammatory cytokine IL-1β and IL-18 is involved in I/R injury
(38), which is critically
dependent on the activation of NLRP3 inflammasome. In the present
study, our results confirmed that NLRP3 inflammasome activated in
the heart of ISO-induced myocardial injury, as measured by cardiac
NLRP3, ASC and caspase-1 expression levels. Clinical study also has
demonstrated that patients with acute myocardial infarction show a
significant increase of IL-1β plasma levels (39). Accordingly, our study found that
ISO remarkably increased IL-1β and IL-18 production in the heart
tissue. Furthermore, Liu et al reported that NLRP3 siRNA and
BAY 11-7082 significantly ameliorated myocardial I/R (16). Collectively, these findings imply
a role of NLRP3 inflammasome in myocardial infarction injury, and
inhibiting NLRP3 inflammasome activation may be a novel therapeutic
target for the treatment of myocardial infarction injury.
Rosuvastatin, is an approved drug for treating
patients with hyperlipidemia and hypercholesterolemia. Given that
rosuvastatin exerts both anti-inflammatory (18,19) and anti-oxidative (20,21) effect, we studied the effect of
rosuvastatin on ISO-induced myocardial infarction injury. We found
that the treatment of rosuvastatin remarkably restored ISO-induced
by increasing antioxidant activities and decreasing lipid
peroxidation, significantly reducing cardiac pro-inflammatory
cytokines production. Importantly, a previous study indicated that
rosuvastatin significantly downregulated NLRP3 expression, and its
downstream cytokines in peripheral blood monocytes of acute
myocardial infarction patients (27). Consistently, our results found
that rosuvastatin remarkably decreased NLRP3, ASC and caspase-1
mRNA and protein levels in the heart of ISO rats implying that
rosuvastatin inhibited cardiac NLRP3 inflammasome activation in ISO
rats. Therefore, we suggest that rosuvastatin alleviates
ISO-induced myocardial infarction injury by attenuating oxidative
stress and via the inhibition of NLRP3 inflammasome.
In conclusion, the present study showed that
rosuvastatin significantly alleviated ISO-induced myocardial
infarction injury in rats. The effect is associated with
attenuation of oxidative stress and inflammation, via the
inhibition of NLRP3 inflammasome. However, further studies are
needed to explore the exact mechanism by which rosuvastatin
inhibits the activation of NLRP3 inflammasome in the heart tissue
of myocardial infraction injury. The results of this study suggest
that the cholesterol-lowering medicine rosuvastatin may have
potential for the prevention and treatment of myocardial
infarction.
Acknowledgments
Not applicable.
Notes
[1]
Funding
No funding was received.
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
YY and KG conceived and designed the study. LJ, YZ
and YH performed the experiments. JC analyzed the data and wrote
the paper. All authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
All animal procedures were done in accordance with
the guidelines for the care and use of laboratory animals approved
by the Ethics Committee for Animal Experimentation of Fudan
University.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion - from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whellan DJ: Heart failure disease
management: Implementation and outcomes. Cardiol Rev. 13:231–239.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nichols M, Townsend N, Scarborough P and
Rayner M: Cardiovascular disease in Europe 2014: Epidemiological
update. Eur Heart J. 35:2929–2933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017. View Article : Google Scholar
|
|
5
|
Sawyer DB, Siwik DA, Xiao L, Pimentel DR,
Singh K and Colucci WS: Role of oxidative stress in myocardial
hypertrophy and failure. J Mol Cell Cardiol. 34:379–388. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neri M, Fineschi V, Di Paolo M, Pomara C,
Riezzo I, Turillazzi E and Cerretani D: Cardiac oxidative stress
and inflammatory cytokines response after myocardial infarction.
Curr Vasc Pharmacol. 13:26–36. 2015. View Article : Google Scholar
|
|
7
|
Kumar M, Kasala ER, Bodduluru LN, Dahiya V
and Lahkar M: Baicalein protects isoproterenol induced myocardial
ischemic injury in male Wistar rats by mitigating oxidative stress
and inflammation. Inflamm Res. 65:613–622. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen M, Wang M, Yang Q, Wang M, Wang Z,
Zhu Y, Zhang Y, Wang C, Jia Y, Li Y, et al: Antioxidant effects of
hydroxysafflor yellow A and acetyl-11-keto-β-boswellic acid in
combination on isoproterenol-induced myocardial injury in rats. Int
J Mol Med. 37:1501–1510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Xie YH, Yang Q, Wang SW, Zhang BL,
Wang JB, Cao W, Bi LL, Sun JY, Miao S, et al: Cardioprotective
effect of paeonol and danshensu combination on
isoproterenol-induced myocardial injury in rats. PLoS One.
7:e488722012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suchal K, Bhatia J, Malik S, Malhotra RK,
Gamad N, Goyal S, Nag TC, Arya DS and Ojha S: Seabuckthorn pulp oil
protects against myocardial ischemia-reperfusion injury in rats
through activation of Akt/eNOS. Front Pharmacol. 7:1552016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang M, Chen J, Zhao J and Meng M:
Etanercept attenuates myocardial ischemia/reperfusion injury by
decreasing inflammation and oxidative stress. PLoS One.
9:e1080242014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abderrazak A, Syrovets T, Couchie D, El
Hadri K, Friguet B, Simmet T and Rouis M: NLRP3 inflammasome: From
a danger signal sensor to a regulatory node of oxidative stress and
inflammatory diseases. Redox Biol. 4:296–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogura Y, Sutterwala FS and Flavell RA: The
inflammasome: First line of the immune response to cell stress.
Cell. 126:659–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu P, Duan L, Chen J, Xiong A, Xu Q,
Zhang H, Zheng F, Tan Z, Gong F and Fang M: Gene silencing of NALP3
protects against liver ischemia-reperfusion injury in mice. Hum
Gene Ther. 22:853–864. 2011. View Article : Google Scholar
|
|
15
|
Shigeoka AA, Mueller JL, Kambo A, Mathison
JC, King AJ, Hall WF, Correia JS, Ulevitch RJ, Hoffman HM and McKay
DB: An inflammasome-independent role for epithelial-expressed Nlrp3
in renal ischemia-reperfusion injury. J Immunol. 185:6277–6285.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao
C, Xin C, Zhu D, Li Y, Yan W, et al: TXNIP mediates NLRP3
inflammasome activation in cardiac microvascular endothelial cells
as a novel mechanism in myocardial ischemia/reperfusion injury.
Basic Res Cardiol. 109:4152014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawaguchi M, Takahashi M, Hata T, Kashima
Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J,
et al: Inflammasome activation of cardiac fibroblasts is essential
for myocardial ischemia/reperfusion injury. Circulation.
123:594–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monetti M, Canavesi M, Camera M, Parente
R, Paoletti R, Tremoli E, Corsini A and Bellosta S: Rosuvastatin
displays anti-atherothrombotic and anti-inflammatory properties in
apoE-deficient mice. Pharmacol Res. 55:441–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Funderburg N, Clagett B, Jiang Y, Debanne
S, Storer N, Labbato D, Juchnowski S, Ferrari B, Lederman M and
McComsey GA: Rosuvastatin reduces immune activation and
inflammation in treated HIV infection. In: Conference on
Retroviruses and Opportunistic Infections; Boston, MA. 2014, Poster
335.
|
|
20
|
Duarte T, da Cruz IB, Barbisan F,
Capelleto D, Moresco RN and Duarte MM: The effects of rosuvastatin
on lipid-lowering, inflammatory, antioxidant and fibrinolytics
blood biomarkers are influenced by Val16Ala superoxide dismutase
manganese-dependent gene polymorphism. Pharmacogenomics J.
16:501–506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang D, Zhang Q, Yang H, Zhang R, Yan W,
Gao H, Wang J, Zhang X, Chen Y and Cao F: Anti-oxidative stress
effect of loading-dose rosuvastatin prior to percutaneous coronary
intervention in patients with acute coronary syndrome: A
prospective randomized controlled clinical trial. Clin Drug
Investig. 34:773–781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ke D, Fang J, Fan L, Chen Z and Chen L:
Regulatory T cells contribute to rosuvastatin-induced
cardioprotection against ischemia-reperfusion injury. Coron Artery
Dis. 24:334–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Z, Cao Y, Qian J, Ma J, Zou Y and Ge
J: Cardioprotection of rosuvastatin against cardiac dysfunction
after coronary microembolization via alleviating inflammatory
induced micro-infarctions. Circulation. 132:A134342015.
|
|
24
|
Ma M, Uekawa K, Hasegawa Y, Nakagawa T,
Katayama T, Sueta D, Toyama K, Kataoka K, Koibuchi N, Kuratsu J, et
al: Pretreatment with rosuvastatin protects against focal cerebral
ischemia/reperfusion injury in rats through attenuation of
oxidative stress and inflammation. Brain Res. 1519:87–94. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaitone SA and Abo-Gresha NM: Rosuvastatin
promotes angiogenesis and reverses isoproterenol-induced acute
myocardial infarction in rats: Role of iNOS and VEGF. Eur J
Pharmacol. 691:134–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo B, Li B, Wang W, Liu X, Liu X, Xia Y,
Zhang C, Zhang Y, Zhang M and An F: Rosuvastatin alleviates
diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK
pathways in a type 2 diabetes rat model. Cardiovasc Drugs Ther.
28:33–43. 2014. View Article : Google Scholar
|
|
27
|
Altaf A, Qu P, Zhao Y, Wang H, Lou D and
Niu N: NLRP3 inflammasome in peripheral blood monocytes of acute
coronary syndrome patients and its relationship with statins. Coron
Artery Dis. 26:409–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar M, Kasala ER, Bodduluru LN, Dahiya
V, Sharma D, Kumar V and Lahkar M: Animal models of myocardial
infarction: Mainstay in clinical translation. Regul Toxicol
Pharmacol. 76:221–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han S, Cai W, Yang X, Jia Y, Zheng Z, Wang
H, Li J, Li Y, Gao J, Fan L, et al: ROS-mediated NLRP3 inflammasome
activity is essential for burn-induced acute lung injury. Mediators
Inflamm. 2015:7204572015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karthikeyan K, Bai BR and Devaraj SN:
Cardioprotective effect of grape seed proanthocyanidins on
isoproterenol-induced myocardial injury in rats. Int J Cardiol.
115:326–333. 2007. View Article : Google Scholar
|
|
31
|
Abbas AM: Cardioprotective effect of
resveratrol analogue isorhapontigenin versus omega-3 fatty acids in
isoproterenol-induced myocardial infarction in rats. J Physiol
Biochem. 72:469–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geng ZH, Huang L, Song MB and Song YM:
Protective effect of a polysaccharide from Salvia miltiorrhiza on
isoproterenol (ISO)-induced myocardial injury in rats. Carbohydr
Polym. 132:638–642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hill MF and Singal PK: Antioxidant and
oxidative stress changes during heart failure subsequent to
myocardial infarction in rats. Am J Pathol. 148:291–300.
1996.PubMed/NCBI
|
|
34
|
Madole MB, Bachewar NP and Aiyar CM: Study
of oxidants and antioxidants in patients of acute myocardial
infarction. Adv Biomed Res. 4:2412015.PubMed/NCBI
|
|
35
|
Patil N, Chavan V and Karnik ND:
Antioxidant status in patients with acute myocardial infarction.
Indian J Clin Biochem. 22:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wattanapitayakul SK and Bauer JA:
Oxidative pathways in cardiovascular disease: Roles, mechanisms,
and therapeutic implications. Pharmacol Ther. 89:187–206. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sandanger Ø, Ranheim T, Vinge LE, Bliksøen
M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G,
Christensen G, et al: The NLRP3 inflammasome is up-regulated in
cardiac fibroblasts and mediates myocardial ischaemia-reperfusion
injury. Cardiovasc Res. 99:164–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pomerantz BJ, Reznikov LL, Harken AH and
Dinarello CA: Inhibition of caspase 1 reduces human myocardial
ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc
Natl Acad Sci USA. 98:2871–2876. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guillén I, Blanes M, Gómez-Lechón MJ and
Castell JV: Cytokine signaling during myocardial infarction:
Sequential appearance of IL-1 beta and IL-6. Am J Physiol.
269:R229–R235. 1995.PubMed/NCBI
|