Introduction
Bone tissue is constantly breaking-down and
building-up to maintain skeleton balance. The bone remodeling
process involves bone formation (osteoblast activities) and bone
resorption (osteoclast activities) (1). Excess osteoclast activity may lead
to adult skeletal osteolytic diseases, including osteoporosis,
rheumatoid arthritis, and periodontal disease (2–4).
Periprosthetic osteolysis (PO) following total hip arthroplasty
(THA) is also a typical osteolytic disease. As a highly successful
procedure, THA has become the standard method to address serious
joint disease. But aseptic loosening, caused by PO, leads
frequently to prosthetic failure. Previous studies have reported
that PO is initiated by inflammatory response to wear debris
(5,6), then excessive osteoclast formation
and bone resorption are stimulated, resulting in osteopenia
(4,7).
Osteoclasts are large multinucleated cells, which
arise from the hematopoietic monocyte/macrophage lineage (8). Macrophage colony-stimulating factor
(M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL)
are required for osteoclast differentiation and formation (9). RANKL induces osteoclast
differentiation by binding to RANK, its cognate receptor (10). Then, downstream signaling pathways
are activated, including nuclear factor-κB (NF-κB), c-Jun
N-terminal kinase (JNK) 1/2, p38, and extracellular
signal-regulated kinase (ERK) 1/2 pathways (10,11). Suppression of these pathways might
provide a potential for treating PO through inhibiting osteoclast
formation or function.
Fraxetin (7, 8-dihydroxy-6-methoxy coumarin;
Fig. 1A), extracted from the
Chinese herb Cortex Fraxini, has anti-inflammatory,
antitumor, antioxidative, antiviral, antihyperglycemic and
neuroprotective effects (12,13). In addition, Kuo et al
(14) reported that fraxetin can
inhibit interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and
anti-first apoptosis signal (Fas) IgM-mediated apoptosis by
suppressing the Fas signal pathway in osteoblastic MG-63 cells.
These findings indicate that fraxetin might be a potential
therapeutic option to prevent osteolytic diseases. However, the
role of fraxetin in osteoclasts has not been reported.
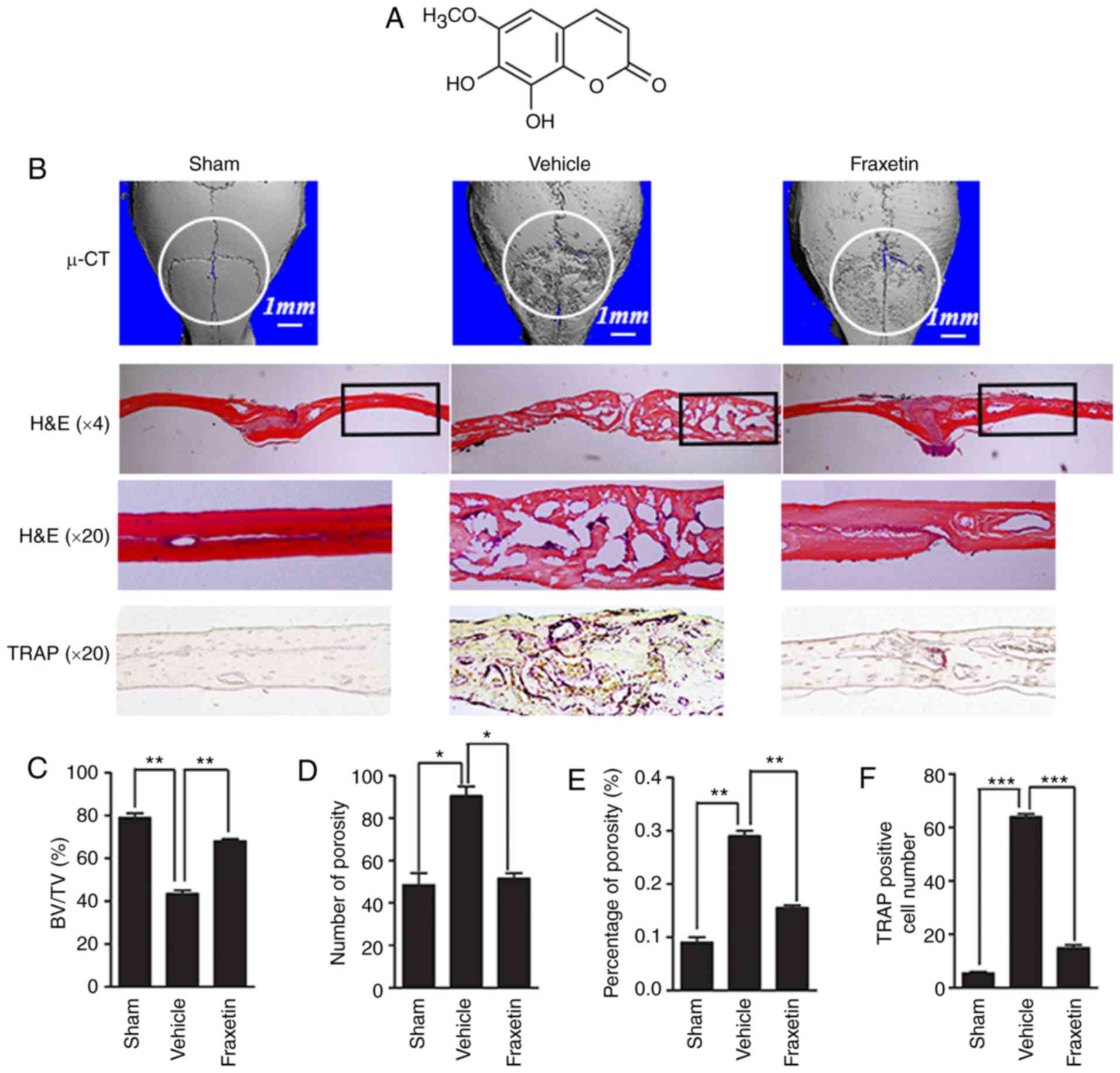 | Figure 1Fraxetin protects against Ti
particle-induced osteolysis of mouse calvarias. (A) Chemical
structure of fraxetin. (B) Representative images from 3D
µ-CT analysis reconstruction (scale bar, 1 mm), H&E
staining (magnification, ×4 and ×20) and TRAP staining
(magnification, ×20). At least three sections per group were
analyzed. (C) Quantified results for BV/TV, (D) number of pores,
(E) % of porosity and (F) osteoclast numbers.
*P<0.05, **P<0.01 and ***P<0.001,
with comparisons indicated by brackets. µ-CT, micro-computed
tomography; H&E, hematoxylin and eosin; TRAP,
tartrate-resistant acid phosphatase; BV, bone volume; TV, tissue
volume. |
The present study aimed to investigate the
therapeutic benefits and mechanism of fraxetin on PO in
vivo, to assess the effect of fraxetin on osteoclastogenesis
and function in vitro, and to determine the mechanisms that
mediate the effects of fraxetin on osteoclast development and
function.
Materials and methods
Reagents
Mouse macrophage RAW264.7 cells were obtained from
the American Type Culture Collection (Manassas, VA, USA). Fetal
bovine serum (FBS) was obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The Cell Counting Kit (CCK)-8
was purchased from Dojindo Molecular Technologies, Inc., (Kumamoto,
Japan). Alpha-modified Eagle’s medium (α-MEM) was obtained from
Gibco; Thermo Fisher Scientific, Inc. Fraxetin, glycine, Tris,
sodium dodecyl sulfate (SDS), NaCl, Acid Phosphatase kit and other
reagents were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). M-CSF and RANKL were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA). The SYBR Premix Ex Taq II and Prime Script
RT reagent kits were purchased from Takara Bio, Inc. (Otsu, Japan).
Antibodies against phosphorylated (p-) JNK1/2 (cat. no. 9251),
total JNK1/2 (cat. no. 9252), p-p38 (cat. no. 9211), total p38
(cat. no. 8690), p-ERK1/2 (cat. no. 9101), total ERK1/2 (cat. no.
9102), MMK6 (cat. no. 9264) and β-actin (cat. no. 4967) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA)
and the working dilution was 1:1,000 for all.
Animals and experimental design
This study obtained ethics approval by the
Institutional Animal Care And Use Committee (IACUC) of Southern
Medical University (SMU; Guangzhou, China), and all experiments
involving animals were performed according to the guidelines of SMU
IACUC. A mouse calvarial osteolysis model was established to
investigate the effects of fraxetin in vivo (15). Commercial pure Titanium (Ti)
particles were obtained from Johnson Matthey (Royston, UK)
(16). The particles were exposed
to 180°C for 6 h, and sterilized with 70% ethanol for 48 h to
remove adherent endotoxins. The particles with an average diameter
of 4.50 mm are similar to wear particles retrieved from
periprosthetic tissues (17,18). The concentration of particles in
subsequent studies was 0.1 mg/ml. Healthy 8 week-old male C57BL/J6
mice, weighing 23±1 g, were purchased from Southern Medical
University Laboratory Animal Center and assigned randomly to three
experimental groups (8 mice per group). The sham group was used as
a negative control. The vehicle group had calvarial osteolysis
mediated by Ti particles. The fraxetin group had calvarial
osteolysis and was treated with fraxetin (5 mg/kg/day). For the
generation of the calvarial osteolysis model, the Ti particles (30
mg) were embedded on the middle suture of the calvaria (19). Two days later, fraxetin or PBS
were locally injected every other day. All animals were housed
under conditions of constant temperature and humidity on a 12-h
light/dark cycle. Food and water were available ad libitum.
After two weeks, the mice were sacrificed, and the calvarias were
excised, fixed in 4% paraformaldehyde for 15 h and stored at 70%
ethanol for micro-computed tomography (µ-CT) analysis.
µ-CT scanning and histological
analysis
The prepared calvarias were analyzed by high
resolution µ-CT. Bones were scanned at an isometric voxel
resolution of 8 µm. The X-ray energy parameters were 80 kV
and 80 µA. A square region of interest (ROI) was selected to
perform the subsequent analyses. Porosity, number of pores, and
bone volume/tissue volume (BV/TV) were studied for the ROI
(20).
After µ-CT scanning, bones were decalcified
in 10% ethylenediaminetetraacetic acid (EDTA; pH 7.4) for 2–3 weeks
prior to embedding in paraffin. Sections with thickness of
4-µm were prepared and stained with tartrate-resistant acid
phosphatase (TRAP) and hematoxylin and eosin (H&E). Stained
sections were observed and photographed on a Leica DM1000 light
microscope. At least 3 fields per slide per group were analyzed.
For calvarias, the osteolysis area was analyzed using Image J
(National Institutes of Health, Bethesda, MD, USA). TRAP-positive
osteoclast numbers were determined.
Osteoclast culture, TRAP staining assay,
resorption pit assay and osteoclastogenesis rescue assay
Primary mouse bone marrow-derived macrophages (BMMs)
were obtained from the bone marrow of long bones. BMMs were
cultured in α-MEM containing 10% FBS, 100 U/ml
penicillin/streptomycin, 2 mM L-glutamine, and 30 ng/ml M-CSF
(complete α-MEM). α-MEM containing 10% FBS, 100 U/ml
penicillin/streptomycin and 2 mM L-glutamine was used for RAW264.7
cell culture.
BMMs were seeded at a density of 8×103
cells/well into a 96-well plate with complete α-MEM, RANKL (50
ng/ml), and fraxetin (0, 3, 6, or 12 µM). For the resorption
pit assay, bovine bone slices were added into the 96-well plate
previously. For the osteoclastogenesis rescue assay, anisomycin
(2.5 ng/ml), a potent activator of p38, was added following the
fraxetin treatment (21,22). After seven days, the mature
osteoclasts were fixed with 4% paraformaldehyde for 30 min and
stained using the Acid Phosphatase kit. Osteoclast-like (OCL) cells
were characterized as TRAP-positive osteoclasts with >3 nuclei.
For the resorption pit assay, mechanical agitation and sonication
were used to remove OCL cells from the bone slices. Resorption pits
were observed using a scanning electron microscope. For each group,
three wells were selected randomly for analysis. Image J software
was used to analyze the numbers and the area of OCL cells and the %
of resorbed bone surface area.
Cytotoxicity assay
The effects of fraxetin on the viability of BMMs
were determined by CCK-8 assay. BMMs were plated at a density of
8,000 cells/well in 96-well plates, and cultured in complete α-MEM
containing fraxetin (0, 6.25, 12.5, 25, 50, 100, 200, 400 and 800
µM) for 48 h. GraphPad Prism (GraphPad Software, Inc., La
Jolla, CA, USA) was used to calculate the half-maximal inhibitory
concentration (IC50).
Western blot analysis
RAW264.7 cells were seeded in 6-well plates at a
density of 5×105 cells/well. First, RAW264.7 cells were
treated with placebo or fraxetin for 2 h. Then, the RAW264.7 cells
were stimulated with RANKL for 0, 5, 10, 20, 30, or 60 min.
Subsequently, cells lysates were collected and western blotting was
performed (23). The Odyssey
infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA) was
used to visualize the antibody reactivity.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
BMMs were seeded at a density of 2×105
cells/well in 6-well plates and cultured in complete α-MEM
supplemented with 50 ng/ml RANKL. Then, the BMMs were treated with
either 0, 3, or 12 µM fraxetin for 5 days, or 12 µM
fraxetin for 0–5 days. A Qiagen RNeasy Mini kit (Qiagen GmbH,
Hilden, Germany) was used to extract total RNA. Reverse
transcription was performed with the Prime Script RT kit. qPCR was
performed using SYBR Premix Ex Taq II with the following specific
primers: TRAP, forward 5′-CTG GAG TGC ACG ATG CCA GCG ACA-3′ and
reverse 5′-TCC GTG CTC GGC GAT GGA CCA GA-3′; nuclear factor of
activated T-cells, cytoplasmic 1 (NFATc1), forward 5′-CCG TTG CTT
CCA GAA AAT AAC A-3′ and reverse 5′-TGT GGG ATG TGA ACT CGG AA-3′;
cathepsin K (CTSK), forward 5′-CTT CCA ATA CGT GCA GCA GA-3′ and
reverse 5′-TCT TCA GGG CTT TCT CGT TC-3′; GAPDH, forward 5′-ACC CAG
AAG ACT GTG GAT GG-3′ and reverse 5′-CAC ATT GGG GGT AGG AAC AC-3′.
The following cycling conditions were used: 40 cycles of
denaturation at 95°C for 5 sec and amplification at 60°C for 24
sec. GAPDH expression was used to normalize transcript levels for
the genes under investigation, and all reactions were run in
triplicate. The 2−ΔΔCq method was used for
quantification (24).
Statistical analysis
Results are expressed as the mean ± standard
deviation. The significance of differences between groups was
analyzed with one-way analysis of variance with a post hoc
Bonferroni test, using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Fraxetin protects against bone loss
induced by wear particles
A Ti particle-induced calvarial osteolysis model was
used to simulate PO. Analysis of parietal bones by µ-CT
revealed extensive bone resorption in mice exposed to Ti particles
(vehicle group; received Ti injection) compared with the negative
controls (sham group; received PBS only injection; Fig. 1B). Bone resorption was lower in
mice administered a 5 mg/kg/day concentration of fraxetin compared
with the vehicle group (Fig. 1B),
demonstrating that fraxetin suppressed osteolysis induced by
Ti-particles. Quantification of bone parameters revealed that
exposure to fraxetin increased bone volume (Fig. 1C) and decreased porosity (Fig. 1D and E) compared with the vehicle
group.
Histological assessment results demonstrated that
fraxetin administration prevented parietal bones from Ti
particle-induced osteolysis. H&E staining revealed few
osteolytic changes in the sham group mice. Multiple osteolytic
changes occurred in the vehicle group, whereas fraxetin
administration reversed opposed this effect (Fig. 1B). Accordingly, TRAP staining
revealed that the number of OCLs was increased following Ti
particle stimulation, while fraxetin administration reversed this
effect (Fig. 1B and F). In
summary, fraxetin could prevent wear particle-induced bone loss by
suppressing osteoclastogenesis.
Fraxetin inhibits osteoclast formation
and function in vitro
Based on the aforementioned in vivo results,
it was hypothesized that fraxetin can inhibit osteoclastogenesis
in vitro. The role and mechanism of fraxetin in normal
osteoclast formation and bone resorption function were therefore
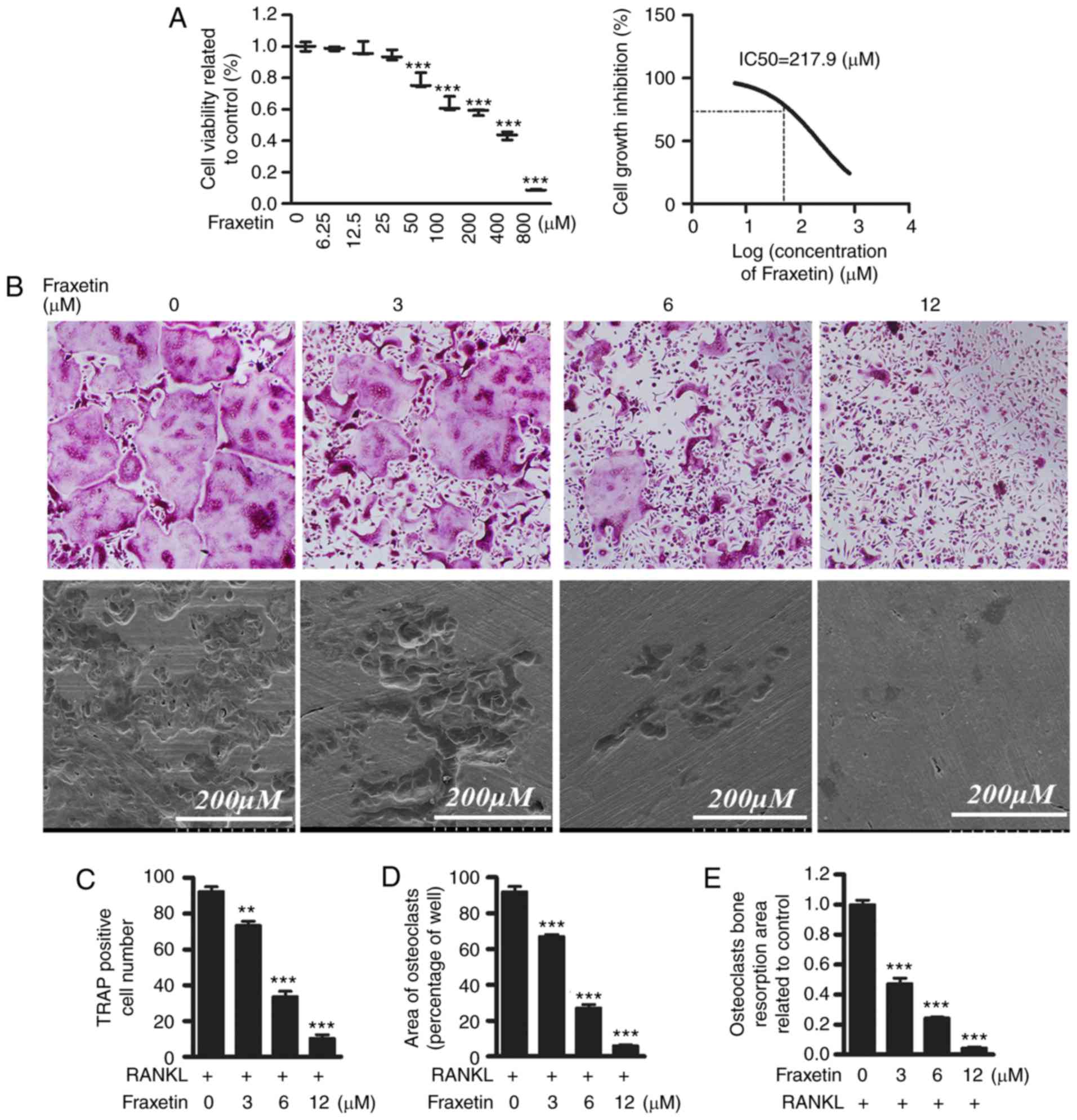
explored further in vitro. First, CCK-8 assays were
performed in order to exclude the possibility that a potential
cytotoxic effect of fraxetin may be responsible for the decrease in
OCL cell numbers. The IC50 value of fraxetin was 217.9
µM at 48 h, suggesting that fraxetin might suppress the
proliferation of BMM cells at concentrations >217.9 µM
(Fig. 2A). Then, differentiated
BMMs were induced into TRAP-positive OCL cells (Fig. 2B). Exposure to fraxetin decreased
osteoclast formation in a dose dependent manner. The numbers of
TRAP-positive OCL cells was suppressed by ~30% in the presence of 3
µM fraxetin (Fig. 2B–D),
and was almost completely suppressed by exposure to fraxetin at
concentrations >12 µM (Fig.
2 B–D). Since the IC50 value of fraxetin was
demonstrated to be 217.9 µM, fraxetin could not have
affected the proliferation of BMMs at doses 6–12 µM,
suggesting that at these latter concentrations of fraxetin impaired
osteoclast formation without cell cytotoxicity.
Bone resorption is the most important function of
osteoclasts. Therefore, bone resorption assays were conducted in
vitro in order to evaluate the effect of fraxetin on bone
resorption. Scanning electron microscopy analysis revealed
osteoclastic bone resorption pits present in BMM cultures in the
presence of RANKL. Exposure of BMMs to 3 µM fraxetin
resulted in a reduction in resorptive activity >50%, and at
fraxetin concentrations >12 µM, resorption was completely
suppressed (Fig. 2B and E). These
results demonstrate that fraxetin administration reduced Ti
particle-induced bone resorption in the presence of RANKL in
vitro.
Fraxetin suppresses osteoclast activity
by downregulating osteoclast-specific gene expression
RT-qPCR results demonstrated that the mRNA
expression levels of TRAP, CTSK and NFATc1 were significantly
decreased following fraxetin treatment, compared with untreated
control cells, in a time- and dose-dependent manner (Fig. 3). These findings suggest that
fraxetin inhibited osteoclast differentiation by suppressing
osteoclast-specific gene expression.
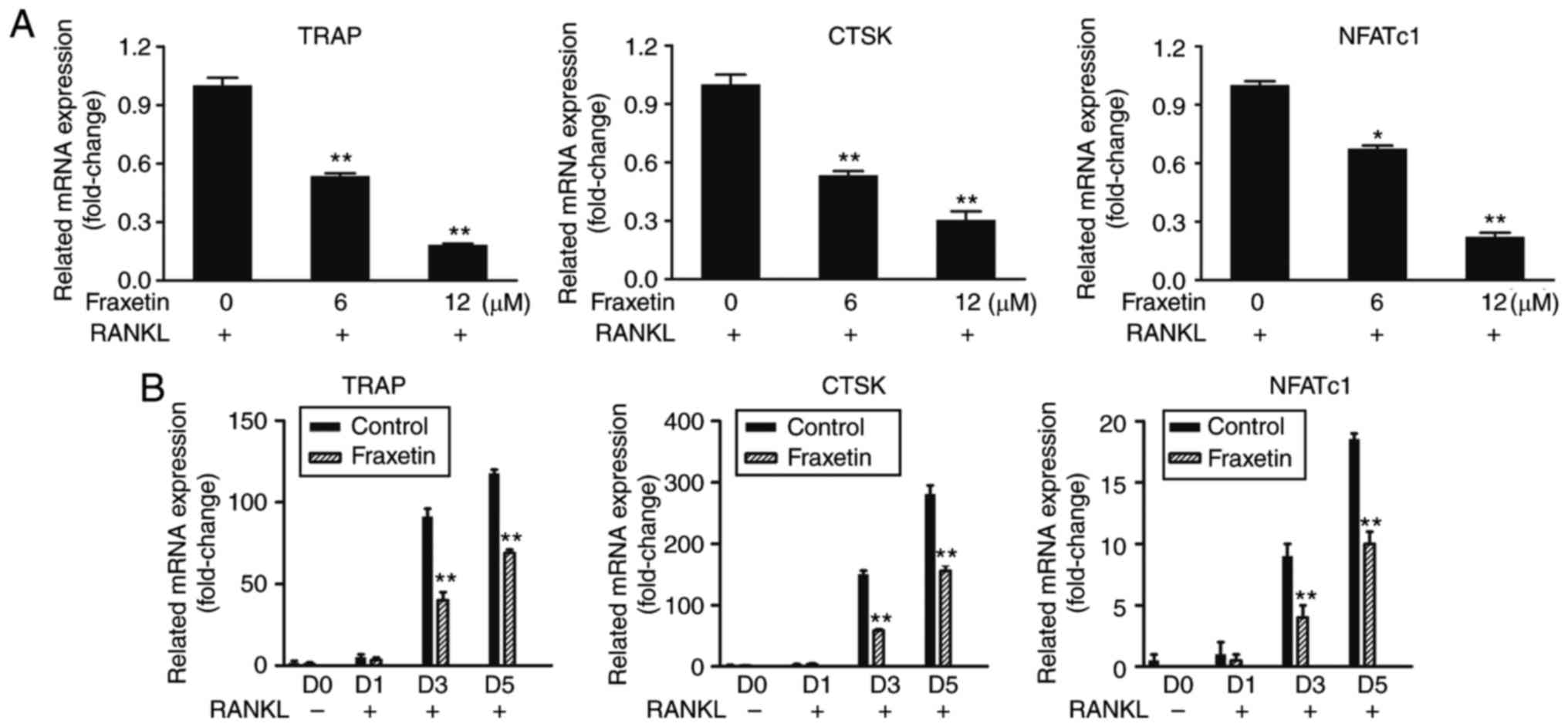 | Figure 3Relative mRNA expression of the
osteoclast-specific genes TRAP, CTSK and NFATc1. (A) BMMs were
cultured with complete α-MEM, 50 ng/ml RANKL, and the indicated
doses of fraxetin for 5 days. (B) BMMs were cultured with complete
α-MEM, 50 ng/ml RANKL and 12 µM fraxetin for 0, 1, 3, and 5
days. *P<0.05, **P<0.01 and
***P<0.001 vs. fraxetin free cells. TRAP,
tartrate-resistant acid phosphatase; CTSK, cathepsin K; NFATc1,
nuclear factor of activated T-cells, cytoplasmic 1; BMMs, bone
marrow-derived macrophages; RANKL, receptor activator of nuclear
factor-κB ligand. |
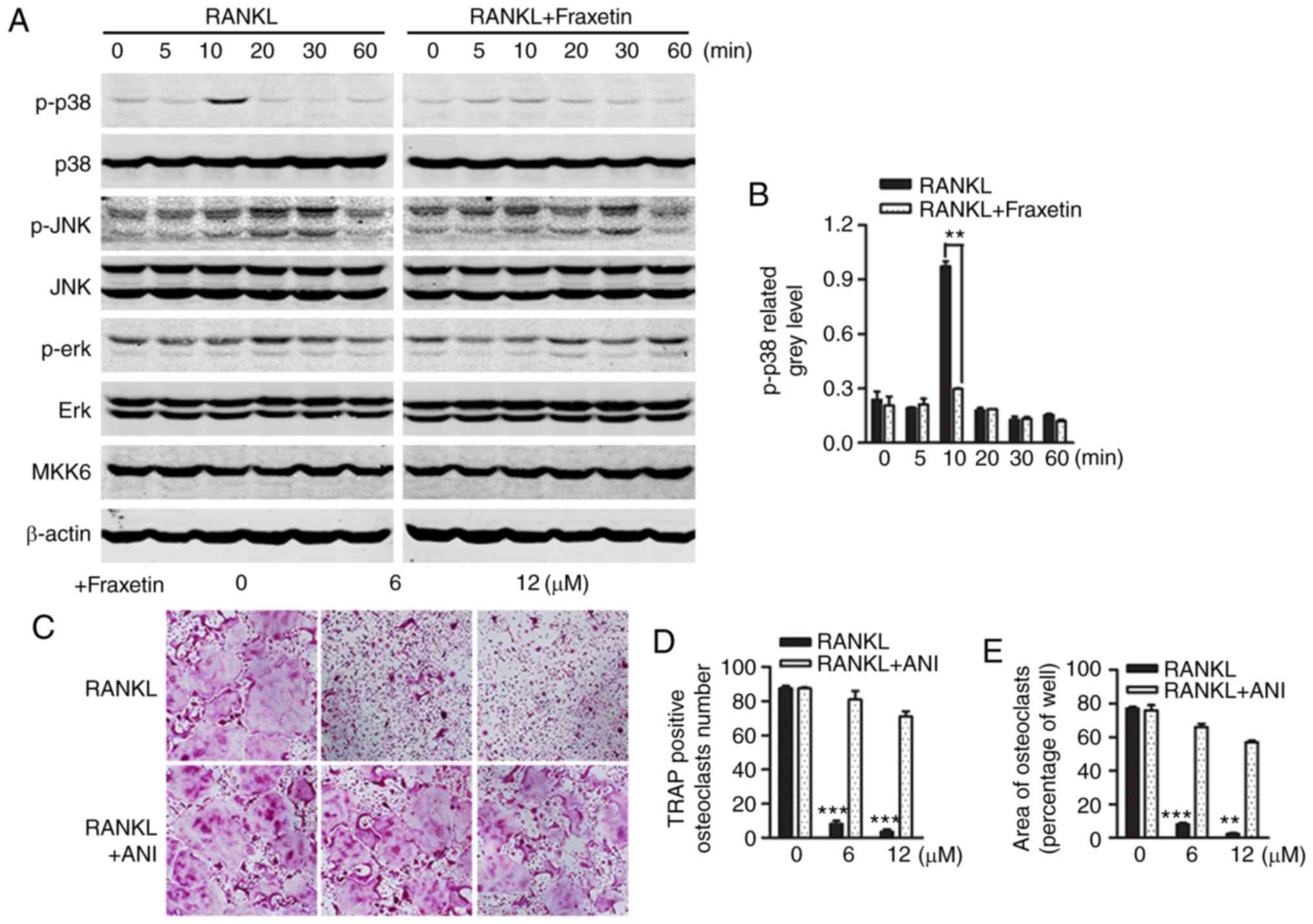
Fraxetin suppresses osteoclast activity
by specifically inhibiting the p38 signaling pathway
Osteoclasts were differentiated from monocytes and
macrophages in response to stimulation with M-CSF and RANKL. The
RAW264.7 cell line has good stability and can substitute for
preosteoclasts in vitro. Western blot analysis was conducted
in RAW264.7 cells in order to investigate the activity of signaling
pathways that determine osteoclast differentiation. Well known,
mitogen-activated protein kinase (MAPK) pathways (namely ERK1/2,
p38 and JNK1/2) have an important role in osteoclast
differentiation downstream of RANK signaling (25). The present western blot results
revealed that stimulation of RAW264.7 cells with RANKL induced peak
p38 phosphorylation within 10 min, and this effect was suppressed
following treatment with 12 µM fraxetin (Fig. 4A). Densitometry analysis of
western blot images confirmed the significant inhibition of p38
phosphorylation by fraxetin (Fig.
4B). To confirm these findings, BMMs exposed to fraxetin were
treated with anisomycin, a p38 agonist (21,22). In accordance with previous
observations, fraxetin inhibited osteoclast formation, whereas
anisomycin precluded this effect (Fig. 4C–E). Conversely, in both the
control and fraxetin-treated groups, ERK and JNK phosphorylation
were induced, and fraxetin treatment did not oppose this effect
(Fig. 4A). Furthermore, MKK6, an
upstream regulator of p38, was not affected by RANKL stimulation or
fraxetin treatment (Fig. 4A).
Taken together, these results demonstrated that fraxetin suppressed
osteoclast differentiation by inhibiting p38 signaling, whereas JNK
and ERK activity was not affected.
Discussion
Previous studies have reported that fraxetin is a
potential anti-osteolytic agent, based on its properties to inhibit
osteoblast apoptosis induced by inflammatory cytokines (14). However, osteoclasts serve an
important role in osteolytic diseases, and the effects of fraxetin
on these cells have not been characterized. In the present study,
fraxetin was demonstrated to suppress PO via inhibition of the
stimulatory effects of RANKL-induced p38 signaling on osteoclast
formation and bone resorption in vitro and vivo.
Thus, the present findings provide a mechanistic justification for
the application of fraxetin to the treatment of PO.
Results from the Ti particle-induced bone loss assay
indicated that the inhibition of osteoclastogenesis by fraxetin
in vivo may have an important role on the therapy of PO.
Additionally, the inhibitory effect of fraxetin on
osteoclastogenesis and bone resorption were further investigated
in vitro. The results demonstrated that fraxetin prevented
the stimulatory effects of RANKL through the p38 cascade. Activated
p38 signaling is a key procedure in bone destruction, while
suppression of p38 activity reduces osteoclast formation and bone
resorption (26,27). p38 is involved in the activation
of activator protein (AP)-1 components (28), but the molecular mechanisms of
their functions are not well understood. A major component of the
transcription factor AP-1 is Fos proto-oncogene (also known as
c-fos), which also induces NFATc1, a master regulator of
osteoclastogenesis, by binding to its promoter region (29,30). The activity of c-fos and NFATc1
has been reported to be regulated by p38 (26,29–32). In addition, the present study
demonstrated that MKK6, the kinase upstream of p38 (33), was unaffected by fraxetin
administration. It was therefore speculated that fraxetin may
prevent the stimulatory effects of RANKL by p38/c-fos/NFATc1
signaling, and subsequently inhibit osteoclastic-specific gene
expression (10).
Ultra-high molecular weight polyethylene, Ti, and
poly-methyl methacrylate (PMMA) are common particles present in
tissues around joint prostheses, which are important for today’s
joint replacement surgeons (34,35). Though metal particles are
relatively less frequently used, osteolysis induced by Ti particles
is typical (36,37). Thus, Ti particle-induced
osteolysis could be representative and suitable for studying the
effect of fraxetin on osteolysis caused by wear debris (16,17). The current results demonstrated
that fraxetin administration prevented Ti particle-induced
osteolysis in a mouse osteolysis model and did not result in any
obvious side effects, confirming that fraxetin inhibits osteoclast
differentiation and function in vivo and suggesting that it
may hold potential therapeutic benefits on PO.
The present study has several strengths. It is the
first that characterized the suppression effects of fraxetin on
osteo-clastogenesis. Chinese herbs are less prone to lead to drug
resistance and have fewer side effects compared with synthetic
drugs. Therefore, investigating the effects of fraxetin further may
be a promising approach to develop treatments for PO. The present
study also presents a limitation. PO is a complex process that
consists of multiple interactions between bone cells and
inflammatory reactions in vivo. However, the present study
only explored the mechanism for osteolytic diseases therapy through
the inhibition of osteoclast activities.
Collectively, the present findings demonstrated that
fraxetin administration improved PO in vitro and in
vivo, through the suppression of osteoclast development and
function via inhibition of the p38 signaling pathway. Therefore,
fraxetin may be a potential agent for the treatment of PO and other
osteolytic diseases.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
DZC designed the study; JCL, ZXW, and ZPM performed
the research; JCL and CZ contributed new reagents or analytical
tools; JCL, CZ and ZXW analyzed data; JCL wrote the study.
Ethics approval and consent to
participate
This study obtained ethics approval by the
Institutional Animal Care and Use Committee (IACUC) of Southern
Medical University (SMU; Guangzhou, China), and all experiments
involving animals were performed according to the guidelines of SMU
IACUC.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Helfrich MH: Osteoclast diseases and
dental abnormalities. Arch Oral Biol. 50:115–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abu-Amer Y, Darwech I and Clohisy JC:
Aseptic loosening of total joint replacements: Mechanisms
underlying osteolysis and potential therapies. Arthritis Res Ther.
9(Suppl 1): S62007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson JM, Rodriguez A and Chang DT:
Foreign body reaction to biomaterials. Semin Immunol. 20:86–100.
2008. View Article : Google Scholar
|
|
6
|
Long M and Rack HJ: Titanium alloys in
total joint replacement-a materials science perspective.
Biomaterials. 19:1621–1639. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holt G, Murnaghan C, Reilly J and Meek RM:
The biology of aseptic osteolysis. Clin Orthop Relat Res.
460:240–252. 2007.PubMed/NCBI
|
|
8
|
Udagawa N, Takahashi N, Akatsu T, Tanaka
H, Sasaki T, Nishihara T, Koga T, Martin TJ and Suda T: Origin of
osteo-clasts: Mature monocytes and macrophages are capable of
differentiating into osteoclasts under a suitable microenvironment
prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci
USA. 87:7260–7264. 1990. View Article : Google Scholar
|
|
9
|
Novack DV: Role of NF-kappaB in the
skeleton. Cell Res. 21:169–182. 2011. View Article : Google Scholar
|
|
10
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng X: RANKing intracellular signaling in
osteoclasts. IUBMB Life. 57:389–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Witaicenis A, Seito LN, da Silveira Chagas
A, de Almeida LD Jr, Luchini AC, Rodrigues-Orsi P, Cestari SH and
Di Stasi LC: Antioxidant and intestinal anti-inflammatory effects
of plant-derived coumarin derivatives. Phytomedicine. 21:240–246.
2014. View Article : Google Scholar
|
|
13
|
Murali R, Srinivasan S and Ashokkumar N:
Antihyperglycemic effect of fraxetin on hepatic key enzymes of
carbohydrate metabolism in streptozotocin-induced diabetic rats.
Biochimie. 95:1848–1854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo PL, Huang YT, Chang CH and Chang JK:
Fraxetin inhibits the induction of anti-Fas IgM, tumor necrosis
factor-alpha and interleukin-1beta-mediated apoptosis by Fas
pathway inhibition in human osteoblastic cell line MG-63. Int
Immunopharmacol. 6:1167–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin S, Park JY, Hong JM, Kim TH, Shin HI,
Park EK and Kim SY: Inhibitory effect of (-)-epigallocatechin
gallate on titanium particle-induced TNF-alpha release and in vivo
osteolysis. Exp Mol Med. 43:411–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu F, Zhu Z, Mao Y, Liu M, Tang T and Qiu
S: Inhibition of titanium particle-induced osteoclastogenesis
through inactivation of NFATc1 by VIVIT peptide. Biomaterials.
30:1756–1762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
von Knoch M, Jewison DE, Sibonga JD,
Sprecher C, Morrey BF, Loer F, Berry DJ and Scully SP: The
effectiveness of polyethylene versus titanium particles in inducing
osteolysis in vivo. J Orthop Res. 22:237–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SS, Woo CH, Chang JD and Kim JH: Roles
of Rac and cytosolic phospholipase A2 in the intracellular
signalling in response to titanium particles. Cell Signal.
15:339–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin A, Cheng TS, Lin Z, Cao L, Chim SM,
Pavlos NJ, Xu J, Zheng MH and Dai KR: Prevention of wear
particle-induced osteolysis by a novel V-ATPase inhibitor
saliphenylhalamide through inhibition of osteoclast bone
resorption. PloS One. 7:e341322012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wedemeyer C, Xu J, Neuerburg C,
Landgraeber S, Malyar NM, von Knoch F, Gosheger G, von Knoch M,
Löer F and Saxler G: Particle-induced osteolysis in
three-dimensional micro-computed tomography. Calcif Tissue Int.
81:394–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cano E, Hazzalin CA and Mahadevan LC:
Anisomycin-activated protein kinases p45 and p55 but not
mitogen-activated protein kinases ERK-1 and -2 are implicated in
the induction of c-fos and c-jun. Mol Cell Biol. 14:7352–7362.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hazzalin CA, Le Panse R, Cano E and
Mahadevan LC: Anisomycin selectively desensitizes signalling
components involved in stress kinase activation and fos and jun
induction. Mol Cell Biol. 18:1844–1854. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin C, Shao Y, Zeng C, Zhao C, Fang H,
Wang L, Pan J, Liu L, Qi W, Feng X, et al: Blocking PI3K/AKT
signaling inhibits bone sclerosis in subchondral bone and
attenuates post-traumatic osteoarthritis. J Cell Physiol.
233:6135–6147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Stevenson DA, Schwarz EL, Carey JC,
Viskochil DH, Hanson H, Bauer S, Weng HY, Greene T, Reinker K,
Swensen J, et al: Bone resorption in syndromes of the Ras/MAPK
pathway. Clin Genet. 80:566–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y
and Kim HH: Induction of c-Fos and NFATc1 during RANKL-stimulated
osteoclast differentiation is mediated by the p38 signaling
pathway. Biochem Biophys Res Commun. 351:99–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zwerina J, Hayer S, Redlich K, Bobacz K,
Kollias G, Smolen JS and Schett G: Activation of p38 MAPK is a key
step in tumor necrosis factor-mediated inflammatory bone
destruction. Arthritis Rheum. 54:463–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lucas JJ, Yamamoto A, Scearce-Levie K,
Saudou F and Hen R: Absence of fenfluramine-induced anorexia and
reduced c-Fos induction in the hypothalamus and central amygdaloid
complex of serotonin 1B receptor knock-out mice. J Neurosci.
18:5537–5544. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharma SM, Bronisz A, Hu R, Patel K,
Mansky KC, Sif S and Ostrowski MC: MITF and PU.1 recruit p38 MAPK
and NFATc1 to target genes during osteoclast differentiation. J
Biol Chem. 282:15921–15929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsumoto M, Kogawa M, Wada S, Takayanagi
H, Tsujimoto M, Katayama S, Hisatake K and Nogi Y: Essential role
of p38 mitogen-activated protein kinase in cathepsin K gene
expression during osteoclastogenesis through association of NFATc1
and PU.1. J Biol Chem. 279:45969–45979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
A 10-year update. Physiol Rev. 92:689–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirakawa K, Bauer TW, Stulberg BN and
Wilde AH: Comparison and quantitation of wear debris of failed
total hip and total knee arthroplasty. J Biomed Mater Res.
31:257–263. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baumann B, Seufert J, Jakob F, Nöth U,
Rolf O, Eulert J and Rader CP: Activation of NF-kappaB signalling
and TNFalpha-expression in THP-1 macrophages by TiAlV- and
polyethylene-wear particles. J Orthop Res. 23:1241–1248.
2005.PubMed/NCBI
|
|
36
|
Masui T, Sakano S, Hasegawa Y, Warashina H
and Ishiguro N: Expression of inflammatory cytokines, RANKL and OPG
induced by titanium, cobalt-chromium and polyethylene particles.
Biomaterials. 26:1695–1702. 2005. View Article : Google Scholar
|
|
37
|
Baumann B, Rader CP, Seufert J, Nöth U,
Rolf O, Eulert J and Jakob F: Effects of polyethylene and TiAlV
wear particles on expression of RANK, RANKL and OPG mRNA. Acta
Orthop Scand. 75:295–302. 2004. View Article : Google Scholar : PubMed/NCBI
|