1. Introduction
Genomic expression and regulation depends on the
canonical cooperation of coding and non-coding genes. Aberrant
activity of either directly leads to biological dysfunction or even
to disease, e.g. cancer. Considering the processes of
carcinogenesis, numerous mechanisms are capable of regulating
cancer development. Among them, non-coding RNA has been gradually
recognized over the past few decades as having a significant role
in cancer (1). In contrast to the
genes that code for proteins, non-coding RNA may regulate gene
expression during or after transcription as well as during
translation. Sharing common features with non-coding RNA, circular
RNA has recently been identified as a novel marker for cancer
(2).
Circular RNAs are a large class of endogenous RNA
occurring in all forms of life, including eukaryotes, prokaryotes,
microbes and viruses. Circular RNA is a type of RNA that is formed
by exon skipping or back-splicing events (3,4).
Unlike the well-known linear RNA, circular RNA is formed with a
covalently closed loop. This loop is formed by binding between the
3′ and 5′ ends in an RNA molecule. This circular feature confers
multiple properties to circular RNAs, several of which have only
been recently identified. In eukaryotic cells, circular RNA was
first identified in the cytoplasm by using electron microscopy in
1979 by Hsu and Coca-Prados (5).
However, this observation attracted little attention at the time,
as the circular RNA form was assumed to be a type of
transcriptional junk produced by mRNA splicing. In the early 1990s,
circular RNA was observed in higher eukaryotic cells (6). It was identified that the DCC netrin
1 receptor, a tumor suppressor gene, was transcribed into a lower
expression of the non-polyadenylated component of cytoplasmic RNA.
In addition, this transcript was indicated to be formed by exons
joining accurately at a consensus splice site in human cells. Nigro
et al (6) put forward the
term ‘exon scrambling’ for the first time, which means that RNA
splicing does not always follow the order predicted from the
positions of exons in the genome but may be spliced at any position
once there is a consensus splice site. Since then, an increasing
number of circular RNAs have been identified in eukaryotic cells,
with the mouse sex-determining region Y (SRY) gene being one of the
most commonly reported genes (7).
SRY is a gene for male determination with only one intron. During
mouse development, SRY genes may be translated into protein whilst
keeping their linear form, and in mature testes, the reverse
sequence located on the 2 sides of the SRY genes enable the
circularization of SRY, which is then stored with a non-coding
pattern in the cytoplasm.
With the wide use of high-throughput RNA-sequencing
technology and bioinformatics tools, an increasing number of
circular RNAs have been identified and characterized. Thus, the
stable expression of this RNA type in cells people has been
increasingly recognized and research now focuses on its role in
controlling or regulating physical and pathological processes. It
has been identified that circular RNA mainly exists in the
cytoplasm and that most circular RNAs are derived from exons, with
only a few are generated from introns. In 2012, Salzman et
al (8) started to develop an
algorithm in pediatric acute lymphoblastic leukemia patients via
using high-throughput RNA sequencing. They identified 1,232 genes
that were transcribed with exon scrambling. In addition, those
identified gene transcripts were also identified in peripheral
blood samples of patients, which further supported the general
feature of circular RNA expression patterns in human cells. Of
note, this group also identified that the expression of circular
RNA is highly evolutionarily conserved, although the circular RNA
expressed varies among different cell types or species. In
addition, circular RNA expression profiles, the ratio between
circular and linear RNA and the pattern of spliced isoforms is
cell-specific (9). Later, Jeck
and Sharpless (4) and Jeck et
al (10) identified
>25,000 circular isoforms that are transcribed from 14% of the
actively expressed genes in fibroblasts. Due to the RNA splicing
pattern, one heterogeneous nuclear RNA may be spliced into numerous
circular isoforms. Due to the lack of 5′ and 3′ ends, circular RNAs
are more resistant to exonuclease-mediated degradation and are more
stable than most linear RNAs in cells. Hence, the circular RNAs are
theoretically expressed at higher levels than their associated
linear RNAs. The abundance of circular RNA has been reported to be
>10-fold of that of the associated linear RNA (4,10).
These results indicate that circular RNAs may serve as novel
biomarkers for various diseases.
The cell is the basic unit of life. While cell
behavior relies on reasonable genomic control, aberrant gene
regulation directly leads to cellular disorder. Cancer is a disease
of genomic instability. While numerous treatments are available,
cancer is a disease class comprising a cell system with dynamic
genomic changes and the ability to evade immune surveillance, which
enables cancer to survive. In addition, this system may
evolutionarily evolve in order to sustain and progress its
malignant phenotype. Circular RNA has been indicated to be involved
in numerous types of cancer. Circular RNA is highly stable and
evolutionarily conserved. Regarding cancer, numerous studies have
reported that circular RNA may be differentially spatiotemporally
expressed according to the different status of cells.
High-throughput RNA sequencing suggested that a variety of circular
RNAs are significantly dysregulated in cancer tissues when compared
with those in adjacent tissues. For instance, circHIPK3 expression
is decreased by ~79.5% in bladder cancer tissues when compared with
that in normal bladder tissues (11). Furthermore, circMTO1 expression
was decreased by 87.4% in hepatocellular carcinoma tissue compared
with that in matching non-tumorous tissues (12). At the same time, the level of
circHIPK3 in bladder cancer patients and that of circMTO1 in
hepatocellular carcinoma patients was highly correlated with the
pathological stage and prognosis. In addition, it has been
determined that circular RNAs are more abundant in exosomes than in
cells, which suggests that circular RNAs may serve as promising
cancer biomarkers (13). Li et
al (14) identified >1,000
circular RNAs in human serum exosomes. Circular RNAs originating
from human cancer cells may enter the circulation via participating
in exosome packing, which may be readily measured. Of note, those
circRNAs measured in exosomes may be used to differentiate between
colon cancer patients and healthy individuals. In addition, several
studies have proven that circular RNAs may be used as biomarkers in
cancer (2,15–17). It is therefore indicated that
circular RNAs may be used as biomarkers in cancer diagnosis and
prognosis. Furthermore, several circular RNAs have been proven to
be ultimately important in controlling cancer cell fate, e.g.
overexpressed ciRS-7, which promotes colon cancer carcinogenesis
via activating the epidermal growth factor
receptor/RAF1/mitogen-activated protein kinase pathway (18,19), while circMYLK promotes bladder
cancer progression through modulation of the vascular endothelial
growth factor A (VEGFA)/VEGF receptor 2 (VEGFR2) signaling pathway
(20). These results suggest that
certain circular RNAs may be regarded as potential therapeutic
targets in cancer. Hence, the present review not only focused on
the role of circular RNA as a biomarker in cancer but also
discussed its biological roles in cancer.
2. Biogenesis of circular RNAs
Compared with the prokaryotic genes, eukaryotic
genes are split by a series of non-coding sequences known as
introns. In eukaryotes, when the gene is transcribed from DNA into
mature mRNA, the introns are removed, leaving just the exons in the
mature mRNA. Subsequently, the remaining exons are translated into
proteins (21). The intron
removal procedure during this process is known as canonical
splicing. With the discovery of circular RNA, it has become
apparent that it may be formed via gene splicing, but in a
non-canonical way. During non-canonical splicing, circular RNA is
synthesized by canonical splicing combined with back-splicing
(22,23).
Similar to linear RNA, circular RNA is also derived
from pre-mRNA. The actual mechanisms of its biogenesis are
presented in Fig. 1. According to
the constituent sections, circular RNA may be classified into four
types, including exonic circular RNA, intronic circular RNA,
exon-intron circular RNA and tRNA intronic circular RNA (22). During pre-mRNA splicing, RNA
binding proteins or the ALU reverse complementary sequence bridge
the two flanking introns, joining them together and allowing the
downstream 5′ end (the splice donor) to be connected to the
upstream 3′ end (the splice acceptor) (10,24,25). Later, by back-splicing in
combination with canonical splicing, the exonic circular RNA is
formed. Furthermore, if only back-splicing occurs with an intron
retained, an exon-intron circular RNA is generated. In addition,
exonic and exon-intron circular RNA may also be generated by exon
skipping (25). A partially
folded pre-mRNA brings the original and non-adjacent exon close to
another exon. Subsequently, exon skipping occurs, during which
flanking regions (containing ALU paring) at the ends of the 2 exons
are joined by canonical splicing, thus forming exonic circular RNA.
Similarly, if an intron is still retained, exon-intron circular RNA
is generated. Intronic circular RNA is derived from the lariat
intron that is excised from pre-mRNA by the canonical splicing
machinery (26). The excised
lariat intron containing a 7-nt GU-rich element near the 5′ splice
site and an 11-nt C-rich element near the 3′ tail of the
branchpoint site may undergo circularization. While this structure
enables the formation of intronic circular RNA, it also helps the
excised lariat intron to escape debranching and exonucleolytic
degradation. tRNA intronic circular RNAs are generated from the
excised tRNA introns that are removed by tRNA splicing enzymes
(27). The 3′,5′-phosphodiester
linkage between the two termini of those excised tRNA introns
generates the tRNA intronic circular RNA.
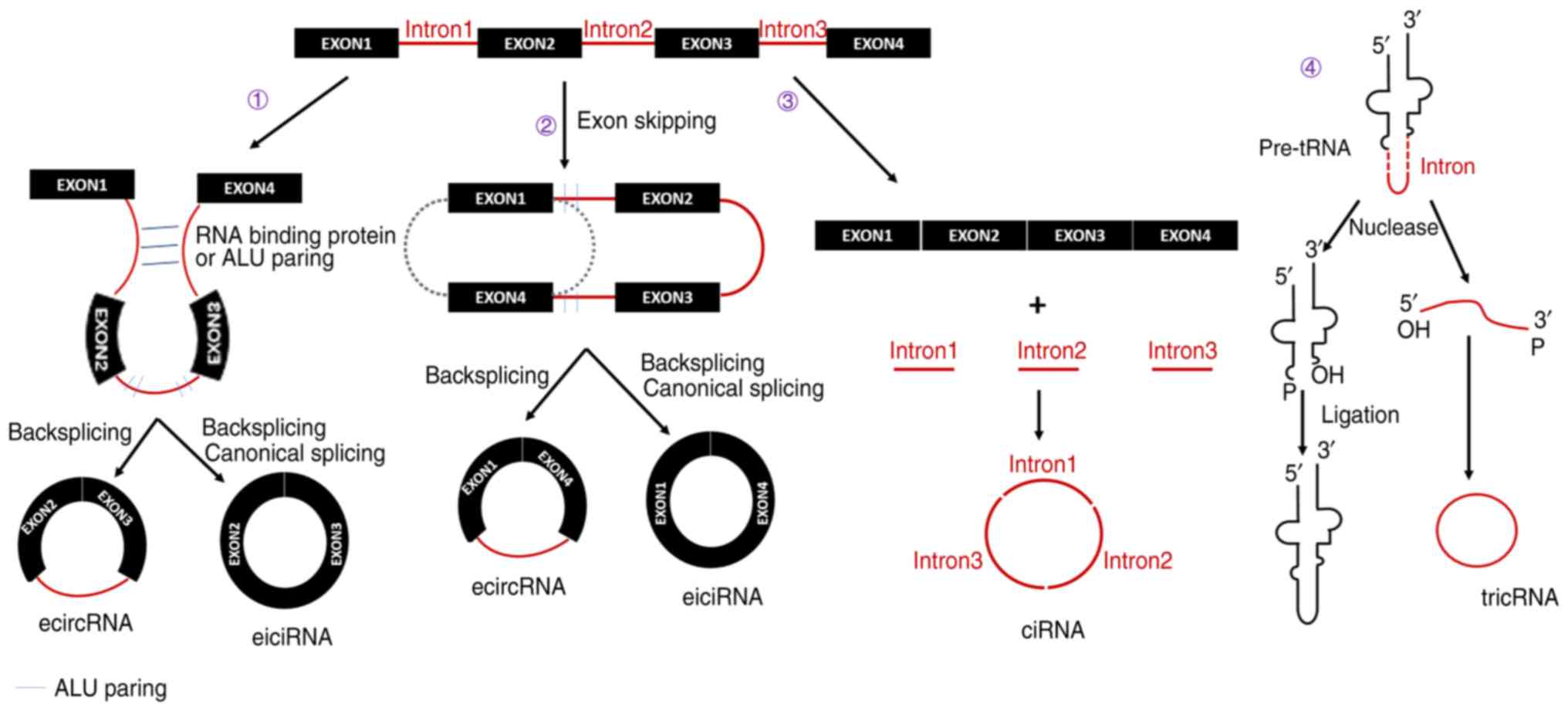 | Figure 1Biogenesis of circular RNA. Circular
RNA may be classified into four types: EcircRNA, ciRNA, eiciRNA and
tricRNA. Furthermore, there are four mechanisms of circular RNA
biogenesis: i) In pre-mRNA splicing, RNA-binding proteins or the
ALU reverse complementary sequence bridge the two flanking introns
and join them together, which allows the downstream 5′ end to be
connected to the upstream 3′ end. ii) A partially folded pre-mRNA
brings flanking regions (contains ALU paring) of the original and
non-adjacent exon close to another exon. Subsequently, exon
skipping occurs to join the ends of the 2 exons. iii) ciRNA is
derived from the lariat intron that is excised from pre-mRNA by the
canonical splicing machinery. iv) tricRNA are generated from the
excised tRNA introns that are removed by tRNA splicing enzymes. The
3′,5′-phosphodiester linkage between the two termini of those
excised tRNA introns generates the tricRNA. ecircRNA, exonic
circular RNA; ciRNA, intronic circular RNA; exon-intron circular
RNA, eiciRNA; tricRNA, tRNA intronic circular RNA. |
3. Function of circular RNAs in cancer
Hundreds of thousands of circular RNAs have been
identified through the widespread use of high-throughput
RNA-sequencing technology. As mentioned above, endogenous circular
RNAs may be classified into 4 types, and each type has different
functions to regulate gene expression. In general, the functions
may be divided into 4 categories: Protein binding, microRNA
sponging, and regulation of transcription and protein translation.
Among the four types of circular RNA, those with exons mainly exist
in the cytoplasm, where they exert post-transcriptional and
translational effects, while circular RNAs with introns are
predominantly retained in the nucleus, where they regulate gene
transcription and splicing. In this chapter, the role of circular
RNA in cancer with regard to the different functions of circular
RNA is highlighted (Table I).
 | Table IPotential circular RNAs in cancer
therapy and determination of their functions and roles. |
Table I
Potential circular RNAs in cancer
therapy and determination of their functions and roles.
| Circular RNA | Cancer type | Dysregulation | Action | Role | (Refs.) |
|---|
| ciRS-7 | Gastric cancer | Upregulated | Sponge for
miR-7 | Oncogene | (30) |
| circPVT1 | Gastric cancer | Downregulated | Sponge for
miR-125 | Inhibits invasion,
migration and angiogenesis | (31) |
| circLARP4 | Gastric cancer | Downregulated | Sponge for
miR-425-5p | Suppresses
migration and growth | (32) |
|
hsa_circ_0006528 | Breast cancer | Downregulated | Sponge for
miR-7-5p | Drug
resistance | (33) |
| circABCB10 | Breast cancer | Upregulated | Sponge for
miR-1271 | Promotes
proliferation and suppresses apoptosis | (34) |
| circTCF25 | Bladder cancer | Upregulated | Sponge for
miR-103a-3p/miR-107 | Promotes
proliferation and migration | (35) |
| circHIPK3 | Bladder cancer | Downregulated | Sponge for
miR-558 | Inhibits invasion,
migration, and angiogenesis | (11) |
| circMYLK | Bladder cancer | Upregulated | Sponge for
miR-29a | Promotes tumor
growth and metastasis | (20) |
|
hsa_circ_0001564 | Osteosarcoma | Upregulated | Sponge for
miR-29c-3p | Promotes
tumorigenicity | (36) |
|
hsa_circ_0016347 | Osteosarcoma | Upregulated | Sponge for
miR-214 | Promotes
proliferation, invasion and metastasis | (37) |
| circUBAP2 | Osteosarcoma | Upregulated | Sponge for
miR-2143 | Promotes growth and
inhibit apoptosis | (38) |
| circGLI2 | Osteosarcoma | Upregulated | Sponge for
miR-125b-5p | Promotes
proliferation, migration and invasion | (39) |
|
hsa_circ_0005986 | Hepatocellular
carcinoma | Downregulated | Sponge for
miR-129-5p | Impedes
progression | (40) |
|
hsa_circRNA_100338 | Hepatocellular
carcinoma | Upregulated | Sponge for
miR-141-3p | Promotes the
metastasis of hepatocellular carcinoma cells | (42) |
| ciRS-7 | Hepatocellular
carcinoma | Upregulated | Sponge for
miR-7 | Promotes
proliferation and invasion | (41) |
| circMTO1 | Hepatocellular
carcinoma | Downregulated | Sponge for
miR-9 | Suppresses
proliferation and invasion | (12) |
|
hsa_circ_000839 | Hepatocellular
carcinoma | Downregulated | Probably sponge for
miR-200b | Suppresses
proliferation and invasion | (43) |
| circITCH | Colorectal
cancer | Upregulated | Sponge for miR-7,
miR-17 and miR-214 | Promotes cancer
progression | (44) |
| ciRS-7 | Colorectal
cancer | Upregulated | Sponge for
miR-7 | Promotes
proliferation and invasion | (19) |
|
hsa_circ_0020397 | Colorectal
cancer | Upregulated | Sponge for
miR-138 | Promotes cancer
progression | (45) |
|
hsa_circ_001569 | Colorectal
cancer | Upregulated | Sponge for
miR-145 | Promotes
proliferation and invasion | (46) |
| circCCDC66 | Colorectal
cancer | Upregulated | Sponge for miR-33b,
miR-93 and miR-185 | Promotes
proliferation, migration and invasion | (47) |
|
hsa_circ_0013958 | Lung
adenocarcinoma | Upregulated | Sponge for
miR-134 | Promotes
proliferation and invasion, and inhibits apoptosis | (48) |
| circITCH | Lung cancer | Upregulated | Sponge for miR-7,
miR-17 and miR-214 | Promotes cancer
progression | (49) |
|
hsa_circ_100290 | Oral cancer | Upregulated | Sponge for
miR-29 | Promotes cell
growth | (50) |
| circTTBK2 | Glioma | Upregulated | Sponge for
miR-217 | Promotes
proliferation, migration and invasion; inhibits apoptosis | (51) |
|
hsa_circRNA_100395 | Papillary thyroid
carcinoma | Upregulated | Sponge for
miR-141-3p/miR-200a-3p | Promotes cancer
progression | (52) |
| circHIAT1 | Renal cancer | Upregulated | Sponge for
miR-195-5p/29a-3p/29c-3p | Enhances migration
and invasion | (53) |
| circITCH | Esophageal squamous
cell carcinoma | Upregulated | Sponge for miR-7,
miR-17 and miR-214 | Promotes cancer
progression | (54) |
| circFBXW7 | Glioma | Downregulated | Protein coding | Impedes
proliferation and induces cell cycle arrest | (57) |
| circFoxo3 | Cancer, especially
breast cancer | Downregulated | Interaction with
p53, MDM2 and Foxo3 | Triggers apoptosis
and promotes tumorigenesis | (59) |
| circZKSCAN1 | Hepatocellular
carcinoma | Downregulated | Cooperation with
ZKSCAN1 mRNA | Represses cancer
progression | (60) |
|
hsa_circ_0067934 | Esophageal squamous
cell carcinoma | Upregulated | Unknown | Promotes cell
growth | (61) |
| circZNF292 | Glioma | Upregulated | Unknown | Promotes
proliferation, migration and invasion; inhibits apoptosis | (62) |
| circAmot11 | Cancer | Upregulated | Induction of c-Myc
nuclear translocation | Promotes
tumorigenesis | (63) |
| circBANP | Colorectal
cancer | Upregulated | Unknown | Promotes gastric
cancer cell growth and migration | (65) |
Circular RNA sequesters micro (mi)RNA to
regulate cancer cells
miRNAs are small non-coding RNAs of ~21 nt in
length. They repress the translation of mRNA via directly targeting
their 3′-untranslated region by base-pairing and then triggering
mRNA cleavage, relying on the degree of complementarity. It has
been identified that circular RNA contains a region with a miRNA
response element, which may act as competing endogenous RNA to
reduce the binding between miRNA and target genes. The first sponge
circular RNA to be characterized was ciRS-7. It was determined that
ciRS-7 shares >73 binding sites with miRNA-7 (28). Their binding strongly decreased
the suppression of the downstream genes of miRNA-7. Ectopic
expression of ciRS-7 significantly mimics the phenotype of miRNA-7
knockdown. Therefore, it has been suggested that circular RNA may
serve as a miRNA antagonist or miRNA sponge. Since then, an
increasing number of circular RNA have been identified as having
roles as miRNA sponges, including circHIPK3, which has been
observed to bind to 9 miRNAs with 18 potential binding sites.
Silencing of circHIPK3 but not HIPK3 mRNA significantly inhibits
human cell growth (29). It may
be concluded that recent evidence supports that circular RNA
regulates various processes in cancer cells via sponging of
miRNAs.
The abovementioned miRNA-7 is a well-known tumor
suppressor, while ciRS-7 is able to act as a sponge for miRNA-7 and
impair the tumor suppressive role of miRNA-7. In gastric cancer, it
has been identified that miRNA-7-induced tumor suppression in
MGC-803 and HGC-27 cells may be blocked by ciRS-7, leading to a
more aggressive oncogenic phenotype via antagonizing the
inactivation of miRNA-7 mediated by the phosphatase and tensin
homologue (PTEN)/phosphoinositide-3 kinase (PI3K)/AKT pathway
(30). CircPVT1 is a transcript
that is derived from the PVT1 gene. It is upregulated in gastric
cancer tissue. Chen et al (31) reported that this transcript may
promote cell proliferation by acting as a sponge for members of the
miRNA-125 family. CircLARP4 was identified to suppress the
proliferation and invasion of gastric cancer cells. It has been
indicated that circLARP4 may act as a sponge for miRNA-424-5p,
which usually targets the large tumor suppressor kinase 1, a core
part of the Hippo signaling pathway, which functions as a tumor
suppressor in gastric cancer (32).
In breast cancer, Gao et al (33) have detected 3,093 circular RNAs,
18 of which are differentially expressed in adriamycin
(ADM)-resistant and native MCF-7 cells. By performing quantitative
real-time polymerase chain reaction (qPCR) analysis, they
identified that Homo sapiens (hsa)_circ_0006528 is
significantly upregulated in ADM-resistant cancer cells. When the
expression of hsa_circ_0006528 was interfered with by small
inhibitory RNA, the sensitivity of breast cancer cells to ADM
decreased significantly. At the same time, downregulation of
hsa_circ_0006528 was associated with an increased expression of
miRNA-7-5p. This suggests a regulatory role for the
hsa_circ_0006528/miR-7-5p/RAF1 axis in ADM-resistant breast cancer
(33). In addition, circABCB10
was reported to promote breast cancer proliferation and suppress
apoptosis via acting as a sponge for miRNA-1271 (34).
The first circular RNA defined in bladder cancer was
circTCF25. By performing microarrays with bladder carcinoma, 469
circular transcripts were identified to be dysregulated compared
with the levels in adjacent normal tissue. Multiple bioinformatic
approaches were used to select circular RNAs that are involved in
cancer-associated pathways via interactions with miRNA. Among those
dysregulated circular RNAs, circTCF25 was indicated to sequester
miRNA-103a-3p/miRNA-107. Overexpression of circTCF25 was associated
with downregulation of miRNA-103a-3p and miRNA-107, increased
expression of cyclin (CCN)-dependent kinase 6 (CDK6) and increased
cell proliferation and migration in vitro and in
vivo. Zhong et al (35) was the first to explore the
interactions between circular RNA and miRNA in bladder cancer.
Since then, further circular RNAs have been identified to have a
regulatory role in bladder cancer via acting as a sponge for
miRNAs. In bladder cancer tissues and cell lines, circHIPK3 is
significantly downregulated and negatively correlates with the
tumor grade and tumor cell invasion as well as lymph node
metastasis. Overexpression of circHIPK3 effectively inhibits
invasion, migration and angiogenesis of bladder cancer cells in
vitro, and suppresses bladder cancer growth and metastasis
in vivo. In addition, it has been identified that circHIPK3
contains two critical binding sites for miRNA-558, which are able
to abundantly sequester miRNA-558 and suppress the expression of
the miRNA-558 target heparanase (11). CircMYLK and VEGFA were reported to
be significantly upregulated in bladder cancer. Upregulation of
circMYLK accelerated cell proliferation, migration and tube
formation, and caused a rearrangement of the cytoskeleton of
bladder cancer cells, while at the same time promoting the growth,
angiogenesis and metastasis of bladder cancer xenografts in
vitro and in vivo. By contrast, knockdown of circ-MYLK
decreased cell proliferation and motility, and induced apoptosis.
Furthermore, upregulation of circMYLK promoted
epithelial-mesenchymal transition. These results suggest that
circMYLK has an important role in bladder carcinogenesis. To
further elucidate the underlying mechanism, it has been indicated
that circMYLK directly binds to miRNA-29a and attenuate the
suppression of its target VEGFA, which promotes cancer progression
through activation of VEGFA/VEGFR2 and the downstream
Ras/extracellular signal-regulated kinase pathway (20).
One of the most significantly overexpressed circular
RNAs in osteosarcoma is hsa_circ_001564. It has been reported that
knockdown of hsa_circ_001564 expression significantly suppressed
the cell proliferation, induced cell-cycle arrest in
G0/G1 phase and promoted apoptosis in
osteosarcoma cell lines (36). A
bioinformatics analysis indicated that hsa_circ_0001564 shares 7
complementary binding regions with miRNA-29c-3p. Ectopic expression
of miRNA-29c-3p expression significantly reversed the oncogenic
effect of hsa_circ_001564. This result indicates that
hsa_circ_0001564 acts as a miRNA-29c-3p sponge to mediate
tumorigenicity in osteosarcoma (36). In addition, hsa_circ_0016347,
circUBAP2 and circGLI2 all have oncogenic roles in osteosarcoma. It
has been demonstrated that hsa_circ_0016347 promotes proliferation,
invasion and metastasis of osteosarcoma cells via sponging
miRNA-214 and upregulating the expression of caspase-1 (37). CircUBAP2 promotes the growth and
reduces the apoptotic rate of osteosarcoma cells via inhibiting the
expression of miRNA-143, thus enhancing the expression and function
of anti-apoptotic B-cell lymphoma 2 (Bcl-2), which is a direct
target of miR-143 (38). In
addition, circGLI2 promotes osteosarcoma cell proliferation,
migration and invasion by targeting miRNA-125b-5p (39).
In hepatocellular carcinoma, hsa_circ_0005986
functions as a miRNA sponge to repress tumorigenesis. The
expression levels of hsa_circ_0005986 in hepatocellular carcinoma
tissues were reported to be significantly lower than those in
adjacent non-tumorous tissues. Furthermore, downregulation of
hsa_circ_0005986 liberates miRNA-129-5p and decreases the
expression levels of the miRNA-129-5p-targeted gene Notch1, thus
accelerating cell proliferation by promoting the
G0/G1 to S phase transition (40). ciRS-7 expression is also
upregulated in hepatocellular carcinoma. It has been reported that
circ-7 promotes the proliferation and invasion of hepatocellular
carcinoma cells through targeting miRNA-7, which directly increases
CCNE1 and PI3K catalytic subunit δ expression. These data suggested
that circ-7 acts as an oncogene partly through targeting miRNA-7 in
hepatocellular carcinoma (41).
hsa_circRNA_100338 has also been indicated to be upregulated in
hepatocellular carcinoma. It acts as an endogenous sponge for
miRNA-141-3p to promote the metastasis of hepatocellular carcinoma
cells (42). By contrast,
circMTO1 acts as a sponge for miRNA-9 to suppress hepatocellular
carcinoma proliferation and invasion via promoting p21 expression
(12). hsa_circ_000839 is another
circular RNA that has been identified to be expressed at high
levels in hepatocellular carcinoma, but its function remains
elusive. Ras homolog family member A (RhoA) is a direct target of
miRNA-200b. Binding with miRNA-200b impacts the invasion and
migration of hepatocellular carcinoma cells, which is initiated by
RhoA. RhoA is positively correlated with hsa_circ_000839, but
inversely correlated with miRNA-200b. However, the correlation
between hsa_circ_000839 and miRNA-200b is negative. This suggests
that miRNA-200b mediates the association between the RhoA gene and
hsa_circ_000839 through endogenous competition (43). Further study is required to
validate the role of hsa_circ_000839 in hepatocellular carcinoma
and to verify the sponging function of hsa_circ_000839.
In colorectal cancer, circITCH expression is usually
low compared with that in the peritumoral tissue. CircITCH has a
sponging action on miRNA-7, miRNA-17 and miRNA-214, which in turn
increases the level of the target of these miRNAs, ITCH. ITCH is a
major protein in the Wnt/β-catenin pathway that has a significant
role in cancer progression. While phosphorylation of dishevelled
segment polarity protein 2 (Dv12) is required for Wnt signaling
stabilization, hyperexpression of ITCH promotes the ubiquitination
and degradation of phosphorylated Dvl2, thereby inhibiting the
Wnt/β-catenin pathway. Hence, these results indicate that circITCH
may have an inhibitory effect on colorectal cancer by regulating
the Wnt pathway (44). Similarly,
Weng et al (19) reported
that ciRS-7 may be a potential miRNA-7 sponge in colorectal cancer.
It has been indicated that elevated ciRS-7 levels in colorectal
cancer may act as a sponge for miRNA-7, thus directly impairing the
tumor-suppressive effect of miRNA-7. Sponging of miRNA-7 then leads
to the activation of the genes downstream of its target, including
as EGFR and RAF1 oncogenes, which finally contribute to a more
malignant phenotype (19).
Another circular RNA, hsa_circ_0020397, is upregulated in
colorectal cancer cells and promotes cancer progression via
sponging of certain miRNAs. It has been indicated that
hsa_circ_0020397 promotes the cell viability and invasion of
colorectal cancer cells and inhibits their apoptosis by enhancing
the expression of the miRNA-138 targets telomerase reverse
transcriptase and programmed death ligand 1 (45). hsa_circ_001569 was also reported
to be upregulated in colorectal cancer, acting as a positive
regulator in cell proliferation and invasion of colorectal cancer
cells by acting as a sponge for miRNA-145, which in turn
upregulates functional targets of miRNA-145, namely E2F5,
Bcl2-associated athanogene 4 and formin-like 2 (46). circCCDC66 has also been proven to
control multiple pathological processes in colon cancer, including
cell proliferation, migration, invasion and anchorage-independent
growth. It has been indicated that circCCDC66 acts as a sponge on
miRNA-33b, miRNA-93 and miRNA-185 via 93 binding sites. In
addition, it protects oncogenes, including DNA methyltransferase 3
β, enhancer of zeste 2 polycomb repressive complex 2 subunit, MYC
and Yes-associated protein 1 from miRNA attack (47). Taken together, these results
highlight the potential oncogenic role of circular RNA in cancer
progression and metastasis.
In addition, in other types of cancer, circular RNA
has also been indicated to have a significant role. In lung
adenocarcinoma, hsa_circ_0013958 is upregulated and promotes cell
proliferation and invasion, while inhibiting cell apoptosis. It has
been indicated that hsa_circ_0013958 acts as a sponge on miRNA-134,
and thus upregulates CCND1, a target of the oncogenic miRNA-134
that has a pivotal role in the development of lung adenocarcinoma
(48). Furthermore, as in
colorectal cancer, circITCH has also been proven to have a tumor
suppressor role in lung cancer. A previous study indicated that
circITCH acts as sponge for oncogenic miRNA-7 and miRNA-214 to
enhance the expression of Itchy E3 ubiquitin protein ligase (ITCH)
and thus suppress the activation of Wnt/β-catenin signalling
(49). In oral cancer,
hsa_circ_100290 promoted cell growth via sponging of miRNA-29,
which liberated CDK6 expression (50). CircTTBK2 is upregulated in glioma
tissues and cell lines. Enhanced expression of circTTBK2 promotes
cell proliferation, migration and invasion, while inhibiting
apoptosis. miRNA-217 is downregulated in glioma tissues and cell
lines. It has been reported that circTTBK2 acts as a miRNA-217
sponge, thus downregulating the expression of HNF1 homeobox B, a
direct target of miRNA-217. This suggests that circTTBK2 has an
oncogenic role in glioma cells (51). In human papillary thyroid
carcinoma, hsa_circRNA_100395 is one of the downregulated circular
RNAs. It has been identified that hsa_circRNA_100395 targets
miRNA-141-3p/miRNA-200a-3p, thus leading to dysregulation of 7
cancer-associated genes: E2F3, glutaminase, CCND2, PTEN, CCNG1,
cell division cycle (CDC)25B and zinc finger protein, FOG family
member 2. These results indicate that hsa_circRNA_100395 is
important in the pathogenesis of human papillary thyroid carcinoma,
but further study is required to elucidate its function and
mechanism of action on miRNA-141-3p/miRNA-200a-3p (52). In renal cancer, circHIAT1 is
downregulated. Wang et al (53) indicated that circHIAT1
participates in androgen receptor (AR)-mediated tumor progression.
In clear-cell renal cell carcinoma, AR suppressed circHIAT1
expression via regulating the expression of HIAT1, the host of
circHIAT1, at the transcriptional level. CircHIAT1 acts as a sponge
for miRNA-195-5p/29a-3p/29c-3p and increases the expression of the
miRNA-targeted gene CDC42 to enhance the migration and invasion of
clear-cell renal cell carcinoma cells. Targeting AR directly leads
to the activation of circHIAT1, which ultimately suppresses cancer
progression. Taken together, these results suggest that circHIAT1
serves as an inhibitor of metastasis via suppressing AR-mediated
migration and invasion of clear-cell renal cell carcinoma cells. In
esophageal squamous cell carcinoma, circITCH has been proven to
suppress cancer progression via the same mechanisms as in
colorectal and lung cancer. CircITCH acts as a sponge for oncogenic
miRNA-7 and miRNA-214 to enhance ITCH expression and thus suppress
the activation of Wnt/β-catenin signalling (54).
Circular RNAs that may be translated into
proteins to regulate cancer cells
Most circular RNAs arise from the protein-coding
regions of genes. Although circular RNAs have been categorized as a
non-coding RNAs, recent studies have indicated that certain
circular RNAs may be translated into proteins in eukaryotic cells.
Wang et al (55) first
reported on the presence of consensus N6-methyladenosine (m6A)
motifs that are enriched in circular RNA. In addition, only a
single m6A site is sufficient to initiate translation in human
cells. Two years later, a study by the same group demonstrated that
the m6A-driven translation of circular RNAs is extensive and that
circular RNA translation requires the participation of the m6A
reader YTH N6-methyladenosine RNA binding protein 3 and the
translation initiation factors eukaryotic translation initiation
factor (eIF) 4 γ 2 and eIF 3 subunit A (56). This discovery extends the current
awareness of the functions of circular RNAs and suggests their
potential role in regulating gene expression. In human cancer,
circFBXW7 was the first circular RNA to be defined as encoding for
protein. It has been reported that circFBXW7 is abundantly
expressed in the normal human brain and encodes for a 21-kDa
protein termed F-box and WD repeat domain containing 7
(FBXW7)-185aa. It has been indicated that the levels of circ-FBXW7
and FBXW7-185aa were significantly reduced in glioblastoma compared
with the levels in paired tumor-adjacent samples. Upregulation of
FBXW7-185aa impedes cell proliferation and induces cell cycle
arrest, while FBXW7-185aa knockdown contributes to more malignant
phenotypes in vivo and in vitro. Furthermore,
FBXW7-185aa is able to reduce the half-life of c-Myc via
antagonizing ubiquitin-specific peptidase 28-induced c-Myc
stabilization (57). This
research further validates the phenomenon that endogenous circular
RNA may be translated into protein in human cells.
Circular RNAs that regulate cancer via
protein-binding patterns
Despite the above studies demonstrating that
circular RNAs act as microRNA sponges and are able to encode for
proteins, other biological functions of circular RNAs in cancer
remain largely elusive. Previously, circFoxo3 was reported to cause
cell cycle arrest by binding with cell cycle protein CDK2 and CDK
inhibitor 1 or p21, resulting in the formation of a ternary
complex. In general, CDK2 facilitates cell cycle entry via
interacting with CCNA and CCNE, while p21 inhibits these
interactions and induces cell cycle arrest. Hence, the ternary
complex formed by the circFoxo3-p21-CDK2 interaction interrupts the
function of CDK2 and then blocks cell cycle progression. This
result, for the first time, put forward the protein-binding
potential of circular RNA (58).
Later, a similar protein-binding pattern was also identified in
human cancer. Du et al (59) demonstrated this in a panel of
human cancer samples and cancer cells. They reported that circFoxo3
was minimally expressed in human cancer, particularly breast
cancer. During cancer cell apoptosis, circFoxo3 expression was
significantly elevated. Silencing of endogenous circ-Foxo3
expression enhanced cancer cell viability, whereas induction of
ectopic circ-Foxo3 triggered stress-induced apoptosis and inhibited
tumor xenograft growth. Furthermore, it has been indicated that
circFoxo3 increased the level of forkhead box (Fox)o3 protein but
repressed that of p53. MDM2 has a significant role in repressing
apoptosis via repression of the expression of Foxo3, p53 and the
Foxo3 downstream molecule p53 upregulated modulator of apoptosis
(Puma). In addition, circFoxo3 has been identified to exert its
function through binding with MDM2, p53 or their complex. By
binding to MDM2 and p53 together, circFoxo3 promotes the
ubiquitination of p53 induced by MDM2 and subsequently leads to p53
degradation. In addition, due to the lower binding affinity between
Foxo3 and circFoxo3, circFoxo3 prevents Foxo3 ubiquitination and
degradation induced by MDM2 binding, resulting in increased levels
of Foxo3 protein and expression of its downstream molecule Puma.
This in turn promotes cell apoptosis (59).
Roles of other circular RNAs in
cancer
hsa_circ_0067934 is upregulated in esophageal
squamous cell carcinoma. In vitro silencing of
hsa_circ_0067934 inhibits the proliferation and migration of
esophageal squamous cell carcinoma cells and blocks cell cycle
progression (60). CircZNF292 has
a critical role in the progression of human glioma. It has been
identified that silencing of circZNF292 significantly suppresses
tube formation by inhibiting cell proliferation and inducing cell
cycle arrest (61). Yang et
al (62) reported that
circAmot11 promotes tumorigenesis by inducing the nuclear
translocation of c-Myc. CircAmotl1 is highly expressed in cancer.
Ectopic expression of circAmotl1 increases the retention of nuclear
c-Myc, which is then able to enhance the stability of c-Myc and
upregulate the targets of c-Myc by increasing the affinity between
c-Myc and the promoter of its targets. CircBANP is overexpressed in
colorectal cancer tissues. Knockdown of circBANP significantly
attenuates colorectal cancer cell proliferation (63). Furthermore, hsa_circ_0000096 is
significantly downregulated in gastric cancer tissues and cell
lines, and is able to promote gastric cancer cell growth and
migration by regulating CCND1, CDK6, matrix metalloproteinase
(MMP)-2 and MMP-9 (64). In
hepatocellular carcinoma, circ-ZKSCAN1 expression is significantly
lower compared with that in matched adjacent normal tissues. It has
been indicated that decreased levels of CircZKSCAN1 are only
associated with tumor size. Silencing of zinc finger with KRAB and
SCAN domains 1 (ZKSCAN1) mRNA and circZKSCAN1 together promotes the
proliferation, migration and invasion of hepatocellular carcinoma
cells, while overexpression of ZKSCAN1 mRNA and circZKSCAN1
represses the progression of hepatocellular carcinoma cells in
vivo and in vitro. However, these effects were not
present if only one of the two molecules was silenced. In addition,
circZKSCAN1 is thought to mediate several cancer-associated
signaling pathways, while ZKSCAN1 mRNA mainly regulates cellular
metabolism. Hence, these results revealed that the two
post-translational products (ZKSCAN1 mRNA and circZKSCAN1) may
cooperate closely to regulate the growth, migration and invasion of
hepatocellular carcinoma cells (65). It is therefore suggested that
circular RNAs hold the potential to be applied as novel therapeutic
targets in cancer.
4. Circular RNAs as potential biomarkers in
cancer
Except for the above biological function of circular
RNAs in cancer, an increasing number of circular RNAs have been
identified as potential biomarkers in cancer. Certain biomarkers
are present in plasma, while others are detected in tissues. To
further clarify the role of circular RNAs in cancer, studies
indicating that circular RNAs may be able to serve as biomarkers in
cancer are further discussed below (Table II).
 | Table IICircular RNAs with potential as
biomarkers in cancer. |
Table II
Circular RNAs with potential as
biomarkers in cancer.
| Circular RNA | Cancer type | Dysregulation | Utility | Correlated or
predicted parameter | Sample | (Refs.) |
|---|
|
hsa_circ_0000745 | Gastric cancer | Downregulated | Diagnosis | Node
metastasis | Plasma | (66) |
|
hsa_circ_0001649 | Gastric cancer | Downregulated | Diagnosis | | Plasma | (67) |
|
hsa_circ_0000190 | Gastric cancer | Downregulated | Diagnosis | Tumor diameter,
lymphatic metastasis, distal metastasis, TNM stage and CA19-9
levels | Plasma | (68) |
|
hsa_circ_0014717 | Gastric cancer | Downregulated | Diagnosis | Tumor stage, distal
metastasis, tissue carcinoembryonic antigen and CA19-9
expression | Gastric juice | (16) |
|
hsa_circ_0004018 | Hepatocellular
carcinoma | Downregulated | Diagnosis | Serum AFP level,
tumor diameter, differentiation, BCLC stage and TNM stage | Tissue | (69) |
|
hsa_circ_0005986 | Hepatocellular
carcinoma | Upregulated | Diagnosis | Chronic hepatitis B
family history, tumor diameter, microvascular invasion and BCLC
stage | Tissue | (40) |
|
hsa_circ_0005075 | Hepatocellular
carcinoma | Downregulated | Diagnosis | Tumor size | Tissue | (70) |
| ciRS-7 | Hepatocellular
carcinoma | Upregulated | Diagnosis | Age <40 years,
serum AFP ≥400 ng/µl and hepatic microvascular invasion | Tissue | (71) |
|
hsa_circ_0001649 | Hepatocellular
carcinoma | Downregulated | Diagnosis | Tumor size and
occurrence of tumor embolus | Tissue | (72) |
|
hsa_circRNA_103809 | Colorectal
cancer | Downregulated | Diagnosis | Lymph node
metastasis, TNM stage and distal metastasis | Tissue | (73) |
|
hsa_circRNA_104700 | Colorectal
cancer | Downregulated | Diagnosis | Lymph node
metastasis, TNM stage and distal metastasis | Tissue | (73) |
|
hsa_circ_0004277 | Acute myeloid
leukemia | Downregulated | Diagnosis | Distinguishing
individuals with cancer from healthy individuals | Tissue | (74) |
|
hsa_circ_0013958 | Lung
adenocarcinoma | Upregulated | Diagnosis | Distinguishing TNM
III-IV vs. TNM I-II | Tissue | (48) |
|
hsa_circ_0067934 | Esophageal squamous
cell carcinoma | Upregulated | Diagnosis | Poor
differentiation | Tissue | (61) |
| hsa_circ_006054,
hsa_circ_100219 and hsa_circ_406697 | Breast cancer | Upregulated | Diagnosis | Combined together
to distinguish individuals with cancer from healthy
individuals | Tissue | (75) |
| circMTO1 | Hepatocellular
carcinoma | Downregulated | Prognosis | Poor overall
survival | Tissue | (12) |
| circFBXW7 | Glioblastoma | Downregulated | Prognosis | Increased overall
survival | Tissue | (57) |
| circRNA_100338 | Hepatocellular
carcinoma | Downregulated | Prognosis | Increased overall
survival, determination of metastasis and tumor grades | Tissue | (42) |
|
hsa_circ_0006633 | Gastric cancer | Upregulated | Diagnosis | Distal metastasis
and tissue carcinoembryonic antigen level | Tissue | (76) |
|
hsa_circ_0058246 | Cancer | Upregulated | Diagnosis | Lymph node
metastasis and recurrence | Tissue | (77) |
| circPVT1 | Gastric cancer | Upregulated | Prognosis | Late T-stage (T4)
tumors and positive neural invasion, shorter overall survival | Tissue | (31) |
| ciRS-7 | Colorectal
cancer | Upregulated | Prognosis | Poorer overall
survival | Tissue | (19) |
Plasma-based diagnostic biomarkers
Based on data from plasma samples from gastric
cancer patients, hsa_circ_0000745 is downregulated in patients with
gastric cancer compared with that in healthy control individuals.
It has been reported that the plasma levels of hsa_circ_0000745 are
correlated with the tumor-node-metastasis stage with an area under
the receiver operating characteristic curve (AUC) of 0.683. At the
same time, combination of the plasma hsa_circ_0000745 levels with
the carcinoembryonic antigen (CEA) levels increased the AUC to
0.775. These results suggest that hsa_circ_0000745 may be used as a
potential diagnostic biomarker for gastric cancer (66). In addition, hsa_circ_0001649 is
significantly downregulated in gastric cancer tissues compared with
that in paired adjacent normal tissues. However, in serum,
hsa_circ_0001649 is significantly upregulated after surgery. The
AUC in the study was 0.834, which suggests that hsa_circ_0001649
holds potential value as a diagnostic biomarker (67). hsa_circ_0000190 is also
downregulated in gastric cancer tissues and plasma samples. The
expression levels of hsa_circ_0000190 are significantly correlated
with the tumor diameter, lymphatic metastasis, distal metastasis,
tumor-nodes-metastasis (TNM) stage and carbohydrate antigen
(CA)19-9 levels in gastric cancer. The AUC for hsa_circ_0000190 in
tissues and plasma reached 0.75 and 0.60, respectively. When
combining tissue and plasma levels, the sensitivity and specificity
reached 0.712 and 0.750, respectively, and at the same time, the
AUC increased to 0.775 (68).
Furthermore, hsa_circ_0014717 was reported to be significantly
downregulated in 77.2% of gastric cancer tissues. Its levels in
gastric cancer tissues are associated with the tumor stage, distal
metastasis, and the tissue levels of CEA and CA19-9. More
importantly, hsa_circ_0014717 may stably exist in human gastric
juice, which suggests that it may serve as a potential human fluid
biomarker. Hence, further study is urgently required to elucidate
the role of hsa_circ_0014717 in gastric juice in predicting the
clinical features of gastric cancer (16).
Tissue-based diagnostic biomarkers
The hsa_circ_0004018 levels in hepatocellular
carcinoma tissue are significantly lower than those in
para-tumorous tissue. It has been suggested that hsa_circ_0004018
levels are correlated with the serum α-fetoprotein (AFP) levels,
tumor diameter, differentiation, the Barcelona Clinic Liver Cancer
(BCLC) stage and the TNM stage. At the same time, comparison of the
levels of hsa_circ_0004018 among hepatocellular carcinoma and
para-tumorous tissues, as well as liver tissues from patients with
chronic hepatitis, indicates a diagnostic value for
hsa_circ_0004018 with an AUC of 0.848 and a sensitivity and
specificity of 0.716 and 0.815, respectively (69). Similarly, hsa_ circ_0005986 is
downregulated in hepatocellular carcinoma. Its low expression has
been proven to be correlated with a family history of chronic
hepatitis B, tumor diameter, microvascular invasion and BCLC stage
(40). By contrast,
hsa_circ_0005075 has been reported to be significantly upregulated
in hepatocellular carcinoma. Elevated hsa_circ_0005075 expression
correlates with the tumor size of hepatocellular carcinoma and has
a good diagnostic potential, with an AUC of 0.94 (70). CiRS-7 is significantly elevated in
hepatocellular carcinoma. In patients with ‘an age of <40 years,
serum AFP ≥400 ng/µl and hepatic microvascular invasion’, a high
correlation with the expression of ciRS-7 was identified with a
cut-off value of 0.135 ng/ml (71). In addition, hsa_circ_0001649
expression is also significantly downregulated in hepatocellular
carcinoma with an AUC of 0.63. Its expression is correlated with
tumor size and the occurrence of tumor embolus (72).
In colorectal cancer, Zhang et al (73) reported that hsa_circRNA_103809 and
hsa_circRNA_104700 levels were significantly decreased compared
with those in normal tissues. Lower expression of
hsa_circRNA_103809 was identified as a significant predictive
factor of lymph node metastasis, TNM stage and distal metastasis.
The AUCs for hsa_circRNA_103809 and hsa_circRNA_104700 were 0.699
and 0.616, respectively. Although their AUCs appear not to be very
good currently, the above data does indicate that
hsa_circRNA_104700 and hsa_circRNA_103809 could serve as biomarkers
for the diagnosis of colorectal cancer (73). Therefore, enlarging the testing
samples is required to furtherly evaluate the potential role of
hsa_circRNA_104700 and hsa_circRNA in cancer diagnosis.
In acute myeloid leukemia (AML), hsa_circ_0004277 is
downregulated, and its levels may be used to distinguish
individuals with AML from healthy individuals with an AUC of 0.957.
In addition, hsa_circ_0004277 displays dynamic expressional changes
according to the progressive stage of AML. Compared with the levels
in healthy controls, lower levels of hsa_circ_0004277 have been
identified in newly diagnosed AML patients without prior treatment.
No significant differences in hsa_circ_0004277 levels were detected
between those patients who achieved complete remission and the
healthy controls, which indicates that the treatment restores
hsa_circ_0004277 expression. However, in relapsed or refractory
patients, downregulated hsa_circ_0004277 expression is observed
again. Hence, these results suggest that hsa_circ_0004277 has a
promising potential to assist with the diagnosis and follow-up of
AML patients (74).
Circular RNA also has a promising role as a
diagnostic biomarker in other types of cancer. In lung
adenocarcinoma, it has been indicated that the expression of
hsa_circ_0013958 is highly associated with TNM stage III/IV
compared with TNM stage I/II, with an AUC of 0.874 and with a
sensitivity and specificity of 0.762 and 0.857, respectively. In
addition, in plasma, the AUC is 0.794, and the sensitivity and
specificity are 0.667 and 0.933, respectively. These results
indicate a promising potential for hsa_circ_0013958 in the
diagnosis of lung adenocarcinoma (48). In esophageal squamous cell
carcinoma, hsa_circ_0067934 is significantly overexpressed compared
with its levels in paired adjacent normal tissues. It has been
reported that higher levels of hsa_circ_0067934 are significantly
associated with poor differentiation of esophageal squamous cell
carcinoma patients, which suggests that hsa_circ_0067934 represents
a novel potential diagnostic biomarker for esophageal squamous cell
carcinoma (60). In breast
cancer, hsa_circ_006054, hsa_circ_100219 and hsa_circ_406697 are
downregulated compared with those in the adjacent non-tumorous
tissue. The combined expression levels of hsa_circ_006054,
hsa_circ_100219 and hsa_circ_406697 have a distinguished potential
for breast cancer diagnosis, with an AUC of 0.82 (75).
Tissue-based prognostic biomarkers
The expression of circMTO1 is significantly
decreased in hepatocellular carcinoma tissues when compared with
that in matched non-tumorous tissues. A Kaplan-Meier survival curve
analysis suggested that a decreased circMTO1 expression is
correlated with a shortened survival of patients. In addition,
76.89 ng/ml the cut-off value of circMTO1 may be used as an
efficient prognostic indicator of poor survival in hepatocellular
carcinoma patients (12). The
ability of circFBXW7 to encode for protein was mentioned above.
Compared with the levels in adjacent normal brain tissues,
circ-FBXW7 expression is significantly lower in glioblastoma
tissues. However, in glioblastoma patients, it has been indicated
that elevated levels of circFBXW7 are associated with an increased
total survival time compared with lower circ-FBXW7 expression. The
median survival time in the higher circFBXW7 expression group was
24.2 months, compared with 11.7 months in the group with lower
circFBXW7 expression (57). This
suggests a novel potential role for circFBXW7 in the prognosis of
glio-blastoma. CircRNA_100338 is upregulated in hepatocellular
carcinoma. As mentioned above, circRNA_100338 promotes cancer
metastasis via sequestering miRNA-141. A clinical study has
identified that hepatocellular carcinoma patients with lower
circRNA_100338 levels had survival rates of 72.0% compared with
42.9% in patients with higher circRNA_100338 levels. Furthermore,
they also indicated that the expression of circRNA_100338 was not
associated with age or sex but was significantly correlated with
numerous clinicopathological parameters of metastasis, including
the TNM stage, vascular invasion and lung metastasis. These results
indicate that circRNA_100338 affects the survival rate of
hepatocellular carcinoma patients by regulating cancer metastasis
(42).
sa_circ_0006633 is downregulated in 79.2% of gastric
cancer tissues compared with those in adjacent non-tumorous
tissues. Downregulation of hsa_circ_0006633 is positively
associated with distal metastasis and tissue CEA levels (76). hsa_circ_0058246 is elevated in
tumor specimens from patients, particularly those with lymph node
metastasis and recurrence (77).
As mentioned above, circPVT1 acts as a sponge for miRNA-125 to
promote cell proliferation in gastric cancer. Chen et al
(31) indicated that lower levels
of circPVT1 are a significant predictive factor for late T-stage
(T4) tumors and neural invasion. In addition, compared with the
patients with higher levels of circPVT1, patients with lower levels
of circPVT1 exhibited significantly shorter overall survival, with
a median survival of 20 vs. 46 months, and shorter progression-free
survival, with a median survival of 17 vs. 36 months. In colorectal
cancer, ciRS-7 has emerged as an independent risk factor for
overall survival. Overexpression of ciRS-7 was associated with
poorer patient survival (P=0.0224 and 0.0061 in the training and
validation cohorts, respectively), which suggest that higher ciRS-7
levels in colorectal cancer predict poorer overall survival
(19).
In a word, circular RNAs serve as novel biomarkers
that may be used in cancer screening based on non-invasive
biomarker detection. Unlike other biomarkers, circular RNAs have
advantages including greater specificity and sensitivity and higher
abundance, while they are just as accessible for detection. Of
note, some studies have reported that the AUC and the
specificity/sensitivity for circular RNA as a biomarker in cancer
were quite poor, however, with regard to its highly stability,
circular RNAs could be used in addition to or in combination with
other markers to enhance the accuracy and/or capacity of diagnosis
and prognosis.
5. Perspectives
Although circular RNA has been known for 3 decades,
it was not until the last 3 years that researchers have begun to
focus on this type of RNA. Circular RNAs are abundant and conserved
endogenous RNAs. Despite being transcribed from the same gene
locus, circular RNA is expressed more abundantly in cells than the
linear form. Circular RNAs have been proven to exhibit tissue or
cell type specificity and may regulate cell behavior through
multiple pathways. In cancer, numerous studies have indicated that
circular RNA may serve as a potential biomarker and therapeutic
target.
It has been demonstrated that differential
expression of certain circular RNA has the capacity to predict the
clinical outcomes of cancer patients. For instance, ciRS-7 and
circHIPK3 are able to predict the tumor grade, metastasis and drug
resistance of cancer patients, while other circular RNAs, including
circFBXW7, circMTO1 and ciRS-7 are prognostic indicators with the
ability to predict parameters including overall survival and
recurrence. In spite of the numerous advantages of circular RNAs as
biomarkers in cancer, several drawbacks, which make the application
of circular RNA difficult at present, still require addressing.
First, studies regarding circular RNA as biomarkers in cancer have
mostly assessed one single circular RNA with hundreds rather than
thousands of samples. Thus, it is more difficult to estimate the
potential of certain circular RNAs to serve as biomarkers in
cancer. To improve this, studies on the ability of multiple
circular RNAs to comprehensively analyze the general situation in
human patients are required. In future studies, an increased sample
size is also desirable. Furthermore, most studies performed qPCR to
compare the expression of circular RNAs between cancerous and
normal tissue. Tissue is a complex and consists of multiple cell
types; hence, qPCR on tissue samples may not accurately reflect the
expression of circular RNA in cancer cells or normal cells, as
cancer cells with decreased expression of circular RNA may be
diluted by adjacent cells with an increased expression to thereby
falsify the results. In situ hybridization is a procedure
that intuitively reflects the real level of circular RNA expression
in cancer cells within a tissue. Hence, in situ
hybridization experiments are urgently required to further verify
the differential expression of further circular RNAs in cancer.
Furthermore, circular RNA has 4 major functions in
cancer: Protein binding, microRNA sponging, and regulation of
transcription and protein translation. It has been suggested that
circular RNA regulates carcinogenesis and cancer progression via
these 3 processes. Aberrant expression of circular RNA directly
interferes with the biological features of cancer cells, including
their proliferation, cell cycle, apoptosis and metastasis. Hence,
these results indicate that circular RNA may serve as a potential
therapeutic target in cancer. However, as only few circular RNAs
have been identified, targeting the known circular RNAs is not
sufficient to control the entire intracellular change. In addition,
interference with one circular RNA may potentially cause cellular
changes beyond the intended one. Therefore, prior to using circular
RNA as a therapeutic target in cancer, further studies are required
elucidate their role in cancer and to identify additional circular
RNAs with a role in cancer.
Acknowledgments
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant nos. U1301221, 81572514, 814723
84, 81402106, 81372729 and 81272808).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
TL conceptualized this review, GZ and MH prepared
the tables, NJ professionally edited the manuscript and YS wrote
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing of interests
The authors declare that they have no competing
interests.
References
|
1
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He J, Xie Q, Xu H, Li J and Li Y: Circular
RNAs and cancer. Cancer Lett. 396:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koch L: RNA: Translated circular RNAs. Nat
Rev Genet. 18:272–273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nigro JM, Cho KR, Fearon ER, Kern SE,
Ruppert JM, Oliner JD, Kinzler KW and Vogelstein B: Scrambled
exons. Cell. 64:607–613. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Capel B, Swain A, Nicolis S, Hacker A,
Walter M, Koopman P, Goodfellow P and Lovell-Badge R: Circular
transcripts of the testis-determining gene Sry in adult mouse
testis. Cell. 73:1019–1030. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salzman J, Gawad C, Wang PL, Lacayo N and
Brown PO: Circular RNAs are the predominant transcript isoform from
hundreds of human genes in diverse cell types. PLoS One.
7:e307332012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar :
|
|
11
|
Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang
C, Liu D, Wang M, Wang L, Zeng F and Jiang G: CircHIPK3 sponges
miR-558 to suppress heparanase expression in bladder cancer cells.
EMBO Rep. 18:1646–1659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dou Y, Cha DJ, Franklin JL, Higginbotham
JN, Jeppesen DK, Weaver AM, Prasad N, Levy S, Coffey RJ, Patton JG
and Zhang B: Circular RNAs are down-regulated in KRAS mutant colon
cancer cells and can be transferred to exosomes. Sci Rep.
6:379822016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li P, Chen S, Chen H, Mo X, Li T, Shao Y,
Xiao B and Guo J: Using circular RNA as a novel type of biomarker
in the screening of gastric cancer. Clin Chim Acta. 444:132–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shao Y, Li J, Lu R, Li T, Yang Y, Xiao B
and Guo J: Global circular RNA expression profile of human gastric
cancer and its clinical significance. Cancer Med. 6:1173–1180.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han YN, Xia SQ, Zhang YY, Zheng JH and Li
W: Circular RNAs: A novel type of biomarker and genetic tools in
cancer. Oncotarget. 8:64551–64563. 2017.PubMed/NCBI
|
|
18
|
Hansen TB, Kjems J and Damgaard CK:
Circular RNA and miR-7 in cancer. Cancer Res. 73:5609–5612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weng W, Wei Q, Toden S, Yoshida K,
Nagasaka T, Fujiwara T, Cai S, Qin H, Ma Y and Goel A: Circular RNA
ciRS-7-A promising prognostic biomarker and a potential therapeutic
target in colorectal cancer. Clin Cancer Res. 23:3918–3928. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong Z, Huang M, Lv M, He Y, Duan C,
Zhang L and Chen J: Circular RNA MYLK as a competing endogenous RNA
promotes bladder cancer progression through modulating VEGFA/VEGFR2
signaling pathway. Cancer Lett. 403:305–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi Y: Mechanistic insights into precursor
messenger RNA splicing by the spliceosome. Nat Rev Mol Cell Biol.
18:655–670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen LL: The biogenesis and emerging roles
of circular RNAs. Nat Rev Mol Cell Biol. 17:205–211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: CircRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL
and Yang L: Complementary sequence-mediated exon circularization.
Cell. 159:134–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moore MJ and Proudfoot NJ: Pre-mRNA
processing reaches back to transcription and ahead to translation.
Cell. 136:688–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu Z, Filonov GS, Noto JJ, Schmidt CA,
Hatkevich TL, Wen Y, Jaffrey SR and Matera AG: Metazoan tRNA
introns generate stable circular RNAs in vivo. RNA. 21:1554–1565.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen
B, Luo Y, Lyu D, Li Y, Shi G, et al: Circular RNA profiling reveals
an abundant circHIPK3 that regulates cell growth by sponging
multiple miRNAs. Nat Commun. 7:112152016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan H, Li T, Jiang Y, Pan C, Ding Y, Huang
Z, Yu H and Kong D: Overexpression of circular RNA ciRS-7 abrogates
the tumor suppressive effect of miR-7 on gastric cancer via
PTEN/PI3K/AKT signaling pathway. J Cell Biochem. 119:440–446. 2018.
View Article : Google Scholar
|
|
31
|
Chen J, Li Y, Zheng Q, Bao C, He J, Chen
B, Lyu D, Zheng B, Xu Y, Long Z, et al: Circular RNA profile
identifies circPVT1 as a proliferative factor and prognostic marker
in gastric cancer. Cancer Lett. 388:208–219. 2017. View Article : Google Scholar
|
|
32
|
Zhang J, Liu H, Hou L, Wang G, Zhang R,
Huang Y, Chen X and Zhu J: Circular RNA_LARP4 inhibits cell
proliferation and invasion of gastric cancer by sponging miR-424–5p
and regulating LATS1 expression. Mol Cancer. 16:1512017. View Article : Google Scholar
|
|
33
|
Gao D, Zhang X, Liu B, Meng D, Fang K, Guo
Z and Li L: Screening circular RNA related to chemotherapeutic
resistance in breast cancer. Epigenomics. 9:1175–1188. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang HF, Zhang XZ, Liu BG, Jia GT and Li
WL: Circular RNA circ-ABCB10 promotes breast cancer proliferation
and progression through sponging miR-1271. Am J Cancer Res.
7:1566–1576. 2017.PubMed/NCBI
|
|
35
|
Zhong Z, Lv M and Chen J: Screening
differential circular RNA expression profiles reveals the
regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in
bladder carcinoma. Sci Rep. 6:309192016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song YZ and Li JF: Circular RNA
hsa_circ_0001564 regulates osteosarcoma proliferation and apoptosis
by acting miRNA sponge. Biochem Biophys Res Commun. 495:2369–2375.
2018. View Article : Google Scholar
|
|
37
|
Jin H, Jin X, Zhang H and Wang W: Circular
RNA hsa-circ-0016347 promotes proliferation, invasion and
metastasis of osteosarcoma cells. Oncotarget. 8:25571–25581.
2017.PubMed/NCBI
|
|
38
|
Zhang H, Wang G, Ding C, Liu P, Wang R,
Ding W, Tong D, Wu D, Li C, Wei Q, et al: Increased circular RNA
UBAP2 acts as a sponge of miR-143 to promote osteosarcoma
progression. Oncotarget. 8:61687–61697. 2017.PubMed/NCBI
|
|
39
|
Li JF and Song YZ: Circular RNA GLI2
promotes osteosarcoma cell proliferation, migration, and invasion
by targeting miR-125b-5p. Tumour Biol. 39:1010428317709991. 2017.
View Article : Google Scholar
|
|
40
|
Fu L, Chen Q, Yao T, Li T, Ying S, Hu Y
and Guo J: Hsa_ circ_0005986 inhibits carcinogenesis by acting as a
miR-129–5p sponge and is used as a novel biomarker for
hepatocellular carcinoma. Oncotarget. 8:43878–43888.
2017.PubMed/NCBI
|
|
41
|
Yu L, Gong X, Sun L, Zhou Q, Lu B and Zhu
L: The circular RNA Cdr1as act as an oncogene in hepatocellular
carcinoma through targeting miR-7 expression. PLoS One.
11:e01583472016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang XY, Huang ZL, Xu YH, Zheng Q, Chen
Z, Song W, Zhou J, Tang ZY and Huang XY: Comprehensive circular RNA
profiling reveals the regulatory role of the
circRNA-100338/miR-141–3p pathway in hepatitis B-related
hepatocellular carcinoma. Sci Rep. 7:54282017. View Article : Google Scholar
|
|
43
|
Wang BG, Li JS, Liu YF and Xu Q:
MicroRNA-200b suppresses the invasion and migration of
hepatocellular carcinoma by downregulating RhoA and circRNA_000839.
Tumour Biol. 39:1010428317719577. 2017. View Article : Google Scholar
|
|
44
|
Huang G, Zhu H, Shi Y, Wu W, Cai H and
Chen X: cir-ITCH plays an inhibitory role in colorectal cancer by
regulating the Wnt/beta-catenin pathway. PloS One. 10:e01312252015.
View Article : Google Scholar
|
|
45
|
Zhang XL, Xu LL and Wang F:
Hsa_circ_0020397 regulates colorectal cancer cell viability,
apoptosis and invasion by promoting the expression of the miR-138
targets TERT and PD-L1. Cell Biol Int. 41:1056–1064. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y,
Yang S, Zeng Z, Liao W, Ding YQ and Liang L: Emerging roles of
circRNA_001569 targeting miR-145 in the proliferation and invasion
of colorectal cancer. Oncotarget. 7:26680–26691. 2016.PubMed/NCBI
|
|
47
|
Hsiao KY, Lin YC, Gupta SK, Chang N, Yen
L, Sun HS and Tsai SJ: Noncoding effects of circular RNA CCDC66
promote colon cancer growth and metastasis. Cancer Res.
77:2339–2350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu X, Wang X, Wei S, Chen Y, Chen Y, Fan
X, Han S and Wu G: hsa_circ_0013958: A circular RNA and potential
novel biomarker for Lung adenocarcinoma. FEBS J. 284:2170–2182.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wan L, Zhang L, Fan K, Cheng ZX, Sun QC
and Wang JJ: Circular RNA-ITCH suppresses lung cancer proliferation
via inhibiting the Wnt/β-catenin pathway. Biomed Res Int.
2016:15794902016. View Article : Google Scholar
|
|
50
|
Chen L, Zhang S, Wu J, Cui J, Zhong L,
Zeng L and Ge S: CircRNA_100290 plays a role in oral cancer by
functioning as a sponge of the miR-29 family. Oncogene.
36:4551–4561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng J, Liu X, Xue Y, Gong W, Ma J, Xi Z,
Que Z and Liu Y: TTBK2 circular RNA promotes glioma malignancy by
regulating miR-217/HNF1β/Derlin-1 pathway. J Hematol Oncol.
10:522017. View Article : Google Scholar
|
|
52
|
Peng N, Shi L, Zhang Q, Hu Y, Wang N and
Ye H: Microarray profiling of circular RNAs in human papillary
thyroid carcinoma. PloS One. 12:e01702872017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang K, Sun Y, Tao W, Fei X and Chang C:
Androgen receptor (AR) promotes clear cell renal cell carcinoma
(ccRCC) migration and invasion via altering the
circHIAT1/miR-195–5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett.
394:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li F, Zhang L, Li W, Deng J, Zheng J, An
M, Lu J and Zhou Y: Circular RNA ITCH has inhibitory effect on ESCC
by suppressing the Wnt/β-catenin pathway. Oncotarget. 6:6001–6013.
2015.PubMed/NCBI
|
|
55
|
Wang Y and Wang Z: Efficient backsplicing
produces translatable circular mRNAs. RNA. 21:172–179. 2015.
View Article : Google Scholar :
|
|
56
|
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang
Y, Jin Y, Yang Y, Chen LL, Wang Y, et al: Extensive translation of
circular RNAs driven by N6-methyladenosine. Cell Res. 27:626–641.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X and Zhao K: Novel role of FBXW7 circular RNA in
repressing glioma tumorigenesis. J Natl Cancer Inst. 110:2018.
View Article : Google Scholar
|
|
58
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang
Z and Yang BB: Induction of tumor apoptosis through a circular RNA
enhancing Foxo3 activity. Cell Death Differ. 24:357–370. 2017.
View Article : Google Scholar :
|
|
60
|
Xia W, Qiu M, Chen R, Wang S, Leng X, Wang
J, Xu Y, Hu J, Dong G, Xu PL and Yin R: Circular RNA
has_circ_0067934 is upregulated in esophageal squamous cell
carcinoma and promoted proliferation. Sci Rep. 6:355762016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang P, Qiu Z, Jiang Y, Dong L, Yang W, Gu
C, Li G and Zhu Y: Silencing of cZNF292 circular RNA suppresses
human glioma tube formation via the Wnt/β-catenin signaling
pathway. Oncotarget. 7:63449–63455. 2016.PubMed/NCBI
|
|
62
|
Yang Q, Du WW, Wu N, Yang W, Awan FM, Fang
L, Ma J, Li X, Zeng Y, Yang Z, et al: A circular RNA promotes
tumorigenesis by inducing c-myc nuclear translocation. Cell Death
Differ. 24:1609–1620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhu M, Xu Y, Chen Y and Yan F: Circular
BANP, an upregulated circular RNA that modulates cell proliferation
in colorectal cancer. Biomed Pharmacother. 88:138–144. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li P, Chen H, Chen S, Mo X, Li T, Xiao B,
Yu R and Guo J: Circular RNA 0000096 affects cell growth and
migration in gastric cancer. Br J Cancer. 116:626–633. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yao Z, Luo J, Hu K, Lin J, Huang H, Wang
Q, Zhang P, Xiong Z, He C, Huang Z, et al: ZKSCAN1 gene and its
related circular RNA (circZKSCAN1) both inhibit hepatocellular
carcinoma cell growth, migration, and invasion but through
different signaling pathways. Mol Oncol. 11:422–437. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huang M, He YR, Liang LC, Huang Q and Zhu
ZQ: Circular RNA hsa_circ_0000745 may serve as a diagnostic marker
for gastric cancer. World J Gastroenterol. 23:6330–6338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li WH, Song YC, Zhang H, Zhou ZJ, Xie X,
Zeng QN, Guo K, Wang T, Xia P and Chang DM: Decreased expression of
Hsa_ circ_00001649 in gastric cancer and its clinical significance.
Dis Markers. 2017:45876982017. View Article : Google Scholar
|
|
68
|
Chen S, Li T, Zhao Q, Xiao B and Guo J:
Using circular RNA hsa_circ_0000190 as a new biomarker in the
diagnosis of gastric cancer. Clin Chim Acta. 466:167–171. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fu L, Yao T, Chen Q, Mo X, Hu Y and Guo J:
Screening differential circular RNA expression profiles reveals
hsa_circ_0004018 is associated with hepatocellular carcinoma.
Oncotarget. 8:58405–58416. 2017.PubMed/NCBI
|
|
70
|
Shang X, Li G, Liu H, Li T, Liu J, Zhao Q
and Wang C: Comprehensive circular RNA profiling reveals that hsa_
circ_0005075, a new circular RNA biomarker, is involved in
hepatocellular crcinoma development. Medicine (Baltimore).
95:e38112016. View Article : Google Scholar
|
|
71
|
Xu L, Zhang M, Zheng X, Yi P, Lan C and Xu
M: The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of
hepatic microvascular invasion in hepatocellular carcinoma. J
Cancer Res Clin Oncol. 143:17–27. 2017. View Article : Google Scholar
|
|
72
|
Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z,
Yang J, Fan J, Liu L and Qin W: Hsa_circ_0001649: A circular RNA
and potential novel biomarker for hepatocellular carcinoma. Cancer
Biomark. 16:161–169. 2016. View Article : Google Scholar
|
|
73
|
Zhang P, Zuo Z, Shang W, Wu A, Bi R, Wu J,
Li S, Sun X and Jiang L: Identification of differentially expressed
circular RNAs in human colorectal cancer. Tumour.
39:1010428317694546. 2017.
|
|
74
|
Li W, Zhong C, Jiao J, Li P, Cui B, Ji C
and Ma D: Characterization of hsa_circ_0004277 as a new biomarker
for acute myeloid leukemia via circular RNA profile and
bioinformatics analysis. Int J Mol Sci. 18:E5972017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lu L, Sun J, Shi P, Kong W, Xu K, He B,
Zhang S and Wang J: Identification of circular RNAs as a promising
new class of diagnostic biomarkers for human breast cancer.
Oncotarget. 8:44096–44107. 2017.PubMed/NCBI
|
|
76
|
Lu R, Shao Y, Ye G, Xiao B and Guo J: Low
expression of hsa_circ_0006633 in human gastric cancer and its
clinical significances. Tumour Biol. 39:1010428317704175. 2017.
View Article : Google Scholar
|
|
77
|
Fang Y, Ma M, Wang J, Liu X and Wang Y:
Circular RNAs play an important role in late-stage gastric cancer:
Circular RNA expression profiles and bioinformatics analyses.
Tumour Biol. 39:1010428317705850. 2017. View Article : Google Scholar
|















