Introduction
The number of diabetic patients worldwide is
expected to reach 642 million by 2040 (1), and the prevalence of diabetic
cardio-myopathy (DCM) among diabetic patients is currently 12%
(2). Diabetes is closely
associated with the onset of coronary heart disease, stroke,
chronic kidney disease, peripheral vascular disease and retinopathy
(3), mainly caused by diabetic
micro-vascular lesions (4).
Abnormal cardiac systolic and diastolic function, cardiomyocyte
apoptosis and fibrosis are observed in prediabetes due to insulin
resistance, abnormal Ca2+ regulation and mitochondrial
dysfunction (5-8), which eventually lead to the onset of
DCM. DCM is a major cause of cardiac function decline in patients
with diabetes mellitus (9,10).
DCM onset occurs early, but its symptoms are often occult, and
treatment efficacy is usually poor (11); however, the detailed molecular
mechanisms underlying this disease remain unclear.
Mitochondria are responsible for energy metabolism,
and cardiomyocytes in particular require mitochondria to provide
energy in order to maintain cardiac function (12). A number of ATP-sensitive potassium
channels (KATP) are present in the mitochondrial
membrane, which are composed of an inward rectifier K+
channel (Kir6.1 subunit) and a sulfonylurea receptor, and play an
important role in cardioprotection by healing ischemic reperfusion
injuries and preventing oxidative stress and apoptosis (13-15). Diazoxide (DZX), being a specific
activator of mitochondrial KATP (mitoKATP)
channels, opens mitoKATP and plays a key role in
cardioprotection and cardiac ischemic preconditioning (13,15).
Foxo1 is an important transcription factor, which is
associated with cell cycle regulation, oxidative stress and
apoptotic gene expression (16).
The upstream regulator of Foxo1, AKT, inhibits Foxo1 activity by
phosphorylating Foxo1 at three conserved phosphorylation sites
(17,18). It was previously reported that
phosphorylation of AKT-Foxo1 was decreased in DCM mice (19), and this phenomenon was closely
associated with the onset of insulin resistance, mitochondrial
dysfunction and cell apoptosis (20,21). There is evidence that the use of
specific mitoKATP channel openers increases p-AKT
expression; however, these studies focused mainly on reperfusion
injury and blood pressure regulation (22-24). It may be hypothesized that opening
of mitoKATP channels regulates the AKT-Foxo1 signaling
pathway, thereby improving cardiac function and inhibiting
apoptosis in DCM.
In the present study, a mouse in vitro and
in vivo model was used to investigate the role of
mitoKATP channel opening in cardiac function and
cardiomyocyte apoptosis, while measuring the expression of p-AKT
and p-Foxo1. The effects of mitoKATP channel opening at
the cellular level were further characterized by mimicking insulin
resistance using the specific AKT inhibitor MK-2206. The aim of the
present study was to elucidate the mechanism of regulation of the
AKT-Foxo1 signaling pathway by mitoKATP channels in
improving cardiac function and inhibiting apoptosis in DCM. This
pathway may represent a novel target for early therapeutic
intervention, and improve the prognosis of patients with diabetes
mellitus.
Materials and methods
Animals and treatment
All animals were treated in strict accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals, and the experimental protocols were approved by
the Ethics Committee of the Chinese PLA General Hospital.
Twenty-week-old male db/db mice (weighing 45-50 g), which were used
as a model of type 2 diabetes, and their lean age-matched
littermates db/m mice (weighing 25-30 g), which were used as
non-diabetic controls, were purchased from Cavens Laboratory Animal
Co., Ltd. (Changzhou, China). All animals were housed under a
light-dark cycle of 12 h, and were allowed free access to standard
food and water. A total of 30 db/db mice were randomly assigned
into three groups: The dimethyl sulfoxide (DMSO) group (n=10),
which received an intraperitoneal injection of 2% DMSO (Amresco,
Washington, DC, USA); the DZX group (n=10), which received an
intraperitoneal injection of DZX (5 mg/kg, Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) dissolved in 2% DMSO; and the DZX plus
5-hydroxydecanoate (5-HD) group (n=10), which received an
intraperitoneal injection of DZX (5 mg/kg) plus 5-HD (5 mg/kg,
Sigma-Aldrich; Merck KGaA) dissolved in 2% DMSO, according to a
previous study (25). A total of
10 db/m mice were used as the control group, and received an
intraperitoneal injection of 2% DMSO. All animals were injected
daily for 4 weeks, and the dosage of vehicle was 10 ml/kg (26).
Echocardiography
Transthoracic echocardiography was performed to
evaluate cardiac function by high-resolution imaging (Vevo 770;
Visual Sonics Inc., Toronto, ON, Canada) at the animal center of
Capital Medical University (Beijing, China). Hemodynamic parameters
were obtained at baseline and after 4 weeks of drug intervention.
The left ventricular ejection fraction (EF), fractional shortening
(FS), left ventricular internal dimension in systole (LVDs), left
ventricular internal dimension in diastole (LVDd), cardiac output
(CO) and left ventricular weight (LVW) were measured. The body
surface area was calculated based on the Meeh-Rubner equation
[A=k'(W2/3)/10,000(k'=9.1)] (27).
Myocyte isolation and cell culture
Primary cultures of neonatal rat ventricular
myocytes were prepared from Sprague-Dawley rats (1-2 days), which
were purchased from Vital River Laboratories (Beijing, China). The
hearts were quickly extracted and immediately washed with D-Hank's
solution (Solarbo, Beijing, China). Straight scissors were used to
mince the hearts into small pieces (1-2 mm3), and
cardiomyocytes were digested with 0.08% trypsin (Amresco) at 37°C
for 6-10 min. The initial cell suspensions were discarded, and the
remaining tissue was digested with 0.08% type II collagenase
(Gibco; Thermo Fisher Scientific Inc., Waltham, MA USA) at 37°C for
6-10 min, and then neutralized with Dulbecco's minimal Essential
medium (HyClone; GE Healthcare, Logan, UT, USA) containing 10%
fetal bovine serum (HyClone; GE Healthcare), until the tissue had
dissolved. All cell suspensions were pelleted by centrifugation at
300 × g for 10 min, and the resulting cardiomyocyte pellet was
resuspended. The cell suspensions were plated into 100-mm cell
culture dishes and incubated for 90 min in an incubator (95%
O2/5% CO2). The cell suspensions were then
collected and plated in 60-mm cell culture dishes at a density of
2-5×105 cells/ml, and 5-bromo-2-deoxyuridine (0.1
mmol/l, Sigma-Aldrich; Merck KGaA) was added into the culture
medium for the first 48 h (28,29). After 48 h, the cultured
cardiomyocytes were divided into five groups for different drug
treatments: Insulin (100 nmol/l, Sigma-Aldrich; Merck KGaA) for 24
h (19), DZX (100 µmol/l)
plus insulin (100 nmol/l) for 24 h, 5-HD (100 µmol/l) plus
DZX (100 µmol/l) plus insulin (100 nmol/l) for 24 h, MK-2206
(5 µmol/l, Selleck Chemicals, Houston, TX, USA) plus DZX
(100 µmol/l) plus insulin (100 nmol/l) for 24 h. DZX, 5-HD
and MK-2206 were applied 30 min in advance according to a
previously published study (30).
The control group received only 2% DMSO.
Blood glucose and N-terminal pro-brain
natriuretic peptide (NT-proBNP) measurements
All mice were fasted for 8 h prior to blood
biochemistry measurements. Blood glucose was detected with a
standard glucometer (Roche Diagnostics GmbH, Mannheim, Germany) in
blood samples obtained from mice tails. NT-proBNP levels in the
serum and culture supernatant were measured by ELISA kit
(Elabscience, Wuhan, China) in blood samples collected from the
eyeballs, according to the manufacturer's instructions. The optical
density of NT-proBNP was measured at a wavelength of 450 nm using
an enzyme-labeled instrument (Epoch; BioTek Instruments, Inc.,
Winooski, VT, USA). CurveExpert 3.1 software (CurveExpert Software,
Chattanooga, TN, USA) was used to establish a standard curve, and
the NT-proBNP concentration of each sample was calculated using the
standard curve. The amount of NT-proBNP in the culture supernatant
was calculated relative to the total protein concentration.
Hematoxylin and eosin staining (H&E)
and TUNEL assay
After 4 weeks of drug treatment, H&E staining
and TUNEL assays were performed to evaluate the pathological
changes in myocardial tissue. Paraformaldehyde 4% (Solarbo) was
used to fix mouse myocardium overnight at 4°C. Paraffin embedding,
tissue sectioning and H&E staining were performed as previously
described (31). Five myocardial
H&E-stained sections were randomly selected from each group.
Cell area measurements were performed on similar myocardial cross
sections, and 50 nucleated cells were randomly selected to measure
the mean cell area (32). The
rate of apoptosis in cardiomyocytes was measured using a TUNEL
assay kit (Roche Diagnostics, Indianapolis, IN, USA) according to
the manufacturer's instructions. Five myocardial TUNEL stained
sections were selected from each group. A similar field of vision
was selected for each image, and Image Pro Plus software (Media
Cybernetics, Inc., Rockville, MD, USA) was used to count the cells.
A selection of 200 cells was randomly chosen to determine the ratio
of TUNEL-stained cells, which was used to determine the rate of
apoptosis (33,34).
Caspase 3 activity assay
Caspase 3 activity was measured using the caspase 3
activity kit (Beyotime Institute of Biotechnology, Shanghai,
China). Lysis buffer was added to the cultured cardiomyocytes at
4°C for ~15 min. The suspension was centrifuged at 4°C for 15 min
(16,000 × g). A 50-µl aliquot of the supernatant extract was
mixed with 10 µl Ac DEVD pNA substrate and 40 µl
detection buffer, and then incubated at 37°C for ~2 h. The
remaining extracts were used to measure protein concentration by
the Bradford protein assay kit (Beyotime Institute of
Biotechnology). P-nitroaniline was measured at a wavelength of 405
nm using an enzyme-labeled instrument (35). The caspase 3 activity was
calculated using the p-nitroaniline absorbance relative to the
total protein concentration.
Protein analysis and immunoblotting
Total protein was extracted from myocardial tissues
and cultured cardiomyocytes using RIPA buffer (Solarbo), and the
protein concentration was measured using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). The total
protein of myocardial tissue samples (70 µg) and cultured
cardiomyocyte samples (50 µg) were separated using 8-12%
SDS-PAGE (optimized to the molecular weight of each target protein)
and transferred to PVDF membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% non-fat milk or 5% BSA in 1X
TBST (Solarbo) for 2 h at room temperature, then incubated
overnight at 4°C with primary antibodies as follows: p-AKT
(1:5,000; rabbit monoclonal, ab81283, Abcam, Cambridge, UK), t-AKT
(1:10,000; rabbit monoclonal, ab179463, Abcam), p-Foxo1 (1:500;
rabbit polyclonal, ab131339, Abcam), t-Foxo1 (1:500; rabbit
polyclonal, ab39670, Abcam), GAPDH (1:30,000; rabbit monoclonal,
ab181602, Abcam), and caspase 3 (1:1,000; rabbit polyclonal, 9662,
Cell Signaling Technology Inc., Danvers, MA, USA). The membranes
were washed in 1X TBST on a shaker at 10 × g for 15 min, and then
incubated at room temperature with HRP-conjugated secondary
antibodies for 60 min. Protein bands were detected using a
chemiluminescent substrate with an imaging system (Tanon, Shanghai,
China), and ImageJ software (National Institutes of Health,
Bethesda, MD, USA) was used to quantify the intensity of the
bands.
RNA isolation and reverse
transcription-quantitative poly- merase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from cultured cardiomyocytes
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Carlsbad, CA, USA), and then reverse-transcribed into cDNA using
the iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). RT-qPCR was performed in a 20-µl
reaction volume containing 3 µl cDNA template, 1 µl
primer mixture, 6 µl ddH2O, 10 µl Power
SYBR Green PCR master mix (Applied Biosystems, Foster City, CA,
USA) in a 7900HT Fast Real-Time PCR System (Thermo Fisher
Scientific, Waltham, MA, USA). The BNP primers used were
AGTCCTTCGGTCTCAAGGCA (F) and CCGATCCGGTCTATCTTGTGC (R), and the
internal control (36β4) primers were CAGAGGTGCTGGACATCACAGAG (F)
and GGCAACAGTCGGGTAGCCAATC (R). The thermal cycling conditions were
carried out according to a previously published study (36). The relative expression of BNP was
calculated relative to 36β4 by the 2−ΔΔCq method.
Myocardial mitochondrial membrane
potential (ΔYm)
ΔYm was measured using fluorescent dye JC-1
(Beyotime Institute of Biotechnology). Cultured cardiomyocytes were
incubated with JC-1 stain for 20 min at 37°C, and carefully washed
twice with ice-cold JC-1 staining buffer (1X). The cells were
immediately visualized under a confocal microscope (FV1000,
Olympus, Tokyo, Japan). JC-1 stain aggregated in the mitochondria
was visible as red fluorescence, while JC-1 outside the
mitochondria was detectable as green fluorescence. The resulting
images were analyzed using Image Pro Plus 6.0 software (Media
Cybernetics, Inc.). ΔYm was determined by calculating the ratio of
red fluorescence to green fluorescence (37).
Statistical analysis
All values were analyzed using SPSS 17.0 software
(SPSS Inc., Chicago, IL, USA), and presented as mean ± standard
deviation. Differences among three or more groups were evaluated by
one-way analysis of variance (ANOVA) followed by the least
significant difference and Dunnett's tests. Differences were
considered statistically significant for P-values <0.05.
Results
Opening of mitoKATP improves
cardiac function in db/db mice
Hemodynamic parameters and serum NT-ProBNP levels
were measured in db/db mice after treatment with DZX. The LVEF, FS
and cardiac index (CI) values were lower, while the serum NT-ProBNP
level increased in db/db mice. DZX-treated mice exhibited increased
LVEF, FS and CI values, and decreased serum NT-ProBNP levels
(P<0.05 in the DZX group vs. the DMSO and 5-HD+DZX groups)
(Table I; Fig. 1A-F). Moreover, DZX exerted no
effect on body weight or blood glucose level (Table I). 5-HD completely blocked the
effects of DZX. These data suggest that opening of
mitoKATP improved cardiac function in db/db mice.
 | Table IEffects of diazoxide on body weight,
blood glucose and serum NT-ProBNP levels in db/db mice. |
Table I
Effects of diazoxide on body weight,
blood glucose and serum NT-ProBNP levels in db/db mice.
| Variables | 0 weeks
| 4 weeks
|
|---|
| Control | DMSO | DZX | 5-HD+DZX | Control | DMSO | DZX | 5-HD+DZX |
|---|
| BW/ΔBW (g) | 26.81±2.82 | 53.42±3.70a | 52.63±2.78a | 53.15±2.20a | 6.53±4.26 | 7.38±4.81 | 6.54±3.39 | 6.49±3.41 |
| GLU/ΔGLU (mM) | 6.45±0.75 | 16.41±3.98a | 18.85±2.90a | 16.94±4.12a | −0.51±1.15 | 0.22±3.26 | −0.69±3.95 | 1.22±4.92 |
| NT-ProBNP
(pg/ml) | – | – | – | – | 78.88±23.71 |
203.31±28.94a,b |
153.65±21.93a |
194.67±25.94a,b |
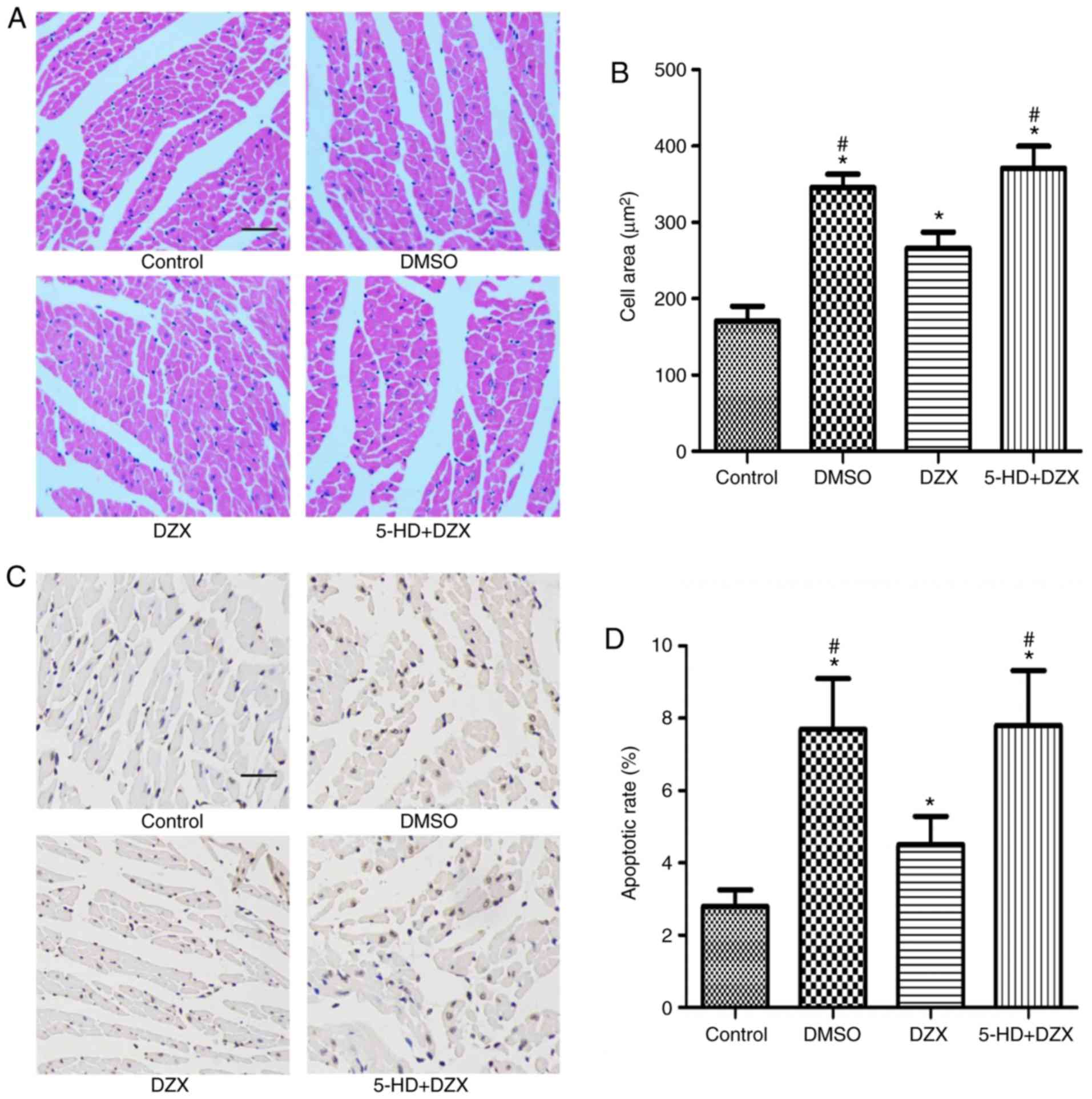
Opening of mitoKATP alleviates
hypertrophy and inhibits apoptosis of cardiomyocytes in db/db
mice
To further explore the effects of DZX treatment on
the pathological changes in myocardial tissue, H&E staining and
TUNEL assays were performed to detect cardiomyocyte hypertrophy and
apoptosis, respectively. The cardiomyocytes of db/db mice were
significantly hypertrophic compared with the control group
(P<0.05). However, hypertrophy was significantly attenuated
following treatment with DZX (P<0.05 in the DZX group vs. the
DMSO and 5-HD+DZX groups) (Fig. 2A
and B). Furthermore, this effect was blocked by treatment with
5-HD.
Similarly, the rate of apoptosis of cardiomyocytes
in db/db mice was significantly higher compared with that in the
control group (P<0.05). DZX decreased the rate of cardiomyocyte
apoptosis (P<0.05 in the DZX group vs. the DMSO and 5-HD+DZX
groups), and its effect was blocked by 5-HD (Fig. 2CA and D). These findings suggest
that opening of mitoKATP attenuated hypertrophic
degeneration and inhibited apoptosis of cardiomyocytes in db/db
mice.
Opening of mitoKATP regulates
the expression of cleaved caspase 3 in db/db mice
To further investigate the effect of
mitoKATP channel opening by DZX on cardiomyocyte
apoptosis in db/db mice, the expression of cleaved caspase 3 was
measured by western blotting in each group. The expression of
cleaved caspase 3 was increased in the DMSO group compared with
that in the control group (P<0.05). DZX treatment decreased the
expression of cleaved caspase 3 (P<0.05 in the DZX group vs. the
DMSO and 5-HD+DZX groups) (Fig. 3A
and B). The regulatory effect of DZX on the expression of
cleaved caspase 3 was blocked by treatment with 5-HD. This suggests
that opening of mitoKATP prevented the progression of
cardiomyocyte apoptosis in db/db mice.
Opening of mitoKATP regulates
the AKT-Foxo1 signaling pathway in db/db mice
In order to determine the effect of
mitoKATP channel opening by DZX on the AKT-Foxo1
signaling pathway, the protein expression of t-AKT, t-Foxo1, p-AKT
and p-Foxo1 was detected by western blotting in each group. The
expression of p-AKT and p-Foxo1 was decreased in the DMSO group
compared with that in the control group (P<0.05). However, DZX
treatment increased the expression of p-AKT and p-Foxo1 (P<0.05
in the DZX group vs. the DMSO and 5-HD+DZX groups), and this effect
was blocked by 5-HD (Fig. 4A and
B). These results suggest that opening of mitoKATP
regulated the AKT-Foxo1 signaling pathway in db/db mice.
Opening of mitoKATP reduces
the level of NT-ProBNP in the culture supernatant and the relative
expression of BNP mRNA in cultured cardiomyocytes
To further characterize the protective effect of
mitoKATP channel opening by DZX in vitro, the
level of NT-ProBNP was detected in the culture supernatant and the
relative expression of BNP mRNA in cultured cardiomyocytes
simulating chronic insulin resistance. The NT-ProBNP level and
relative expression of BNP mRNA were increased in cells mimicking
insulin resistance compared with that in the control group
(P<0.05). DZX treatment decreased the NT-ProBNP level and the
relative expression of BNP mRNA (Fig.
5A and B), whereas its effect was blocked by 5-HD. These data
indicate that opening of mitoKATP decreased the
expression of heart failure markers during insulin resistance.
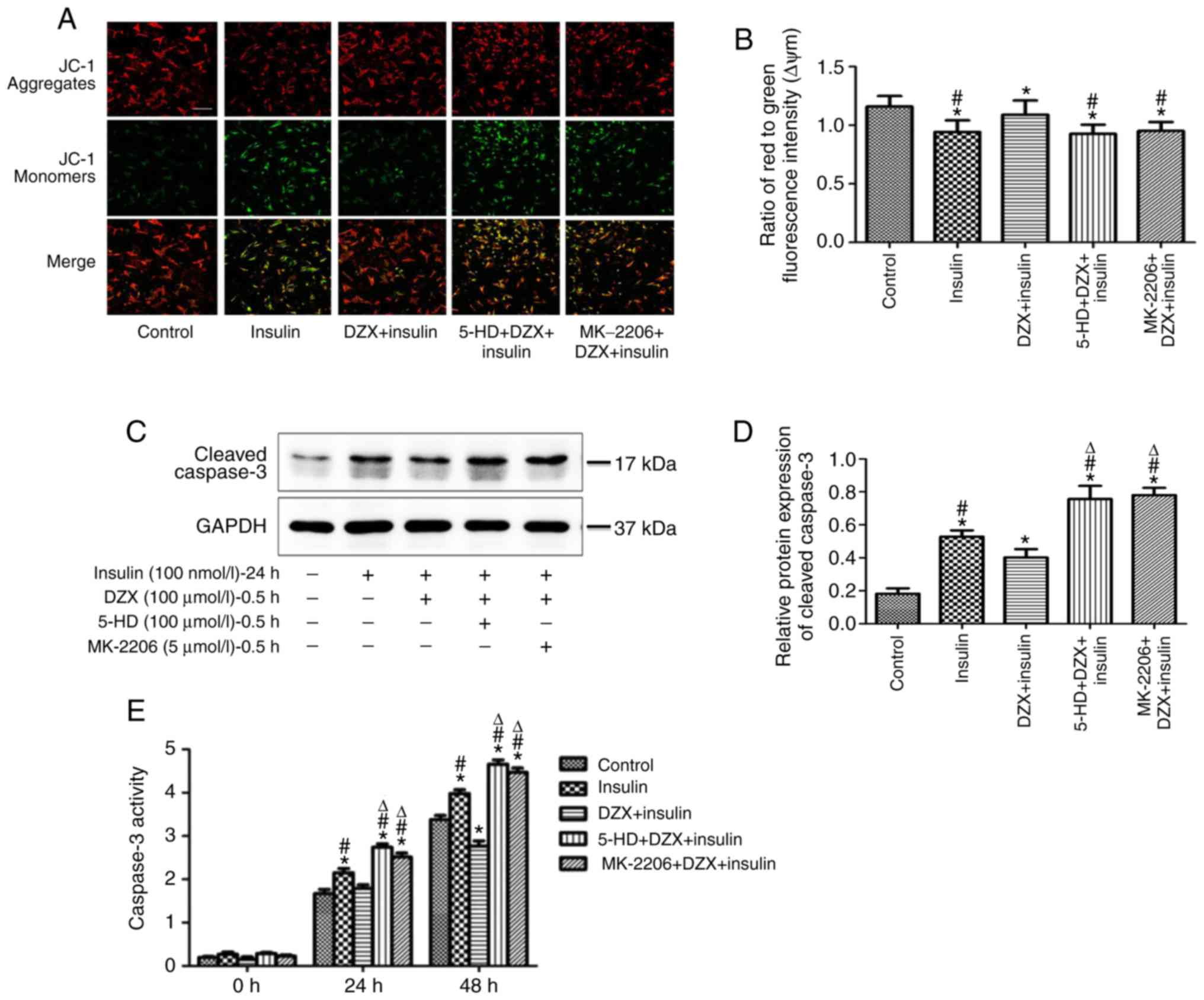
Opening of mitoKATP regulates
the ΔYm, cleaved caspase 3 expression and caspase 3 activity in
cultured cardiomyocytes
To further explore the role of mitoKATP
channel opening on energy metabolism, ΔYm was measured in each
group, and was found to be decreased in cells mimicking insulin
resistance compared with that in the control group (P<0.05). DZX
treatment resulted in increased ΔYm, and its effects were blocked
by 5-HD (Fig. 6A and B).
Similarly, the expression of cleaved caspase 3 and
the activity of caspase 3 were increased in cells mimicking insulin
resistance. DZX treatment significantly decreased the expression of
cleaved caspase 3 and reduced caspase 3 activity (Fig. 6C-E). The effect of DZX was blocked
by 5-HD. These results suggest that opening of mitoKATP
not only improved the energy metabolism of cardiomyocytes, but also
attenuated the apoptosis of cardiomyocytes during insulin
resistance.
The protective effects and apoptosis
inhibition via opening mitoKATP are mediated by
regulation of the AKT-Foxo1 signaling pathway during insulin
resistance in cultured cardiomyocytes
Opening mitoKATP channels with DZX
treatment similarly increased the expression of p-AKT and p-Foxo1
in cultured cardiomyocytes (P<0.05 in the DZX group vs. the DMSO
and 5-HD+DZX groups) during induced insulin resistance, and this
effect was blocked by 5-HD (Fig. 7A
and B).
To determine whether the protective effects and
inhibition of apoptosis observed following DZX treatment were a
result of the regulation of the AKT-Foxo1 signaling pathway, the
effects of DZX treatment on heart failure marker expression, ΔYm
and apoptosis were evaluated after treatment with MK-2206.
Treatment with MK-2206 prior to treatment with DZX inhibited the
increase of p-AKT and p-Foxo1 expression, the increase in ΔYm, the
inhibition of apoptosis, including decreased cleaved caspase 3
expression and activity, and the decrease of culture supernatant
NT-ProBNP and BNP mRNA expression that were induced by
mitoKATP channel opening (Figs. 5–7). This indicates that the opening of
mitoKATP exerts protective effects and inhibits
apoptosis via regulating the AKT-Foxo1 signaling pathway during
insulin resistance.
Discussion
Taken together, the data of the present study
indicate that DZX treatment mediated the opening of
mitoKATP channels and attenuated the development of
cardiac dysfunction, as evidenced by decreased levels of serum
NT-ProBNP in db/db mice. DZX treatment also appeared to inhibit
apoptosis and increase the expression of p-AKT and p-Foxo1 both
in vivo (in db/db mice) and in vitro (in
cardiomyocytes simulating insulin resistance); furthermore, these
effects were blocked by the specific AKT inhibitor MK-2206.
DCM is mainly caused by sustained hyperglycemia and
hyperinsulinemia, which eventually lead to the decline of cardiac
systolic and diastolic function (38,39). In the present study, cardiac
dysfunction was observed in db/db mice, which was characterized by
the decrease of LVEF, FS and CI values, and the increase of the
serum NT-ProBNP level. The results were consistent with those of
previous studies (19). Opening
of mitoKATP channels by DZX treatment increased the
values of LVEF, FS and CI, while it decreased the serum NT-ProBNP
level. It was also observed that opening of mitoKATP
channels by DZX treatment decreased NT-ProBNP levels in the culture
supernatant, and decreased the relative expression of BNP mRNA in
cells simulating insulin resistance in vitro. Taken
together, the in vivo and in vitro data confirmed
that opening of mitoKATP channels improved cardiac
function and decreased the expression of heart failure markers in
DCM, which, to the best of our knowledge, has not been previously
reported.
Stable ΔYm is key to energy synthesis (40). A decrease in ΔYm affects energy
synthesis, leading to cell dysfunction (41), while possibly either initiating
apoptosis or promoting the onset of apoptosis (42). In the present study, the ΔYm was
found to be decreased in cells simulating insulin resistance in
vitro, resulting in altered metabolism in cardiomyocytes
(43), which led to a series of
pathological changes, ultimately leading to apoptosis. The opening
of mitoKATP channels increased the ΔYm and decreased the
expression of cleaved caspase 3. This suggests that
mitoKATP channel opening improves the energy metabolism,
which may inhibit the onset of apoptosis during simulated insulin
resistance. This phenomenon may have resulted in the improved
cardiac function observed in DZX-treated db/db mice (44).
Foxo1 is an important transcription factor that
promotes the oxidative stress response and induces the expression
of pro-apoptotic genes (45). The
phosphorylation of Foxo1 by p-AKT promotes its transfer out of the
nucleus, which inhibits its transcriptional activity, improving
energy metabolism and inhibiting apoptosis (46). It was previously reported that the
expression of p-AKT and p-Foxo1 decreased in DCM (19). In the present study, decreased
p-AKT and p-Foxo1 expression was observed during simulated insulin
resistance both in vivo and in vitro. However, DZX
treatment resulted in increased expression of p-AKT and p-Foxo1.
These data suggest that opening of mitoKATP channels
regulates the AKT-Foxo1 signaling pathway.
Increased p-Foxo1 expression improves the energy
metabolism of the mitochondria and inhibits the onset of apop-tosis
(19,45,46). Opening of mitoKATP
channels also plays an important role in maintaining mitochondrial
function (47,48). In the present study, cells were
pre-treated with the specific AKT inhibitor MK-2206 in order to
elucidate the role of mitoKATP channels in the AKT-Foxo1
signaling pathway. It was observed that MK-2206 treatment inhibited
the increase in p-AKT and p-Foxo1 expression, increased ΔYm,
inhibited apoptosis and decreased the culture supernatant NT-ProBNP
and BNP mRNA expression levels that were induced by DZX treatment.
Therefore, it may be concluded that the improvement in cardiac
function and inhibition of apoptosis observed as a result of
mitoKATP channel opening occurs via regulation of the
AKT-Foxo1 signaling pathway during DCM.
The proposed mechanism by which mitoKATP
channel opening improves cardiac function in DCM is summarized in
Fig. 8. The expression of p-AKT
and p-Foxo1 decreases during insulin resistance, and the
transcription factor Foxo1 is overexpressed, leading to a decrease
in ΔYm, inhibition of energy metabolism and an increase in
apoptotic gene expression, ultimately leading to a decline in
cardiac function. When mitoKATP channels open, the
expression of p-AKT and p-Foxo1 increases and p-Foxo1 is
transferred out of the nucleus, inhibiting the transcriptional
activity of Foxo1, which increases ΔYm, improves energy metabolism
and inhibits apoptosis, thus improving cardiac function.
There were certain limitations to the present study.
Opening of mitoKATP was shown to improve cardiac
function and inhibit cardiomyocyte apoptosis in diabetic mice, and
the underlying mechanism was associated with the regulation of
AKT-Foxo1 by opening of mitoKATP. However, the
regulatory mechanisms linking mitoKATP and the AKT-Foxo1
signaling pathway, as well as the detailed binding sites of inward
rectifier potassium channel and Foxo1, remain to be further
elucidated in future studies.
In summary, opening of mitoKATP channels
regulates the AKT-Foxo1 signaling pathway, which improves cardiac
function and inhibits apoptosis during DCM. MitoKATP may
therefore be an attractive potential therapeutic target for
DCM.
Funding
This study was funded by the National Natural
Science Foundation of China (grant nos. 81570349 and 81200157).
Availability of data and materials
The data generated and analyzed in the present study
are available from the corresponding author upon reasonable
request.
Authors' contributions
PD researched the data and wrote the manuscript. JW,
LW and FS researched the data. YL and YD analyzed and interpreted
the data. SW and SZ wrote and reviewed the manuscript. QZ designed
and supervised the research, wrote and critically revised the
manuscript. All authors have read and approved the final version of
this manuscript.
Ethics approval and consent to
participate
All animals were treated in strict accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals, and the experimental protocols were approved by
the Ethics Committee of the Chinese PLA General Hospital, Beijing,
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests to disclose.
Acknowledgments
Not applicable.
References
|
1
|
Cefalu WT, Buse JB, Tuomilehto J, Fleming
GA, Ferrannini E, Gerstein HC, Bennett PH, Ramachandran A, Raz I,
Rosenstock J and Kahn SE: Update and next steps for real-world
translation of interventions for type 2 diabetes prevention:
Reflections from a diabetes care editors' expert forum. Diabetes
Care. 39:1186–1201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertoni AG, Hundley WG, Massing MW, Bonds
DE, Burke GL and Goff DC Jr: Heart failure prevalence, incidence,
and mortality in the elderly with diabetes. Diabetes Care.
27:699–703. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parrinello CM, Matsushita K, Woodward M,
Wagenknecht LE, Coresh J and Selvin E: Risk prediction of major
complications in individuals with diabetes: The atherosclerosis
risk in communities study. Diabetes Obes Metab. 18:899–906. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coll-de-Tuero G, Mata-Cases M,
Rodriguez-Poncelas A, Pepió JM, Roura P, Benito B and Franch-Nadal
J: Prevalence and associated variables in a random sample of 2642
patients of a Mediterranean area. BMC Nephrol. 13:872012.
View Article : Google Scholar
|
|
5
|
Demmer RT, Allison MA, Cai J, Kaplan RC,
Desai AA, Hurwitz BE, Newman JC, Shah SJ, Swett K, Talavera GA, et
al: Association of impaired glucose regulation and insulin
resistance with cardiac structure and function: Results from
ECHO-SOL (Echocardiographic Study of Latinos). Circ Cardiovasc
Imaging. 9:e0050322016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nunes S, Soares E, Fernandes J, Viana S,
Carvalho E, Pereira FC and Reis F: Early cardiac changes in a rat
model of prediabetes: Brain natriuretic peptide overexpression
seems to be the best marker. Cardiovasc Diabetol. 12:442013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bugger H and Abel ED: Molecular mechanisms
of diabetic cardiomyopathy. Diabetologia. 57:660–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huynh K, Bernardo BC, McMullen JR and
Ritchie RH: Diabetic cardiomyopathy: Mechanisms and new treatment
strategies targeting antioxidant signaling pathways. Pharmacol
Ther. 142:375–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ernande L and Derumeaux G: Diabetic
cardiomyopathy: Myth or reality. Arch Cardiovasc Dis. 105:218–225.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pappachan JM, Varughese GI, Sriraman R and
Arunagirinathan G: Diabetic cardiomyopathy: Pathophysiology,
diagnostic evaluation and management. World J Diabetes. 4:177–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karnafel W: Diabetic cardiomyopathy.
Pathophysiology and clinical implications. Przegl Lek. 57(Suppl 4):
S9–S11. 2000.in Polish.
|
|
12
|
Guzun R, Kaambre T, Bagur R, Grichine A,
Usson Y, Varikmaa M, Anmann T, Tepp K, Timohhina N, Shevchuk I, et
al: Modular organization of cardiac energy metabolism: Energy
conversion, transfer and feedback regulation. Acta Physiol (Oxf).
213:84–106. 2015. View Article : Google Scholar
|
|
13
|
Cuong DV, Kim N, Joo H, Youm JB, Chung JY,
Lee Y, Park WS, Kim E, Park YS and Han J: Subunit composition of
ATP-sensitive potassium channels in mitochondria of rat hearts.
Mitochondrion. 5:121–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slocinska M, Lubawy J, Jarmuszkiewicz W
and Rosinski G: Evidences for an ATP-sensitive potassium channel
(KATP) in muscle and fat body mitochondria of insect. J Insect
Physiol. 59:1125–1132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akao M, Ohler A, O'Rourke B and Marbán E:
Mitochondrial ATP-sensitive potassium channels inhibit apoptosis
induced by oxidative stress in cardiac cells. Circ Res.
88:1267–1275. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szydłowski M, Jabłońska E and Juszczyński
P: FOXO1 transcription factor: A critical effector of the PI3K-AKT
axis in B-cell development. Int Rev Immunol. 33:146–157. 2014.
View Article : Google Scholar
|
|
17
|
Tzivion G, Dobson M and Ramakrishnan G:
FoxO transcription factors; Regulation by AKT and 143-3 proteins.
Biochim Biophys Acta. 1813:1938–1945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xin Z, Ma Z, Jiang S, Wang D, Fan C, Di S,
Hu W, Li T, She J and Yang Y: FOXOs in the impaired heart: New
therapeutic targets for cardiac diseases. Biochim Biophys Acta.
1863:486–498. 2017. View Article : Google Scholar
|
|
19
|
Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng
H, Dostal DE, White MF, Baker KM and Guo S: Myocardial loss of IRS1
and IRS2 causes heart failure and is controlled by p38alpha MAPK
during insulin resistance. Diabetes. 62:3887–3900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kandula V, Kosuru R, Li H, Yan D, Zhu Q,
Lian Q, Ge RS, Xia Z and Irwin MG: Forkhead box transcription
factor 1: Role in the pathogenesis of diabetic cardiomyopathy.
Cardiovasc Diabetol. 15:442016. View Article : Google Scholar :
|
|
21
|
Palomer X, Salvadó L, Barroso E and
Vázquez-Carrera M: An overview of the crosstalk between
inflammatory processes and metabolic dysregulation during diabetic
cardiomyopathy. Int J Cardiol. 168:3160–3172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue Y, Xie N, Cao L, Zhao X, Jiang H and
Chi Z: Diazoxide preconditioning against seizure-induced oxidative
injury is via the PI3K/Akt pathway in epileptic rat. Neurosci Lett.
495:130–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grossini E, Molinari C, Caimmi PP, Uberti
F and Vacca G: Levosimendan induces NO production through p38 MAPK,
ERK and Akt in porcine coronary endothelial cells: Role for
mitochondrial K(ATP) channel. Br J Pharmacol. 156:250–261. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J, Tian W, Ma X, Guo J, Shi Q, Jin Y,
Xi J and Xu Z: The molecular mechanism underlying morphine-induced
Akt activation: Roles of protein phosphatases and reactive oxygen
species. Cell Biochem Biophys. 61:303–311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lemos Caldas FR, Rocha Leite IM, Tavarez
Filgueiras AB, de Figueiredo Júnior IL, Gomes Marques, de Sousa TA,
Martins PR, Kowaltowski AJ and Fernandes Facundo H: Mitochondrial
ATP-sensitive potassium channel opening inhibits
isoproterenol-induced cardiac hypertrophy by preventing oxidative
damage. J Cardiovasc Pharmacol. 65:393–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diehl KH, Hull R, Morton D, Pfister R,
Rabemampianina Y, Smith D, Vidal JM and van de Vorstenbosch C;
European Federation of Pharmaceutical Industries Association and
European Centre for the Validation of Alternative Methods: A good
practice guide to the administration of substances and removal of
blood, including routes and volumes. J Appl Toxicol. 21:15–23.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spiers DE and Candas V: Relationship of
skin surface area to body mass in the immature rat: A
reexamination. J Appl Physiol Respir Environ Exerc Physiol.
56:240–243. 1984.PubMed/NCBI
|
|
28
|
Vidyasekar P, Shyamsunder P, Santhakumar
R, Arun R and Verma RS: A simplified protocol for the isolation and
culture of cardiomyocytes and progenitor cells from neonatal mouse
ventricles. Eur J Cell Biol. 94:444–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ehler E, Moore-Morris T and Lange S:
Isolation and culture of neonatal mouse cardiomyocytes. J Vis Exp.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia Y, Javadov S, Gan TX, Pang T, Cook MA
and Karmazyn M: Distinct KATP channels mediate the antihypertrophic
effects of adenosine receptor activation in neonatal rat
ventricular myocytes. J Pharmacol Exp Ther. 320:14–21. 2007.
View Article : Google Scholar
|
|
31
|
Yang C, Zhang W, Liu X, Liang Y, Li P,
Zhang Y and Yuan Y: The influence of the single different radiation
dose and time on the microscopic structure and ultrastructure of
Balb/c mice]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
28:979–982. 2014.In Chinese.
|
|
32
|
Zhu LA, Fang NY, Gao PJ, Jin X and Wang
HY: Differential expression of alpha-enolase in the normal and
pathological cardiac growth. Exp Mol Pathol. 87:27–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jian J, Xuan F, Qin F and Huang R: The
antioxidant, anti-inflammatory and anti-apoptotic activities of the
Bauhinia Championii flavone are connected with protection against
myocardial ischemia/reperfusion injury. Cell Physiol Biochem.
38:1365–1375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei K, Liu L, Xie F, Hao X, Luo J and Min
S: Nerve growth factor protects the ischemic heart via attenuation
of the endoplasmic reticulum stress induced apoptosis by activation
of phosphatidylinositol 3-kinase. Int J Med Sci. 12:83–91. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Y, Zhu W, Wang Z, Yuan W, Sun Y, Liu H
and Du Z: Combinatorial microRNAs suppress hypoxia-induced
cardio-myocytes apoptosis. Cell Physiol Biochem. 37:921–932. 2015.
View Article : Google Scholar
|
|
36
|
Liu X, Duan P, Hu X, Li R and Zhu Q:
Altered KATP channel subunits expression and vascular reactivity in
spontaneously hypertensive rats with age. J Cardiovasc Pharmacol.
68:143–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Jameel MN, Li Q, Mansoor A, Qiang
X, Swingen C, Panetta C and Zhang J: Stem cells for myocardial
repair with use of a transarterial catheter. Circulation. 120(Suppl
11): S238–S246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ward ML and Crossman DJ: Mechanisms
underlying the impaired contractility of diabetic cardiomyopathy.
World J Cardiol. 6:577–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fuentes-Antrás J, Picatoste B,
Gómez-Hernández A, Egido J, Tuñón J and Lorenzo Ó: Updating
experimental models of diabetic cardiomyopathy. J Diabetes Res.
2015:6567952015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kadenbach B: Intrinsic and extrinsic
uncoupling of oxidative phosphorylation. Biochim Biophys Acta.
1604:77–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brand MD and Nicholls DG: Assessing
mitochondrial dysfunction in cells. Biochem J. 435:297–312. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gomez-Cabrera MC, Sanchis-Gomar F,
Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C and Viña J:
Mitochondria as sources and targets of damage in cellular aging.
Clin Chem Lab Med. 50:1287–1295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kevelaitis E, Oubenaissa A, Mouas C,
Peynet J and Menasche P: Opening of mitochondrial potassium
channels: A new target for graft preservation strategies.
Transplantation. 70:576–578. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ali M, Mehmood A, Anjum MS, Tarrar MN,
Khan SN and Riazuddin S: Diazoxide preconditioning of endothelial
progenitor cells from streptozotocin-induced type 1 diabetic rats
improves their ability to repair diabetic cardiomyopathy. Mol Cell
Biochem. 410:267–279. 2015. View Article : Google Scholar
|
|
45
|
Monsalve M and Olmos Y: The complex
biology of FOXO. Curr Drug Targets. 12:1322–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maiese K, Chong ZZ, Hou J and Shang YC:
The 'O' class: Crafting clinical care with FoxO transcription
factors. Adv Exp Med Biol. 665:242–260. 2009. View Article : Google Scholar
|
|
47
|
Kim MY, Kim MJ, Yoon IS, Ahn JH, Lee SH,
Baik EJ, Moon CH and Jung YS: Diazoxide acts more as a PKC-epsilon
activator, and indirectly activates the mitochondrial K(ATP)
channel conferring cardioprotection against hypoxic injury. Br J
Pharmacol. 149:1059–1070. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Katoh H, Nishigaki N and Hayashi H:
Diazoxide opens the mitochondrial permeability transition pore and
alters Ca2+ transients in rat ventricular myocytes. Circulation.
105:2666–2671. 2002. View Article : Google Scholar : PubMed/NCBI
|