Introduction
Acute pancreatitis (AP) is a sudden inflammation of
the pancreas and the most common reason for hospitalization among
non-malignant gastrointestinal disorders in the United States
(1,2). AP is commonly triggered by various
risk factors, such as alcohol abuse, duct obstruction, obesity,
smoking, drug toxicity and metabolic disorders (3,4).
This condition arises from pancreatic acinar cell injury by the
activation of proteolytic pancreatic enzymes, including amylase,
lipase, and trypsin (5). Injured
acinar cells produce pro-inflammatory cytokines such as interleukin
(IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (6). These cytokines damage the acinar
cell, causing systemic inflammatory response syndrome, which is a
subset of cytokine storm (5-8).
Although AP is a critical disease, few studies have investigated
effective therapeutic treatments for AP.
Icariin (ICA) is a natural flavonoid glycoside
isolated from plants in the Epimedium genus (Yin-Yang-Huo)
of the Berberidaceae family and a well-known traditional Chinese
medicine for treating male sexual dysfunctions, such as erectile
dysfunction and premature ejaculation (9,10).
ICA, the major and active compound of Epimedium, has been
associated with many biological and pharmacological effects, such
as anti-osteoporotic (11),
antioxidative (12),
anti-inflammatory (13),
anti-cancer (14),
anti-depressant (15) and
neuro-protective effects (16).
Previous studies have reported that ICA ameliorates various
diseases, including atherosclerosis, asthma and erectile
dysfunction (17-19); however, the therapeutic effects of
ICA in acute pancreatitis require further investigation.
The purpose of this study was to investigate the
effects of ICA against cerulein-induced AP. Pancreas weight/body
weight (PW/BW) ratio, serum amylase and lipase levels, histological
appearance of the pancreas and lung, myeloperoxidase (MPO) activity
and pro-inflammatory cytokine levels were evaluated to determine
the attenuating effects of ICA on the severity of cerulein-induced
AP. Furthermore, we evaluated the activation of mitogen activated
protein kinases (MAPKs, indicated by MAPKs phosphorylation) and
nuclear factor κB (NF-κB, indicated by Iκ-Bα degradation) to
determine the inhibitory mechanisms of ICA on AP.
Materials and methods
Chemicals and reagents
ICA, dimethyl sulfoxide (DMSO), cerulein, NaCl,
hexadecyl-trimethyl-ammonium bromide (HTAB), and Tris-HCl were
purchased from Sigma-Aldrich (Merck KGaA). ICA stock solutions were
using DMSO. Easy-Blue™ total RNA extraction kit was purchased from
iNtRON Biotechnology. Anti-phosphorylated extracellular
signal-regulated kinase (ERK)1/2 (1:1,000; cat. no. 9101L),
phosphorylated c-Jun N-terminal kinase (JNK 1/3; 1:1,000; cat. no.
9251L), and p38 (1:1,000; cat. no. 9211L) antibodies and SB203580
(p38 inhibitor) were purchased from Cell signaling Technology, Inc.
Total MAPK antibodies against ERK 1/2 (1:1,000; sc-93), JNK 1/3
(1:1,000; sc-474) and p38 (1:1,000; sc-535), inhibitory κ-Bα
(Iκ-Bα; 1:1,000; sc-371), and β-actin antibody (1:1,000; sc-1615)
were obtained from Santa Cruz Biotechnology, Inc. In addition,
horseradish peroxidase (HRP)-conjugated secondary antibodies,
including chicken anti-rabbit IgG-HRP (1:5.000; sc-516087) and
chicken anti-goat IgG-HRP (1:10.000; sc-516086) were purchased from
Santa Cruz Biotechnology, Inc.
Ethical aspects
All experiments were performed in accordance with
the animal care regulations of Wonkwang University set forth and
approved by the Wonkwang University Animal Ethics Committee.
Animal models
All experiments were performed according to the
protocols approved by the Animal Care Committee of Wonkwang
University. C57BL/6 mice (n=324, 6-8 weeks old, female, weighing
15-20 g), were purchased from Orient Bio. All animals were bred and
housed in standard shoebox cages in a climate-controlled room with
an ambient temperature of 23±2°C under a 12 h light/dark cycle for
7 days. Animals were fed standard laboratory chow, and water ad
libitum. Mice were randomly assigned to control or experimental
groups.
Experimental design
AP was induced via six intraperitoneal injections
(i.p.) of cerulein (50 µg/kg) at intervals of 1 h. Control
animals were administered DMSO under the same conditions. In the
pre-treatment groups, ICA (5, 10 or 20 mg/kg, i.p.), SB203580 (1
mg/kg i.p., n=6) or DMSO (i.p.) were administered 1 h before the
first cerulein injection, in the experimental (n=6) and the control
group (n=6) respectively. Mice were sacrificed 6 h after the last
cerulein injection. In the post-treatment groups, ICA (20 mg/kg,
n=6) or DMSO (control group, n=6) were administered 1, 3 and 5 h
after the first cerulein injection. Mice were sacrificed 6 h after
the last cerulein injection. Blood (withdrawn from the heart), the
pancreas, and the lung were rapidly removed and stored at −80°C for
further analysis. For the detection of MAPKs and NF-κB, mice were
pretreated with ICA (10 or 20 mg/kg) or DMSO 1 h before the
intraperitoneal injection of cerulein (50 µg/kg), and
sacrificed at 0, 15, 30 and 60 min after cerulein injection,
respectively. For immunofluorescence, mice were pretreated with ICA
(20 mg/kg) or DMSO 1 h before the intraperitoneal injection of
cerulein (50 µg/kg), and sacrificed at 30 min after cerulein
injection. Mice were sacrificed via CO2 asphyxiation.
The CO2 flow rate displaced 20% of the cage volume per
minute. To ensure death following CO2 asphyxiation,
cervical dislocation was performed. The pancreas was rapidly
removed and stored at −80°C for further analysis.
Histological analysis
The appearance of the entire pancreas and the
semi-quantitative index based on edema and inflammation were
examined from each treatment group. The pancreas and lung tissues
were fixed in 4% formalin solution at room temperature overnight,
embedded in paraffin, cut into 4 µm sections, stained with
hematoxylin for 8 min and eosin for 2 min overnight. Samples were
examined under a light microscope. Tissue sections from each
sample, representing a minimum of 100 fields were examined and
scored on a scale of 0-3 (0 corresponding to normal appearance and
3 corresponding to severe disease), based on the presence of
interstitial edema and interstitial inflammation. In the assessment
of the lung, the sections were examined for the presence of wall
thickening and inflammation. The levels of edema, and
pro-inflammatory cell infiltration were scored by pathologists in a
blinded manner who were unware of the study design; scores were set
based on a scale of 0 (normal) to 3 (severe) (20). In brief, a score of 0 presented
the absence of edema and inflammatory cells, 1 indicated focal
edema between lobules and 10 inflammatory cells; 2 suggested
diffuse edema between lobules and 10-20 inflammatory cells, while a
score of 3 reflected diffuse edema between acinar cells and >20
inflammatory cells.
Measurement of serum amylase and lipase
levels
Blood samples for the determination of serum amylase
and lipase levels were obtained 6 h after the last injection of
cerulein. Mice were sacrificed via CO2 asphyxiation
followed by cervical dislocation; blood samples were then withdrawn
from the heart. Amylase and lipase activities were determined by an
assay kit of Bio Assay Systems.
MPO activity
Sequestration of neutrophils within the pancreas and
lung was evaluated by measuring the MPO activity within tissues.
Briefly, tissue samples were weighed, homogenized with 20 mM
phosphate buffer (pH 7.4), and centrifuged at 10,000 × g for 10
min, 4°C. The pellets were then re-suspended in 50 mM phosphate
buffer (pH 6.0) containing 0.5 % HTAB. The samples were centrifuged
at 10,000 × g for 5 min, 4°C, and mixed with 80 mM sodium phosphate
buffer (pH 5.4) containing 1.6 mM TMB. The mixture was incubated at
37°C for 110 sec, and the reaction was terminated with 2 M
H2SO4. Tissue MPO activity was determined by
measuring the absorbance at 450 nm with a Versa Max microplate
reader (Molecular Devices, LLC) and was expressed as U/mg
protein.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
mRNA transcripts of mouse pancreatic tissues were
analyzed by RT-qPCR. Total RNA was isolated from the mouse pancreas
using Easy-Blue™ and subjected to RT using ABI cDNA synthesis kit
according to the manufacturer's protocols (Applied Biosystems;
Thermo Fisher Scientific, Inc.). For each sample, triplicate test
reactions and a control reaction lacking reverse transcriptase were
analyzed for the expression of the gene of interest, and the
results were normalized to those of the 'housekeeping' hypoxanthine
phosphoribosyltransferase (HPRT) mRNA. qPCR was performed using a
standard protocol from the TaqMan™ Universal Master Mix II, no UNG
(Applied Biosystems; Thermo Fisher Scientific, Inc.) on
StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). qPCR was performed at 50°C for 2 min and
95°C for 10 min, followed by 60 cycles of amplification at 95°C for
10 sec and 60°C for 30 sec. Forward, reverse, and probe
oligonucleotide primers for multiplex real-time TaqMan PCR were
purchased from Applied Biosystems (Thermo Fisher Scientific, Inc.).
The forward, reverse and probe oligonucleotide primers for
multiplex real-time TaqMan PCR were as follows: For mouse TNF-α
forward, 5′-TCT CTT CAA GGG ACA AGG CTG-3′, reverse, 5′-ATA GCA AAT
CGG CTG ACG GT-3′; mouse IL-1β forward, 5′-TTG ACG GAC CCC AAA AGA
T-3′, reverse, 5′-GAA GCT GGA TGC TCT CAT CTG-3′; mouse IL-6
forward, 5′-TTC ATT CTC TTT GCT CTT GAA TTA GA-3′, reverse, 5′-GTC
TGA CCT TTA GCT TCA AAT CCT-3′; mouse HPRT (forward, 5′-GAC CGG TCC
CGT CAT GC-3′; reverse, 5′-CAT AAC CTG GTT CAT CAT CGC TAA-3′).
Western blotting
Pancreatic tissues were homogenized and lysed on ice
with radioimmunoprecipitation assay lysis buffer (iNtRON
Biotechnology). Then, the lysates were boiled in 62.5 mM Tris-HCl
buffer, pH 6.8, containing 2% SDS, 20% glycerol, and 10%
2-mercaptoethanol. Proteins were separated on a 10% SDS-PAGE and
transferred to a nitrocellulose membrane. The membrane was blocked
with 5% skim milk in phosphate-buffered saline with Tween-20 for 2
h at room temperature, followed by incubation with primary
antibodies overnight at 4°C. After washing three times, the
membrane was incubated with secondary antibodies for 1 h at room
temperature. The proteins were visualized using an enhanced
chemiluminescence detection system (GE Healthcare) according to the
manufacturer's protocols. The bands were detected and quantified by
using Quantity One software (version 4.5.2; Bio-Rad Laboratories,
Inc.).
Immunofluorescence
Immunofluorescence for the analysis of
phosphorylated p38 was performed on pancreas tissue. The pancreas
tissues were embedded in frozen section compound, cut into 9
µm sections, fixed in 100% methanol at −20°C for 5 min The
tissues were incubated with the primary antibodies against
phosphorylated p38 (1:250) at 4°C overnight followed by the
fluorescence labeled secondary antibodies Alexa Fluor®
488 goat anti rabbit (1:2,000; A27034; Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 2 h. Nuclei were counter
stained with DAPI (5 ng/ml) at room temperature for 5 min. Stained
sections were visualized using a confocal laser microscope (Olympus
Corporation).
Statistical analysis
Data were expressed as the mean ± standard error of
the mean. Statistical significance of intergroup differences was
evaluated using two-way analysis of variance, with time and dose as
variables, followed by a Duncan's post-hoc test. All statistical
analyses were performed using SPSS version 10.0 statistical
analysis software (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference. All experiments
were conducted in triplicate.
Results
Effects of ICA on the severity of
cerulein-induced AP
To evaluate the prophylactic effects of ICA against
cerulein-induced AP, histological examination of the pancreas and
lung was carried out. In the DMSO-treated mice, histological
features of the pancreas and lung showed typical normal
architecture. In the DMSO-treated mice with AP, histological
features of damaged pancreas tissue were observed, as characterized
by interstitial edema and inflammatory cell infiltration. However,
treatment with ICA ameliorated the pancreatic histological damage
in a dose-dependent manner (Fig.
1B). In addition, histological features of the lung in
cerulein-induced AP were observed; lung injury was characterized by
alveolar wall thickening and inflammatory cell infiltration.
Nevertheless, lung injury was significantly reduced by treatment
with ICA compared with the control (Fig. 1D and E). In addition, to assess
the effects of ICA on the severity of cerulein-induced AP, PW/BW
ratio, serum amylase and lipase levels were measured. In the
cerulein-induced AP mice, the PW/BW ratio was increased than in the
control mice due to pancreatic edema; however, cerulein-induced
increases in the PW/BW ratio was inhibited by ICA treatment in a
dose-dependent manner (Fig. 1F).
Furthermore, the levels of serum digestive enzymes, including
amylase and lipase were significantly increased in the
cerulein-induced AP mice than in the control mice; the levels of
both enzymes were decreased by ICA treatment (Fig. 1G and H).
 | Figure 1Effects of ICA on the severity of
cerulein-induced AP. (A) Scheme for pretreatment experiment. Mice
were pretreated with ICA (5, 10 or 20 mg/kg) or DMSO 1 h prior to
the induction of AP with cerulein (50 µg/kg). Mice were
sacrificed 6 h after the last cerulein injection. Representative
H&E-stained sections of the (B) pancreas and (D) lung (original
magnification, ×200). Histological scores for (C) pancreatic edema
and inflammation, (E) pulmonary wall thickening and inflammation.
(F) PW/BW ratio, serum levels of (G) amylase and (H) lipase. The
groups were treated as indicated in the experimental protocol. Data
are presented as the mean ± standard error of the mean, n=6.
Results are representative of three experiments.
*P<0.05 vs. DMSO treatment alone;
†P<0.05 vs. cerulein treatment alone. Scale bar, 20
µm. AP, acute pancreatitis; DMSO, dimethyl sulfoxide; ICA,
icariin; PW/BW ratio, pancreas weight/body weight ratio. |
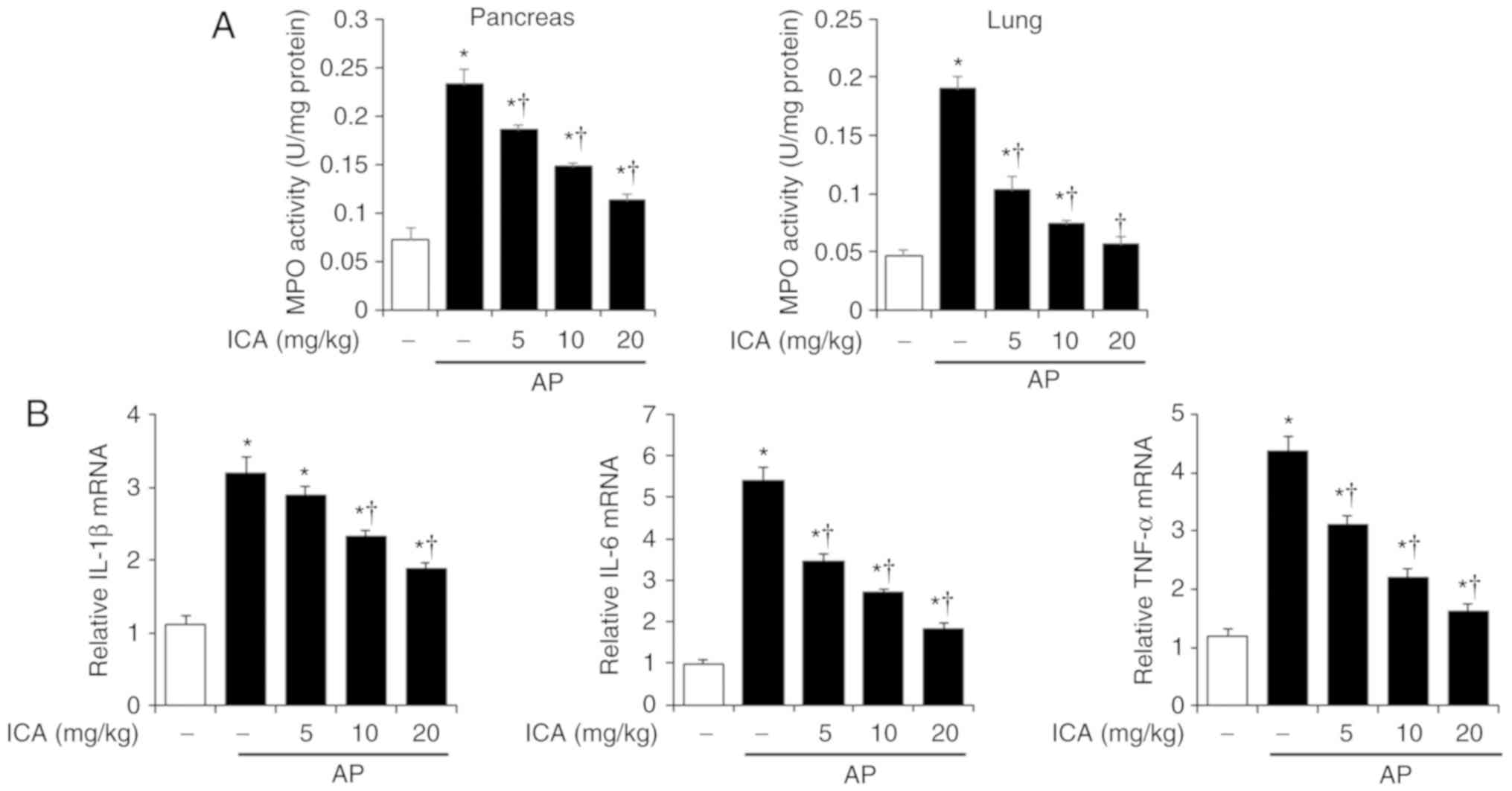
Effects of ICA on MPO activity and
pancreatic cytokine production in cerulein-induced AP
Neutrophil migration into target tissue is increased
when inflammation occurs (21).
Therefore, we examined MPO activity in the pancreas and lung after
the induction of AP, in order to measure neutrophil infiltration.
As shown in Fig. 2A, MPO activity
was significantly higher in cerulein-induced AP mice than in
control mice; however, the increase of MPO activity was inhibited
by ICA treatment in the pancreas and the lung in a dose dependent
manner. Of note, the production of pro-inflammatory cytokines,
including IL-1β, IL-6 and TNF-α were determined to increase during
cerulein-induced AP (22). Thus,
we examined the pancreatic mRNA levels of IL-1β, IL-6 and TNF-α. As
shown in Fig. 2B, the expression
levels of these pro-inflammatory cytokines significantly increased
in cerulein-induced AP mice compared with the control; however, ICA
treatment inhibited IL-1β, IL-6 and TNF-α expression in a
dose-dependent manner.
Effects of ICA on the activation of MAPK
and NF-κB in cerulein-induced AP
To investigate the inhibitory mechanisms of ICA on
AP, the activation of MAPKs and NF-κB was examined in the pancreas.
Mice were administered ICA (20 mg/kg) or DMSO for 1 h and then
stimulated with cerulein for 0, 15, 30 and 60 min. Cerulein
treatment triggered the phosphorylation of MAPKs and the
degradation of Iκ-Bα. However, administration of ICA inhibited the
phosphorylation of p38, but not of ERK 1/2, JNK, and degradation of
Iκ-Bα (Fig. 3A). Consistent with
Fig. 3A, p38 phosphorylation was
also inhibited by ICA as determined via immunofluorescence staining
(Fig. 3C). To determine whether
the inhibition of p38 activation was responsible for the effects of
ICA, we administered ICA at different concentrations (10 or 20
mg/kg). As shown in Fig. 3D and
E, the phosphorylation of p38 was inhibited by ICA in a
dose-dependent manner.
 | Figure 3Effects of ICA on the activation of
MAPK. (A) Scheme for applied in the study. Mice were pretreated
with ICA (20 mg/kg) or DMSO (control) 1 h prior to the induction of
AP with cerulein (50 µg/kg). Mice were sacrificed at 0, 15,
30 and 60 min after cerulein treatment. Pancreatic tissues were
harvested for western blot analysis to detect (B) phosphorylation
of MAPKs and degradation of Iκ-Bα. β-actin was used as a loading
control. (C) Quantitative analysis of western blot. (D)
Phosphorylation of p38 at 30 min after cerulein treatment was
detect by immunofluorescence staining. (E) After 30 min of cerulein
treatment, phosphorylation of p38 in different concentrations of
ICA (10 or 20 mg/kg) was detected by western blot analysis. (F)
Quantitative analysis of western blot. Results are representative
of three experiments. *P<0.05 vs. DMSO + cerulein
treatment. Scale bar, 40 µm. ERK, extracellular
signal-regulated kinase; ICA, icariin; IF, immunofluorescence;
Iκ-Bα, inhibitory κ-Bα; JNK, c-Jun N-terminal kinase; p,
phosphorylated. |
Effects of P38 on the severity in
cerulein-induced AP
To determine whether inhibition of p38 could affect
the reduction of inflammatory responses, a p38 inhibitor (SB203580,
1 mg/kg) was employed, after which the severity of AP was
evaluated. As presented in Fig.
4, inhibition of p38 by SB203580 suppressed the histological
damage to the pancreas and lung, and reduced the PW/BW ratio, and
the levels of amylase and lipase compared with AP mice (Fig. 4). These observations were similar
to the effects of ICA (Fig. 1).
Additionally, administration of SB203580 significantly reduced MPO
activity, and the mRNA levels of IL-1β, IL-6 and TNF-α compared
with in AP mice (Fig. 5).
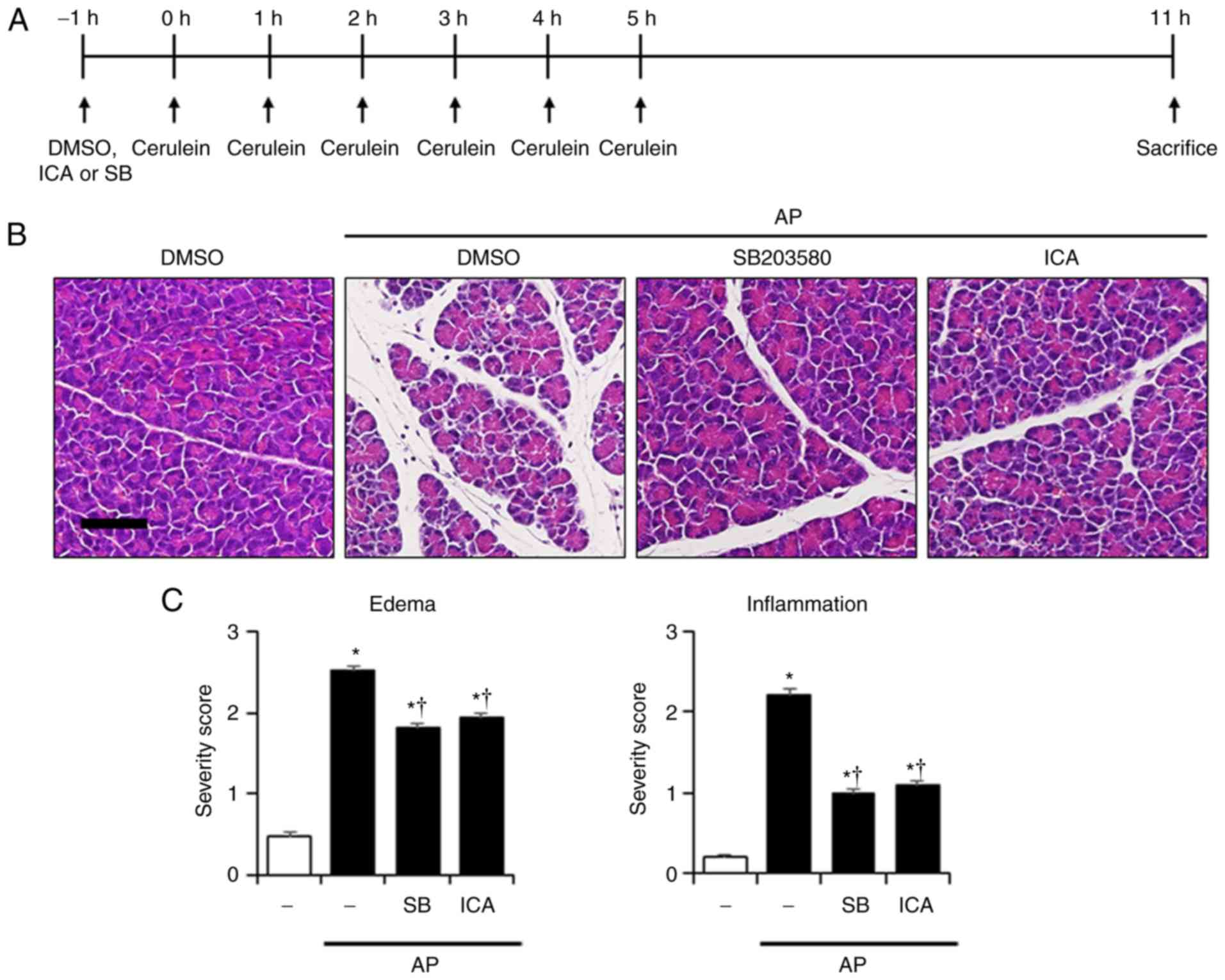 | Figure 4Effects of p38 inhibition by SB203580
on the severity of cerulein-induced AP. (A) Scheme for p38
inhibitor experiment. Mice were pretreated with ICA (20 mg/kg),
SB203580 (1 mg/kg) or DMSO 1 h prior to the induction of AP with
cerulein (50 µg/kg). Mice were sacrificed 6 h after the last
cerulein injection. H&E-stained sections of the (B) pancreas
and (D) lung (original magnification, ×200). Histological scores
for (C) pancreatic edema and inflammation, (E) pulmonary wall
thickening and inflammation. (F) PW/BW ratio, and serum levels of
(G) amylase and (H) lipase. Data are presented as the mean ±
standard error of the mean, n=6. Results are representative of
three experiments. *P<0.05 vs. DMSO treatment alone;
†P<0.05 vs. cerulein treatment alone. Scale bar, 20
µm. AP, acute pancreatitis; DMSO, dimethyl sulfoxide; ICA,
icariin; PW/BW ratio, pancreas weight/body weight ratio. |
 | Figure 5Effects of p38 inhibition by SB203580
on MPO activity and pancreatic cytokine production in
cerulein-induced AP. Mice were pretreated with ICA (20 mg/kg),
SB203580 (1 mg/kg) or DMSO 1 h prior to the induction of AP with
cerulein (50 µg/kg). Mice were sacrificed 6 h after the last
cerulein injection. (A) Pancreas and lung MPO activity was measured
6 h after the last injection of cerulein. (B) mRNA levels of
pancreatic IL-1β, IL-6 and TNF-α were quantified by reverse
transcription-quantitative polymerase chain reaction. Data are
presented as the means ± standard error of the mean, n=6. Results
are representative of three experiments. *P<0.05 vs.
DMSO treatment alone, †P<0.05 vs. cerulein treatment
alone. AP, acute pancreatitis; ICA, icariin; IL-1β, interleukin-1β;
IL-6, interleukin-6; MPO, myeloperoxidase; TNF-α, tumor necrosis
factor-α. |
Therapeutic effects of ICA in
cerulein-induced AP
To examine the therapeutic effects of ICA in
cerulein-induced AP, we applied ICA after the onset of AP.
Post-treatment of ICA at 1 h but not 3 and 5 h after first cerulein
injection significantly reduced the PW/BW ratio, serum amylase and
lipase activities, and histological injury of the pancreas and lung
compared with the AP mice, which suggests that ICA could exhibit
therapeutic properties in the early phase of AP (Fig. 6).
 | Figure 6Therapeutic effects of ICA against
cerulein-induced AP. (A) Scheme for posttreatment experiment. Mice
were treated with ICA (20 mg/kg) or DMSO 1, 3 or 5 h after the
first cerulein injection. Mice were sacrificed 6 h after the last
cerulein injection. Representative H&E-stained sections of the
(B) pancreas and (D) lung (original magnification, ×200).
Histological scores for (C) pancreatic edema and inflammation, (E)
pulmonary wall thickening and inflammation. (F) PW/BW ratio, and
serum levels of (G) amylase and (H) lipase. Data are presented as
the mean ± standard deviation, n=6. Results are representative of
three experiments. *P<0.05 vs. DMSO treatment alone;
†P<0.05 vs. cerulein treatment alone. Scale bar, 20
µm. AP, acute pancreatitis; DMSO, dimethyl sulfoxide; ICA,
icariin; PW/BW ratio, pancreas weight/body weight ratio. |
Discussion
AP is an acute inflammatory disease that occurs in
the pancreas, and its incidence is ~300 in 1 million (23). The symptoms of AP vary from mild
inflammation of the pancreas to severe inflammatory reactions with
multiple organ failure (24). The
mortality rate of mild AP is ~10% and most of them are self-healing
without complications (25). The
mortality rate of severe AP is about 20-30%, which is caused by
complications of multiple organ failure, such as liver dysfunction
and pulmonary dysfunction (26).
In general, AP is treated via temporary symptomatic relief,
including antibiotics or analgesics, and in severe cases, the
necrotic pancreatic tissue is removed through surgery. However,
current therapies have limitations such as AP-associated
complications and high mortality (27). Therefore, agents for the
prevention and treatment of AP are urgently required.
The diagnosis of AP is based on the increasing
levels of serum amylase and lipase. This is due to the fact that
the levels of these digestive enzymes, which increase during AP,
contribute to acinar cell injury, further promoting the
inflammatory process, including the release of cytokines and
chemokines (28,29). Serum amylase concentration
increases at least three times in comparison with the upper limit
of the normal range (30). It
starts to rise within a few hours after the onset of symptoms and
reverts to the normal level within 3-5 days. Due to these features
(serum amylase elevation in early AP and short half-life of
amylase), the measurement of serum amylase level may be a suitable
diagnosis criterion in early presentation. Additionally, serum
lipase concentration is increased at least four times of the normal
level in cerulein-induced AP (31). It remains high for a longer period
of time than the levels of amylase at 8-14 days (31). Therefore, serum lipase analysis
may have an advantage over serum amylase levels as of its delayed
presentation. In the present study, serum amylase and lipase levels
increased in mice with cerulein-induced AP; however, ICA treatment
inhibited the elevation of serum amylase and lipase levels, which
suggests the therapeutic effect of ICA on AP was mediated by the
reduction in the levels of these enzymes.
Neutrophils have a central role in the development
of AP by mediating local tissue damage and remote organ injury
(32). They are recruited to
sites of infection or inflammation, and in regions of pancreatitis
(32). Infiltration and activated
neutrophils prolong lifespan of neutrophils by several days and
release inflammatory mediators, which further worsen the severity
of AP (33,34). As inflammation continues,
neutrophil accumulation increases in which 'neutrophil swarmimg'
occurs; this promotes the progression of local pancreatic
inflammation to multiple organ dysfunction syndrome (MODS) by
overwhelming inflammatory responses (35). Neutrophil depletion by
anti-neutrophil serum was reported to inhibit the severity of
cerulein-induced AP (36). Thus,
the measurement of neutrophil count may be regarded as a diagnosis
index of AP. In this study, we assessed neutrophil infiltration
into the pancreas and lung during cerulein-induced AP by measuring
MPO activity. ICA treatment was determined to inhibit the activity
of MPO in the pancreas and lung in AP. This suggested that the
inhibitory effects of ICA on neutrophil infiltration could
contribute to the amelioration of AP and AP-associated lung
injury.
Severe AP often leads to MODS, and the most common
and earliest target organ is the lung (37). Additionally, acute lung injury
(ALI) and acute respiratory distress syndrome (ARDS) the more
severe form of ALI, are the common complications of AP (38). ALI occurred in 10-25% of AP
patients and has been linked with ≤60% of AP-associated
mortalities, particularly in elderly patients (39,40). Generally, AP-associated mild lung
injury recovers rapidly; however, secondary lung infection, which
leads to the release of lung-derived inflammatory mediators across
the injured epithelial barrier into the circulation, gives rise to
ARDS and mortality (41-43). The variety of effectors that
originate from the inflammatory effects of AP are involved in the
pathogenesis of secondary lung infection (44-46). Pancreatic mediators, such as
pro-inflammatory cytokines and inflammatory cells, are
representative of secondary lung infection pathogenesis (44-48). In the present study, our results
showed that ICA treatment inhibited the elevation of pancreatic
cytokines, which could be promote ALI. In addition, histological
lung injury and lung MPO activity were decreased by ICA during AP
in our study. Overall, ICA was proposed to exhibit protective
activities in mice against AP-associated ALI via the inhibition of
pancreatic mediators, such as pro-inflammatory cytokines and
neutrophil infiltration.
In regards to the anti-inflammatory activities of
ICA, which were determined to be mediated by MAPKs and the NF-κB
pathway in a mouse model (49),
we investigated the effects of ICA on the activation of MAPKs and
NF-κB in cerulein-induced AP. The important signaling pathways,
MAPKs and NF-κB, are known to involve various inflammatory
regulators in the pancreas (50).
In the present study, cerulein activated MAPK and NF-κB in the
pancreas as expected, although, only cerulein-induced activation of
p38 was inhibited by ICA treatment. These results are in line with
that of a previous study where the anti-inflammatory activity of
ICA was mainly mediated by the deactivation of p38 MAPK; however,
the model of inflammation differed (13). In addition, we reported that the
administration of SB203580, a p38 inhibitor, improved the severity
of AP similar to ICA. These results suggest that the administration
of ICA could reduce the severity of cerulein-induced AP via
inhibition of p38.
In the present study, we have demonstrated that ICA
exhibited protective and therapeutic effects against
ceru-lein-induced AP and AP-associated lung injury. Furthermore,
ICA inhibited the activation of p38 MAPK in cerulein-induced AP.
Collectively, our findings suggest that ICA is a potential
therapeutic agent for the treatment of AP.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Research
Foundation of Korea grant funded by the Korea government (MEST;
grant nos. NRF-2017R1C1B2010031, NRF-2017R1D1A1B03032371 and
NRF-2017R1A5A2015805).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DUK, GSB, HJS and SJP made substantial contributions
to the design of the study. DUK, GSB, MJK, JWC and DGK performed
the experiments. MJK and DGK contributed to the statistical
analysis of data. DUK and GSB wrote the manuscript. All authors
reviewed the results and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All experiments were carried out in accordance with
the animal care regulations set forth and approved by the Wonkwang
University Animal Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peery AF, Crockett SD, Barritt AS, Dellon
ES, Eluri S, Gangarosa LM, Jensen ET, Lund JL, Pasricha S, Runge T,
et al: Burden of gastrointestinal, liver, and pancreatic diseases
in the United States. Gastroenterology. 149:1731–1741. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maléth J and Hegyi P: Ca2+ toxicity and
mitochondrial damage in acute pancreatitis: Translational overview.
Philos Trans R Soc Lond B Biol Sci. 371:201504252016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bettaieb A, Koike S, Chahed S, Bachaalany
S, Griffey S, Sastre J and Haj FG: Pancreatic protein tyrosine
phosphatase 1B deficiency exacerbates acute pancreatitis in mice.
Am J Pathol. 186:2043–2054. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yadav D and Lowenfels AB: The epidemiology
of pancreatitis and pancreatic cancer. Gastroenterology.
144:1252–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minkov GA, Halacheva KS, Yovtchev YP and
Gulubova MV: Pathophysiological mechanisms of acute pancreatitis
define inflammatory markers of clinical prognosis. Pancreas.
44:713–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Büchler MW, Gloor B, Müller CA, Friess H,
Seiler CA and Uhl W: Acute necrotizing pancreatitis: Treatment
strategy according to the status of infection. Ann Surg.
232:619–626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bae GS, Heo KH, Park KC, Choi SB, Jo IJ,
Seo SH, Kim DG, Shin JY, Kang DG, Lee HS, et al: Apamin attenuated
cerulein-induced acute pancreatitis by inhibition of JNK pathway in
mice. Dig Dis Sci. 58:2908–2917. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chakraborty M, Hickey AJ, Petrov MS,
Macdonald JR, Thompson N, Newby L, Sim D, Windsor JA and Phillips
AR: Mitochondrial dysfunction in peripheral blood mononuclear cells
in early experimental and clinical acute pancreatitis.
Pancreatology. 16:739–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li LS, Luo YM, Liu J, Zhang Y, Fu XX and
Yang DL: Icariin inhibits pulmonary hypertension induced by
monocrotaline through enhancement of NO/cGMP signaling pathway in
rats. Evid Based Complement Alternat Med.
2016:79154152016.PubMed/NCBI
|
|
10
|
Hsueh TY, Ho JK, Lin LC, Chiu AW, Lin CH
and Tsai TH: Herb-drug interaction of Epimedium extract on the
pharmaco-kinetic of dapoxetine in rats. J Chromatogr B Analyt
Technol Biomed Life Sci. 1014:64–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen G, Wang C, Wang J, Yin S, Gao H,
Xiang LU, Liu H, Xiong Y, Wang P, Zhu X, et al: Antiosteoporotic
effect of icariin in ovariectomized rats is mediated via the
Wnt/β-catenin pathway. Exp Ther Med. 12:279–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang ZS, Xiao HJ, Qi T, Hu ZM, Li H, Chen
DL, Xu YL and Chen J: Antioxidative protective effect of icariin on
the FeSO4/H2O2-damaged human sperm
based on confocal raman micro-spectroscopy. J Huazhong Univ Sci
Technolog Med Sci. 34:755–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong L, Liu J, Wang J, Luo Q, Zhang H, Liu
B, Xu F, Pang Q, Liu Y and Dong J: Icariin inhibits TNF-α/IFN-γ
induced inflammatory response via inhibition of the substance P and
p38-MAPK signaling pathway in human keratinocytes. Int
Immunopharmacol. 29:401–407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan HL, Chan KG, Pusparajah P, Saokaew S,
Duangjai A, Lee LH and Goh BH: Anti-cancer properties of the
naturally occurring aphrodisiacs: Icariin and its derivatives.
Front Pharmacol. 7:1912016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu B, Xu C, Wu X, Liu F, Du Y, Sun J, Tao
J and Dong J: Icariin exerts an antidepressant effect in an
unpredictable chronic mild stress model of depression in rats and
is associated with the regulation of hippocampal neuroinflammation.
Neuroscience. 294:193–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YJ, Zheng HY, Huang XX, Han SX, Zhang
DS, Ni JZ and He XY: Neuroprotective effects of icariin on brain
metabolism, mitochondrial functions, and cognition in
triple-transgenic Alzheimer's disease mice. CNS Neurosci Ther.
22:63–73. 2016. View Article : Google Scholar
|
|
17
|
Wang Y, Wang YS, Song SL, Liang H and Ji
AG: Icariin inhibits atherosclerosis progress in Apoe null mice by
downregulating CX3CR1 in macrophage. Biochem Biophys Res Commun.
470:845–850. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei Y, Liu B, Sun J, Lv Y, Luo Q, Liu F
and Dong J: Regulation of Th17/Treg function contributes to the
attenuation of chronic airway inflammation by icariin in
ovalbumin-induced murine asthma model. Immunobiology. 220:789–797.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu T, Xin H, Li WR, Zhou F, Li GY, Gong
YQ, Gao ZZ, Qin XC, Cui WS, Shindel AW and Xin ZC: Effects of
icariin on improving erectile function in streptozotocin-induced
diabetic rats. J Sex Med. 8:2761–2772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ethridge RT, Chung DH, Slogoff M, Ehlers
RA, Hellmich MR, Rajaraman S, Saito H, Uchida T and Evers BM:
Cyclooxygenase-2 gene disruption attenuates the severity of acute
pancreatitis and pancreatitis-associated lung injury.
Gastroenterology. 123:1311–1322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MJ, Bae GS, Choi SB, Jo IJ, Kim DG,
Shin JY, Lee SK, Kim MJ, Shong HJ and Park SJ: Lupeol protects
against cerulein-induced acute pancreatitis in mice. Phytother Res.
29:1634–1639. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Que RS, Cao LP, Ding GP, Hu JA, Mao KJ and
Wang GF: Correlation of nitric oxide and other free radicals with
the severity of acute pancreatitis and complicated systemic
inflammatory response syndrome. Pancreas. 39:536–540. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andersson R, Andersson B, Haraldsen P,
Drewsen G and Eckerwall G: Incidence, management and recurrence
rate of acute pancreatitis. Scand J Gastroenterol. 39:891–894.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson CD, Besselink MG and Carter R:
Acute pancreatitis. BMJ. 349:g48592014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mossad DE, Dinh BV, Markert RJ, Musleh MN
and Agrawal S: Predictors of in hospital mortality in acute
pancreatitis. JOP J Pancreas. 18:465–469. 2017.
|
|
26
|
Popa CC, Badiu DC, Rusu OC, Grigorean VT,
Neagu SI and Strugaru CR: Mortality prognostic factors in acute
pancreatitis. J Med Life. 9:413–418. 2016.PubMed/NCBI
|
|
27
|
Kimura W and Mössner J: Role of
hypertriglyceridemia in the pathogenesis of experimental acute
pancreatitis in rats. Int J Pancreatol. 20:177–184. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raraty MG, Murphy JA, Mcloughlin E, Smith
D, Criddle D and Sutton R: Mechanisms of acinar cell injury in
acute pancreatitis. Scand J Surg. 94:89–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cotton PB, Lehman G, Vennes J, Geenen JE,
Russell RC, Meyers WC, Liqoury C and Nickl N: Endoscopic
sphincterotomy complications and their management: An attempt at
consensus. Gastrointest Endosc. 37:383–393. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Winslet M, Hall C, London NJ and
Neoptolemos JP: Relation of diagnostic serum amylase levels to
aetiology and severity of acute pancreatitis. Gut. 33:982–986.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tietz NW and Shuey DF: Lipase in serum-the
elusive enzyme: An overview. Clin Chem. 39:746–756. 1993.PubMed/NCBI
|
|
32
|
Montecucco F, Mach F, Lenglet S, Vonlaufen
A, Gomes Quinderé AL, Pelli G, Burger F, Galan K, Dallegri F,
Carbone F, et al: Treatment with Evasin-3 abrogates
neutrphil-mediated inflammation in mouse acute pancreatitis. Eur J
Clin Invest. 44:940–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Merza M, Hartman H, Rahman M, Hwaiz R,
Zhang E, Renström E, Luo L, Mörgelin M, Regner S and Thorlacius H:
Neutrophil extracellular traps induce trypsin activation,
inflammation, and tissue damage in mice with severe acute
pancreatitis. Gastroenterology. 149:1920–1931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mantovani A, Cassatella MA, Costantini C
and Jaillon S: Neutrophils in the activation and regulation of
innate and adaptive immunity. Nat Rev Immunol. 11:519–531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang ZW, Meng XX and Xu P: Central role of
neutrophil in the pathogenesis of severe acute pancreatitis. J Cell
Mol Med. 19:2513–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sandoval D, Gukovskaya A, Reavey P,
Gukovsky S, Sisk A, Braquet P, Pandol SJ and Poucell-Hatton S: The
role of neutrophils and platelet-activating factor in mediating
experimental pancreatitis. Gastroenterology. 111:1081–1091. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El-Menyar A, Thani E, Zakaria A, Zarour A,
Tuma M, AbdulRahman H, Parchani A, Peralta R and Latifi R: Multiple
organ dysfunction syndrome (MODS): Is it preventable or inevitable.
Int J Clin Med. 3:722–730. 2012. View Article : Google Scholar
|
|
38
|
Elder AS, Saccone GT and Dixon DL: Lung
injury in acute pancreatitis: Mechanisms underlying augmented
secondary injury. Pancreatology. 12:49–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shields CJ, Winter DC and Redmond HP: Lung
injury in acute pancreatitis: Mechanisms, prevention, and therapy.
Curr Opin Crit Care. 8:158–163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Westerloo DJ, Schultz MJ, Bruno MJ, de
Vos AF, Florquin S and van der Poll T: Acute pancreatitis in mice
impairs bacterial clearance from the lungs, whereas concurrent
pneumonia prolongs the course of pancreatitis. Crit Care Med.
32:1997–2001. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou MT, Chen CS, Chen BC, Zhang QY and
Andersson R: Acute lung injury and ARDS in acute pancreatitis:
Mechanisms and potential intervention. World J Gastroenterology.
16:2094–2099. 2010. View Article : Google Scholar
|
|
43
|
Aeffner F, Bolon B and Davis IC: Mouse
models of acute respiratory distress syndrome: A review of
analytical approaches, pathologic features, and common
measurements. Toxicol Pathol. 43:1074–1092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jaffray C, Yang J and Norman J: Elastase
mimics pancreatitis-induced hepatic injury via inflammatory
mediators. J Surg Res. 90:95–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee WL and Downey GP: Neutrophil
activation and acute lung injury. Curr Opin Crit Care. 7:1–7. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lopez-Font I, Gea-Sorlí S, de-Madaria E,
Gutiérrez LM, Pérez-Mateo M and Closa D: Pancreatic and pulmonary
mast cells activation during experimental acute pancreatitis. World
J Gastroenterol. 16:3411–3417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fritz S, Hackert T, Hartwig W, Rossmanith
F, Strobel O, Schneider L, Will-Schweiger K, Kommerell M, Büchler
MW and Werner J: Bacterial translocation and infected pancreatic
necrosis in acute necrotizing pancreatitis derives from small bowel
rather than from colon. Am J Surg. 200:111–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matull WR, Pereira SP and O'donohue JW:
Biochemical markers of acute pancreatitis. J Clin Pathol.
59:340–344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ma P, Zhang S, Su X, Qiu G and Wu Z:
Protective effects of icariin on cisplatin-induced acute renal
injury in mice. Am J Transl Res. 7:2105–2014. 2015.PubMed/NCBI
|
|
50
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|