Introduction
The degradation of the extracellular matrix (ECM)
serves an important in various diseases; for instance, in cancer,
ECM degradation contributes to tumor invasion or metastasis
(1). The matrix
metalloproteinases (MMPs) are important members of the
zinc-dependent endopeptidase family and have been classified into
eight subgroups, which include collagenases, matrilysin,
metalloelastase, gelatinases, enamelysin and stromelysins (2). These enzymes target several
components of the ECM, such as collagen, elastin and fibronectin;
thus, they serve a crucial role in its degradation (3). In addition, MMPs are involved in a
variety of physiological and pathological processes, including
angiogenesis, tissue remodeling, and tumor invasion and metastasis
(4-6). Among the MMPs, MMP-2 and MMP-9 are
gelatinases, and have been reported to serve an important role in
tumor invasion and metastasis through their ability to degrade an
essential ECM component, type IV collagen (7). Notably, previous reports have
established that the increased MMP-9 expression in cancer cells is
associated with a marked increase in tumor invasion and metastasis,
which is mediated by ECM degradation (8-10).
Therefore, the targeted inhibition of MMP-9 expression in cancer
cells presents a promising approach to suppress ECM degradation,
and restrain tumor invasion and metastasis (1,11,12).
Brown algae constitute a rich source of bioactive
compounds, which have recently attracted increasing attention due
to their biomedical and pharmaceutical potential (13,14). As of the health-beneficial effects
of these bioactive compounds, brown algae have been used worldwide,
including Korea, Japan, China, and European countries, as a
functional food, medicinal ingredients and gelling agents (15,16). Among brown algae, Sargassum
thunbergii is one of the main species in biomass, as well as an
important pharmaceutical resource (17,18). Accordingly, extracts and bioactive
compounds, including polysaccharides, phlorotannins, flavonoids and
proteins, which are derived from S. thunbergii have been
extensively investigated (18).
Furthermore, numerous studies demonstrated that these extracts and
compounds exhibit a broad spectrum of biological activities,
including pro-osteoblastogenic, antitumor, anti-inflammatory,
antioxidative, anti-MMP and antiadipogenic effects (19-28).
Indole derivatives can be produced through chemical
synthesis or can be isolated from several natural resources,
including Actinobacteria, algae, cruciferous vegetables, fungal and
marine sponges (29-32). They exhibit a variety of
biological activities, including antitumor, antioxidative,
anti-inflammatory and anticonvulsant properties (33-35). In addition, the indole derivative
indole-6-carboxaldehyde (I6CA) has been shown to exert
anti-adipogenic effects in 3T3-L1 adipo-cytes (26). Although previous reports have
highlighted their biological potential, to the best of our
knowledge, no study has reported the MMP inhibitory activity of
indole derivatives and their underlying mechanism of action.
Therefore, the present study aimed to utilize
phorbol 12-myristate 13-acetate (PMA), a compound that can activate
MMP-9, to mimic the conditions in cancer cells, and investigate the
inhibitory effects of I6CA isolated from S. thunbergii on
MMP-9. Furthermore, we aimed to determine the mechanism underlying
the effects of I6CA in HT1080 cells.
Materials and methods
Materials
The brown alga S. thunbergii was collected
from the coast of Jeju Island, Korea. The HT1080 cell line was
obtained from the American Type Culture Collection. Dulbecco's
modified Eagle's medium (DMEM), fetal bovine serum (FBS),
penicillin/streptomycin/amphotericin (10,000 U/ml, 10,000
μg/ml, and 2,500 μg/ml, respectively),
phosphate-buffered saline (PBS), and 0.25%
trypsin-ethyl-enediaminetetraacetic acid (EDTA) were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.).
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), gelatin (type A), PMA, dimethyl sulfoxide (DMSO) and silica
gel were purchased from Sigma-Aldrich (Merck KGaA).
The specific anti-MMP-9 rabbit polyclonal antibody
(ab38898) was purchased from Abcam. Rabbit polyclonal antibodies
against extracellular signal-regulated kinase (ERK) 1/2
(sc-292838), p38 (sc-7149), and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; sc-25778), mouse monoclonal antibodies
against phosphorylated (p)-ERK 1/2 (sc-7383), c-Jun N-terminal
kinase (JNK) 1/2 (sc-7345), p-JNK 1/2 (sc-6254), p-p38 (sc-7973),
and nuclear factor-κB (NF-κB) p65 (sc-8008), and horseradish
peroxidase-conjugated donkey polyclonal secondary antibody against
goat IgG (sc-2020) and goat poly-clonal secondary antibody against
mouse IgG (sc-2031) were all purchased from Santa Cruz
Biotechnology, Inc. The anti-inhibitor of NF-κB α (IκBα) rabbit
polyclonal (cat. no. 9242) and anti-p-IκBα rabbit monoclonal (cat.
no. 2859) antibodies were purchased from Cell Signaling Technology,
Inc. The Alexa Fluor® 546-conjugated goat polyclonal
anti-mouse IgG (H+L) cross-absorbed secondary antibody (A-11030),
Alexa Fluor 633-conjugated goat polyclonal anti-rabbit IgG (H+L)
cross-adsorbed secondary antibody (A-21070), and Hoechst 33342
nucleic acid stain (H1399) were purchased from Invitrogen (Thermo
Fisher Scientific, Inc.). Coomassie Brilliant Blue R-250 was
purchased Biosesang. Other common analytical-grade chemicals and
reagents used in this study were commercially available.
Extraction and isolation of I6CA from S.
thumbergii
After collection, all algal samples were washed
three times with tap water to remove salt, sand and epiphytes
attached to the surface, and were carefully rinsed again with fresh
water. The samples were stored at -20°C in a medical refrigerator
until use.
The frozen algae were lyophilized and homogenized
into powder. The powdered S. thumbergii (2 kg) was extracted
three times with 80% methanol, and the methanol extract was
centrifuged at 3,500 x g for 30 min at 4°C and then filtered with
Whatman No. 1 (Whatman Ltd.) filter paper to remove the residue.
The filtrate was evaporated at 40°C to obtain a methanol extract,
which was then suspended in distilled water (DW) and partitioned
using chloroform. The chloroform fraction (2 g) was subjected to
silica column chromatography by stepwise elution with a
chloroform-methanol solution (30:1→1:1, v/v) to separate the active
fraction (12.3 mg) in chloroform fraction. Silica gel (230-400
mesh) was packed in a glass column (Pyrex, 300x38 mm, Corning Inc.)
and a flow of solvent by gravity was allowed during the
purification.
The active fractions were further separated by a
Sephadex LH-20 column saturated with 100% methanol. Next, the
active compounds were purified by reversed-phase high performance
liquid chromatography (HPLC) using a Waters HPLC system (Alliance
2690; Waters Corporation) equipped with a Waters 996 photodiode
array detector and a C18 column (J'sphere ODSH80, 250x4.6 mm, 4
μm; YMC) by stepwise elution with methanol-water gradient
(ultraviolet absorbance detection wavelength, 296 nm; flow rate, 1
ml/min; injected volume, 30 μl). The HPLC eluting conditions
were as follows: 5-70% methanol for 40 min to 100% methanol for 20
min, followed by 10 min re-equilibration time of the column. HPLC
anlaysis was performed in a room kept at 24±1°C.
Finally, the purified compound was identified by
comparing their 1H- and 13C-nuclear magnetic resonance (NMR)
spectra with reported data (36).
NMR spectra (data not shown) were recorded on a ZEOL 600 MHz NMR
spectrometer (JEOL). The chemical structure of I6CA isolated from
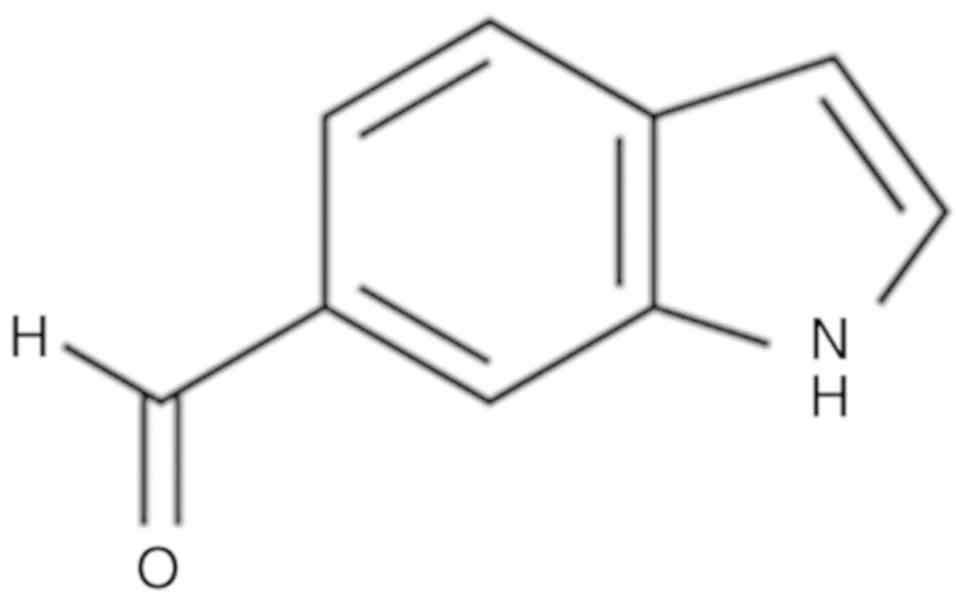
S. thunbergii is shown in Fig.
1. For cell culture experiments, I6CA was dissolved in DMSO,
and the final concentration of DMSO in culture medium was adjusted
to ~0.01%.
Cell culture
HT1080 cells were routinely cultured in complex
medium (DMEM supplemented with 10% heat-inactivated FBS and 1%
penicillin/streptomycin/amphotericin). Cultures were maintained at
37°C, 5% CO2 in a humidified atmosphere. The cells were
subcultured every 3 days using trypsin-EDTA for 3 min for cell
detachment.
Cell viability assay
HT1080 cells were seeded in a 96-well plate at a
density of 104 cells/well in 100
μl of complete medium. The next day, HT1080 cells were
treated, in the absence or presence of serum, with increasing
concentrations of I6CA (100, 200 and 400 μM) in the presence
or absence of PMA (10 ng/ml) at 37°C. After 24 h of incubation, 20
μl of MTT stock solution (1 mg/ml in PBS) was added to each
well. After 2 h of incubation at 37°C, the supernatant was removed,
and in each well, the formazan crystals were dissolved in 100
μl of DMSO. Then, the absorbance was measured at 540 nm
using a Powerwave XS2 microplate reader (BioTek Instruments, Inc.).
The relative cell viability was calculated based on the quantity of
MTT converted to formazan by the untreated and PMA only-treated
cells (100% viability). The data are expressed as the mean
percentage of the viable cells ± standard deviation of triplicate
experiments.
Analysis of MMP-9 gelatinolytic activity
by gelatin zymography
The gelatinolytic activity of MMP-9, secreted from
HT1080 cells, was determined by gelatin zymography (37-39). HT1080 cells grown in 6-well plate
at a density of 105 cells/well in
2 ml of complete medium were treated with I6CA (100, 200 and 400
μM) in serum-free medium in the presence or absence of PMA
(10 ng/ml) for 24 h at 37°C. Cell culture supernatants were
collected and their protein contents were measured using a
bicinchoninic assay (BCA) protein assay kit (Thermo Fisher
Scientific, Inc.) with a standard curve of a range of bovine serum
albumin concentrations in DW (0-1 mg/ml; Thermo Fisher Scientific,
Inc.). 20 μl of cell culture supernatants were subjected to
electrophoresis on 10% SDS-polyacrylamide gels containing 0.25%
gelatin. The gel was washed in 2.5% Triton X-100 at room
temperature to remove SDS and then incubated overnight at 37°C in
developing buffer (50 mM Tris-HCl at pH 7.5, 200 mM NaCl, 5 mM
CaCl2·2H2O, and 0.02% Brij-35). Finally, the
gel was stained at room temperature with Coomassie Blue staining
solution (1% Coomassie Brilliant Blue R-250, 45% methanol, and 10%
acetic acid) for 30 min and destained using the same solution
without dye. Negative staining (clear bands against a blue
background) indicated proteolysis and, therefore, gelatinolytic
activity. The MMP-9 gelatinolytic activity was quantified by
measuring the band intensities using ImageJ 1.8.0. software
(National Institutes of Health). The normalization of loading
protein was conducted via electrophoresis with 10%
SDS-polyacrylamide gels after which Coomassie staining to analyze
the amount of total loading protein.
Western blot analysis
HT1080 cells were lysed for 30 min in lysis buffer
(20 mM Tris-HCl at pH 7.4, 5 mM EDTA, 10 mM
Na4P2O7, 100 mM NaF, 2 mM
Na3VO4, 1% NP-40, 10 mg/ml aprotinin, 10
mg/ml leupeptin, and 1 mM PMSF) and then cell debris was removed by
centrifugation for 15 min at 16,000 x g, 4°C. The protein
concentration of the cell lysates was determined using a BCA
protein assay kit. Next, 30 μg of proteins were separated by
electrophoresis using 10% SDS-PAGE and transferred onto an Amersham
Protran Premium 0.45 μm nitrocellulose blotting membrane (GE
Healthcare Life Sciences). The membrane was blocked for 2 h at room
temperature with 5% nonfat dry milk in TBS-T (25 mM Tris-HCl at pH
7.4, 137 mM NaCl, 2.65 mM KCl, 0.05% Tween-20) and then incubated
for 24 h at 4°C with primary antibodies (1:1,000 dilution). After
washing with TBS-T, the membrane was incubated for 2 h at room
temperature with secondary antibody (1:5,000 dilution). The bands
were visualized using a CAS-400SM Davinch-Chemi image™ system
(Davinch-K Co. Ltd.).
Immunofluorescence staining and confocal
microscopy
HT1080 cells were seeded onto a sterilized 24x24 mm,
thickness No. 1 Deckglaser microscope cover glass (Paul Marienfel
GmbH & Co. KG). The cover glass was then transferred into a 5
cm cell culture dish, and the cells were treated for 30 min at 37°C
with I6CA in the presence or absence of PMA (10 ng/ml). For
immunofluorescence staining, the cells were washed three times with
PBS and fixed for 20 min at room temperature with cold 100%
methanol. After removing the methanol and washing three times with
PBS, the fixed cells were permeabilized for 20 min at room
temperature with 0.5% Tween-20 in PBS. Subsequently, the
permeabilized cells were incubated overnight at 4°C with a mouse
anti-NF-κB (p65) primary antibody (1:200 diluted in TBS-T). Cells
were washed three times with TBS-T and incubated for 2 h at 4°C
with an Alexa Fluor 546-conjugated goat polyclonal anti-mouse IgG
(H + L) cross-absorbed secondary antibody. Finally, the nuclei were
counterstained with Hoechst 33342 (20 ng/ml in PBS) for 5 min at
room temperature. The cells were observed under an LSM 700 confocal
microscope (Carl Zeiss AG) using a x40 water immersion objective
lens. Images were acquired and processed using the ProgRes
CapturePro 2.10.0.1 software (Carl Zeiss AG). Pseudo-colors were
applied to the images using ImageJ 1.8.0 software, and NF-κB p65
and nuclei were depicted in red and blue, respectively.
Statistical analysis
All quantitative data are presented in as the mean ±
standard deviation of at least three independent experiments that
were conducted using fresh reagents. The statistical significance
of the differences observed between groups was assessed by analysis
of variance, followed by Duncan's multiple range test. All
statistical analyses were performed using the SPSS Statistics 12.0
software (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
I6CA does not affect the viability of
HT1080 cells
We first assessed the cytotoxicity of I6CA purified
from S. thunbergii extract using an MTT assay. HT1080 cells
were treated for 24 h with increasing concentrations of I6CA in the
presence or absence of PMA and serum. Following the MTT assay, we
found no evidence of a cytotoxic effect of I6CA and PMA on HT1080
cells, even at the highest concentration of 400 μM (Fig. 2). Therefore, it was concluded that
these concentrations had no impact on cell viability and could be
used to conduct subsequent experiments.
I6CA inhibits MMP-9 secretion and
expression in HT1080 cells
Next, we examined the inhibitory effects of I6CA on
the secretion and protein expression of MMP-9 in HT1080 cells.
Using gelatin zymography, we assessed the gelatinolytic activity of
MMP-9 in the cell culture supernatant of PMA-stimulated HT1080
cells. Notably, we reported that I6CA inhibited the gelatinolytic
activity of MMP-9 in a dose-dependent manner (Fig. 3A). To determine whether I6CA
inhibited MMP-9 expression, we performed western blot analysis. Our
data demonstrated that the expression levels of MMP-9 exhibited a
similar trend to the gelatinolytic activity in the cell culture
supernatant (Fig. 3B).
I6CA suppresses mitogen-activated protein
kinases (MAPKs) activation in PMA-stimulated HT1080 cells
To investigate the mechanism mediating the
inhibition of MMP-9 expression by I6CA, we used western blotting to
analyze whether I6CA could regulate the activation of MAPKs in
PMA-stimulated HT1080 cells. MAPK activation is mediated by
phosphorylation (8). As shown in
Fig. 4A, the phosphorylation of
the three MAPKs, JNK, ERK and p38 MAPK, was significantly promoted
in PMA-stimulated HT1080 cells, compared with in untreated cells.
Of note, I6CA treatment significantly suppressed the
phosphorylation of JNK and ERK, but not that of p38 MAPK, in
response to PMA stimulation.
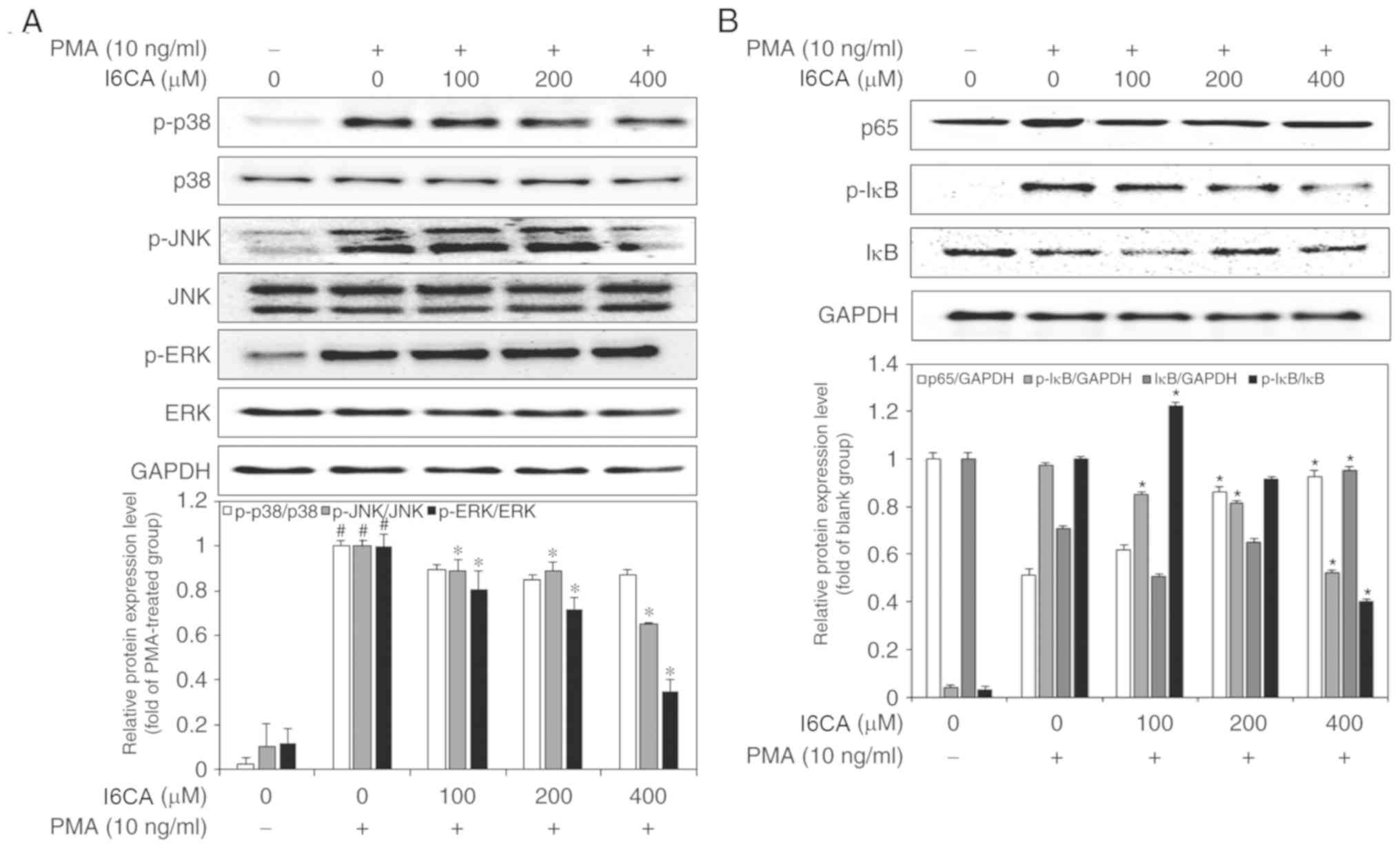 | Figure 4Inhibitory effects of I6CA on
MMP-9-related signaling pathway. Effects of I6CA on phosphorylation
of three members of the MAPK family, (A) JNK, ERK and p38 MAPK, and
the levels of activation of two essential players in the NF-κB
pathway, (B) IκBα and NF-κB p65. The ratio between the densities of
the bands corresponding to the proteins of interest and loading
control was used to determine their relative expression. The
expression data was normalized against the ratio calculated for
PMA-stimulated HT1080 cells (arbitrarily set to 1-fold). All data
are presented as the mean values ± standard deviation of triplicate
experiments. *P<0.05 vs. PMA-stimulated HT1080 cells.
#P<0.05 vs. un-stimulated HT1080 cells. ERK,
extracellular signal-regulated kinase; I6CA,
indole-6-carboxaldehyde; MMP-9, matrix metalloproteinase-9; IκBα,
inhibitor of κBα; JNK, c-Jun N-terminal kinase; p, phosphorylated;
PMA, phorbol 12-myristate 13-acetate. |
I6CA inhibits IκBα degradation and
prevents NF-κB nuclear translocation in PMA-stimulated HT1080
cells
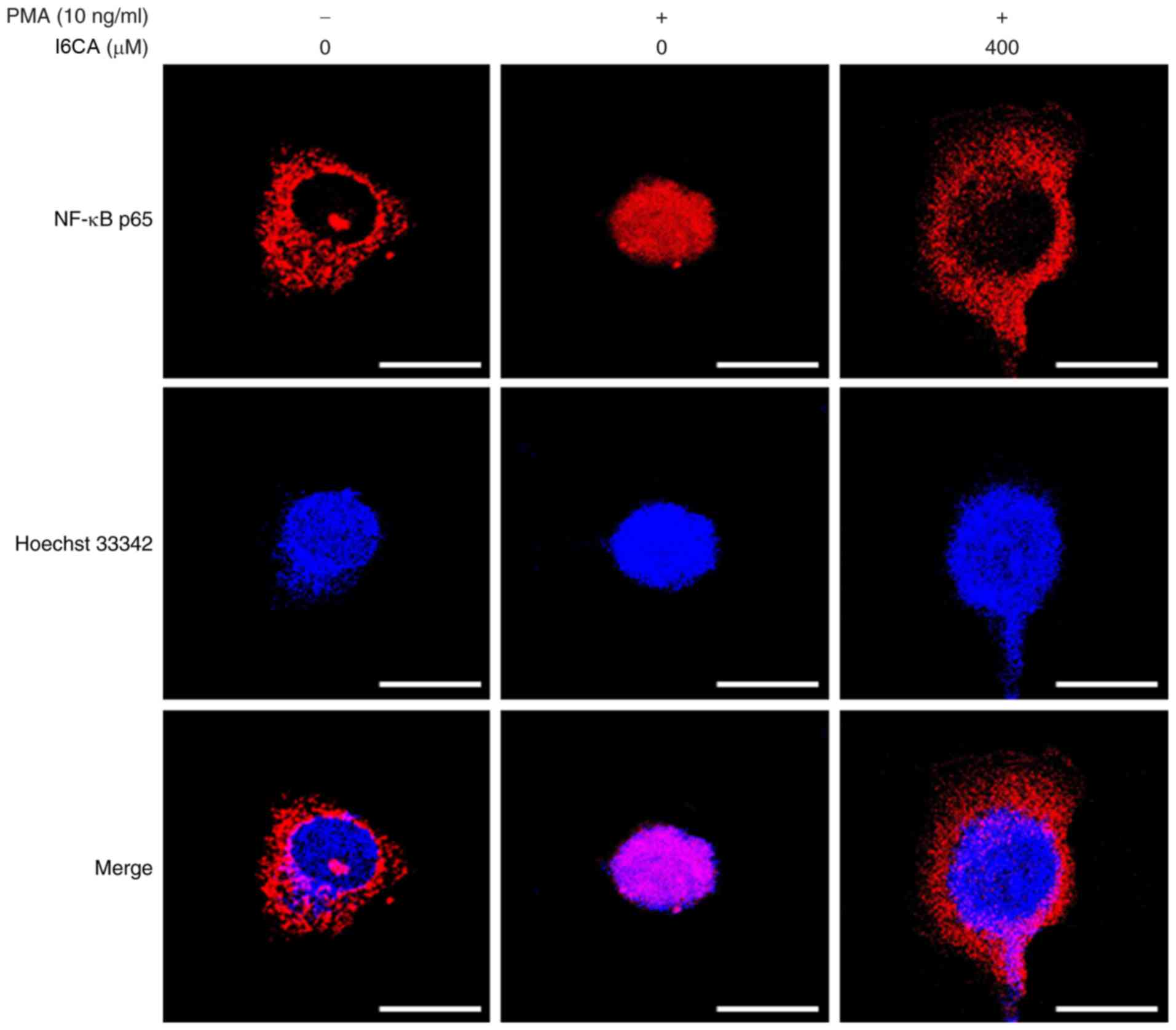
We examined whether I6CA decreases nuclear
translocation of the NF-κB p65 subunit. As presented in Fig. 4B, western blotting was conducted
to analyze the phosphorylation and degradation of IκBα, an
essential step in the nuclear translocation of NF-κB p65 subunit
(10). We determined that PMA
stimulation induced the phosphorylation and degradation of IκBα,
and NF-κB nuclear translocation in HT1080 cells, whereas I6CA
treatment suppressed these effects induced by PMA. To confirm these
results, we directly monitored the nuclear translocation of the
NF-κB p65 subunit using immunofluorescence staining and confocal
microscopy. When compared with untreated cells, the amount of NF-κB
p65 detected in the nuclei of HT1080 cells increased following PMA
stimulation (Fig. 5). Conversely,
I6CA inhibited the nuclear translocation of NF-κB, as indicated by
a reduced level of NF-κB p65 detected in the nuclei of HT1080 cells
treated with both I6CA and PMA.
Discussion
The incidence of cancer-associated mortality is
steadily increasing, and tumor metastasis, the formation of
secondary tumors in distant organs, constitutes the leading cause
of these deaths (40,41). Tumor metastasis is a fundamental
property of malignant cells and occurs via a series of sequential
events that, in the long term, involves the migration and invasion
of neighboring tissues, tumor cell intravasation and survival in
the circulation system (blood and lymph), and extravasation and
proliferation to form secondary tumors in distant organs (42). Tumor cells must degrade the ECM to
migrate and invade surrounding tissues and organs; the suppression
of ECM degradation is a crucial step in the prevention of tumor
metastasis (1). It is widely
recognized that MMP-2 and MMP-9 can degrade various components of
the ECM and, therefore, are vital regulators of ECM degradation
(43,44). With increasing interest in
exploring the applications of medicinal substances extracted from
plants and other organisms, various compounds have been evaluated
for their potential effects as inhibitors of MMP-2 and MMP-9
activities (45-47).
Indole derivatives extracted from various natural
resources, including marine algae, have attracted increasing
attention, and numerous studies have investigated their biological
effects (29-32). Among indole derivatives, a recent
study has shown that I6CA could inhibit obesity-related
adipogenesis (26); however, the
analysis of the biological effects of I6CA remains limited.
Therefore, we purified I6CA from S. thunbergii for further
investigation, focusing on its potential effects as an inhibitor of
MMP-9, and the underlying mechanisms. In this report, we presented
data indicating that I6CA significantly inhibited MMP-9 secretion
and expression in PMA-stimulated HT1080 cells. Comparing the
observed inhibitory effect of I6CA on MMP-9 with those reported for
other bioactive compounds, we noted that bisphosphonates,
carboxylated chitooligosaccharides, various cardiovascular drugs
and flavonoids were less potent than I6CA purified from S.
thunbergii (1,48-50). However, the methanol extract of
the red alga Corallina pilulifera appeared to be a more
potent inhibitor of MMP-9 than I6CA (51).
The MAPK and NF-κB pathways are generally recognized
for their role in the regulation of various physiological
processes, including cell proliferation, apoptosis and invasion
(3,7,52).
According to previous studies, MAPK and NF-κB pathways are known to
promote MMP-9 expression in PMA-stimulated HT1080 cells (42,53). The MAPK pathways involve the
phosphorylation and subsequent activation of MAPK family members,
including JNK, ERK, and p38 MAPK (54). In the NF-κB pathway, IκBα binds
and retains in the cytosol the inactivated form of the canonical
p65/p50 heterodimer, which is activated and translocates to the
nucleus upon IκBα phosphorylation, ubiquitination and degradation
(55-58). In previous studies, PMA has been
shown to activate the MAPK and NF-κB pathways in HT1080 cells
(7,8,59-61). Therefore, the activation state of
the MAPK and NF-κB pathways is often assessed to analyze the
mechanism of regulation of MMP-9 expression (8,62).
To unravel the mechanisms of MMP-9 inhibition by I6CA, we examined
the activation state of the MAPK and NF-κB pathways in
PMA-stimulated HT1080 cells. Our results indicated that I6CA
suppressed the phosphorylation of JNK and ERK, but not that of p38
MAPK, in response to PMA stimulation. In addition, I6CA inhibited
the phosphorylation and degradation of IκBα, and NF-κB p65 nuclear
translocation in PMA-stimulated HT1080 cells. Previous studies
reported the MMP inhibitory effects of various bioactive compounds,
such as sulfated polysaccharides and polyphenolic compounds
isolated from brown algae (63-65). Based on our findings, we proposed
that I6CA, an indole derivative isolated from a brown alga, is a
potent MMP-9 inhibitor that acts via the suppression of the MAPK
and NF-κB pathways.
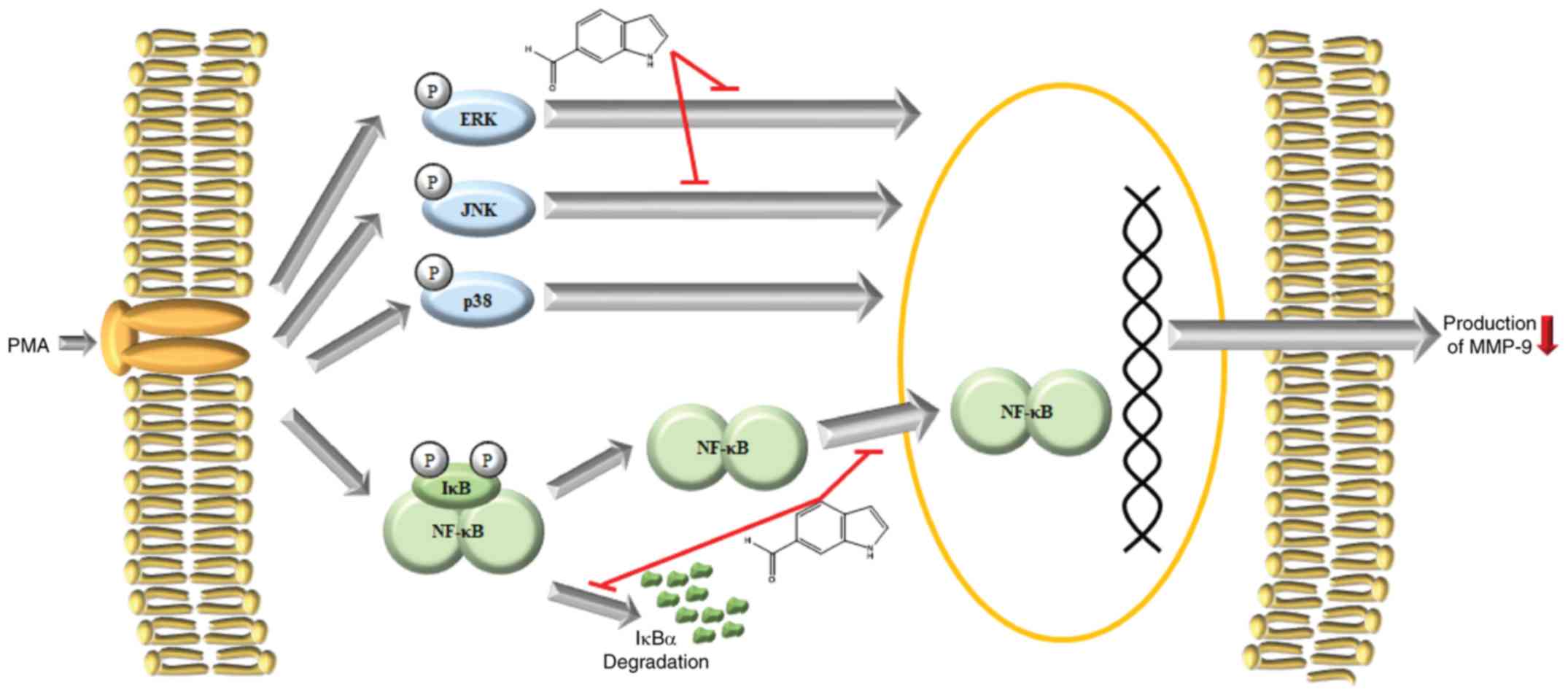
In conclusion, we demonstrated that I6CA purified
from S. thunbergii significantly inhibited the secretion and
protein expression of MMP-9 in PMA-stimulated HT1080 cells. These
effects were determined to be mediated via the suppression of
phosphorylation and activation of JNK and ERK, IκBα phosphorylation
and degradation, and NF-κB p65 nuclear translocation (Fig. 6). These findings may further our
understanding of the mechanism of action of I6CA in the inhibition
of MMP-9. Of note, we investigated only the inhibitory effects of
I6CA on MMP-9. Therefore, we plan to perform in vivo
experiments in the future to verify whether I6CA inhibits ECM
degradation through MMP-9 inhibition, suppressing metastasis.
Acknowledgements
Not applicable.
Funding
This research was supported by a research grant from
the Marine Biotechnology Program (grant. no. 20150220) of the
Ministry of Oceans and Fisheries of Republic of Korea and partially
supported by a research grant from the Korea Institute of Ocean
Science and Technology (grant. no. PE99722).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
THK, SJH and SCK performed the experiments. SJH
extracted and isolated indol-6-carboxaldehyde. MY, WSP, IWC and WKJ
conceived and designed the study. All authors have reviewed and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rajapakse N, Kim MM, Mendis E, Huang R and
Kim SK: Carboxylated chitooligosaccharides (CCOS) inhibit MMP-9
expression in human fibrosarcoma cells via down-regulation of AP-1.
Biochim Biophys Acta. 1760:1780–1788. 2006. View Article : Google Scholar
|
|
2
|
Konttinen YT, Ainola M, Valleala H, Ma J,
Ida H, Mandelin J, Kinne RW, Santavirta S, Sorsa T, López-Otín C
and Takagi M: Analysis of 16 different matrix metalloproteinases
(MMP-1 to MMP-20) in the synovial membrane: Different profiles in
trauma and rheumatoid arthritis. Ann Rheum Dis. 58:691–697. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nguyen VT, Qian ZJ and Jung WK: Abalone
Haliotis discus hannai intestine digests with different molecule
weights inhibit MMP-2 and MMP-9 expression in human fibrosarcoma
cells. Fish Aquat Sci. 15:137–143. 2012.
|
|
4
|
Kessenbrock K, Wang CY and Werb Z: Matrix
metalloproteinases in stem cell regulation and cancer. Matrix Biol.
44-46:184–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown GT and Murray GI: Current
mechanistic insights into the roles of matrix metalloproteinases in
tumour invasion and metastasis. J Pathol. 237:273–281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bae WY, Choi JS, Kim JE, Park C and Jeong
JW: Zingerone suppresses angiogenesis via inhibition of matrix
metallopro-teinases during tumor development. Oncotarget.
7:47232–47241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen VT, Qian ZJ, Ryu B, Kim KN, Kim D,
Kim YM, Jeon YJ, Park WS, Choi IW, Kim GH, et al: Matrix
metalloproteinases (MMPs) inhibitory effects of an octameric
oligopeptide isolated from abalone Haliotis discus hannai. Food
Chem. 141:503–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nguyen VT, Qian ZJ, Lee B, Heo SJ, Kim KN,
Jeon YJ, Park WS, Choi WII, CH J, Ko SC, et al: Fucoxanthin
derivatives from Sargassum siliquastrum inhibit matrix
metalloproteinases by suppressing NF-κB and MAPKs in human
fibrosarcoma cells. Algae. 29:355–366. 2014. View Article : Google Scholar
|
|
9
|
Kim YS, Kang HR, Jang SW and Ko JS:
Celastrol inhibits breast cancer cell invasion via suppression of
NF-ĸB-mediated matrix metalloproteinase-9 expression. Cell Physiol
Biochem. 28:175–184. 2011. View Article : Google Scholar
|
|
10
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9 in
human cervical and ovarian cancer cell lines by cytokines, inducers
and inhibitors. Oncol Rep. 23:605–614. 2010.PubMed/NCBI
|
|
11
|
Elewa MA, Al-Gayyar MM, Schaalan MF, Abd
El Galil KH, Ebrahim MA and El-Shishtawy MM: Hepatoprotective and
anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma
and its relation to vascular invasion markers. Clin Exp Metastasis.
32:479–493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang H, Jang SW, Park JH and Shim S:
Glaucine inhibits breast cancer cell migration and invasion by
inhibiting MMP-9 gene expression through the suppression of NF-κB
activation. Mol Cell Biochem. 403:85–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ko SC, Lee M, Lee JH, Lee SH, Lim Y and
Jeon YJ: Dieckol, a phlorotannin isolated from a brown seaweed,
Ecklonia cava, inhibits adipogenesis through AMP-activated protein
kinase (AMPK) activation in 3T3-L1 preadipocytes. Environ Toxicol
Pharmacol. 36:1253–1260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deniaud-Bouët E, Hardouin K, Potin P,
Kloareg B and Hervé C: A review about brown algal cell walls and
fucose-containing sulfated polysaccharides: Cell wall context,
biomedical properties and key research challenges. Carbohydr Polym.
175:395–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SH and Jeon YJ: Anti-diabetic effects
of brown algae derived phlorotannins, marine polyphenols through
diverse mechanisms. Fitoterapia. 86:129–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung WK, Heo SJ, Jeon YJ, Lee CM, Park YM,
Byun HG, Choi YH, Park SG and Choi IW: Inhibitory effects and
molecular mechanism of dieckol isolated from marine brown alga on
COX-2 and iNOS in microglial cells. J Agric Food Chem.
57:4439–4446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao X, Lee JR, Park SK, Kim NG and Choi
HG: Detrimental effects of sediment on attachment, survival and
growth of the brown alga Sargassum thunbergii in early life stages.
Phycol Res. 67:77–81. 2019. View Article : Google Scholar
|
|
18
|
Ren B, Chen C, Li C, Fu X, You L and Liu
RH: Optimization of microwave-assisted extraction of Sargassum
thunbergii polysaccharides and its antioxidant and hypoglycemic
activities. Carbohydr Polym. 173:192–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JA, Karadeniz F, Ahn BN, Kwon MS, Mun
OJ, Bae MJ, Seo Y, Kim M, Lee SH, Kim YY, et al: Bioactive quinone
derivatives from the marine brown alga Sargassum thunbergii induce
anti-adipogenic and proosteoblastogenic activities. J Sci Food
Agric. 96:783–790. 2016. View Article : Google Scholar
|
|
20
|
Jin W, Zhang W, Liu G, Yao J, Shan T, Sun
C and Zhang Q: The structure-activity relationship between
polysaccharides from Sargassum thunbergii and anti-tumor activity.
Int J Biol Macromol. 105:686–692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang JY, Khan MN, Park NH, Cho JY, Lee MC,
Fujii H and Hong YK: Antipyretic, analgesic, and anti-inflammatory
activities of the seaweed Sargassum fulvellum and Sargassum
thunbergii in mice. J Ethnopharmacol. 116:187–190. 2008. View Article : Google Scholar
|
|
22
|
Park PJ, Heo SJ, Park EJ, Kim SK, Byun HG,
Jeon BT and Jeon YJ: Reactive oxygen scavenging effect of enzymatic
extracts from Sargassum thunbergii. J Agric Food Chem.
53:6666–6672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ou M, Sun X, Liang J, Liu F, Wang L, Wu X
and Tu J: A poly-saccharide from Sargassum thunbergii inhibits
angiogenesis via downregulating MMP-2 activity and VEGF/HIF-1α
signaling. Int J Biol Macromol. 94:451–458. 2017. View Article : Google Scholar
|
|
24
|
Kim JA, Kong CS, Seo YW and Kim SK:
Sargassum thunbergii extract inhibits MMP-2 and -9 expressions
related with ROS scavenging in HT1080 cells. Food Chem.
120:418–425. 2010. View Article : Google Scholar
|
|
25
|
Li YX, Wijesekara I, Li Y and Kim SK:
Phlorotannins as bioactive agents from brown algae. Process
Biochem. 46:2219–2224. 2011. View Article : Google Scholar
|
|
26
|
Kang MC, Ding Y, Kim EA, Choi YK, de
Araujo T, Heo SJ and Lee SH: Indole derivatives isolated from brown
alga Sargassum thunbergii inhibit adipogenesis through AMPK
activation in 3T3-L1 preadipocytes. Mar Drugs. 15:1192017.
View Article : Google Scholar :
|
|
27
|
Joung EJ, Gwon WG, Shin T, Jung BM, Choi
JS and Kim HR: Anti-inflammatory action of the ethanolic extract
from Sargassum serratifolium on lipopolysaccharide-stimulated mouse
peritoneal macrophages and identification of active components. J
Appl Phycol. 29:563–573. 2017. View Article : Google Scholar
|
|
28
|
Kang MC, Lee HG, Choi HD and Jeon YJ:
Antioxidant properties of a sulfated polysaccharide isolated from
an enzymatic digest of Sargassum thunbergii. Int J Biol Macromol.
132:142–149. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Wang JD, Liu CX, Yuan JH, Wang XJ
and Xiang WS: A new prenylated indole derivative from endophytic
actinobacteria Streptomyces sp neau-D50. Nat Prod Res. 28:431–437.
2014. View Article : Google Scholar
|
|
30
|
Zhang MZ, Chen Q and Yang GF: A review on
recent developments of indole-containing antiviral agents. Eur J
Med Chem. 89:421–441. 2015. View Article : Google Scholar
|
|
31
|
Patel H, Darji N, Pillai J and Patel B:
Recent advance in anti-cancer activity of indole derivatives. Int J
Drug Res Tech. 2:225–230. 2017.
|
|
32
|
Longeon A, Copp BR, Quévrain E, Roué M,
Kientz B, Cresteil T, Petek S, Debitus C and Bourguet-Kondracki ML:
Bioactive indole derivatives from the South Pacific marine sponges
Rhopaloeides odorabile and Hyrtios sp. Mar Drugs. 9:879–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simoni D, Romagnoli R, Baruchello R,
Rondanin R, Rizzi M, Pavani MG, Alloatti D, Giannini G, Marcellini
M, Riccioni T, et al: Novel combretastatin analogues endowed with
antitumor activity. J Med Chem. 49:3143–3152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bandgar BP, Kinkar SN, Chavan HV, Jalde
SS, Shaikh RU and Gacche RN: Synthesis and biological evaluation of
asymmetric indole curcumin analogs as potential anti-inflammatory
and antioxidant agents. J Enzyme Inhib Med Chem. 29:7–11. 2014.
View Article : Google Scholar
|
|
35
|
Mandour AH, El-Sawy ER, Shaker KH and
Mustafa MA: Synthesis, anti-inflammatory, analgesic and
anticonvulsant activities of 1,8-dihydro-1-ary1-8-alkyl pyrazolo
(3,4-b) indoles. Acta Pharm. 60:73–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu GH, Choo SJ, Kim YH, Ryoo IJ, Seok SJ,
Ahn JS and Yoo ID: Secondary metabolites of Volvariella bombycina
and their inhibitory effects on melanogenesis. J Microbiol
Biotechnol. 20:78–81. 2010.PubMed/NCBI
|
|
37
|
Chirumbolo S and Bjoklund G: Quercetin
affecting gelatinases in rat aortas: Some comments.
Atherosclerosis. 275:444–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cho HJ, Lee S, Park SJ, Lee YD, Jeong K,
Park JH, Lee YS, Kim B, Jeong HS and Kim S: Tumor
microenvironment-responsive fluo-rogenic nanoprobe for ratiometric
dual-channel imaging of lymph node metastasis. Colloid Surf B
Biointerfaces. 179:9–16. 2019. View Article : Google Scholar
|
|
39
|
Panwar P, Butler GS, Jamroz A, Azizi P,
Overall CM and Brömme D: Aging-associated modifications of collagen
affect its degradation by matrix metalloproteinases. Matrix Biol.
65:30–44. 2018. View Article : Google Scholar
|
|
40
|
Heron M and Anderson RN: Changes in the
leading cause of death: Recent patterns in heart disease and cancer
mortality. NCHS Data Brief. 1–8. 2016.PubMed/NCBI
|
|
41
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chung TW, Choi HJ, Lee JY, Jeong HS, Kim
CH, Joo M, Choi JY, Han CW, Kim SY, Choi JS and Ha KT: Marine algal
fucoxanthin inhibits the metastatic potential of cancer cells.
Biochem Biophys Res Commun. 439:580–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee SJ, Cho SC, Lee EJ, Kim S, Lee SB, Lim
JH, Choi YH, Kim WJ and Moon SK: Interleukin-20 promotes migration
of bladder cancer cells through extracellular signal-regulated
kinase (ERK)-mediated MMP-9 protein expression leading to nuclear
factor (NF-κB) activation by inducing the up-regulation of
p21(WAF1) protein expression. J Biol Chem. 288:5539–5552. 2013.
View Article : Google Scholar
|
|
44
|
Zhang J, Zhu X, Li H, Li B, Sun L, Xie T,
Zhu T, Zhou H and Ye Z: Piperine inhibits proliferation of human
osteosarcoma cells via G2/M phase arrest and metastasis by
suppressing MMP-2/-9 expression. Int Immunopharmacol. 24:50–58.
2015. View Article : Google Scholar
|
|
45
|
Liao YF, Rao YK and Tzeng YM: Aqueous
extract of Anisomeles indica and its purified compound exerts
anti-metastatic activity through inhibition of NF-κB/AP-1-dependent
MMP-9 activation in human breast cancer MCF-7 cells. Food Chem
Toxicol. 50:2930–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang KL, Hsia SM, Chan CJ, Chang FY, Huang
CY, Bau DT and Wang PS: Inhibitory effects of isoliquiritigenin on
the migration and invasion of human breast cancer cells. Expert
Opin Ther Targets. 17:337–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen YT, Kao CJ, Huang HY, Huang SY, Chene
CY, Line YS, Wen ZH and Wang HMD: Astaxanthin reduces MMP
expressions, suppresses cancer cell migrations, and triggers
apoptotic caspases of in vitro and in vivo models in melanoma. J
Func Foods. 31:20–31. 2017. View Article : Google Scholar
|
|
48
|
Boissier S, Ferreras M, Peyruchaud O,
Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM and
Clézardin P: Bisphosphonates inhibit breast and prostate carcinoma
cell invasion, an early event in the formation of bone metastases.
Cancer Res. 60:2949–2954. 2000.PubMed/NCBI
|
|
49
|
Kim MH: Flavonoids inhibit
VEGF/bFGF-induced angiogenesis in vitro by inhibiting the
matrix-degrading proteases. J Cell Biochem. 89:529–538. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rival Y, Benéteau N, Chapuis V,
Taillandier T, Lestienne F, Dupont-Passelaigue E, Patoiseau JF,
Colpaert FC and Junquéro D: Cardiovascular drugs inhibit MMP-9
activity from human THP-1 macrophages. DNA Cell Biol. 23:283–292.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ryu BM, Qian ZJ, Kim MM, Nam KW and Kim
SK: Anti-photoaging activity and inhibition of matrix
metallopro-teinase (MMP) by marine red alga, Corallina pilulifera
methanol extract. Radiat Phys Chem. 78:98–105. 2009. View Article : Google Scholar
|
|
52
|
Dai Z, Lei P, Xie J and Hu Y: Antitumor
effect of resveratrol on chondrosarcoma cells via phosphoinositide
3-kinase/AKT and p38 mitogen-activated protein kinase pathways. Mol
Med Rep. 12:3151–3155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Toufaily C, Charfi C and Annabi B and
Annabi B: A role for the cavin-3/matrix metalloproteinase-9
signaling axis in the regulation of PMA-activated human HT1080
fibrosarcoma cell neoplastic phenotype. Cancer Growth Metastasis.
7:43–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Im NK, Jang WJ, Jeong CH and Jeong GS:
Delphinidin suppresses PMA-induced MMP-9 expression by blocking the
NF-κB activation through MAPK signaling pathways in MCF-7 human
breast carcinoma cells. J Med Food. 17:855–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim YS, Ahn CB and Je JY:
Anti-inflammatory action of high molecular weight Mytilus edulis
hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-κB
and MAPK pathways. Food Chem. 202:9–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rothgiesser KM, Erener S, Waibel S,
Lüscher B and Hottiger MO: SIRT2 regulates NF-κB-dependent gene
expression through deacetylation of p65 Lys310. J Cell Sci.
123:4251–4258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zerfaoui M, Errami Y, Naura AS, Suzuki Y,
Kim H, Ju J, Liu T, Hans CP, Kim JG, Abd Elmageed ZY, et al:
Poly(ADP-Ribose) polymerase-1 is a determining factor in
Crm1-mediated nuclear export and retention of p65 NF-kappa B upon
TLR4 stimulation. J Immunol. 185:1894–1902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL,
Chao CH, Yamaguchi H, Yang NK, Ding Q, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li L, Wang Y, Qi B, Yuan D, Dong S, Guo D,
Zhang C and Yu M: Suppression of PMA-induced tumor cell invasion
and migration by ginsenoside Rg1 via the inhibition of
NF-κB-dependent MMP-9 expression. Oncol Rep. 32:1779–1786. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kießling MK, Nicolay JP, Schlör T, Klemke
CD, Süss D, Krammer PH and Gülow K: NRAS mutations in cutaneous T
cell lymphoma (CTCL) sensitize tumors towards treatment with the
multikinase inhibitor Sorafenib. Oncotarget. 8:45687–45697. 2017.
View Article : Google Scholar
|
|
61
|
Litvinov IV, Cordeiro B, Fredholm S, Ødum
N, Zargham H, Huang Y, Zhou Y, Pehr K, Kupper TS, Woetmann A and
Sasseville D: Analysis of STAT4 expression in cutaneous T-cell
lymphoma (CTCL) patients and patient-derived cell lines. Cell
Cycle. 13:2975–2982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim A, Yim NH, Im M, Jung YP, Kim T and Ma
JY: Suppression of the invasive potential of highly malignant tumor
cells by KIOM-C, a novel herbal medicine, via inhibition of NF-κB
activation and MMP-9 expression. Oncol Rep. 31:287–297. 2014.
View Article : Google Scholar
|
|
63
|
Alassali A, Cybulska I, Brudecki GP,
Farzanah R and Thomsen MH: Methods for upstream extraction and
chemical characterization of secondary metabolites from algae
biomass. Adv Tech Biol Med. 4:1632016.
|
|
64
|
Zorofchian Moghadamtousi S, Karimian H,
Khanabdali R, Razavi M, Firoozinia M, Zandi K and Abdul Kadir H:
Anticancer and antitumor potential of fucoidan and fucoxanthin, two
main metabolites isolated from brown algae. ScientificWorldJournal.
2014:7683232014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Thomas NV, Manivasagan P and Kim SK:
Potential matrix metalloproteinase inhibitors from edible marine
algae: A review. Environ Toxicol Pharmacol. 37:1090–1100. 2014.
View Article : Google Scholar : PubMed/NCBI
|