Introduction
Atherosclerosis is a chronic multifactorial vessel
disease, promoted by several risk factors, and is the main cause of
cardiovascular disease, which is a primary cause of mortality
worldwide, accounting for ~18 million mortalities per year (~31% of
all mortalities) (1,2). It is well understood that
endothelial cells (ECs), macrophages and smooth muscle cells are
all involved in atherosclerosis (3). The process of endothelial injury and
its dysfunction serves vital roles in the development of
atherosclerosis and deserves more attention (4). Endothelial cell dysfunction, which
manifests in lesion-prone areas of the arterial vasculature, occurs
throughout the early stages of atherosclerosis and serves a role in
plaque regression and plaque instability (5). Increasing evidence has demonstrated
that multiple pathways and physiopathological processes are also
involved in the development of atherosclerosis, such as
inflammation, apoptosis, lipid deposition, foam cell formation,
oxidative stress and necrosis (6–8).
Oxidized low-density lipoprotein (ox-LDL) can destroy endothelial
function and enhance endothelial apoptosis, leading to endothelial
injury, thus contributing to the development and pathogenesis of
atherosclerosis (9). Oxidative
stress and inflammation interact to promote vascular wall LDL
oxidation, resulting in a high level of ox-LDL, which leads to
apoptosis of vascular endothelial cells and subsequent endothelial
injury (7,9). Human umbilical vein endothelial
cells (HUVECs) are among one of the most popular models used for
ECs in vitro because human umbilical veins are relatively
more available compared with other types of blood vessels (10). Therefore, interventions for
endothelial injury and oxidative stress induced by ox-LDL may be
utilized as effective novel therapeutic strategies for the
treatment of atherosclerosis.
Geniposide, a major compound extracted from the
gardenia fruit, is a type of traditional Chinese medicine for the
treatment of inflammation, brain disorders and hepatic disease
(11). Geniposide exhibits a
broad spectrum of pharmacological actions involving anti-apoptosis
(12), anti-inflammatory injury
(13), anti-oxidative stress
(14), anti-cancer (15) and anti-angiogenesis effects
(16). Previous in vitro
and in vivo studies have focused on the potential
cardioprotective effects of geniposide, particularly in
atherosclerosis (17–19). Cheng et al (17) demonstrated that geniposide can
decrease plaque size and mitigate atherosclerosis-associated
inflammatory damage by regulating the miR-101-associated signaling
pathway. Another study also demonstrated that the combination of
Scutellaria baicalensis georgi with geniposide exerts
inflammation-regulatory effects and inhibits atherosclerotic
lesions (19). However, the
mechanisms underlying the protective effects of geniposide on
anti-oxidative stress and anti-inflammatory response in
atherosclerosis are poorly understood.
MicroRNAs (miRNAs/miRs), endogenous short non-coding
RNAs, ~22 nucleotides in length, are well established as the
mediators of post-transcriptional regulation in a wide array of
biological processes (20). Among
them, miR-21, one of the major dynamically modulated miRNAs,
elicits various pathophysiological processes implicated in the
development of various diseases, such as neurodegenerative diseases
and cancer, and serves vital roles in cardiovascular diseases
(21–23). Previous studies have confirmed
that miR-21 in endothelial cells is significantly increased in
atherosclerotic plaques and that it causes pharmacological effects
by directly targeting the PTEN tumor suppressor gene, indicating a
pivotal effect of the miR-21/PTEN pathway in atherosclerosis
(24,25). A number of reports have indicated
that the miR-21/PTEN pathway regulates important functions of ECs,
apoptosis and inflammatory responses during atherosclerosis
(26,27). However, the exact actions of the
miR-21/PTEN pathway in the progression of atherosclerosis and in
the cardioprotection of geniposide remain unknown.
Hence, the present study aimed to investigate
whether the miR-21/PTEN pathway contributes to the antioxidant and
anti-inflammatory actions of geniposide in ox-LDL injury in HUVECs.
Overall, the present study provided crucial evidence that
geniposide may be an active component in preventing the progression
of atherosclerosis.
Materials and methods
Cell culture and treatment
HUVECs were supplied by the American Type Culture
Collection and cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% (v/v) fetal bovine serum (Thermo Fisher
Scientific, Inc.) and 100 μg/ml penicillin/streptomycin
(Beyotime Institute of Biotechnology) in a humidified atmosphere
with 5% CO2 at 37°C. HUVECs were incubated with ox-LDL
(25, 50 or 100 μg/ml; Beijing Solarbio Life Science Company;
cat. no. H7950; dissolved in culture medium) for 24 h at 37°C to
establish an in vitro atherosclerotic cell model. The
control group was treated with same amount of culture medium
instead of ox-LDL.
To illustrate the physiological effect of geniposide
on ox-LDL injury, HUVECs were pretreated with different
concentrations of geniposide (5, 10, 20, 40 or 80 μM;
Shanghai Pureone Biotechnology; purity >98%; cat. no. P0118) for
30 min and then treated with ox-LDL (50 μg/ml) for 24 h at
37°C. To demonstrate the role of the miR-21/PTEN pathway in the
cardioprotection of geniposide during atherosclerosis, HUVECs were
transfected with miR-21 mimic, miR-21 inhibitor or miRNA negative
control (miR-NC) and then treated with geniposide (40 μM)
for 1 h followed by ox-LDL (50 μg/ml) for 24 h at 37°C.
Transfection
In order to inhibit or enhance miR-21 level, miR-21
mimic (100 nM), miR-21 inhibitor (100 nM) or miRNA NC (100 nM)
(Guangzhou RiboBio Co., Ltd.) were transfected into HUVECs using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer’s protocol. The
sequences were as follows: miR-21 mimic sense,
5′-UAGCUUAUCAGACUGAUGUUGA-3′; miR-21 mimic anti-sense,
5′-AACAUCAGUCUGAUAAGCUAUU-3′; miR-21 inhibitor,
5′-UCAACAUCAGUCUGAUAAGCUA-3′; and miR-21 negative control (miR-NC)
sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense,
5′-ACGUGACACGUUCGGAGAATT-3′. Following transfection for 6 h, the
medium was changed and HUVECs were subjected to treatment with
ox-LDL (50 μg/ml) for a further 24 h at 37°C and the cells
were cultured for 48 h. Transfection efficiency was determined
using reverse transcription-quantitative PCR (RT-qPCR).
Cell viability assay
The cell viability of HUVECs was measured using a
MTT assay kit (Beyotime Institute of Biotechnology; cat. no.
ST316). Briefly, HUVECs were seeded onto 96-well plates at a
density of 1×104/well. Following treatment as
aforementioned, MTT solution (5 mg/ml, 20 μl) was added to
each well. Subsequently, cells were incubated for a further 4 h at
37°C, and 150 μl DMSO was added to dissolve the precipitate.
The optical density (OD) at 570 nm was measured using a microplate
reader.
Lactate dehydrogenase (LDH) release
assay
The LDH release from cell to supernatant was
determined using a LDH Cytotoxicity assay kit (Beyotime Institute
of Biotechnology; cat. no. C0016), according to the manufacturer’s
protocol. Briefly, following the aforementioned treatments, the
supernatants were collected and centrifuged at 500 × g for 5 min at
room temperature to measure LDH release. The OD value was measured
at 490 nm using a microplate reader.
Flow cytometry analysis
Apoptosis in treated HUVECs was detected using an
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
apoptosis detection kits (BD Biosciences; cat. no. BD 556547),
following the manufacturer’s protocol. In brief, HUVECs were
treated as aforementioned, and the culture medium and the cells
were collected. Following two PBS washes, the cells were
resuspended in 1X binding buffer (100 μl) at a density of
1×105 cells/ml, and then double stained with 5 μl
Annexin V-FITC and 5 μl PI for 15 min in the dark. The
percentage of apoptotic cells was determined using a FACScan flow
cytometer and analyzed using FCS Express software (version 1.2; BD
Biosciences). Each experiment was performed in triplicate.
Mitochondrial membrane potential (MMP)
determination
The MMP in HUVECs cells was detected using a
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1) staining kit (Beyotime Institute of Biotechnology;
cat. no. C2006). The cells were harvested, washed twice with PBS
and resuspended in 10 μM JC-1 solution at 37°C for 20 min.
Next, the JC-1 staining solution was removed and the cells were
washed and resuspended in PBS. The fluorescence values were then
analyzed with a FACSCanto flow cytometer and FCS Express software
(version 1.2, BD Biosciences). The loss of MMP was reflected by the
decrease in red fluorescence from the JC-1 aggregates and the
increase in green fluorescence from the JC-1 monomers.
Caspase-3 activation analysis
The caspase-3 activity in HUVECs was analyzed using
a caspase-3 assay kit (Sigma-Aldrich; Merck KGaA; cat. no. CASP3F).
Briefly, cells were seeded onto 6-well plates at a density of
1×105 cells/well, treated as aforementioned, trypsinized
and analyzed for 10 min on ice. Next, the cells lysates were mixed
with caspase-3 substrate (DEVD-AFC; 1 μM) and detection
buffer at 37°C for 2 h. The relative fluorescence intensity of each
well was detected using a microplate reader at wavelengths of 400
nm excitation and 505 nm emission.
Intracellular reactive oxygen species
(ROS) production determination
The intracellular ROS generation was analyzed by
2′,7′-dichlorofluorescindiacetate (DCFH-DA; Beyotime Institute of
Biotechnology), a ROS-sensitive fluorescent dye, followed by flow
cytometry. Briefly, following relevant treatment or transfection,
cells were treated with RIPA lysis buffer and washed with cold PBS
twice. Next, cells were stained with 10 μM
2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) in the dark for
20 min at 37°C, and the fluorescence intensity was detected using a
FACSCalibur flow cytometer (488 nm excitation/521 nm emission) and
analyzed with FCS Express software (version 1.2; BD
Biosciences).
Oxidative stress markers detection
HUVECs were seeded onto 6-well plates with
1×106 cells/well. Following pretreatment as
aforementioned, cells were lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology). Following centrifugation at 4°C,
12,000 × g for 10 min, protein concentration was assayed using a
BCA Protein assay kit (Beyotime Institute of Biotechnology).
Malondialdehyde (MDA) content was determined using a MDA assay kit
(Beyotime Institute of Biotechnology; cat. no. S0131) in accordance
with the manufacturer’s protocol. The enzymatic activities of
superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) and
catalase (CAT) were determined using a Superoxide Dismutase assay
kit (Nanjing Jiancheng Bioengineering Institute; cat. no. A001-2),
Cellular Glutathione Peroxidase assay kit (Beyotime Institute of
Biotechnology; cat. no. S0056), and Catalase assay kit (Beyotime
Institute of Biotechnology; cat. no. A007-1), respectively.
RT-qPCR
Total RNA from treated HUVECs was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). For
measuring the expression of miR-21, the first strand of
complementary DNA (cDNA) was synthesized using PrimeScript Reverse
Transcriptase (Takara Bio, Inc.) at 30°C for 10 min, reverse
transcription at 42°C for 45 min, heat preservation at 72°C for 15
min, followed by cooling on ice. Subsequently, the cDNA was used
for qPCR using Taqman Universal Master mix II (Applied Biosystems;
Thermo Fisher Scientific, Inc.) following the manufacturer’s
instructions. The qPCR thermocycling conditions were: 94°C for 90
sec for an initial denaturation, 30 denaturation cycles at 95°C for
10 sec, followed by annealing and elongation at 60°C for 45 sec;
and final extension at 72°C for 5 min. For measuring the expression
of PTEN, the first strand of cDNA was obtained by SuperScript III
Platinum SYBR-Green One-Step RT-qPCR kit (Invitrogen; Thermo Fisher
Scientific, Inc.) in a 15-min incubation at 50°C. qPCR was
performed FastStart Universal SYBR-Green Master (Invitrogen; Thermo
Fisher Scientific, Inc.). qPCR was performed on an Applied
Biosystems (ABI PRISM) 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The qPCR conditions
were: Pre-denaturation at 95°C for 3 min and 40 cycles of
denaturation at 94°C for 30 sec and annealing at 60°C for 45 sec.
The primer sequences for RT-qPCR were as follows: miR-21 forward,
5′-GGGGTAGCTTATCAGACTGATG-3′ and reverse,
5′-TGTCGTGGAGCGGCAATTG-3′; PTEN forward,
5′-GCAATATGTTCATAACGATGGCTGTGG-3′ and reverse,
5′-GAACTGGCAGGTAGAAGGCAACTC-3′; β-actin forward,
5′-AGGGAAATCGTGCGTGAC-3′ and reverse, 5′-CGCTCATTGCCGATAGTG-3′; and
U6 forward, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGTA-3′. All primers were obtained from Sangon
Biotech Co., Ltd. U6 was used for normalizing the level of miR-21
and β-actin was used to normalize the levels of PTEN. The
experiment was performed in triplicate. The relative expression was
quantified using the 2−ΔΔCq method (28).
Western blotting
Whole cell extracts from HUVECs treated with
geniposide (40 μM) for 1 h followed by treatment with ox-LDL
(50 μg/ml) for 24 h were extracted using RIPA lysis buffer
(Beyotime Institute of Biotechnology) supplemented with
phenylmethanesul-fonyl (PMSF; Beyotime Institute of Biotechnology),
and the protein concentration was quantified using a bicinchoninic
acid kit (Beyotime Institute of Biotechnology). Proteins (30
μg/lane) were separated by SDS-PAGE on a 12% gel and
transferred to polyvinylidene fluoride membranes (EMD Millipore).
Following blocking with 5% skimmed milk for 2 h at room
temperature, membranes were incubated with primary antibodies
against PTEN (cat. no. 9188), Bax (cat. no. 5023), Bcl-2 (cat. no.
15071), Nox2 (cat. no. 4312) and GAPDH (cat. no. 5174) (all
1:2,000; all purchased from Cell Signaling Technology, Inc.) at 4°C
overnight. Following three 10-min PBS washes, membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; Santa Cruz Biotechnology, Santa Cruz, Inc; cat. no.
sc-2357) at room temperature for 90 min, and the bands were
visualized with an enhanced chemiluminescence reagent (GE
Healthcare). GAPDH served as the internal control for total
protein. The relative expression of each protein was normalized to
endogenous control GAPDH using the Image Lab™ 4.4 software (Bio-Rad
Laboratories, Inc.).
Quantitative detection of IL-1β, IL-6,
TNF-α and IL-10 levels by enzyme-linked immunosorbent assay
(ELISA)
HUVECs were seeded onto 6-well plates with
1×106 cells/well and treated as aforementioned. The
levels of inflammation-associated cytokines in the supernatant were
measured using ELISA kits (PeproTech, Inc.), including kits for
IL-1β (cat. no. 900-M91), IL-6 (cat. no. 900-K50), TNF-α (cat. no.
900-K73) and IL-10 (cat. no. 900-M53). Briefly, culture medium was
collected and centrifuged at 300 × g for 10 min at 4°C to obtain
the supernatants for the measurement of IL-1β, IL-6, TNF-α and
IL-10 levels in accordance with the manufacturer’s instructions.
The supernatants of different concentrations standards (100
μl) were added to pre-coated enzyme plates separately. Then,
detection antibody as part of the aforementioned kits (50
μl; 1:100) was added to each well and incubated at room
temperature for 90 min. After washing six times, streptavidin
labeled with horseradish peroxidase (1:100) was added to each pore
and incubated at room temperature for 30 min. Following incubation
with chromogenic substrate (TMB; 100 μl; included in the
kits) at room temperature for 5–30 min in dark, termination
solution (100 μl) was added to each well. The absorbance at
450 nm was read with a microplate reader.
Statistical analysis
The data were analyzed by GraphPad Prism 6 software
(GraphPad Software, Inc.). All quantitative data were performed in
triplicate and expressed as the mean ± standard deviation.
Statistical comparisons between various groups were performed with
one-way analysis of variance analysis followed by a Tukey’s
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Geniposide attenuates HUVECs injury and
promotes the miR-21/PTEN pathway in HUVECs exposed to ox-LDL
Firstly, the damage of ox-LDL to HUVECs was
investigated. The MTT assay results demonstrated that ox-LDL
significantly decreased the viability of HUVECs in a
concentration-dependent manner (Fig.
1A). Since 50 μg/ml ox-LDL was the lowest concentration
to reduce cell viability to ~ 60% compared with the control group,
this concentration was selected for subsequent study. To illustrate
the physiological effect of geniposide on injury induced by ox-LDL
in HUVECs, HUVECs were pretreated with different concentrations of
geniposide (5, 10, 20, 40 and 80 μM) for 30 min and then
treated with ox-LDL (50 μg/ml) for 24 h. The MTT assay
results demonstrated that pretreatment with geniposide (10, 20, 40
and 80 μM for 30 min) led to a significant suppression of
ox-LDL-induced decrease in cell viability (Fig. 1B), while 5 μM geniposide
had no effect on the ox-LDL-induced downregulation of cell
viability. Additionally, the LDH release analysis results (Fig. 1C) indicated that ox-LDL (50
μg/ml) significantly increased LDH activity compared with
the control, whereas these effects were significantly reversed by
geniposide (5, 10, 20, 40 and 80 μM). Notably, MTT and LDH
release analysis results revealed that 40 μM geniposide
markedly attenuated ox-LDL injury in HUVECs, which was considered
as an appropriate concentration for subsequent studies.
Furthermore, the effects of geniposide on the miR-21/PTEN pathway
in ox-LDL-treated HUVECs were investigated. As presented in
Fig. 1, ox-LDL (50 μg/ml)
significantly decreased miR-21 level (Fig. 1D) and increased PTEN expression
(Fig. 1E and F) compared with the
control group, whereas these effects were reversed by geniposide
(40 μM). Geniposide treatment alone had no effects on miR-21
and PTEN. These results suggest that the miR-21/PTEN pathway may
contribute to the cardioprotection of geniposide against
ox-LDL-induced injury in HUVECs.
 | Figure 1Effect of geniposide and ox-LDL on
cell viability, LDH activity and miR-21/PTEN pathway in HUVECs. (A)
HUVECs were treated with ox-LDL (25, 50 and 100 μg/ml) for
24 h, and the cell viability was detected by MTT assay. (B) HUVECs
were pretreated with geniposide (5, 10, 20, 40 and 80 μM)
for 30 min followed by treatment with ox-LDL (50 μg/ml) for
24 h, and the cell viability was detected by MTT assay. (C) The LDH
activity was determined by a commercially available Cytotoxicity
LDH assay kit. HUVECs were pretreated with geniposide (40
μM) for 1 h followed by treatment with ox-LDL (50
μg/ml) for 24 h. (D) The level of miR-21 was detected by
reverse transcription-quantitative PCR. (E) The expression of PTEN
was measured by western blot analysis. (F) Quantitative
densitometric analysis of PTEN expression. Data are shown as mean ±
standard deviation, n=3. *P<0.05,
**P<0.01 vs. control group; #P<0.05,
##P<0.01 vs. ox-LDL alone group. GEN, geniposide;
ox-LDL, oxidized low-density lipoprotein; PTEN, phosphate and
tension homolog; miR, microRNA; LDH, lactate dehydrogenase; HUVECs,
human umbilical vein endothelial cells. |
Geniposide inhibits apoptosis induced by
ox-LDL in HUVECs
The impacts of geniposide on apoptosis were
subsequently investigated. As presented in Fig. 2, Annexin V-FITC/PI staining
results showed that geniposide pretreatment significantly reversed
the ox-LDL-induced upregulation of the apoptotic rate (Fig. 2A and B). Bax and Bcl-2, members of
the Bcl-2 protein family, are involved in mitochondria-mediated
apoptosis (29). Western blotting
confirmed that ox-LDL-induced a significant increase in Bax (a
pro-apoptotic protein) expression and a significant decrease in
Bcl-2 (an anti-apoptotic protein) expression, whereas these effects
were both significantly reversed by geniposide (Fig. 2C). Caspase-3 is an effector enzyme
involved in cell apoptosis (30).
The results further demonstrated that the ox-LDL-induced
upregulation of caspase-3 activity was also significantly
attenuated by geniposide (Fig.
2D). In addition, ox-LDL resulted in a reduced MMP, which was
also significantly reversed by geniposide (Fig. 2E). Geniposide alone had no effects
on apoptosis in HUVECs. Overall, these results suggested that
geniposide attenuated ox-LDL-induced apoptosis in HUVECs.
miR-21/PTEN pathway mediates the
protection of geniposide against ox-LDL-induced injury in
HUVECs
To further confirm the role of the miR-21/PTEN
pathway in the beneficial action of geniposide, HUVECs were
transfected with miR-21 mimic or inhibitor, in order to overexpress
or knockdown miR-21 expression. RT-qPCR results revealed that the
miR-21 mimic-transfected cells demonstrated significantly increased
miR-21 level, whereas the miR-21 inhibitor significantly decreased
miR-21 level in HUVECs compared with the miR-NC transfection group
(Fig. 3A). Studies have revealed
that PTEN is the negatively regulated target of miR-21 (31,32). Western blot analysis revealed that
the miR-21 mimic led to a significant downregulation of PTEN
expression, whereas the miR-21 inhibitor led to a significant
upregulation of PTEN expression (Fig.
3B and C), indicating enhancement of the miR-21/PTEN pathway
induced by miR-21 mimic and inhibition of the miR-21/PTEN pathway
induced by miR-21 inhibitor. In addition, miR-21 mimic further
aggravated geniposide-induced increase in cell viability (Fig. 3D) and the decrease in LDH activity
(Fig. 3E), whereas the miR-21
inhibitor had the opposite effects. The geniposide-induced the
decreases in caspase-3 activity (Fig.
3F) and apoptosis rate (Fig. 3G
and H) were also exacerbated by miR-21 mimic and blocked by
miR-21 inhibitor, compared with miR-NC transfection group.
Furthermore, the geniposide-induced downregulation of Bax
expression and upregulation of Bcl-2 expression (Fig. 3I and K) were promoted by miR-21
mimic and reversed by miR-21 inhibitor under ox-LDL condition.
Overall, these results indicated that the miR-21/PTEN pathway
mediated the protection of geniposide against ox-LDL-induced injury
in HUVECs.
 | Figure 3Effects of miR-21 upregulation or
downregulation on the miR-21/PTEN pathway and the protection of
geniposide against ox-LDL injury in HUVECs. HUVECs were transfected
with miR-21 mimic, miR-21 inhibitor or miR-NC and then treated with
geniposide (40 μM) for 1 h followed by ox-LDL (50
μg/ml) treatment for 24 h. (A) miR-21 level was quantified
by reverse transcription-quantitative PCR assay. (B) PTEN
expression was measured by western blot analysis. (C) Quantitative
analysis of PTEN expression. **P<0.01 vs. miR-NC
transfection group. (D) The viability of HUVECs was detected by MTT
assay. (E) The activity of LDH activity was evaluated by a LDH
commercially available Cytotoxicity LDH Assay kit. (F) The activity
of caspase-3 was assayed by a caspase-3 assay kit. (G) The
apoptosis rate was analyzed by Annexin V-FITC/PI Apoptosis
Detection kit. (H) Quantitative densitometric analysis of apoptosis
rate was determined by flow cytometry. (I) The expressions of Bax
and Bcl-2 were determined by western blot analysis. Quantitative analysis of (J) Bax and (K) Bcl-2
expression. Data are shown as mean ± standard deviation, n=3.
**P<0.01 vs. control group; #P<0.05,
##P<0.01 vs. ox-LDL treatment group;
&P<0.05, &&P<0.01 vs.
ox-LDL + miR-21 co-treatment group. GEN, geniposide; ox-LDL,
oxidized low-density lipoprotein; miR-21 M, miR-21 mimic; miR-21 I,
miR-21 inhibitor; miR-NC, miRNA negative control; NS, no
significance; LDH, lactate dehydrogenase; HUVECs, human umbilical
vein endothelial cells; FITC, fluorescein isothiocyanate; PI,
propidium iodide. |
miR-21/PTEN pathway contributes to the
inhibition of geniposide on ox-LDL-induced oxidative stress in
HUVECs
To further investigate the role of the miR-21/PTEN
pathway on the impact of geniposide on oxidative stress under
ox-LDL condition, oxidative stress markers including ROS
generation, MDA content and Nox2 expression were detected. As shown
in Fig. 4, DCFH-DA staining
results (Fig. 4A) showed that
geniposide pretreatment obviously attenuated ox-LDL-induced
increase in ROS generation (Fig.
4B), MDA content (Fig. 4C)
and Nox2 expression (Fig. 4D and
E) in HUVECs compared with the ox-LDL group. However, these
effects were exacerbated by miR-21 mimic and reversed by miR-21
inhibitor. Additionally, geniposide eliminated ox-LDL-induced
decreases in antioxidant enzyme activities, including SOD (Fig. 4F), GSH-PX (Fig. 4G) and CAT (Fig. 4H), which were strengthened by
miR-21 mimic and reversed by miR-21 inhibitor. Overall, these
results demonstrated that miR-21 is involved in the anti-oxidative
stress of geniposide in ox-LDL injury.
 | Figure 4Effects of geniposide oxidative
stress in the presence or absence of miR-21 mimic or inhibitor in
ox-LDL-treated HUVECs. HUVECs were transfected with miR-21 mimic or
miR-21 inhibitor or miR-NC and then treated with geniposide (40
μM) for 1 h prior to ox-LDL (50 μg/ml) treatment for
24 h. (A) The ROS production was analyzed by DCFH-DA staining.
Magnification, x200. (B) Quantitative densitometric analysis of ROS
level was determined. (C) The MDA content was measured by a
commercial kit. (D) Nox2 expression was measured by western blot
analysis. (E) Quantitative densitometric analysis of Nox2
expression. (F) SOD, (H) GSH-Px and (G) CAT activities were
determined by commercial kits. Data are shown as mean ± standard
deviation, n=3. *P<0.05, **P<0.01 vs.
control group. #P<0.05, ##P<0.01 vs.
ox-LDL treatment group. &P<0.05,
&&P<0.01 vs. ox-LDL + GEN + miR-NC
co-treatment group. GEN, geniposide; ox-LDL, oxidized low-density
lipoprotein; miR-21 M, miR-21 mimic; miR-21 I, miR-21 inhibitor;
miR-NC, miRNA negative control; NS, no significance; ROS, reactive
oxygen species; MDA, malondialdehyde; Nox2, NADPH oxidase 2; SOD,
peroxide dismutase; GSH-PX, glutathione peroxidase; CAT, catalase;
HUVECs, human umbilical vein endothelial cells. |
miR-21/PTEN contributes to
geniposide-induced anti-inflammatory action in ox-LDL-treated
HUVECs
Finally, the effects of geniposide on ox-LDL-induced
inflammatory response in HUVECs were further explored. As shown in
Fig. 5, the ELISA results
revealed that geniposide significantly reversed the ox-LDL-induced
upregulation of pro-inflammatory cytokines, such as IL-1β (Fig. 5A), IL-6 (Fig. 5B) and TNF-α (Fig. 5C), compared with the ox-LDL alone
group. However, these actions of geniposide were aggravated by
miR-21 mimic and suppressed by miR-21 inhibitor. In addition, the
geniposide-induced increase in the anti-inflammatory cytokine IL-10
level was also further significantly promoted by miR-21 mimic and
mitigated by miR-21 inhibitor (Fig.
5D). Overall, these results demonstrated that geniposide
inhibited the inflammatory response induced by ox-LDL via the
upregulation of miR-21/PTEN.
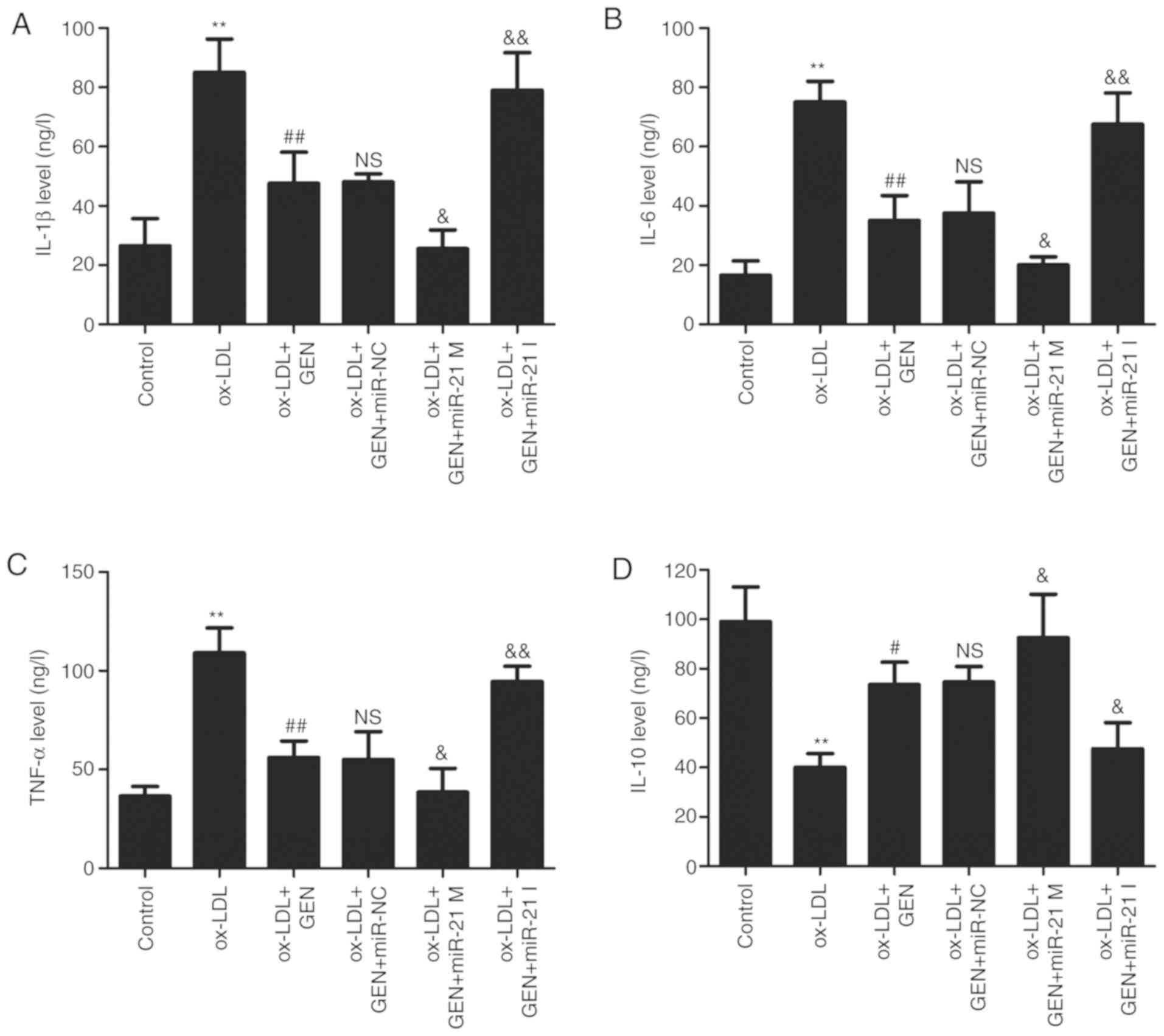 | Figure 5Effects of geniposide inflammatory
cytokine levels in the presence or absence of miR-21 M or miR-21 I
in ox-LDL-treated HUVECs. HUVECs were transfected with miR-21 mimic
M, miR-21 I or miR-NC and then treated with geniposide (40
μM) for 1 h prior to ox-LDL (50 μg/ml) treatment for
24 h. The levels of (A) IL-1β, (B) IL-6, (C) TNF-α and (D) IL-10 in
the culture supernatant were detected by enzyme-linked
immunosorbent assay kits. Data are shown as means ± standard
deviation, n=3. **P<0.01 vs. control group.
#P<0.05, ##P<0.01 vs. ox-LDL treatment
group; &P<0.05, &&P<0.01
vs. ox-LDL + GEN + miR-NC co-treatment group. GEN, geniposide;
ox-LDL, oxidized low-density lipoprotein; miR-21 M, miR-21 mimic;
miR-21 I, miR-21 inhibitor; miR-NC, miRNA negative control; NS, no
significance. |
Discussion
The present study first demonstrated that geniposide
protects against atherosclerotic damage by inhibiting oxidative
stress and inflammation, via enhancing the miR-21/PTEN pathway in
in vitro experiments.
The pathogenesis of atherosclerosis has been
extensively studied, such as endothelial cell damage, apoptosis,
inflammatory response, oxidative stress and calcium overload
(33,34). Geniposide, an iridoid glucoside,
is involved in maintaining multiple beneficial actions depending on
its anti-inflammatory and anti-oxidative activities (17,35,36). Emerging evidence has revealed the
protective effects of geniposide on the development of
atherosclerosis (17,37,38). Consistent with these previous
studies, the present study also found that geniposide pretreatment
attenuated ox-LDL-induced HUVECs injury and apoptosis. It is
understood that excessive apoptosis contributes to cell death
invariably following atherosclerosis (39). These results indicated that
geniposide protects HUVECs against ox-LDL injury via inhibiting
apoptosis.
miR-21, a highly expressed miRNA in the heart, has a
crucial function of modulating normal biological processes of the
myocardial tissue (40).
Accumulating evidence also suggests that the miR-21/PTEN pathway
exhibits critical actions on cell survival and death in the
cardiovascular system (24,41,42). As previously reported, miR-21
expression is decreased, whereas PTEN is upregulated following high
glucose (HG) treatment in endothelial cells, implying that
miR-21/PTEN could be an effective therapeutic target for the
treatment of HG-induced vascular injury (42). Another study revealed that the
decrease in miR-21 level is accompanied by the increase of nitrogen
dioxide PTEN expression under hypoxia/reperfusion condition in
cardiomyocytes, and miR-21/PTEN pathway activation counteracted the
anti-apoptotic effect of trimetazidine, an anti-ischemic and
antioxidant agent, on hypoxia/reperfusion injury (25). However, to the best of our
knowledge, the association between geniposide and miR-21/PTEN has
not yet been reported. The present study found that ox-LDL
treatment significantly resulted in inhibition of the miR-21/PTEN
pathway, which is consistent with previous studies (24,43). Notably, these effects were
reversed by geniposide pretreatment, indicating geniposide induced
the activation of the miR-21/PTEN pathway under ox-LDL condition.
Additionally, miR-21 mimic transfection further promoted
geniposide-induced protection against ox-LDL injury, while miR-21
inhibitor transfection remarkably attenuated geniposide-induced
protection against ox-LDL injury. Overall, these results suggested
that the miR-21/PTEN pathway mediated geniposide-induced
cardioprotection against ox-LDL injury. In addition, studies have
demonstrated the roles of the miR-21/PTEN pathway in tumor growth,
migration and invasion (44,45). Combined with previous studies
regarding the protection of geniposide against tumor (15,46), miR-21/PTEN pathway may contribute
to the antitumor effect of geniposide.
Excess ROS generation-induced oxidative stress has
emerged as a critical, final universal mechanism in atherosclerosis
(47). An imbalance in the
oxidant (non-enzymatic and enzymatic, MPO and NADH
oxidase)/anti-oxidant mechanisms (GSH, Vitamins, Gpx, Prdx, SOD and
PON) induces excess ROS production, leading to a state of
atherogenic plaque formation (48). Geniposide exerts beneficial
activities in human diseases by inhibiting oxidative stress injury
(49–51). Geniposide decreases intracellular
ROS accumulation and enhances the antioxidant enzymatic SOD and CAT
activities in H2O2-treated melanocytes
(49). Geniposide also enhances
mitochondrial function via attenuating oxidative stress and MDA,
and enhancing the antioxidant defense system, resulting in the
inhibition of cardiomyocyte apoptosis and protection against
hypoxia/reoxygenation injury (14). However, the antioxidant
characteristic of geniposide in atherosclerosis has not been
demonstrated. The present study found that geniposide mitigated the
ox-LDL-induced upregulation of ROS generation and MDA content, and
the downregulation of SOD, GSH-Px and CAT activities, inhibiting
oxidative stress and promoting antioxidant defense system. Notably,
the present study further found that miR-21 mimic exacerbated the
inhibition of geniposide on oxidative stress, whereas miR-21
inhibitor blocked the inhibition of geniposide in ox-LDL-treated
HUVECs, which was consistent with previous studies associated with
the anti-oxidative activity of miR-21 (52,53). These results indicated that
miR-21/PTEN contributed to anti-oxidative stress effects of
geniposide in atherosclerosis.
Inflammation plays an important role during every
phase of atherosclerosis development, and an increasing body of
evidence suggests that inflammation resolution may become a
potential approach in treatment of atherosclerosis (54,55). A recent study reported that
geniposide inhibits arterial plaque formation and eliminates the
inflammatory response in ApoE−/− mice via regulating the
miR-101/MKP-1/p38 pathway (17).
Likewise, the present study revealed that geniposide pretreatment
decreased pro-inflammatory cytokine (IL-1β, IL-6 and TNF-α) levels
and increased the anti-inflammatory cytokine (IL-10) level in
ox-LDL-treated HUVECs. miR-21 has been demonstrated to play an
important role in the induction and resolution of inflammatory
responses (56). The elimination
of PTEN induced by dysregulated miR-21 promotes inflammatory
responses (57). The present
study further found that miR-21 mimic strengthened the
anti-inflammatory effect of geniposide under ox-LDL condition in
HUVECs, whereas the miR-21 inhibitor abolished the
anti-inflammatory effect of geniposide. Taken together, these
results indicated that the miR-21/PTEN pathway is involved in the
anti-inflammatory effect of geniposide in atherosclerosis.
The present study also has some limitations.
Firstly, PTEN overexpression or knockdown should be considered to
directly confirm the role of the miR-21/PTEN pathway in the
anti-oxidant and anti-inflammatory effects of geniposide in
atherosclerosis. Secondly, the present study was only an in
vitro study, and the detailed underlying mechanism should be
further demonstrated in animal models of atherosclerosis.
The present study provides evidence that geniposide
protects against ox-LDL-induced endothelial injury, with
suppressing oxidative stress and inflammatory response, which is
associated with the enhancement of miR-21/PTEN pathways. These
findings suggest that geniposide may exhibit clinical potential in
the prevention of atherosclerosis, and these outcomes opened a new
view of research regarding the role of the miR-21/PTEN pathway in
the potential protective role of geniposide in a variety of
diseases related to oxidative stress and inflammation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
SZ designed and performed the experiments, and wrote
the manuscript. YS and KZ analyzed the data. YG, JC and LQ designed
the experiments and analyzed the data. LH performed the
experiments. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fitridge R and Thompson M: Mechanisms of
Vascular Disease: A Reference Book for Vascular Specialists
[Internet]. University of Adelaide Press; Adelaide, AU: 2011
|
|
2
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American Heart Association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P, Bornfeldt KE and Tall AR:
Atherosclerosis: Successes, surprises, and future challenges. Circ
Res. 118:531–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee DY and Chiu JJ: Atherosclerosis and
flow: Roles of epigenetic modulation in vascular endothelium. J
Biomed Sci. 26:562019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Back M, Yurdagul A Jr, Tabas I, Oorni K
and Kovanen PT: Inflammation and its resolution in atherosclerosis:
Mediators and therapeutic opportunities. Nat Rev Cardiol.
16:389–406. 2019.PubMed/NCBI
|
|
7
|
Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang
J, Yuan Q, Yu H, Xu W and Xie X: New insights into oxidative stress
and inflammation during diabetes mellitus-accelerated
atherosclerosis. Redox Biol. 20:247–260. 2019. View Article : Google Scholar
|
|
8
|
Libby P, Buring JE, Badimon L, Hansson GK,
Deanfield J, Bittencourt MS, Tokgözoğlu L and Lewis EF:
Atherosclerosis. Nat Rev Dis Primers. 5:562019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trpkovic A, Resanovic I, Stanimirovic J,
Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D and Isenovic ER:
Oxidized low-density lipoprotein as a biomarker of cardiovascular
diseases. Crit Rev Clin Lab Sci. 52:70–85. 2015. View Article : Google Scholar
|
|
10
|
Cao Y, Gong Y, Liu L, Zhou Y, Fang X,
Zhang C, Li Y and Li J: The use of human umbilical vein endothelial
cells (HUVECs) as an in vitro model to assess the toxicity of
nanoparticles to endothelium: A review. J Appl Toxicol.
37:1359–1369. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shangguan WJ, Zhang YH, Li ZC, Tang LM,
Shao J and Li H: Naringin inhibits vascular endothelial cell
apoptosis via endoplasmic reticulum stress and
mitochondrialmediated pathways and promotes intraosseous
angiogenesis in ovariectomized rats. Int J Mol Med. 40:1741–1749.
2017.PubMed/NCBI
|
|
12
|
Cai L, Li CM, Tang WJ, Liu MM, Chen WN,
Qiu YY and Li R: Therapeutic effect of penta-acetyl geniposide on
adjuvant-induced arthritis in rats: Involvement of inducing
synovial apoptosis and inhibiting NF-κB signal pathway.
Inflammation. 41:2184–2195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan T, Shi X, Chen H, Chen R, Wu D, Lin Z,
Zhang J and Pan J: Correction to: Geniposide suppresses
interleukin-1β-induced inflammation and apoptosis in rat
chondrocytes via the PI3K/Akt/NF-κB signaling pathway.
Inflammation. 42:404–405. 2019. View Article : Google Scholar
|
|
14
|
Jiang YQ, Chang GL, Wang Y, Zhang DY, Cao
L and Liu J: Geniposide prevents Hypoxia/Reoxygenation-Induced
apoptosis in H9c2 Cells: Improvement of mitochondrial dysfunction
and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell
Physiol Biochem. 39:407–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Habtemariam S and Lentini G: Plant-derived
anticancer agents: Lessons from the pharmacology of geniposide and
its aglycone, genipin. Biomedicines. 6:pii: E39. 2018. View Article : Google Scholar
|
|
16
|
Koo HJ, Lee S, Shin KH, Kim BC, Lim CJ and
Park EH: Geniposide, an anti-angiogenic compound from the fruits of
Gardenia jasminoides. Planta Med. 70:467–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng S, Zhou F, Xu Y, Liu X, Zhang Y, Gu
M, Su Z, Zhao D, Zhang L and Jia Y: Geniposide regulates the
miR-101/MKP-1/p38 pathway and alleviates atherosclerosis
inflammatory injury in ApoE(−/−) mice. Immunobiology. 224:296–306.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang B, Liao PP, Liu LH, Fang X, Li W and
Guan SM: Baicalin and geniposide inhibit the development of
atherosclerosis by increasing Wnt1 and inhibiting dickkopf-related
protein-1 expression. J Geriatr Cardiol. 13:846–854.
2016.PubMed/NCBI
|
|
19
|
Bhaskaran M and Mohan M: MicroRNAs:
History, biogenesis, and their evolving role in animal development
and disease. Vet Pathol. 51:759–774. 2014. View Article : Google Scholar :
|
|
20
|
Kumarswamy R, Volkmann I and Thum T:
Regulation and function of miRNA-21 in health and disease. RNA
Biol. 8:706–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Juźwik CA, Drake S, Zhang Y, Paradis-Isler
N, Sylvester A, Amar-Zifkin A, Douglas C, Morquette B, Moore CS and
Fournier AE: microRNA dysregulation in neurodegenerative diseases:
A systematic review. Prog Neurobiol. 182:1016642019. View Article : Google Scholar
|
|
22
|
Cheng Y and Zhang C: MicroRNA-21 in
cardiovascular disease. J Cardiovasc Transl Res. 3:251–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jazbutyte V and Thum T: MicroRNA-21: From
cancer to cardiovascular disease. Curr Drug Targets. 11:926–935.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li FP, Lin DQ and Gao LY: LncRNA TUG1
promotes proliferation of vascular smooth muscle cell and
atherosclerosis through regulating miRNA-21/PTEN axis. Eur Rev Med
Pharmacol Sci. 22:7439–7447. 2018.PubMed/NCBI
|
|
25
|
Yang Q, Yang K and Li AY: Trimetazidine
protects against hypoxia-reperfusion-induced cardiomyocyte
apoptosis by increasing microRNA-21 expression. Int J Clin Exp
Pathol. 8:3735–3741. 2015.PubMed/NCBI
|
|
26
|
Canfrán-Duque A, Rotllan N, Zhang X,
Fernández-Fuertes M, Ramírez-Hidalgo C, Araldi E, Daimiel L, Busto
R, Fernández-Hernando C and Suárez Y: Macrophage deficiency of
miR-21 promotes apoptosis, plaque necrosis, and vascular
inflammation during atherogenesis. EMBO Mol Med. 9:1244–1262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chipont A, Esposito B, Challier I,
Montabord M, Tedgui A, Mallat Z, Loyer X and Potteaux S:
Microrna-21 deficiency alters the survival of ly-6clo monocytes in
apoe−/− mice and reduces early-stage
atherosclerosis-brief report. Arterioscler Thromb Vasc Biol.
39:170–177. 2019. View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−D elta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang
Z, Gao X, Chen Z, Xue H and Li G: Immunosuppressive effects of
hypoxia-induced glioma exosomes through myeloid-derived suppressor
cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene.
37:4239–4259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo Q, Cai Z, Tu J, Ling Y, Wang D and Cai
Y: Total flavonoids from Smilax glabra Roxb blocks
epithelial-mesenchymal transition and inhibits renal interstitial
fibrosis by targeting miR-21/PTEN signaling. J Cell Biochem.
120:3861–3873. 2019. View Article : Google Scholar
|
|
33
|
Emini Veseli B, Perrotta P, De Meyer GRA,
Roth L, Van der Donckt C, Martinet W and De Meyer GRY: Animal
models of atherosclerosis. Eur J Pharmacol. 816:3–13. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olvera Lopez E and Jan A: Cardiovascular
Disease. StatPearls Treasure Island (FL): 2019
|
|
35
|
Ma ZG, Kong CY, Song P, Zhang X, Yuan YP
and Tang QZ: Geniposide protects against obesity-related cardiac
injury through AMPKalpha- and Sirt1-Dependent Mechanisms. Oxid Med
Cell Longev. 2018:6053727. 2018. View Article : Google Scholar
|
|
36
|
Zhang Z, Wang X, Zhang D, Liu Y and Li L:
Geniposide-mediated protection against amyloid deposition and
behavioral impairment correlates with downregulation of mTOR
signaling and enhanced autophagy in a mouse model of Alzheimer’s
disease. Aging (Albany NY). 11:536–548. 2019. View Article : Google Scholar
|
|
37
|
Liao P, Liu L, Wang B, Li W, Fang X and
Guan S: Baicalin and geniposide attenuate atherosclerosis involving
lipids regulation and immunoregulation in ApoE−/− mice. Eur J
Pharmacol. 740:488–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu L, Liao P, Wang B, Fang X, Li W and
Guan S: Oral administration of baicalin and geniposide induces
regression of atherosclerosis via inhibiting dendritic cells in
ApoE-knockout mice. Int Immunopharmacol. 20:197–204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paone S, Baxter AA, Hulett MD and Poon
IKH: Endothelial cell apoptosis and the role of endothelial
cell-derived extracellular vesicles in the progression of
atherosclerosis. Cell Mol Life Sci. 76:1093–1106. 2019. View Article : Google Scholar
|
|
40
|
Oyama Y, Bartman CM, Gile J and Eckle T:
Circadian microRNAs in Cardioprotection. Curr Pharm Des.
23:3723–3730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Q, Yang K and Li A: microRNA-21
protects against ischemia-reperfusion and
hypoxia-reperfusion-induced cardiocyte apoptosis via the
phosphatase and tensin homolog/Akt-dependent mechanism. Mol Med
Rep. 9:2213–2220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang JY, Ma J, Yu P, Tang GJ, Li CJ, Yu
DM and Zhang QM: Reduced beta 2 glycoprotein I prevents high
glucose-induced cell death in HUVECs through miR-21/PTEN. Am J
Transl Res. 9:3935–3949. 2017.PubMed/NCBI
|
|
43
|
Nariman-Saleh-Fam Z, Vahed SZ,
Aghaee-Bakhtiari SH, Daraei A, Saadatian Z, Kafil HS, Yousefi B,
Eyvazi S, Khaheshi I, Parsa SA, et al: Expression pattern of
miR-21, miR-25 and PTEN in peripheral blood mononuclear cells of
patients with significant or insignificant coronary stenosis. Gene.
698:170–178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qiang Z, Meng L, Yi C, Yu L, Chen W and
Sha W: Curcumin regulates the miR-21/PTEN/Akt pathway and acts in
synergy with PD98059 to induce apoptosis of human gastric cancer
MGC-803 cells. J Int Med Res. 47:1288–1297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H, Feng X, Zhang M, Liu A, Tian L,
Bo W, Wang H and Hu Y: Long non-coding RNA CASC2 upregulates PTEN
to suppress pancreatic carcinoma cell metastasis by downregulating
miR-21. Cancer Cell Int. 19:182019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ma J and Ding Y: Geniposide suppresses
growth, migration and invasion of MKN45 cells by down-regulation of
lncRNA HULC. Exp Mol Pathol. 105:252–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kattoor AJ, Pothineni NVK, Palagiri D and
Mehta JL: Oxidative stress in atherosclerosis. Curr Atheroscler
Rep. 19:422017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Khosravi M, Poursaleh A, Ghasempour G,
Farhad S and Najafi M: The effects of oxidative stress on the
development of atherosclerosis. Biol Chem. 400:711–732. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu W, Zhao Y, Kong Y, Zhang W, Ma W, Li W
and Wang K: Geniposide prevents H2O2 -induced
oxidative damage in melanocytes by activating the PI3K-Akt
signalling pathway. Clin Exp Dermatol. 43:667–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu W, Li G, Holscher C and Li L:
Neuroprotective effects of geniposide on Alzheimer’s disease
pathology. Rev Neurosci. 26:371–383. 2015. View Article : Google Scholar
|
|
51
|
Shin D, Lee S, Huang YH, Lim HW, Lee Y,
Jang K, Cho Y, Park SJ, Kim DD and Lim CJ: Protective properties of
geniposide against UV-B-induced photooxidative stress in human
dermal fibroblasts. Pharm Biol. 56:176–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Magenta A, Dellambra E, Ciarapica R and
Capogrossi MC: Oxidative stress, microRNAs and cytosolic calcium
homeostasis. Cell Calcium. 60:207–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pereira-da-Silva T, Coutinho Cruz M,
Carrusca C, Cruz Ferreira R, Napoleao P and Mota Carmo M:
Circulating microRNA profiles in different arterial territories of
stable atherosclerotic disease: A systematic review. Am J
Cardiovasc Dis. 8:1–13. 2018.PubMed/NCBI
|
|
54
|
Fredman G and Tabas I: Boosting
Inflammation Resolution in Atherosclerosis: The Next Frontier for
Therapy. Am J Pathol. 187:1211–1221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu MY, Li CJ, Hou MF and Chu PY: New
Insights into the Role of Inflammation in the Pathogenesis of
Atherosclerosis. Int J Mol Sci. 18:pii: E2034. 2017. View Article : Google Scholar
|
|
56
|
Sheedy FJ: Turning 21: Induction of miR-21
as a key switch in the inflammatory response. Front Immunol.
6:192015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yue S, Rao J, Zhu J, Busuttil RW,
Kupiec-Weglinski JW, Lu L, Wang X and Zhai Y: Myeloid PTEN
deficiency protects livers from ischemia reperfusion injury by
facilitating M2 macrophage differentiation. J Immunol.
192:5343–5353. 2014. View Article : Google Scholar : PubMed/NCBI
|



















