Contents
Introduction
RANKL/RANK system
RANKL/RANK signaling is involved in oral SCC
cell-induced osteoclastogenesis
Osteoclast function regulated by RANKL/RANK
signaling
The role of RANKL/RANK signaling on bone invasion by
oral SCCs
Conclusions and perspectives
Introduction
Malignant tumors of the oral cavity, 95% of which
are squamous cell carcinoma (SCCs), account for approximately 30%
of all head and neck cancers (1).
Although oral SCC represents 1–2% of all human malignancies in
Japan, it is the sixth most common cancer worldwide, with more than
500,000 new cases diagnosed each year. The most common sites for
SCCs are the tongue and gingiva (1–3).
Carcinoma of the mandibular gingiva, in particular,
is associated with bone invasiveness in many patients (Fig. 1A and B) (4–6).
Gingival SCC may eventually directly invade the mandible, a feature
associated with a worse prognosis. The presence of mandibular
invasion is an important criterion for deciding whether surgical
intervention is necessary (7).
According to the American Joint Committee on Cancer Classification,
mandibular invasion is the most advanced primary stage (T4) and
overall stage (IV) for these tumors. The 5-year survival of
patients with stage IV oral lesions has been demonstrated to be
39%, as compared with 53, 68 and 70% for stages III, II and I,
respectively (8).
The invasion of bone by oral SCCs may be associated
with an increase in both osteoclastic and osteoblastic activity.
Before invasion of the mandible, SCCs in close proximity to bone
initially induce deposition of new bone, especially on the
periosteal surface. Two distinct types of invasion of the mandible
by oral SCCs have been described (6,8,9). In
the erosive pattern the tumor advances on a broad front separated
from bone by a layer of connective tissue and osteoclasts are
present in the region between bone and stromal tissue. In contrast,
the infiltrative pattern of bone invasion is associated with finger
and islands of tumor tissue, which invades cancellous spaces with
higher osteoclastic activity (Fig.
1C) (10,11). Cases exhibiting features of both
patterns are designated as having a mixed pattern. The infiltrative
type showed significantly higher rates of positive bone margin and
primary site recurrence than the erosive types. In fact, the 3-year
disease-free survival of patients with infiltrative and erosive
patterns was 30 and 73%, respectively (12). Since these clinicopathological
studies indicate that bone invasion by gingival SCC is a critical
event that determines prognosis, it is important to develop a
therapeutic approach to prevent the bone invasion process.
Mandibulectomy, if necessary, has a major influence
on the patient’s quality of life (QOL) and is a critical
determinant of postoperative functional outcome (Fig. 1D). Patients with advanced oral SCC
have a high mortality, but treatment is complicated by the
disruption of speech and swallowing after surgical resection
(6–11). It is generally agreed that patients
with mandibular invasion should be treated surgically, although the
extent of mandibular resection required remains controversial.
Resection of the mandibular bone leads to physical damage and
frequently also to psychological problems for patients (6–11).
The ability of oral SCCs to invade the maxilla or
mandibular bone is a critical factor, which, because it leads to
metastasis, affects the prognosis of patients (Fig. 1C) (3). Although controversial, bone
destruction that occurs with oral SCC invasion is thought to be
mediated by osteoclasts rather than by the carcinoma itself
(5). Recent studies have
established that bone resorption by osteoclasts is an important
step in the process of bone invasion and metastasis in several
types of malignancy, indicating that a full understanding of the
regulation of osteoclastogenesis by oral SCC cells is necessary to
prevent bone invasion by oral SCC cells. Several in vitro
and animal experiments using human OSCC cells have shown that tumor
cells produce prostaglandins and several cytokines, including
interleukin-6 (IL-6), IL-11, TNFα and parathyroid hormone-related
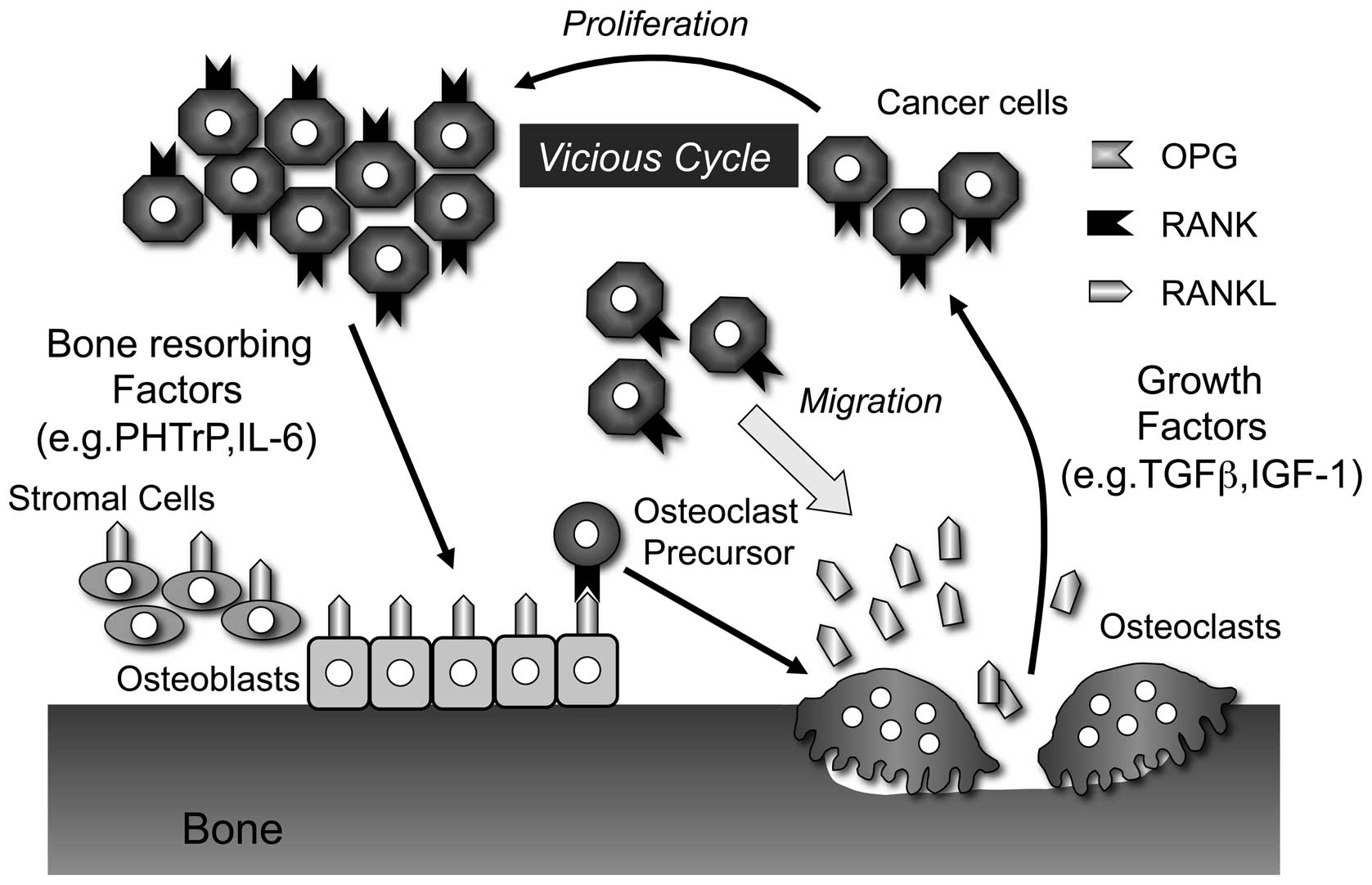
protein (PTHrP) (9,13). Indeed, bone is also a storehouse
for a variety of cytokines and growth factors and thus provides an
extremely fertile environment for cell growth once cancer cells
arrest there (Fig. 2) (13). Shibahara et al have reported
that the invasive tumors showed a high level of expression of IL-6,
IL-11, TNFα and PTHrP (14). In
contrast, expression of TGF-β, IL-1α/β and IL-18 were not different
between invasive and non-invasive tumors, suggesting that various
cancer-derived cytokines, such as IL-6, IL-11, TNFα and PTHrP, play
important roles in bone invasion by oral SCC (14).
Three proteins crucial for osteoclast development
and activation are the receptor activator of NF-κB ligand (RANKL),
its receptor, RANK and its decoy receptor, osteoprotegerin (OPG)
(15–19). In vitro, the RANKL/RANK
signaling pathway, together with macrophage colony-stimulation
factor (M-CSF), regulates osteoclast differentiation from
monocyte/macrophage progenitors (15,16),
whereas the addition of OPG into these culture systems prevents
osteoclastogenesis (17,18). Furthermore, these proteins are
known to be involved in both normal and pathological bone
metabolism. IL-6, IL-11, TNFα and PTHrP released from oral SCCs
also induce RANKL expression in osteoblasts or bone marrow stromal
cells (19). Thus, RANKL levels
are increased in osteolytic lesions associated with malignant
tumors, whereas OPG levels are increased in osteoblastic lesions
(20). This suggests that blocking
RANKL/RANK with soluble RANK (sRANK) or OPG inhibits
osteoclastogenesis and successfully prevents bone destruction by
cancers (21–23). Therefore, in this review, we first
summarize the RANKL/RANK signaling pathway and its effects on
osteoclastogenesis. We also describe recent discoveries on the role
of RANKL/RANK in bone invasion by oral SCC, based on recent
findings from our lab and others. Finally, we discuss the
possibility that RANKL inhibition might successfully prevent bone
invasion by oral SCC.
RANKL/RANK system
In the past decade, great progress has been made in
understanding bone biology and, in particular, the molecular
mechanisms of osteoclast development. Osteoclasts, which are
present only in bone, are large, multinucleate cells with the
capacity to resorb mineralized tissue (19,24,25).
Osteoclastic bone resorption consists of multiple steps: the
proliferation of osteoclast precursors belong to hematopoietic
cells, differentiation of progenitors into mononuclear
preosteoclasts (pOCs), fusion of pOCs into multinucleate
osteoclasts, clear zone (actin ring) and ruffled border formation
(activation), and apoptosis. It has been proposed that osteoblasts
or bone marrow stromal cells are involved in osteoclastogenesis
through a mechanism involving cell-to-cell contact with osteoclast
precursors. This hypothesis was proven by the discovery of RANKL, a
member of TNF ligand family (19,24,25).
In 1997, Simonet et al reported the discovery
of OPG, an inhibitor of bone resorption (18). OPG is a member of the TNF receptor
family but, unlike all other members of the family, lacks a
transmembrane domain and represents a secreted TNF receptor
(Fig. 3). Tsuda et al
independently isolated the same protein as ‘osteoclast inhibitory
factor (OCIF)’, a heparin-binding molecule from the conditioned
media of human fibroblast cultures, and showed that its cDNA
sequence is identical to that of OPG (17). OPG strongly inhibits osteoclast
formation in cocultures of mouse bone marrow cells and primary
osteoblasts (POBs) induced by osteotropic factors such as 1α
dihydroxyvitamin D3 or PGE2. The administration of OPG to
ovariectomized rats causes an increase in bone volume and mineral
density associated with a decreased number of osteoclasts (18). Furthermore, OPG-deficient mice
exhibit severe osteoporosis caused by enhanced osteoclast
formation, suggesting that OPG is a physiological regulator of
osteoclast development (26).
A recombinant soluble form of RANKL, together with
M-CSF, induces osteoclastogenesis from mouse bone marrow cells or
spleen cells in the absence of osteoblasts (15,16).
RANKL was first cloned during a search for apoptosis regulatory
genes in mouse T cell hybridoma and was named TNF-related
activation-induced cytokine (TRANCE) (27). Two other groups also isolated
ligands of OCIF and OPG, respectively, which turned out to be
identical to TNF-related activation-induced cytokine (TRANCE)
(15,16). RANKL is a type II transmembrane
protein of the TNF ligand family that is expressed in cells such as
osteoblasts and T cells (Fig. 3).
RANKL-deficient mice exhibit typical osteopetrosis due to lack of
osteoclasts, suggesting that RANKL is an absolute requirement for
osteoclast development (28).
Anderson et al cloned a new member of the TNF
receptor family, termed ‘RANK’, from a cDNA library of human
dendritic cells. The mouse homolog was also isolated from the fetal
mouse liver cDNA library (29).
RANK is a transmembrane heterotrimer on the surface of
hematopoietic osteoclast progenitors, mature osteoclasts, dendritic
cells, and mammary gland epithelial cells (Fig. 3). It fails to bind other members of
the TNF ligand family, such as Fas ligand, CD40 ligand, TNFα or
TRAIL (19). A soluble form of
RANK prevents RANKL-induced osteoclastogenesis (30). RANK-deficient mice have a complete
block in osteoclast development that can be rescued by
transplantation of bone marrow cells from wild-type mice,
indicating that they have an intrinsic defect in osteoclast
function (31).
RANK, RANKL and OPG are involved in not only
physiological but also pathological conditions of bone metabolism.
Bone resorption is a major pathological factor in chronic
inflammatory diseases such as periodontitis, osteoporosis and
arthritis. It is now clear that an imbalance in the RANKL-OPG ratio
is crucial for initiating the bone loss associated with these
conditions (24,25). Therefore, inhibition of RANKL/RANK
signaling might be effective for preventing inflammatory bone
destruction. In fact, denosumab, a human monoclonal antibody
against RANKL, has been shown to effectively reduce RANK signaling
and thus osteoclast activity. In large, randomized, phase III
studies, it has been demonstrated to prevent fractures and bone
loss and improve the bone mineral density in various cancerous and
non-cancerous settings (32).
RANKL/RANK signaling is involved in oral SCC
cell-induced osteoclastogenesis
The dysregulation of functional equilibrium in the
RANKL/RANK/OPG triad is responsible for osteolysis associated with
the development of malignant tumors in bone sites. Recent studies
have shown that administration of OPG or sRANK prevents bone
metastasis by cancer cells in vivo(21–23).
These findings suggest that the RANKL/RANK system contributes to
bone metastasis by cancer cells. However, it remains controversial
whether cancer cells directly express RANKL on their surface and to
what extent this expression contributes to osteoclast
formation.
The expression of RANKL has already been detected in
several tumor cell types and can be considered a key factor in bone
remodeling associated with bone metastases (33–37).
Several oral SCC cells in patients and oral SCC cell lines express
RANKL (38–40). Recently, Chuang et al
compared RANKL expression between buccal SCC without bone invasion
(25 cases) and gingival SCC with invasion (15 cases). There were no
differences between the immunohistochemical expression of RANKL in
cases of buccal and gingival SCC (41). This suggests that, in cases of
human buccal SCC without bone invasion, tumor cells do possess the
potential to induce osteoclastogenesis through the RANKL/RANK
pathway if triggered under appropriate conditions. It is possible
that close proximity of the cancer to the jawbone may be a
prerequisite.
It has been reported that cancer cells expressing
RANKL are able to induce osteoclastogenesis even in the absence of
other accessory cells (38,42).
On the other hand, not all cancers express RANKL, and cell-to-cell
contact between cancer cells and host cells does not always lead to
RANKL expression (43,44). We showed in a previous study that
BHY cells, which were highly invasive to the mandibular bone when
inoculated into the masseter of nude mice, expressed RANKL on their
cell surface but failed to induce osteoclastogenesis in cocultures
of mouse bone marrow cells (BMCs) and BHY cells (39). However, adding BHY cells to a
coculture of mouse POBs and BMCs markedly induced
osteoclastogenesis in the absence of osteotropic factors.
Consistent with these results, HSC-2 cells, which do not express
RANKL, also induced osteoclast formation in cocultures of mouse
BMCs and POBs without any osteotropic factors (39). The addition of BHY cells suppressed
mouse OPG mRNA expression and protein production by POBs. This
finding is consistent with the observation that BHY cells did not
enhance osteoclastogenesis in cocultures of BMCs and POBs from
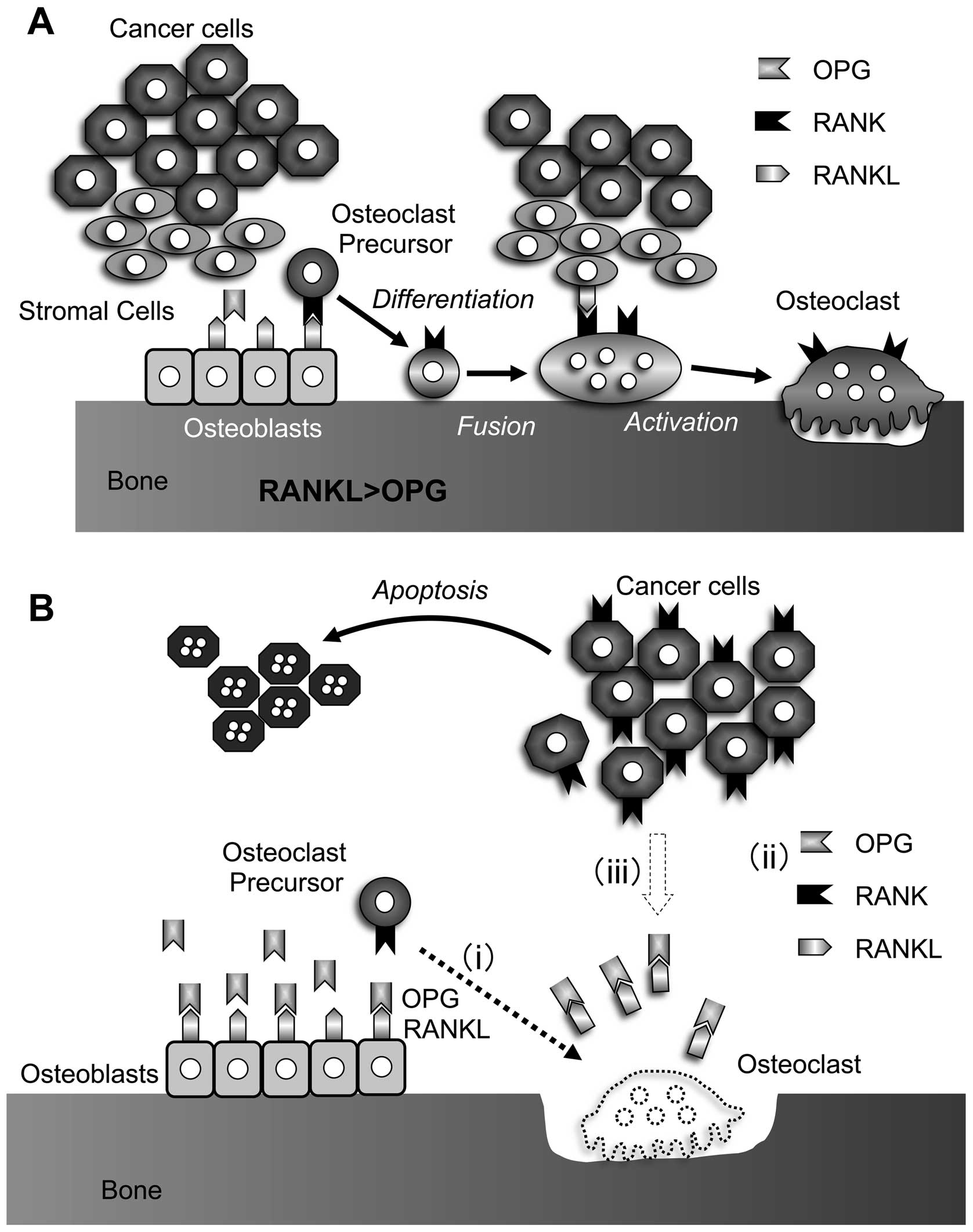
OPG-deficient mice. Furthermore, immunohistochemical analysis
showed a reduction of OPG expression in stromal cells from
osteolytic lesions as compared to normal lesions. Therefore, oral
SCC-induced suppression of OPG expression in POBs, rather than
expression of RANKL in oral SCC cells, appears critical for
osteoclastogenesis (Fig. 4A)
(39).
Osteoclast function regulated by RANKL/RANK
signaling
Despite the importance of oral SCC for
osteoclastogenesis, its roles in osteoclast function, such as
multinucleation, survival and pit-forming activity, is not fully
understood. Therefore, we also examined the effect of oral SCCs on
osteoclast function using BHY cells (45). Osteoclasts are terminally
differentiated cells, with a short life span, that undergo rapid
apoptosis in the absence of cytokines such as M-CSF, IL-1, TNFα or
RANKL. Both BHY cells and CM from BHY cells support osteoclast
survival by suppressing expression of Bim, a member of the BH
(Bcl-2 homology) 3-only family of pro-apoptotic proteins (45). Soluble factors, such as IL-1β, TNFα
or RANKL, from BHY cells might contribute to the survival of
osteoclasts. M-CSF, IL-1 and RANKL induced both the survival and
multinucleation of prefusion osteoclasts (46). BHY cells induced not only the
survival but also the multinucleation of prefusion osteoclasts.
Although the CM from BHY cells also induced multinucleation of
prefusion osteoclasts, it did so less efficiently. Furthermore,
adding BHY cells, but not the CM of BHY cells, induced pit-forming
activity of osteoclasts. Adding OPG abrogated the activity
(45). Thus, oral SCC cells
regulate not only osteoclast formation but also function (Fig. 4A).
The role of RANKL/RANK signaling on bone
invasion by oral SCCs
The addition of OPG dramatically suppresses oral SCC
regulation of both osteoclast differentiation and function in
vitro, suggesting that RANKL/RANK inhibition might be an
effective therapeutic approach for inhibiting oral SCC-induced bone
invasion. In support of this notion, recent studies in rodent
models of breast and prostate cancer have established that
inhibition of RANKL/RANK signal decreases bone lesion development
and tumor growth in bone (21–23).
Alternatively, it has been reported that functional RANK is
expressed on some bone-associated tumor cells (21–23,54).
Indeed, the migration of RANK-positive tumor cells is induced by
RANKL stimulation. Thus, these observations suggest that increased
RANKL expression in the tumor-bone environment is a promoting
factor for bone tumor development (Fig. 2) (21–23,47).
We injected B88 human oral SCC cells into the
masseter region of nude mice to establish an animal model of oral
SCC bone invasion and then determine whether OPG prevents bone
invasion by oral SCC in vivo. Treatment with OPG for 3 weeks
decreased bone invasion by B88 cells and reduced the number of
tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts.
OPG decreased tumor burden and increased cell death in B88 cells,
whereas B88 cell mitosis was unchanged. Thus, the suppressive
effect of OPG on B88 tumor burden is attributed to increased cell
death rather than inhibition of proliferation. However, OPG did not
affect apoptosis and proliferation of B88 cells in vitro,
suggesting that effects of OPG on apoptosis in B88 cells are
restricted to the bone environment (48).
Consistent with other types of cancer, tumor tissue
from oral SCC patients and oral SCC cell lines, including BHY and
B88, express RANK. Chuang et al reported that RANK
expression was observed in oral SCC, but not normal mucosal tissue
(41). RANK might become
upregulated in cancer cells. Although tumor cells were closely
associated with bone in controls, tumors were farther from the bone
in mice treated with OPG. To support these results, RANKL enhanced
B88 cell migration in a modified chemotaxis chamber equipped with a
gelatin-coated filter. This effect was inhibited by OPG (48).
Taken together, RANKL/RANK inhibition suppresses
bone invasion by inhibiting osteoclastogenesis and cancer cell
migration and by inducing apoptosis of cancer cells via indirect
anticancer action in vivo (Fig.
4B).
Conclusions and perspectives
Bone is a good environment for the progression of
bone invasion. It has been suggested that oral SCC cells release
soluble factors that activate osteoclast differentiation and
function directly or indirectly via osteoblasts (Fig. 2) (13). During bone destruction, osteoclasts
release various growth factors, including insulin-like growth
factor and transforming growth factor β (Fig. 2). This cycle has been proposed to
explain tumor development in bone. The inhibition of osteoclast
differentiation and function by blocking RANKL/RANK constitutes a
potentially novel approach to maintaining skeletal integrity.
Indeed, blocking RANKL/RANK with sRANK or OPG successfully prevents
the development of bone invasion (Fig.
4).
A phase I study testing recombinant OPG in patients
with multiple myeloma- or breast carcinoma-related bone metastases
is currently in progress (49).
Thus far, OPG has no side-effects when administered as a single
subcutaneous injection to patients (50), and it has been used subcutaneously
to treat bone metastases from multiple myeloma and breast cancer
cells (23,51). The RANKL inhibitor denosumab, a
human monoclonal antibody against human RANKL, has also developed
and is tested in the clinic. Denosumab was generally well tolerated
throughout clinical trials without the patients having detectable
anti-denosumab antibodies (32).
In the case of mandibular bone invasion, it is possible to treat
the cancer with OPG locally because oral SCC cells invade from
cortical bone, and the tumor and defect area are at the surface of
the body. However, recent reports showed that the observed
incidence of osteonecrosis of the jaw was comparable to that with
bisphosphonates (52,53). Therefore, it is important to
carefully consider the dosage and schedule of administration to
avoid any side-effects of local injection of OPG or denosumab
before the realization of the clinical application.
A great deal of progress has been made in
understanding the pathogenesis of oral SCC. In addition, new
approaches have been developed in the fields of molecular biology,
cancer genetics, and cancer biology to examine the cellular and
molecular mechanisms of bone invasion by oral SCCs (54). In particular, the discovery of the
RANKL/RANK/OPG triad was a breakthrough in our knowledge of
osteoclast biology. The development of targeted approaches against
the RANKL/RANK system will contribute to preventing bone invasion
by oral SCCs.
Acknowledgements
This study was supported by a
Grant-in-Aid from Kyushu Dental College Internal Grants (to E.J.),
the Ministry of Education, Culture, Sports, Science and Technology
of Japan (to M.S. 70549261) and a Grant-in-Aid from Kyushu Dental
College Alumni Association Grants (to M.S.).
References
|
1
|
Haddad RI and Shin DM: Recent advances in
head and neck cancer. N Engl J Med. 359:1143–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen EE, Haraf DJ, Kunnavakkam R, et al:
Epidermal growth factor receptor inhibitor gefitinib added to
chemoradiotherapy in locally advanced head and neck cancer. J Clin
Oncol. 28:3336–3343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jimi E, Furuta H, Matsuo K, Tominaga K,
Takahashi T and Nakanishi O: The cellular and molecular mechanisms
of bone invasion by oral squamous cell carcinoma. Oral Dis.
17:462–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ash CS, Nason RW, Abdoh AA and Cohen MA:
Prognostic implications of mandibular invasion in oral cancer. Head
Neck. 22:794–798. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Semba I, Matsuuchi H and Miura Y:
Histomorphometric analysis of osteoclastic resorption in bone
directly invaded by gingival squamous cell carcinoma. J Oral Pathol
Med. 25:429–435. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Totsuka Y, Usui Y, Tei K, Fukuda H, Shindo
M, Iizuka T and Amemiya A: Mandibular involvement by squamous cell
carcinoma of the lower alveolus: analysis and comparative study of
histologic and radiologic features. Head Neck. 13:40–50. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shibahara T, Noma H, Takasaki Y and Nomura
T: Repair of the inferior alveolar nerve with a forearm cutaneous
nerve graft after ablative surgery of the mandible. J Oral
Maxillofac Surg. 58:714–717. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah J and Lydiatt WM: Buccal mucosa,
alveolus, retromolar trigone, floor of mouth, hard palate, and
tongue tumors. Comprehensive Management of Head and Neck Tumors.
Thawley SE: 2nd edition. WB Saunders; Philadelphia, PA: pp.
686–693. 1999
|
|
9
|
Carter RL, Tsao SW, Burman JF, Pittam MR,
Clifford P and Shaw HJ: Patterns and mechanisms of bone invasion by
squamous carcinomas of the head and neck. Am J Surg. 146:451–455.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Müller H and Slootweg PJ: Mandibular
invasion by oral squamous cell carcinoma. Clinical aspects. J
Craniomaxillofac Surg. 18:80–84. 1990.PubMed/NCBI
|
|
11
|
Totsuka Y, Usui Y, Tei K, Kida M,
Mizukoshi T, Notani K and Fukuda H: Results of surgical treatment
for squamous carcinoma of the lower alveolus: segmental vs.
marginal resection. Head Neck. 13:114–120. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong RJ, Keel SB, Glynn RJ and Varvares
MA: Histological pattern of mandibular invasion by oral squamous
cell carcinoma. Laryngoscope. 110:65–72. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guise TA and Mundy GR: Cancer and bone.
Endocr Rev. 19:18–54. 1998.
|
|
14
|
Shibahara T, Nomura T, Cui NH and Noma H:
A study of osteoclast-related cytokines in mandibular invasion by
squamous cell carcinoma. Int J Oral Maxillofac Surg. 34:789–793.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yasuda H, Shima N, Nakagawa N, et al:
Osteoclast differentiation factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar
|
|
16
|
Lacey DL, Timms E, Tan HL, et al:
Osteoprotegerin ligand is a cytokine that regulates osteoclast
differentiation and activation. Cell. 93:165–176. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuda E, Goto M, Mochizuki S, Yano K,
Kobayashi F, Morinaga T and Higashio K: Isolation of a novel
cytokine from human fibroblasts that specifically inhibits
osteoclastogenesis. Biochem Biophys Res Commun. 234:137–142. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simonet WS, Lacey DL, Dunstan CR, et al:
Osteoprotegerin: a novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee Y, Schwarz E, Davies M, et al:
Differences in the cytokine profiles associated with prostate
cancer cell induced osteoblastic and osteolytic lesions in bone. J
Orthop Res. 21:62–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller RE, Branstetter D, Armstrong A, et
al: Receptor activator of NF-κB ligand inhibition suppresses bone
resorption and hypercalcemia but does not affect host immune
responses to influenza infection. J Immunol. 179:266–274. 2007.
|
|
22
|
Armstrong AP, Miller RE, Jones JC, Zhang
J, Keller ET and Dougall WC: RANKL acts directly on RANK-expressing
prostate tumor cells and mediates migration and expression of tumor
metastasis genes. Prostate. 68:92–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Canon JR, Roudier M, Bryant R, Morony S,
Stolina M, Kostenuik PJ and Dougall WC: Inhibition of RANKL blocks
skeletal tumor progression and improves survival in a mouse model
of breast cancer bone metastasis. Clin Exp Metastasis. 25:119–129.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar
|
|
25
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizuno A, Amizuka N, Irie K, et al: Severe
osteoporosis in mice lacking osteoclastogenesis inhibitory
factor/osteoprotegerin. Biochem Biophys Res Commun. 247:610–615.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong BR, Rho J, Arron J, et al: TRANCE is
a novel ligand of the tumor necrosis factor receptor family that
activates c-Jun N-terminal kinase in T cells. J Biol Chem.
272:25190–25194. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kong YY, Yoshida H, Sarosi I, et al: OPGL
is a key regulator of osteoclastogenesis, lymphocyte development
and lymph-node organogenesis. Nature. 397:315–323. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anderson DM, Maraskovsky E, Billingsley
WL, et al: A homologue of the TNF receptor and its ligand enhance
T-cell growth and dendritic-cell function. Nature. 390:175–179.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jimi E, Akiyama S, Tsurukai T, et al:
Osteoclast differentiation factor acts as a multifunctional
regulator in murine osteoclast differentiation and function. J
Immunol. 163:434–442. 1999.PubMed/NCBI
|
|
31
|
Dougall WC, Glaccum M, Charrier K, et al:
RANK is essential for osteoclast and lymph node development. Genes
Dev. 13:2412–2424. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Body JJ, Facon T, Coleman RE, et al: A
study of the biological receptor activator of nuclear factor-κB
ligand inhibitor, denosumab, in patients with multiple myeloma or
bone metastases from breast cancer. Clin Cancer Res. 12:1221–1228.
2006.
|
|
33
|
Bendre M, Gaddy D, Nicholas RW and Suva
LJ: Breast cancer metastasis to bone: it is not all about PTHrP.
Clin Orthop Relat Res. 415:S39–S45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thomas RJ, Guise TA, Yin JJ, Elliott J,
Horwood NJ, Martin TJ and Gillespie MT: Breast cancer cells
interact with osteoblasts to support osteoclast formation.
Endocrinology. 140:4451–4458. 1999.PubMed/NCBI
|
|
35
|
Brown JM, Corey E, Lee ZD, True LD, Yun
TJ, Tondravi M and Vessella RL: Osteoprotegerin and RANK ligand
expression in prostate cancer. Urology. 57:611–616. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sezer O, Heider U, Zavrski I, Kühne CA and
Hofbauer LC: RANK ligand and osteoprotegerin in myeloma bone
disease. Blood. 101:2094–2098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yaccoby S, Wezeman MJ, Henderson A, Kühne
CA and Hofbauer LC: Cancer and the microenvironment:
myeloma-osteoclast interactions as a model. Cancer Res.
64:2016–2023. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagai M, Kyakumoto S and Sato N: Cancer
cells responsible for humoral hypercalcemia express mRNA encoding a
secreted form of ODF/TRANCE that induces osteoclast formation.
Biochem Biophys Res Commun. 269:532–536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tada T, Jimi E, Okamoto M, Ozeki S and
Okabe K: Oral squamous cell carcinoma cells induce osteoclast
differentiation by suppression of osteoprotegerin expression in
osteoblasts. Int J Cancer. 116:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kayamori K, Sakamoto K, Nakashima T, et
al: Roles of interleukin-6 and parathyroid hormone-related peptide
in osteoclast formation associated with oral cancers: significance
of interleukin-6 synthesized by stromal cells in response to cancer
cells. Am J Pathol. 176:968–980. 2010. View Article : Google Scholar
|
|
41
|
Chuang FH, Hsue SS, Wu CW and Chen YK:
Immunohistochemical expression of RANKL, RANK, and OPG in human
oral squamous cell carcinoma. J Oral Pathol Med. 38:753–759. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Farrugia AN, Atkins GJ, To LB, et al:
Receptor activator of nuclear factor-κB ligand expression by human
myeloma cells mediates osteoclast formation in vitro and correlates
with bone destruction in vivo. Cancer Res. 63:5438–5445. 2003.
|
|
43
|
Giuliani N, Bataille R, Mancini C,
Lazzaretti M and Barillé S: Myeloma cells induce imbalance in the
osteoprotegerin/osteoprotegerin ligand system in the human bone
marrow environment. Blood. 98:3527–3533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ohshiba T, Miyaura C, Inada M and Ito A:
Role of prostaglandin E produced by osteoblasts in osteolysis due
to bone metastasis. Biochem Biophys Res Commun. 300:957–964. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tada T, Shin M, Fukushima H, Okabe K,
Ozeki S, Okamoto M and Jimi E: Oral squamous cell carcinoma cells
modulate osteoclast function by RANKL-dependent and -independent
mechanisms. Cancer Lett. 274:126–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jimi E, Nakamura I, Duong LT, Ikebe T,
Takahashi N, Rodan GA and Suda T: Interleukin 1 induces
multinucleation and bone-resorbing activity of osteoclasts in the
absence of osteoblasts/stromal cells. Exp Cell Res. 247:84–93.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jones DH, Nakashima T, Sanchez OH, et al:
Regulation of cancer cell migration and bone metastasis by RANKL.
Nature. 440:692–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shin M, Matsuo K, Tada T, et al: The
inhibition of RANKL/RANK signaling by osteoprotegerin suppresses
bone invasion by oral squamous cell carcinoma cells.
Carcinogenesis. 32:1634–1640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Body JJ, Greipp P, Coleman RE, et al: A
phase I study of AMGN-0007, a recombinant osteoprotegerin
construct, in patients with multiple myeloma or breast carcinoma
related bone metastases. Cancer. 97:887–892. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bekker PJ, Holloway D, Nakanishi A,
Arrighi M, Leese PT and Dunstan CR: The effect of a single dose of
osteoprotegerin in postmenopausal women. J Bone Miner Res.
16:348–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roodman GD and Dougall WC: RANK ligand as
a therapeutic target for bone metastases and multiple myeloma.
Cancer Treatment Rev. 34:143–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Steger GG and Bartsch R: Denosumab for the
treatment of bone metastases in breast cancer: evidence and
opinion. Ther Adv Med Oncol. 3:233–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kyrgidis A and Toulis KA:
Denosumab-related osteonecrosis of the jaws. Osteoporos Int.
22:369–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|