Introduction
Brain cancer is one of the most devastating central
nervous system pathologies and recent studies suggest that cancer
stem cells (CSCs) are the most important oncogenic cells in brain
cancer (1–3). Despite the importance of tumorigenic
CSCs in the pathogenesis of brain cancer, increasing evidence
supports a role for the microenvironment or stroma of brain cancer
as an additional significant factor (4–7).
Accordingly, the microenvironment including astrocytes, microglia
and endothelial cells and the stroma, composed of non-neural cells,
may support critical tumorigenic roles, such as initiation,
progression (1,2) and metastasis of cancers (5). The importance of the cancer
microenvironment has received increased scrutiny since the ‘seed
and soil’ hypothesis (8) has been
revisited (9,10). Accordingly, we have taken a keen
interest in the tumor microenvironment, especially mesenchymal
stem-like cells (MSLCs), which resemble bone marrow mesenchymal
stem cells (BM-MSCs), as components of the tumor microenvironment
(11,12).
Evidence suggests that glioblastomas are maintained
by glioma CSCs (gCSCs) (3,13,14)
and, further, that understanding the microenvironment of gliomas is
important for grasping glioma biology (15–18).
After Lang et al first mentioned the isolation of
mesenchymal stem cells (MSCs) from glioma specimens [Lang et
al, Neuro-Oncol 9: abs. 596, 2007; Lang et al, J Clin
Oncol 26 (Suppl 15): abs. 2001, 2008], MSLCs received considerable
research attention and a recent series of studies have reported the
isolation of MSCs/MSLCs from mouse normal brains (19), mouse orthotopic glioma specimens
(11) and Korean glioma specimens
(12). Furthermore, a very recent
study investigated the relationship between gCSCs and glioma stroma
MSLCs (GS-MSLCs) in glioblastoma (7). Similarly, we performed a series of
studies examining the presence of gCSCs and their relationship
(3,6).
Although meningioma is one among the most common
brain tumors also in Korea (20),
little is known about meningioma cell biology. The recent
successful isolation and characterization of CSCs from meningioma
has provided a better understanding of meningioma biology (21–23).
Components of the meningioma stroma are also likely important, as
supported by previous studies (24–27).
Because meningioma is a mesenchymal tumor (28,29),
it is reasonable to suppose that meningiomas have a higher
frequency of MSLCs. Despite the increased interest in meningioma to
the best of our knowledge, there are no studies on meningioma
stroma MSLCs (MS-MSLCs). In this study, we hypothesized that cells
similar to BM-MSCs exist in meningioma specimen and tested this
hypothesis based on cell morphology, differentiation potential,
surface antigens and lack of oncogenicity. In addition, we sought
to verify possible locations of MS-MSLCs.
Materials and methods
Single cell isolation and MS-MSLC
culture
Specimens from patients with human meningioma were
freshly obtained from the operating room with the approval of
Institutional Review Boards of our institutes. Informed consent was
provided according to the Declaration of Helsinki.
Neuropathologists diagnosed these surgical specimens according to
World health Organization (WHO) classification (30). Candidate MS-MSLCs were isolated
from meningioma specimens within 60 min of meningioma removal using
mechanical dissociation methods proven effective for MSC isolation
from bone marrow (31,32), normal brain (19) and gliomas (12,28).
Briefly, surgical specimens were minced and dissociated with a
scalpel in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12
(DMEM/F-12; Mediatech, Manassas, VA, USA) and then passed through a
series of cell strainers with a 100-μm nylon mesh (BD
Falcon, Franklin Lakes, NJ, USA). Cell suspensions were washed
twice in minimal essential medium-α (MEMα; Mediatech, Herndon, VA,
USA) and single-cell suspensions were placed in a 10-cm2
cell culture dish at a density of 2×106
cell/cm2. These cells were cultured in complete MSC
medium consisting of MEMα, 10% fetal bovine serum (FBS; Lonza,
Basel, Switzerland), 2 mM L-glutamine (Mediatech) and
antibiotic-antimycotic solution (100X, Gibco, Invitrogen Korea,
Seoul, Korea). After 24 h, non-adherent cells were removed by
washing twice with phosphate-buffered saline (PBS; Mediatech) and
the adherent cells were cultured until they reached confluence. The
cells were then trypsinized (0.25% trypsin with 0.1% EDTA) and
sub-cultured at a density of 5,000 cells/cm2. The cells
were cultured continuously through 3–4 passages, consistent with
their role as progenitor/stem cells. Cell cultures were observed
with an IX71 inverted phase-contrast microscope (Olympus, Tokyo,
Japan) to determine their morphology. Images of cells were obtained
at each passage using a DP70 Digital Microscope Camera (Olympus)
equipped with DP Controller software (Olympus).
Flow cytometry analysis
To investigate the surface antigen expression
profile, candidate MS-MSLCs were first counted and washed in PBS
(Mediatech) by centrifugation, after which pellets were resuspended
in fluorescent-activated cell sorting (FACS) buffer (PBS with 10%
FBS) at a concentration of 5×105 cells/100 μl.
These single-cell suspensions were incubated at 4°C for 30 min with
phycoerythrin-, fluorescein isothiocyanate (FITC)-, Alexa Fluor
647-, or allophycocyanin-conjugated antibodies against CD105 (0.25
μg/100 μl; eBioscience, San Diego, CA, USA), CD45 (5
μg/100 μl; BD Pharmingen, San Diego, CA, USA), CD73
(5 μg/100 μl; BD Pharmingen), CD90 (0.25
μg/100 μl; eBioscience), CD31 (0.5 μg/100
μl; eBioscience) and nerve/glial antigen 2 (NG2, 2.5
μg/100 μl; R&D Systems, Minneapolis, MN, USA).
All antibody solutions were prepared in FACS buffer. For the
detection of NG2 proteoglycan, a FITC-conjugated secondary NG2
antibody (Millipore, Billerica, MA, USA) was used following primary
antibody incubation (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). FACS analysis was performed using a FACS Vantage SE (BD
Biosciences) flow cytometry system equipped with FlowJo software
(Tree Star, Inc., Ashland, OR, USA) and 30,000 events were recorded
for each sample. Because we merely sought to show the presence of
MSLCs among heterogeneous cells instead of isolating a uniform
population of MSCs (33),
heterogeneous cell populations in which FACS showed that >10% of
cells expressed surface antigen were considered positive and those
with <5% by FACS were considered negative (12).
Mesenchymal differentiation
To determine the mesenchymal differentiation
potential of candidate MS-MSLCs, we used a proven trilineage
differentiation test identical to that described previously
(12,31,32).
Briefly, we tested the capacity of candidate MS-MSLCs to
differentiate along adipogenic, osteogenic and chondrogenic
lineages. For adipogenic differentiation, MS-MSLCs were seeded in a
6-well plate at a density of 4×104 cells/cm2
in complete MSC medium. At confluence, cell differentiation was
induced with adipogenic differentiation medium from the adipogenic
differentiation BulletKit (Lonza Walkersville, Walkersville, MD,
USA). These cells were fed with fresh medium every 3–4 days for 3
weeks. In control experiments, cells were incubated for the same
period of time in complete MSC medium. On day 21, the cells were
washed in PBS (Mediatech) and fixed in 10% formalin (Fisher
Scientific, Fair Lawn, NJ, USA) for 1 h at room temperature. After
fixation, the cells were rinsed with deionized water several times,
after which of 60% isopropanol (Pharmco-AAPER, Brookfield, CT, USA)
was added and cells were allowed to sit for 5 min. Oil red O
solution (Sigma) was then added to each well. After 5 min, the
cells were rinsed with deionized water and briefly counter-stained
with hematoxylin (Sigma). For osteogenic differentiation, candidate
MS-MSLCs were plated at a density of 3×104
cells/cm2 in a 6-well plate. The next day, the medium
was replaced with osteogenic differentiation medium from the
osteogenic differentiation BulletKit (Lonza Walkersville). These
cells were fed with fresh medium every 3–4 days for 3 weeks. In
control experiments, cells were incubated for the same period of
time in complete MSC medium. On day 21, cell cultures were washed
twice with PBS (Mediatech) and fixed in 70% ice-cold ethanol
(Pharmco-AAPER) for 1 h, followed by washing with deionized water.
The cells were stained with 40 mM Alizarin Red (pH 4.2; Sigma) for
10 min at room temperature with rotation, followed by washing with
deionized water five times. For chondrogenic differentiation,
candidate MS-MSLCs were trypsinized and washed in serum-containing
medium. Aliquots of 2.5×105 cells suspended in 0.5 ml of
medium were placed in 15-ml conical polypropylene tubes (SPL,
Pocheon, Gyeonggi, Korea). The cells were then gently centrifuged
for 5 min at 150 x g and left at the bottom of the tubes, which
were placed in an incubator with caps loosened to permit gas
exchange. The cells formed small pellets that were cultured for 3
weeks in chondrogenic differentiation medium from the chondrogenic
differentiation BulletKit (Lonza Walkersville) supplemented with 20
μg/ml of transforming growth factor (TGF)-β3 (Ontogeny
Research Products, Cambridge, MA, USA). Every 3–4 days, the cells
were fed with fresh medium. In control experiments, the cells were
incubated for the same period of time in complete MSC medium. These
pellets were fixed in 10% formalin for 1 h at room temperature,
then embedded in paraffin sections and stained with toluidine blue
(Sigma) for proteoglycans and glycosaminoglycans.
Animal subjects
Four-to-eight-week-old male athymic nude mice
(Central Laboratory Animal Inc., Seoul, Korea) were used to assess
the tumorigenicity of candidate MS-MSLCs. Mice were housed in
micro-isolator cages under sterile conditions and observed for ≥1
week before study initiation to ensure proper health. Lighting,
temperature and humidity were controlled centrally. All
experimental procedures were approved by our Institutional Animal
Care and Use Committee. The body weights of mice were checked
daily. If body weight decreased by >15% compared with the
original body weight, mice were euthanized as proscribed by the
approved protocol. The brain was dissected and placed in formalin
for pathological studies.
Orthotopic meningioma xenografting of
candidate MS-MSLCs
Mice were anesthetized with a solution of Zoletil
(30 mg/kg; Virbac Korea, Seoul, Korea) and xylazine (10 mg/kg;
Bayer Korea, Seoul, Korea) delivered intraperitoneally. Candidate
MS-MSLCs were implanted into the right frontal lobe of nude mice
using a guide-screw system within the skull, as described
previously (34). Mice received
5×105 candidate MS-MSLCs via a Hamilton syringe (Dongwoo
Science Co., Seoul, Korea) inserted to a depth of 4.5 mm. Each
sample of candidate MSLCs was injected into three mice
simultaneously using a multiple microinfusion syringe pump (Harvard
Apparatus, Holliston, MA, USA) at a speed of 0.5 μl/min, as
previously described (11,12,19,34,35).
At least 180–200 days after injection, mouse brains were carefully
removed, sectioned, stained with hematoxylin and eosin (H&E)
and examined for tumors.
Meningioma tissue preparation and
immunofluorescence labeling
The possible location of MS-MSLCs in human
meningioma specimens was investigated using double
immunofluorescence labeling. Meningioma specimens were immediately
removed and post-fixed in 4% paraformaldehyde at 4°C overnight.
After dehydration with 30% sucrose in PBS, meningioma specimens
were frozen with OCT compound (Sakura Finetek USA. Inc., Torrance,
CA, USA) at −80°C. Frozen sections were processed for
immunofluorescence labeling using goat anti-human CD105 (1:100;
R&D Systems), rabbit anti-human CD31 (1:50, an endothelial cell
marker; Abcam, MA, USA) and rabbit anti-human NG2 antibodies
(1:100, a pericyte marker; Millipore, Danvers, MA, USA). Alexa
Fluor 488- and Alexa Fluor 555-conjugated goat anti-rabbit IgG
antibodies (1:2,000; Invitrogen, CA, USA) were used as secondary
antibodies. Samples were mounted in DAPI
(4′,6-diamidino-2-phenylindole)-containing Vectashield mounting
medium (H-1200; Sunil Technopia, Seongnam, Korea) to stain nuclei
and were examined under a fluorescence inverted microscope (IX71;
Olympus) equipped with DP Controller software (Olympus).
Statistical analyses
Data are expressed as means ± standard deviations.
Survival curves for MS-MSLC-implanted mice were obtained using the
Kaplan-Meier method. SPSS version 18.0KO software (SPSS Korea,
Seoul, Korea) was used for calculations.
Results
Step 1: selection of MS-MSLCs by
adherence to plastic
MS-MSLCs were obtained from a total of 20 meningioma
specimens (10 WHO grade I and 10 WHO grade II) and grown under MSC
culture conditions, as described previously (12,31,32).
Candidate MS-MSLCs with general properties of human BM-MSCs and
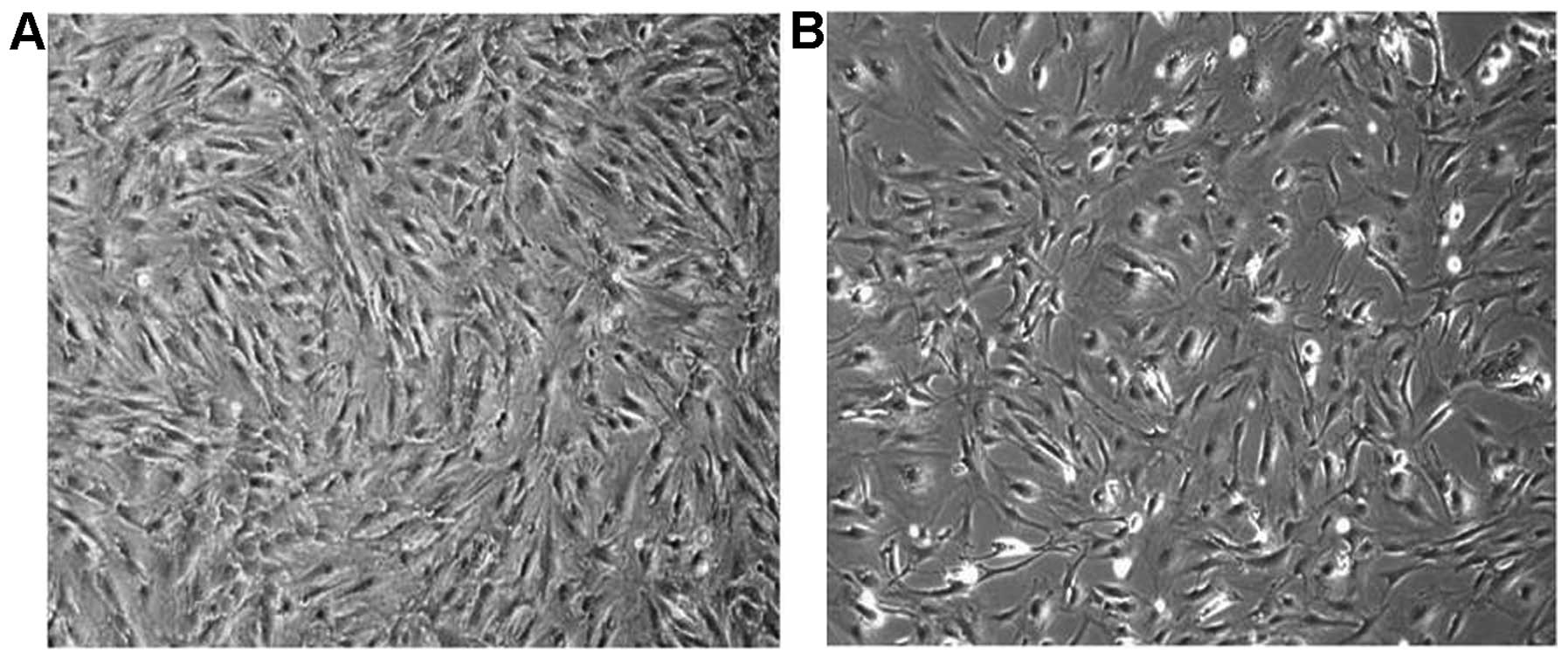
MSLCs, characterized by their spindle shape and ability to adhere
to plastic, were selected from WHO grade II (Fig. 1A) and grade I (Fig. 1B) meningiomas. Five of the ten WHO
grade II meningioma samples and 2 of the 10 WHO grade I meningioma
samples passed step 1 and were selected for characterization
(Table I). Although the proportion
of spindle-shaped, adherent cells in each of these selected
specimens was different, their morphology showed little difference
between WHO grade I and II.
 | Table I.Step 1: selection of candidate
MS-MSLCs based on adherence to plastic under MSC culture
conditions. |
Table I.
Step 1: selection of candidate
MS-MSLCs based on adherence to plastic under MSC culture
conditions.
| MS-MSLCs | Age | Sex | WHO grade | Pathology | Adherence to
plastic | Pass step 1 |
|---|
| MS-MSLC0519 | 75 | M | II | Atypical
meningioma | No | No |
| MS-MSLC0824 | 56 | F | II | Atypical
meningioma | No | No |
| MS-MSLC0831 | 70 | F | II | Atypical
meningioma | Yes | Yes |
| MS-MSLC0907 | 72 | F | II | Atypical
meningioma | No | No |
| MS-MSLC1013 | 77 | M | II | Atypical
meningioma | No | No |
| MS-MSLC1208 | 69 | M | II | Atypical
meningioma | Yes | Yes |
| MS-MSLC0525 | 52 | F | II | Atypical
meningioma | No | No |
| MS-MSLC0817 | 48 | F | II | Atypical
meningioma | Yes | Yes |
| MS-MSLC0802 | 55 | M | II | Atypical
meningioma | Yes | Yes |
| MS-MSLC1025 | 55 | F | II | Atypical
meningioma | Yes | Yes |
| MS-MSLC0614 | 37 | M | I | Transitional
meningioma | No | No |
| MS-MSLC0603 | 69 | F | I | Meningothelial
meningioma | No | No |
| MS-MSLC0622 | 30 | F | I | Meningothelial
meningioma | Yes | Yes |
| MS-MSLC0629 | 82 | F | I | Meningothelial
meningioma | No | No |
| MS-MSLC0223 | 26 | M | I | Meningothelial
meningioma | Yes | Yes |
| MS-MSLC0405 | 65 | M | I | Microcystic
meningioma | No | No |
| MS-MSLC0928 | 51 | F | I | Secretory
meningioma | No | No |
| MS-MSLC0608 | 46 | F | I | Meningothelial
meningioma | No | No |
| MS-MSLC0627 | 20 | F | I | Meningothelial
meningioma | No | No |
| MS-MSLC1112 | 37 | F | I | Fibrous
meningioma | No | No |
Step 2: selection of MS-MSLCs based on
surface antigen expression
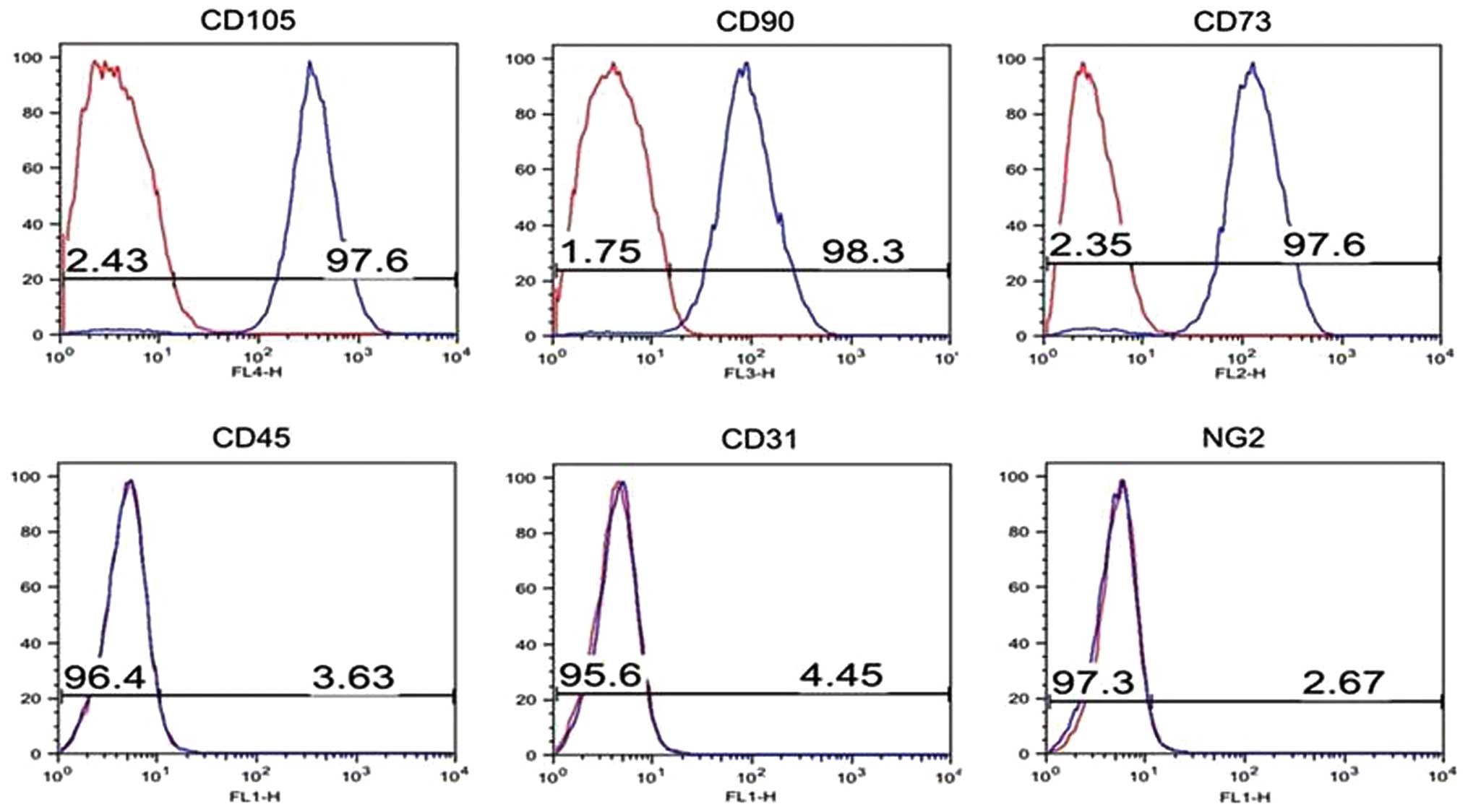
Flow cytometry analysis was used to assess surface
antigen expression in spindle-shaped cells that adhered to plastic
under MSC/MSLC culture conditions. Although there are no specific
pathognomonic markers for human BM-MSCs, it is generally agreed
that CD105, CD90 and CD73 are positive markers and CD45 is negative
marker for most MSLCs (12,32,33).
Using these criteria, we tested whether candidate cells are
MS-MSLCs (Fig. 2). Because MSLCs
from mice normal brains (19),
glioma xenografts (11) and Korean
glioma specimens (12) are found
around vessels, CD31, a marker of endothelial cells and NG2, a
marker of pericyte were additionally used to discriminate MS-MSLCs
and vessel-related cells. MS-MSLCs were negative for CD31 and NG2
(Fig. 2), as expected for these
non-endothelial, non-pericyte cells. Of the meningioma specimens
that passed step 1, three of five WHO grade II and one of two WHO
grade I specimens showed proper surface antigen expression
(Table II).
 | Table II.Step 2: selection of candidate
MS-MSLCs based on surface marker expression.a |
Table II.
Step 2: selection of candidate
MS-MSLCs based on surface marker expression.a
| MS-MSLCs | WHO grade | Pathology | CD105 (%) | CD90 (%) | CD73 (%) | CD45 (%) | CD31 (%) | NG2 (%) | Pass step 2 |
|---|
| MS-MSLC0831 | II | Atypical
meningioma | Failed to
subculture | No |
| MS-MSLC1208 | II | Atypical
meningioma | 95.97 | 2.10 | 98.50 | 1.13 | 1.24 | 5.94 | No |
| MS-MSLC0802 | II | Atypical
meningioma | 97.40 | 83.70 | 95.50 | 1.78 | 0.40 | 0.51 | Yes |
| MS-MSLC0817 | II | Atypical
meningioma | 92.10 | 11.10 | 91.10 | 5.80 | 0.68 | 0.34 | Yes |
| MS-MSLC1025 | II | Atypical
meningioma | 98.1 | 27.7 | 94.2 | 0.32 | 0.33 | 3.29 | Yes |
| MS-MSLC0223 | I | Meningothelial
meningioma | 97.60 | 98.30 | 97.60 | 3.63 | 4.45 | 2.97 | Yes |
| MS-MSLC0622 | I | Meningothelial
meningioma | 97.29 | 34.84 | 1.35 | 1.35 | 1.19 | 1.61 | No |
Step 3: selection of MS-MSLCs based on
mesenchymal differentiation
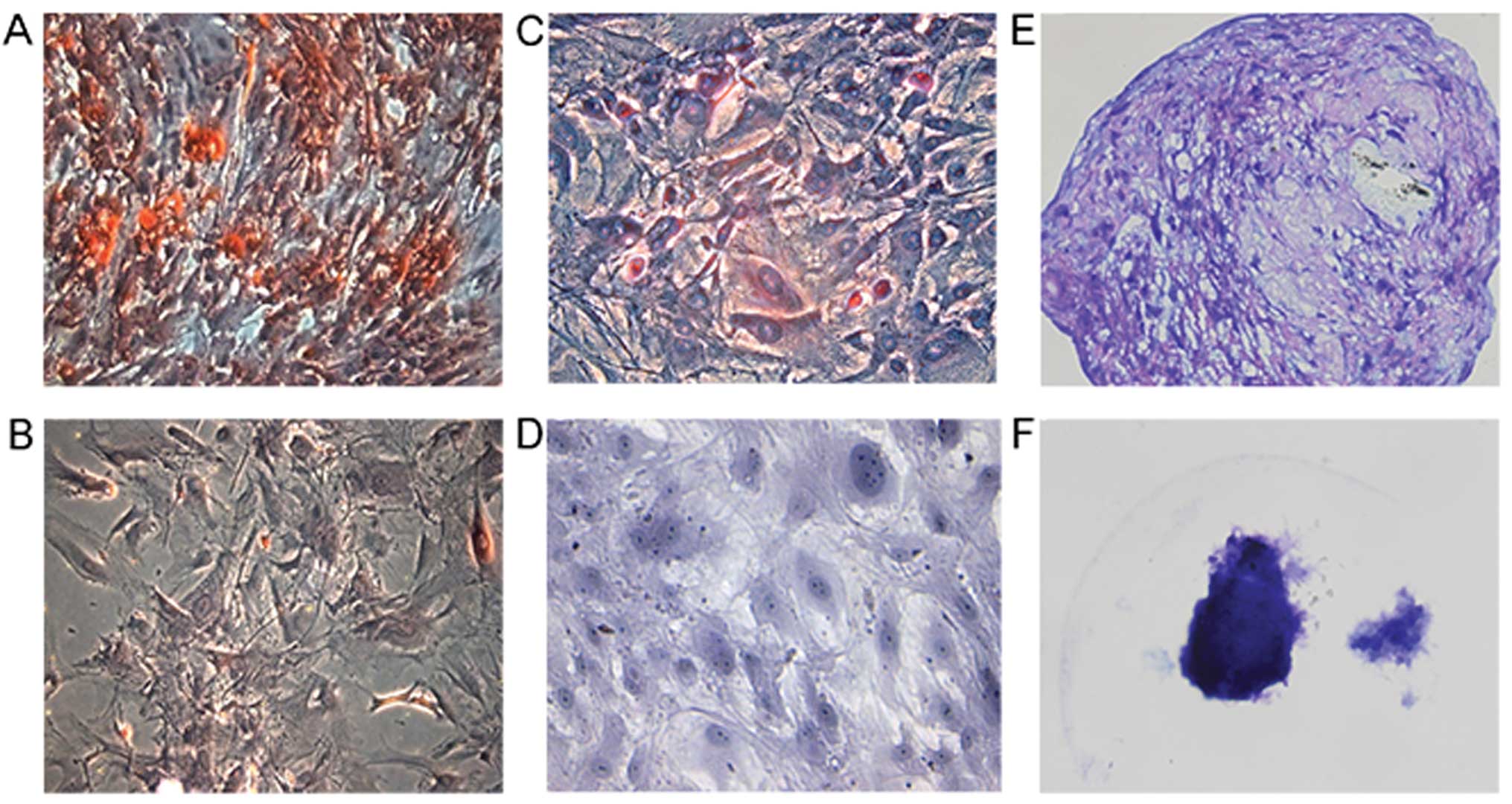
MSCs/MSLCs exhibit trilineage - osteocyte, adipocyte
and chondrocyte - differentiation capacity (11,12,19,33).
To validate the mesenchymal trilineage differentiation potential of
MS-MSLCs, we tested candidate cells that passed steps 1 and 2 for
their ability to differentiate into osteocytes, adipocytes and
chondrocytes when cultured in induction medium (Fig. 3A, C and E). Differentiation into
only two of the three cell types meant failure to pass step 3. No
trilineage differentiation was observed in control medium (Fig. 3B, D and F). Among the selected
candidate MS-MSLCs, only WHO grade II meningioma cells satisfied
the criterion of trilineage differentiation potential (Table III).
 | Table III.Step 3: selection of MS-MSLCs based
on in vitro mesenchymal differentiation.a |
Table III.
Step 3: selection of MS-MSLCs based
on in vitro mesenchymal differentiation.a
| MS-MSLCs | WHO grade | Pathology | Osteogenesis | Adipogenesis | Chondrogenesis | Pass step 3 |
|---|
| MS-MSLC0802 | II | Atypical
meningioma | Yes | Yes | Yes | Yes |
| MS-MSLC0817 | II | Atypical
meningioma | Yes | Yes | Yes | Yes |
| MS-MSLC1025 | II | Atypical
meningioma | Yes | Yes | Yes | Yes |
| MS-MSLC0223 | I | Meningothelial
meningioma | Yes | No | Yes | No |
Step 4: selection of MS-MSLCs based on in
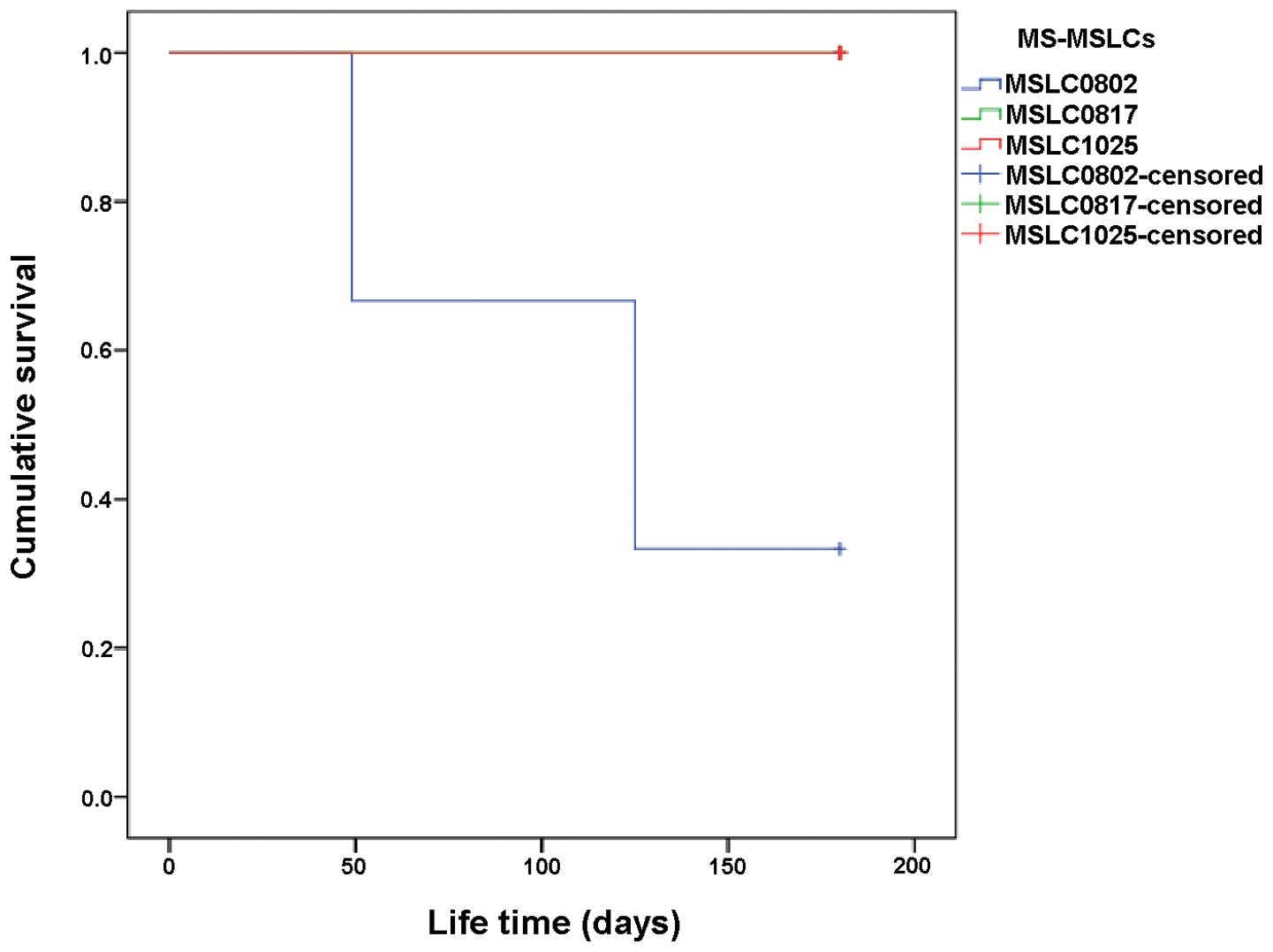
vivo non-tumorigenicity
Unlike CSCs, MSCs/MSLCs are not tumorigenic in
vivo. To satisfy this criterion, most mice intracranially
implanted with candidate MS-MSLCs that passed steps 1, 2 and 3
should survive for more than 6 months. Tests of the three candidate
MS-MSLCs from WHO grade II meningioma samples that passed steps 1,
2 and 3 showed that mice implanted with candidate MS-MSLCs from two
samples (MS-MSLC0817 and MS-MSLC1025) survived for >6 months
(Fig. 4), whereas those implanted
with the third sample (MS-MSLC0802) died ∼4 months later (Table IV). Notably, however, mice in the
group implanted with MS-MSLC0802 meningioma cells that failed to
survive >4 months died from infection and not because of a
tumor. Accordingly, two groups of MS-MSLCs (MS-MSLC0817 and
MS-MSLC1025) isolated from meningioma specimens passed step 4, the
final test for MS-MSLC selection, convincingly demonstrating no
tumorigenicity or general toxicity (Table V).
 | Table IV.Step 4: selection of candidate
MS-MSLCs based on tumorigenicity. |
Table IV.
Step 4: selection of candidate
MS-MSLCs based on tumorigenicity.
| MS-MSLCs | WHO grade | Pathology | Tumorigenesis | Pass step 4 |
|---|
| MS-MSLC0802a | II | Atypical
meningioma | No | No |
| MS-MSLC0817 | II | Atypical
meningioma | No | Yes |
| MS-MSLC1025 | II | Atypical
meningioma | No | Yes |
 | Table V.Final success rate for isolation of
MS-MSLCs according to selection step (1–4). |
Table V.
Final success rate for isolation of
MS-MSLCs according to selection step (1–4).
| Pathology | Step 1 Plastic
adherence % | Step 2 Surface
antigen expression % | Step 3 Mesenchymal
differentiation % | Step 4 No
tumorigenesis % | Final success rate
of MS-MSLCs isolation % |
|---|
| WHO grade II | 50 (5/10) | 60 (3/5) | 100 (3/3) | 66.7 (2/3) | 20 (2/10) |
| Atypical MNG | 50 (5/10) | 60 (3/5) | 100 (3/3) | 66.7 (2/3) | 20 (2/10) |
| WHO grade I | 20 (2/10) | 50 (1/2) | 0 (0/1) | 0 (0/1) | 0 (0/10) |
| Meningothelial
MNG | 60 (3/5) | 50 (1/2) | 0 (0/1) | 0 (0/1) | 0 (0/2) |
| Microcystic
MNG | 0 (0/1) | | | | 0 (0/1) |
| Secretory
MNG | 0 (0/1) | | | | 0 (0/1) |
Immunofluorescence detection of CD31, NG2
and CD105
The results of steps 1–4 corroborate the hypothesis
that MSLCs exist in meningioma specimens, although the question of
where MS-MSLCs are located remained. Previous studies of MSLCs in
normal mouse brains (19), mouse
glioma xenografts (11) and Korean
glioma specimens (12) have
suggested that these cells were located in a perivascular site. To
verify that MS-MSLCs might also be located in a perivascular niche,
we analyzed meningioma specimens for expression of the markers
CD105, CD31 and NG2 by immunofluorescence. CD105, a surface marker
present in most MSCs/MSLCs, was selected for establishing the
presence of MS-MSLCs. To determine whether CD105-positive cells
were near endothelial cells, we performed double-immunofluorescence
labeling for the endothelial cell markers, CD31. Histological
analyses suggested that some CD105-positive cells were closely
associated with CD31-positive cells (Fig. 5A). To determine whether
CD105-positive cells were associated with pericytes, we performed
double-immunofluorescence labeling for CD105 and the pericyte
marker NG2. Similar to the results obtained with CD105 and CD31
double-immunofluorescence labeling, some CD105-positive cells were
intimately associated with NG2-positive cells (Fig. 5B). Accordingly, we infer that some
CD105-positive candidate MS-MSLCs are located in the perivascular
niche (Fig. 5, arrows). However,
not all CD105-positive cells were found near vessels. These cells
may be niche-independent cells (Fig.
5, arrowheads).
Discussion
Considerable recent evidence supports the presence
of MSCs in various human tissues (19,36).
Other studies have also reported the existence of MSCs or MSLCs in
the stroma of brain and other tumors (6,19,37–39),
although little information about the function of these cells is
available. In the present study, we successfully isolated MS-MSLCs
from meningioma specimens with plastic adherence properties
(Fig. 1 and Table I) and a surface antigen profile
(Fig. 2 and Table II) similar to those of BM-MSCs. In
addition, these cells exhibited mesenchymal trilineage
differentiation capacity (Fig. 3
and Table III) and the absence of
tumorigenicity (Fig. 4 and
Table IV). We also found evidence
for localization of a subset of these MS-MSLCs to perivascular
areas.
Ultimately, MS-MSLCs that satisfied all four
criteria (adherence to plastic, surface antigen expression,
mesenchymal differentiation and non-tumorigenicity) were isolated
from 2 of 10 WHO grade II meningioma specimens (20%). In a previous
study, Korean GS-MSLCs (KGS-MSLCs) were isolated from 1 of 5 WHO
grade II Korean glioma specimens (20%), but not from WHO grade I
specimens (0/1) (12). Consistent
with this, both meningioma specimens that yielded MS-MSLCs in the
present study were WHO grade II (Table
V). Because meningiomas are mesenchymal tumors (28,29)
and gliomas are neuroepithelial in origin, we initially anticipated
that the rate of isolation of MSLCs from meningiomas would be
higher; however, this turned out not to be the case. Despite the
fact that meningiomas and gliomas are histologically different
tumors, MSLC isolation rates were similar (20%) and only WHO grade
II tumors yielded MSLCs that satisfied all criteria. Thus, although
sample sizes were small (e.g., only 1 WHO grade I and 5 grade II
gliomas in the previous study), the results of our study taken
together with the previous report (12) suggest that the frequency of MSLC
isolation depends on WHO grade rather than cancer type.
Malignant meningiomas are highly aggressive and
easily recur after surgical treatment (40), so understanding the mechanism of
meningioma recurrence is highly important. Studies have shown that
arachnoid cells, which share similar properties with meningioma
cells, are a significant factor in recurrence (41) demonstrating, for example, that
arachnoid membranes containing arachnoid cells and clusters of
cancer cells are closely related to meningioma recurrence (42). These studies suggest that cancer
cells near arachnoid cells and a perivascular site might follow the
mechanism by which arachnoid cells preferentially locate around
perivascular areas and penetrate into the brain (41,43).
The results of our double-immunofluorescence labeling for CD31, NG2
and CD105 might be consistent with localization of MS-MSLCs in a
vascular niche, possibly indicating that MSLCs follow a mechanism
similar to that of penetrating arachnoid cells, although our data
do not provide direct evidence for this, such a mechanism could be
a crucial determinant of meningioma recurrence. We are currently
following the progression-free survival of the two different
patient groups: those from whom MS-MSLCs could be isolated and
those from whom they could not. These follow-up observations could
show the prognostic value of MS-MSLCs.
Although the origin of meningioma is unclear, it is
believed that arachnoid cells are the most likely source (44); thus, most meningiomas occur near
cerebral meninges. To evaluate in vivo tumorigenicity, we
used an intracranial meningioma mouse model, implanting candidate
MS-MSLCs into the right frontal lobe of a nude mouse. Although the
use of this xenograft model might be questioned because cells were
intracranially injected, these cells were usually injected into the
subdural space near cerebral meninges, where meningiomas are
typically found. Because, in the intracranial xenograft model
system, tumors form within the brain, they might show some
differences in characteristics. However, others have tested the
tumorigenesis of WHO grade II meningioma using intracranial
injection mouse models (21).
Accordingly, we adopted this intracranial xenograft system
(21) to evaluate the in
vivo tumorigenicity of meningioma-derived MSLCs (Fig. 4).
CD105 (endoglin) is an endothelial cell protein that
binds TGF-β (45). CD105 is also
expressed on immature blood vessels, whereas CD31 is an endothelial
cell marker that is not expressed on immature vessels. For this
reason, CD105 can be used as a single marker to verify angiogenesis
(46,47). However, endothelial cells are not
the only cells that express CD105; BM-MSCs are also highly
CD105-positive. Hence, CD105 is frequently used as a marker for
MSCs/MSLCs and a tool for isolating MSLC populations from specimens
(12,48,49).
Although CD105 is used to screen for MSCs, there is no single
marker for these cells. Because of this, it is impossible to
confirm that CD105-positive cells are MSCs. Following the minimal
requirement for defining MSCs (33), we used the surface markers CD90,
CD73 and CD45 (12) to define
MSLCs (Fig. 2). To distinguish
MS-MSLCs from endothelial cells and pericytes, we used CD31, a
marker of endothelial cells and NG2, a marker of pericytes, as
negative surface markers (Table
II).
The results of double-immunofluorescence labeling
for CD31, NG2 and CD105 in this study showed that CD105-positive
cells were located in two different sites (Fig. 5). Some clusters of these cells were
situated near endothelial cells (Fig.
5A, arrows) and pericytes (Fig.
5B, arrows), whereas others were located inside the meningioma
stroma (Fig. 5, arrowheads). This
outcome indirectly suggests the possible location of MS-MSLCs as
the meningioma stroma and perivascular areas. Although our study is
the first to show the successful isolation and characterization of
MSLCs from meningioma specimens, there is no direct method to
definitively establish their location. Another question is the
uncertain origin of putative MS-MSLCs near blood vessels. These
cells could be innate meningioma stroma MSLCs or circulating MSLCs
derived from bone marrow. Resolving this question will require
further studies to validate the origin of cells situated near the
vascular niche.
Recent studies demonstrated the isolation and
characterization of meningioma stem-like cells (21,23).
The relationship between these so-called meningioma CSCs (mCSCs)
and the MS-MSLCs isolated from meningiomas and described in the
present study is not clear. One report on gliomas suggests a
relationship between gCSCs and GS-MSLCs (7). In that study, GS-MSLCs were proposed
to influence gCSCs and make gliomas more aggressive by promoting
angiogenesis (7). On the basis of
this relationship, we postulate that MS-MSLCs are related to mCSCs,
although further study will be required to verify this hypothesis.
Mesenchymal tumors share a molecular signature with MSCs (50), indicating a close relationship
between meningiomas and mesenchymal molecular signatures. This
suggests that the mesenchymal molecular features of meningiomas
might be derived from MSLCs in the meningioma stroma. The
intriguing possibility of a connection between the mesenchymal
molecular signatures of meningiomas and MS-MSLCs, which was not
directly addressed in the present study, is currently under
investigation in our laboratory.
According to the ‘seed and soil’ hypothesis
(8), CSCs are considered the seed
and the tumor microenvironment is considered the soil (9,10).
Within the tumor, CSCs are identified by virtue of their
self-renewal, differentiation and tumorigenicity in orthotopic
xenografts (3,13,14),
whereas other cells in the tumor microenvironment might be thought
of as elements that are significant for the biologic behavior of
CSCs (4,7). The seed and soil hypothesis is
crucially important for understanding the mechanism of metastasis
(8,9). According to our studies, MS-MSLCs
might be considered an important part of meningiomas and could be a
new cell source of the meningioma microenvironment. In addition,
these MS-MSLCs might be the key to unlocking the relationship
between mCSCs and meningioma stroma cells. Investigating the
biological relationship between MS-MSLCs and mCSCs in the context
of the seed and soil concept is a fertile avenue for future
research.
Acknowledgements
This research was supported by the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (NRF-2013R1A1A2006427) and a grant from the
National R&D Program for Cancer Control, Ministry for Health,
Welfare and Family Affairs, Republic of Korea (1020340).
References
|
1.
|
Fomchenko EI and Holland EC: Stem cells
and brain cancer. Exp Cell Res. 306:323–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Galderisi U, Cipollaro M and Giordano A:
Stem cells and brain cancer. Cell Death Differ. 13:5–11. 2006.
View Article : Google Scholar
|
|
3.
|
Kong BH, Park NR, Shim JK, et al:
Isolation of glioma cancer stem cells in relation to histological
grades in glioma specimens. Childs Nerv Syst. 29:217–229. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Xouri G and Christian S: Origin and
function of tumor stroma fibroblasts. Semin Cell Dev Biol.
21:40–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shin GY, Shim JK, Lee JH, et al: Changes
in the biological characteristics of glioma cancer stem cells after
serial in vivo subtransplantation. Childs Nerv Syst. 29:55–64.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kong BH, Shin HD, Kim SH, et al: Increased
in vivo angiogenic effect of glioma stromal mesenchymal
stem-like cells on glioma cancer stem cells from patients with
glioblastoma. Int J Oncol. 42:1754–1762. 2013.
|
|
8.
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 133:571–573. 1889.
View Article : Google Scholar
|
|
9.
|
Fidler IJ and Poste G: The ‘seed and soil’
hypothesis revisited. Lancet Oncol. 9:8082008.
|
|
10.
|
Mendoza M and Khanna C: Revisiting the
seed and soil in cancer metastasis. Int J Biochem Cell Biol.
41:1452–1462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kim SM, Kang SG, Park NR, et al: Presence
of glioma stroma mesenchymal stem cells in a murine orthotopic
glioma model. Childs Nerv Syst. 27:911–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kim YG, Jeon S, Sin GY, et al: Existence
of glioma stroma mesenchymal stemlike cells in Korean glioma
specimens. Childs Nerv Syst. 29:549–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
14.
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hoelzinger DB, Demuth T and Berens ME:
Autocrine factors that sustain glioma invasion and paracrine
biology in the brain microenvironment. J Natl Cancer Inst.
99:1583–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Oliver L, Olivier C, Marhuenda FB, Campone
M and Vallette FM: Hypoxia and the malignant glioma
microenvironment: regulation and implications for therapy. Curr Mol
Pharmacol. 2:263–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kanamori M, Kawaguchi T, Berger MS and
Pieper RO: Intracranial microenvironment reveals independent
opposing functions of host alphaVbeta3 expression on glioma growth
and angiogenesis. J Biol Chem. 281:37256–37264. 2006. View Article : Google Scholar
|
|
18.
|
Stewart PA, Farrell CL and Del Maestro RF:
The effect of cellular microenvironment on vessels in the brain.
Part 1: vessel structure in tumour, peritumour and brain from
humans with malignant glioma. Int J Radiat Biol. 60:125–130. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kang SG, Shinojima N, Hossain A, et al:
Isolation and perivascular localization of mesenchymal stem cells
from mouse brain. Neurosurgery. 67:711–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lee CH, Jung KW, Yoo H, Park S and Lee SH:
Epidemiology of primary brain and central nervous system tumors in
Korea. J Korean Neurosurg Soc. 48:145–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hueng DY, Sytwu HK, Huang SM, Chang C and
Ma HI: Isolation and characterization of tumor stem-like cells from
human meningiomas. J Neurooncol. 104:45–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Rath P, Miller DC, Litofsky NS, et al:
Isolation and characterization of a population of stem-like
progenitor cells from an atypical meningioma. Exp Mol Pathol.
90:179–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hu D, Wang X, Mao Y and Zhou L:
Identification of CD105 (endoglin)-positive stem-like cells in
rhabdoid meningioma. J Neurooncol. 106:505–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Mosnier JF, Perret AG, Scoazec JY and
Brunon J: Expression of beta2 integrins and macrophage-associated
antigens in meningeal tumours. Virchows Arch. 436:131–137. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kimura Y, Matsumae M and Tsutsumi Y:
Pericellular deposition of basement membrane material in myxoid
meningioma: immunohistochemical evidence for unbalanced production
of type IV collagen and laminin. Pathol Int. 48:53–57. 1998.
View Article : Google Scholar
|
|
26.
|
Shamah SM, Alberta JA, Giannobile WV, et
al: Detection of activated platelet-derived growth factor receptors
in human meningioma. Cancer Res. 57:4141–4147. 1997.PubMed/NCBI
|
|
27.
|
Nystrom SH: Fine structure of tumour
stroma and blood vessel stroma in human supratentorial menigioma.
Nature. 194:587–588. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Majumdar K, Mandal S, Thakkar R, Saran RK
and Srivastava AK: Meningeal osteochondroma simulating meningioma
with metaplastic change: a rare golf-ball-like lesion of
non-meningothelial mesenchymal origin. Brain Tumor Pathol. Mar
2–2013.(Epub ahead of print).
|
|
29.
|
Celebre A, Wu MY, Danielson B, et al:
Anaplastic meningioma with extensive single-cell infiltration: a
potential role for epithelial-mesenchymal transformation in the
progression of a meningothelial tumour? Histopathology.
62:1111–1114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Mareschi K, Biasin E, Piacibello W,
Aglietta M, Madon E and Fagioli F: Isolation of human mesenchymal
stem cells: bone marrow versus umbilical cord blood. Haematologica.
86:1099–1100. 2001.PubMed/NCBI
|
|
32.
|
Lennon DP and Caplan AI: Isolation of
human marrow-derived mesenchymal stem cells. Exp Hematol.
34:1604–1605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
34.
|
Lal S, Lacroix M, Tofilon P, Fuller GN,
Sawaya R and Lang FF: An implantable guide-screw system for brain
tumor studies in small animals. J Neurosurg. 92:326–333. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Nakamizo A, Marini F, Amano T, et al:
Human bone marrow-derived mesenchymal stem cells in the treatment
of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|
|
36.
|
da Silva Meirelles L, Chagastelles PC and
Nardi NB: Mesenchymal stem cells reside in virtually all post-natal
organs and tissues. J Cell Sci. 119:2204–2213. 2006.PubMed/NCBI
|
|
37.
|
El-Haibi CP and Karnoub AE: Mesenchymal
stem cells in the pathogenesis and therapy of breast cancer. J
Mammary Gland Biol Neoplasia. 15:399–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Hall B, Andreeff M and Marini F: The
participation of mesenchymal stem cells in tumor stroma formation
and their application as targeted-gene delivery vehicles. Handb Exp
Pharmacol. 180:263–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Feigin I: Mixed mesenchymal tumors:
meningioma and nerve sheath tumor. J Neuropathol Exp Neurol.
37:459–470. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Ng HK, Tse CC and Lo ST: Meningiomas and
arachnoid cells: an immunohistochemical study of epithelial
markers. Pathology. 19:253–257. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Kamitani H, Masuzawa H, Kanazawa I and
Kubo T: Recurrence of convexity meningiomas: tumor cells in the
arachnoid membrane. Surg Neurol. 56:228–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Lopes CA and Mair WG: Tubular structures
in arachnoid cells. Acta Neuropathol. 27:363–368. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Ohnishi Y, Iwatsuki K, Morii E, et al:
Histopathological study of spinal meningioma originating from the
arachnoid villi. Brain Tumor Pathol. 28:77–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Cheifetz S, Bellon T, Cales C, et al:
Endoglin is a component of the transforming growth factor-beta
receptor system in human endothelial cells. J Biol Chem.
267:19027–19030. 1992.PubMed/NCBI
|
|
46.
|
Behrem S, Zarkovic K, Eskinja N and Jonjic
N: Endoglin is a better marker than CD31 in evaluation of
angiogenesis in glioblastoma. Croat Med J. 46:417–422.
2005.PubMed/NCBI
|
|
47.
|
Barresi V and Barresi G: Endoglin: a
marker of neoplasias or rather of neo-angiogenesis? Head Neck.
32:970–971. 2010.PubMed/NCBI
|
|
48.
|
Yen BL, Huang HI, Chien CC, et al:
Isolation of multipotent cells from human term placenta. Stem
Cells. 23:3–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Crisan M, Chen CW, Corselli M, Andriolo G,
Lazzari L and Peault B: Perivascular multipotent progenitor cells
in human organs. Ann NY Acad Sci. 1176:118–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Galie M, Konstantinidou G, Peroni D, et
al: Mesenchymal stem cells share molecular signature with
mesenchymal tumor cells and favor early tumor growth in syngeneic
mice. Oncogene. 27:2542–2551. 2008. View Article : Google Scholar : PubMed/NCBI
|