Introduction
Angiogenesis, a co-operative process with vascular
endothelial cells (ECs) and pericytes, is essential for tumour
growth and expansion because the blood vessels supply malignant
cells with sufficient oxygen and nutrition. Interruption of this
process, therefore, could be an effective strategy for preventing
malignant tumours. The putative angiogenic factor vascular
endothelial growth factor (VEGF) is the best known to be involved
in the growth and development of colorectal cancer (CRC) and its
hepatic metastases (1–3). Anti-VEGF monoclonal antibody,
bevacizumab (BV) is already clinically feasible in combination with
conventional chemotherapies as the first anti-angiogenic drug that
is proven to bring better prognosis of patients with colorectal
cancer (CRC) in a phase III randomized controlled trial (4).
Besides VEGF, other important endothelial growth
factors are angiopoietins (Angs), which are ligands for the
endothelium-specific tyrosine kinase receptor Tie2 (5). Angs play a role in normal vascular
development and in embryonic angiogenesis. Among 4 subtypes (Ang1,
2, 3 and 4), the best-characterized are Ang1 and its natural
antagonist, Ang2. Ang1 is widely expressed in normal adult tissues,
while Ang2 is expressed primarily at sites of vascular remodeling,
such as the ovaries, uterus and placenta (5). Angiogenesis requires migration and
remodeling of ECs derived from pre-existing blood vessels and
regulation of the perivascular microenvironment. Thus, Ang2
destabilizes pre-existing vessels by weakening interactions between
ECs and periendothelial supporting cells (PESCs) (3), also called vascular pericytes. Ang1
subsequently acts, via the Tie2 receptor, to remodel the primitive
vessels and to help maintain and stabilize mature vessels (6). Recent preclinical studies have shown
that angiopoietin 2 may be another promising target against colon
cancer through inhibition of either Ang2 (7–9) or
Ang2+Ang1 (9,10).
There are two aspects of angiogenesis, i.e. growth
and differentiation of the vessels. Although vascular growth (EC
proliferation) has been examined by estimating vessel count and
vessel size so far, little is known about vascular differentiation,
i.e., vacuole or lumen formation, especially in vivo. In the
present study, we investigated the effects of Ang2 on vascular
growth and differentiation, in vitro and in vivo. We
first examined in vitro effects of Ang2 inhibition or
addition of Ang2 using HUVECs. Secondly we examined growth and
differentiation of tumour-associated vessels when xenografts
derived from a colon cancer were treated by the Ang2 inhibitor
L1-10. Effect of VEGF inhibition was also examined to discriminate
Ang2 specific action on the tumour-associated vessels. Our data
provide a novel aspect that Ang2 may play an essential role in
in vivo vascular differentiation, and therefore support a
rationale for Ang2-targeted therapy against colon cancer.
Materials and methods
Cell lines
Human umbilical vascular endothelial cell (HUVEC)
was purchased from Takara Bio Co. (Shiga, Japan). Human colon
cancer cells (HCT116, DLD1, SW480) were purchased from the American
Type Culture Collection (Manassas, VA). KM12SM (11) was a kind gift from Professor T.
Minamoto (Cancer Research Institute, Kanazawa University, Kanazawa,
Japan). Colon cancer cells were grown in DMEM supplemented with 10%
fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml
streptomycin in 5% CO2 at 37°C. HUVEC were grown on
MCDB131 culture medium (Chlorella Inc., Tokyo, Japan) supplemented
with 10% FBS, antibiotics, and 10 ng/ml basic fibroblast growth
factor.
Attached collagen gel culture
HUVECs (2×106 cells/ml) were cultured on
0.03% type I collagen (Cellmatrix I-A, Nitta Gelatin Inc., Osaka,
Japan) coated dishes. Collagen solution (0.3%) was diluted by
Medium 199 (Life Technologies, Carlsbad, CA) and reconstruction
buffer (50 mM NaOH, 260 mM NaHCO3, 200 mM HEPES,
according to the Nitta Gelatin manual). After collagen coating,
cell suspension was seeded, then medium containing appropriate
concentration of reagent such as VEGF, Ang2 and L1-10 was added,
and 24 h later, same volume of PBS, 1/25 volume of Collagenase N-2
(Nitta Gelatin Inc.) solution were added into the well, incubated
at 37°C with mild shaking. Suspension was spun down, pellet was
resuspended with same volume of PBS and 1/50 volume of Collagenase
N-2. After 10 min incubation at 37°C, pellet was lysed and
supernatant was used for western blot analysis (12).
Collagen gel matrix culture
In vitro formation of tubular structures by
HUVEC was examined using collagen gel matrix culture. Collagen gel
(0.06%) layer was made (base layer). Collagen gel (0.06%) suspended
with HUVECs was added onto base layer, and immediately polymerized
at 37°C. Medium containing appropriate concentration of reagent
such as VEGF, Ang2 or L1-10 was added. Twenty-four or 48 h later,
HUVECs were harvested as mentioned above or observed under an
inverted microscope. Cell concentration of HUVECs for western blot
analysis and morphogenesis were 1×106 cells/ml and
3×106 cells/ml, respectively.
Reagents and antibodies
Human recombinant VEGF and mouse IgG was obtained
from IBL Co. Ltd. (Gunma, Japan). Ang2 was purchased from (R&D
Systems, Minneapolis, MN). The following antibodies were used at
appropriate concentrations as recommended by the manufacturers:
antibodies for angiopoietin 2 (C-19, Santa Cruz Biotechnology,
Santa Cruz, CA), VEGF (A20, Santa Cruz Biotechnology), Tie2 (H-176
for immunoprecipitation, C-20 for western blot analysis, Santa Cruz
Biotechnology), phosphorylated Tie2 (Tyr992, #4221, Cell Signaling
Technology, Danvers, MA), actin (Sigma-Aldrich, St. Louis, MO),
Rac1 (Cytoskeleton, Denver, CO), CDC42 (BD Biosciences, San Jose,
CA), CD31 and α smooth muscle actin (Dako, Glostrup, Denmark).
L1-10
L1-10, an Ang2 neutralizing peptibody
(genetically-engineered peptide-Fc fusion protein) was kindly
donated by Amgen Inc. (Seattle, WA). L1-10 is a specific inhibitor
of angiopoietin-2, and inhibits interactions between Tie2 in
endothelial cells and human or mouse angiopoietin-2 (7,13,14).
Binding activity of L1-10 to Ang2 was
measured by ELISA
Recombinant human angiopoietin-2 (R&D Systems)
was immobilized on a plate. After blocking with 1% BSA, 1 pM
recombinant human Tie2/Fc Chimera (R&D Systems) was added, from
500 to 0.02 nM of L1-10 and recombinant human IgG1 Fc (R&D
Systems). Molecular weight of L1-10 was assumed as 62.5 kDa and
that of IgG Fc was 26.6 kDa. Anti-Tie2 monoclonal antibody (BD
Biosciences) at 0.25 μg/ml was added. After washing, 0.05
μg/ml anti-mouse IgG (Goat IgG) Fab’ conjugated with HRP
(IBL Co. Ltd.) was added. Color development was done by incubating
with tetramethyl benzi-dine solution for 30 min. Absorbance at 450
nm was measured by microplate reader.
Western blot analysis
Western blot analysis was performed as we described
previously (12). Briefly, the
protein samples (25 μg) were separated by 10 or 12.5% PAGE
followed by electroblotting onto a polyvinylidene difluoride (PVDF)
membrane. The membrane was incubated with the primary antibodies at
the appropriate concentrations (1:100 for Ang2, 1:200 for VEGF and
Tie2, 1:250 for CDC42 and 1:1,000 for Rac1 actin and phosphorylated
Tie2). The protein bands were detected using the Amersham enhanced
chemiluminescence detection system (GE Healthcare, Buckinghamshire,
UK).
To detect phosphorylated Tie2, lysates of HUVEC were
immunoprecipitated with an anti-Tie2 antibody (H-176, Santa Cruz
Biotechnology). Immunocomplexes were recovered on Protein
A-Sepharose (GE Healthcare) and separated by SDS-PAGE, transferred
to blotting membrane as described above, then probed with
anti-phosphorylated Tie2 antibody (#4221, Cell Signaling
Technology).
Measurement of Ang2 and VEGF secretion in
culture medium
Each cell line was cultured until about 70%
confluence in DMEM supplemented with FBS. The medium was then
replaced with new medium without FBS, collected 24 h later, and
stored at −80°C. Ang2 and VEGF levels were analyzed using the
QuantikineHuman Angiopoietin-2 immunoassay kit (R&D Systems)
and the Human VEGF assay kit (IBL Co. Ltd.), respectively.
Animals
Female 4-week-old athymic nude mice were purchased
from Nihon CREA Inc. (Tokyo, Japan) and were housed under
pathogen-free conditions in microisolator cages with irradiated
rodent chow and water available ad libitum. The experimental
protocol was approved by the Ethics Review Committee for Animal
Experimentation of Osaka University School of Medicine.
Subcutaneous xenograft model
The most actively secreting VEGF and Ang2 cell line
was KM12SM. KM12SM cells at 80–90% confluence was used in
experiments. A total of 1×106 cells (5×106
cells/0.1 ml DMEM without FBS) was subcutaneously inoculated in the
right flank of each mouse. The doses of each drug were based on the
results of preliminary experiments. Mice were randomly assigned to
the groups.
Single agent treatment: L1-10 or anti-VEGF
antibody treatment was started immediately after inoculation.
There were four mice in each group. L1-10 was administered
subcutaneously into the left flank skin at 2 mg/kg, 5 mg/kg every
two days. Anti-VEGF antibody (200 μg, IBL Co. Ltd.), was
administered intraperitoneally every three days. Control groups for
each drug were administered mouse immunoglobulin (IgG) in the same
manner as the experimental group. Treatment was continued for 18
days. Mice were sacrificed at day 20, and tumours were harvested
for histological examinations.
Combination treatment with early administration:
L1-10 and anti-VEGF antibody injections were initiated immediately
after inoculation. There were five mice in each group.
Anti-VEGF antibody (200 μg) was administered
intraperitoneally every three days. Combination group was
administered 5 mg/kg of L1-10 subcutaneously every two days and 200
μg of anti-VEGF antibody was administered intraperitoneally
every three days. Control group was administered 200 μg of
mouse IgG intraperitoneally every three days. Treatment was
continued to day 18. Mice were sacrificed at day 20, and tumours
were harvested for histological examinations.
Combination treatment with late administration:
L1-10 and anti-VEGF antibody treatment were initiated 5 days after
inoculation. There were four mice in each group. Dose was 10
mg/kg for L1-10, 150 μg for anti-VEGF antibody, 150
μg for IgG, and combination (L1-10 and anti-VEGF antibody)
treatment was applied. Treatment was continued to day 31. At day 34
mice were sacrificed.
After inoculation of KM12SM cells into nude mice,
control IgG, anti-VEGF antibody and L1-10 were administered as
summarized in Table I.
 | Table I.Administration schedules of
combination treatment. |
Table I.
Administration schedules of
combination treatment.
| Group | Drug | Route | Interval
(days) | Dose
|
|---|
| Early | Late |
|---|
| Control | IgG | i.p. | 3 | 200 μg | 150 μg |
| VEGF | Anti-VEGF
antibody | i.p. | 3 | 200 μg | 150 μg |
| Combination | Anti-VEGF
antibody | i.p. | 3 | 200 μg | 150 μg |
| L1-10 | s.c. | 2 | 5 mg/kg | 10 mg/kg |
Evaluation of antitumour activity
Tumour size and body weight were measured every two
days. Tumour size was measured by an electronic caliper. Tumour
volume was calculated according to the following formula: length
(mm) × width2 (mm)/2. Mice were sacrificed at the final
day of experiment. Gross autopsy findings were noted.
Immunohistochemistry
Immunostaining was done as described previously
(3). Briefly, after
deparaffinization, heat antigen retrieval was done in 10 mM citrate
buffer (pH 6.0) at 95°C for 40 min. The slides were then processed
for immunohistochemistry using the Vectastain Elite avidin-biotin
complex kit (Vector Laboratories, Burlingame, CA). Primary
antibodies were applied to sections at a dilution of 1:750 for CD31
and incubated overnight at 4°C. For the negative control,
non-immunized immunoglobulin G (Vector Laboratories) was used as a
substitute for the primary antibody.
Double-staining of endothelial cells and pericytes
was performed with anti-CD31 antibody and anti-α-SMA antibody,
respectively. First, CD31 staining which yields a brown color was
performed. After removal of the CD31 antibody by thorough washing
in 0.1 M glycine solution (pH 2.2) for 1 h, mouse monoclonal
anti-human SMA antibody at a dilution of 1:200 was applied to the
section for 2 h at room temperature. This step was followed by
incubation with anti-mouse secondary antibody conjugated with a
dextran backbone containing alkaline phosphatase (EnVision AP;
Dako) for 30 min. Color development (deep pink) based on alkaline
phosphatase activity was achieved using fuchsin solution.
Image analysis
CD31-stained samples were used for image analyses.
Outer and inner contours of vessels at 100-times magnification in
one microscopic field were measured with WinROOF program Ver.5.5.0
(Mitani Corporation, Fukui, Japan). Outer contours were expressed
as surface area of vessel and inner contours were expressed as
surface area of lumen.
Statistical analyses
Data are expressed as mean ± SD. Statistical
analysis was performed using the StatView J-4.5 program (Abacus
Concepts Inc., Berkeley, CA). The mean tumour volume of each
treatment group was compared by Student’s t-test. A p-value
<0.05 was considered statistically significant.
Results
Ang2 and VEGF expression in colon cancer
cells
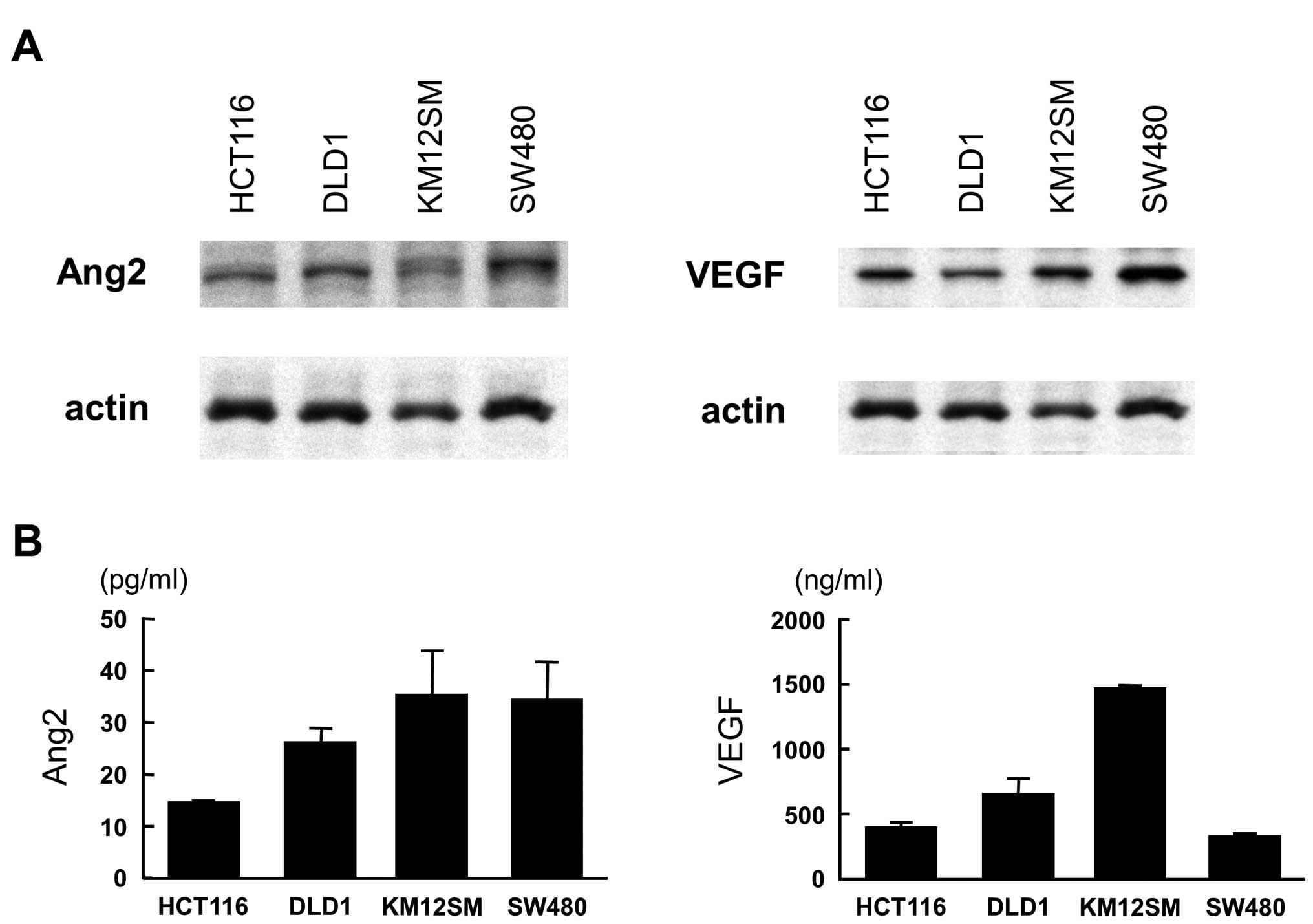
Western blot analysis showed that HCT116, DLD1,
KM12SM and SW480 colon cancer cell lines exclusively displayed
intense expression of both Ang2 and VEGF (Fig. 1A). ELISA showed various levels of
Ang2 and VEGF in the culture medium and KM12SM was found to release
high level of both Ang2 and VEGF (Fig.
1B).
Inhibition of binding affinity between
Ang2 and Tie2 by L1-10 peptibody
In vitro immunoreaction test indicated that
the L1-10 peptibody (L1-10) blocked the binding of Ang2 and Tie2 at
more than 0.1 nM in a dose-dependent manner, while addition of
human IgG1 Fc at various concentrations did not affect Ang2-Tie2
binding affinity (Fig. 2A). To
examine the activation status of the Tie2 receptor, phosphorylation
on Tyr992 of the Tie2 receptor was examined using the HUVEC with
early exposure (15 min) by Ang2 or L1-10. Although Tie2 expression
did not change, Ang2 inhibited phosphorylation of Tie2 receptor at
200 ng/ml. This dephosphorylation by Ang2 was abolished with
addition of L1-10 at 30 ng/ml. (Fig.
2B).
Effects of VEGF, Ang2 and L1-10 on growth
and tube formation of HUVECs
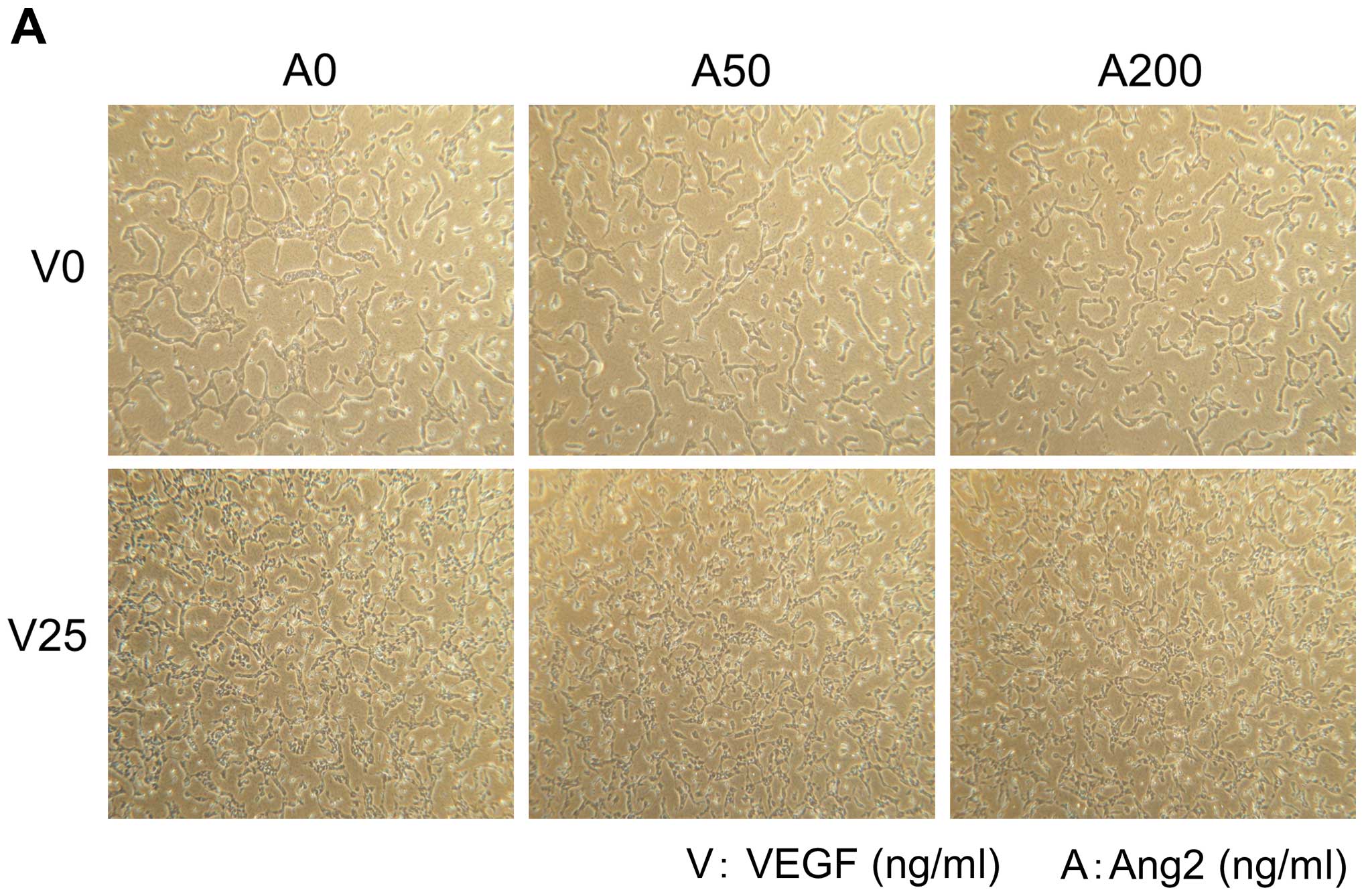
Addition of Ang2 or L1-10 did not enhance cell
proliferation and tube formation of HUVECs (Fig. 3A and B, upper panels). On the other
hand, addition of VEGF at 25 ng/ml enhanced growth and it resulted
in increased number of tube formation of HUVECs (Fig. 3A and B, lower panels).
We then examined protein expression of Rac1 and
CDC42 since these molecules are reportedly shown to be involved in
process of tube formation of HUVECs (15,16).
As shown in Fig. 3C, VEGF enhanced
expression of both the Rac1 and the CDC42 proteins, while Ang2 at
various concentrations did not affect the expression of the two
proteins (Fig. 3C).
Ang2 inhibition by L1-10 treatment
reduced tumour formation in nude mice
Subcutaneous injection of L1-10 (at 2 mg/kg or 5
mg/kg) into different sites of KM12SM xenografts inhibited tumour
formation dose-dependently (Fig.
4A). In the 5 mg/kg group, tumour formation was significantly
inhibited from day 10 compared to that of control group. In the 2
mg/kg group, tumour formation was inhibited in later phase of
experiment period. Histopathological examination of xenografts
revealed that when compared to control IgG treatment (Fig. 4Ba), tumour vessels in L1-10 treated
mice extended like a pine needle and lumen formation was scarcely
noted (Fig. 4Bb). Double staining
for CD31 and α-SMA showed that vascular endothelial cells were
tightly covered with pericytes in L1-10 treated group, whereas only
partial recruitment of pericytes was found in the control group. In
treatment with VEGF neutralizing antibody, tumour vessels decreased
and endothelial cells were relatively covered with pericytes
(Fig. 4Bc).
 | Figure 4.Growth curve of KM12SM xenograft
model. (A) Subcutaneous injection of L1-10 (at 2 or 5 mg/kg) into
different sites of KM12SM xenografts inhibited tumour formation in
a dose-dependent manner. (B) Histopathological examination of
xenografts (a) control IgG treatment, (b) L1-10 treatment, (c)
treatment with anti-VEGF neutralizing antibody. Anti-VEGF antibody
was administered into peritoneal cavity at 200 μg on days 0,
3, 6, 9, 12, 15 and 18. Mice were sacrificed on day 20. Double
staining for CD31 (brown) and α-SMA (pink) was performed to label
vascular endothelial cells and vascular pericytes, respectively.
Magnifications (left panel, right panel): (a) ×100, ×400; (b) ×100,
×200; and (c) ×200, ×400. |
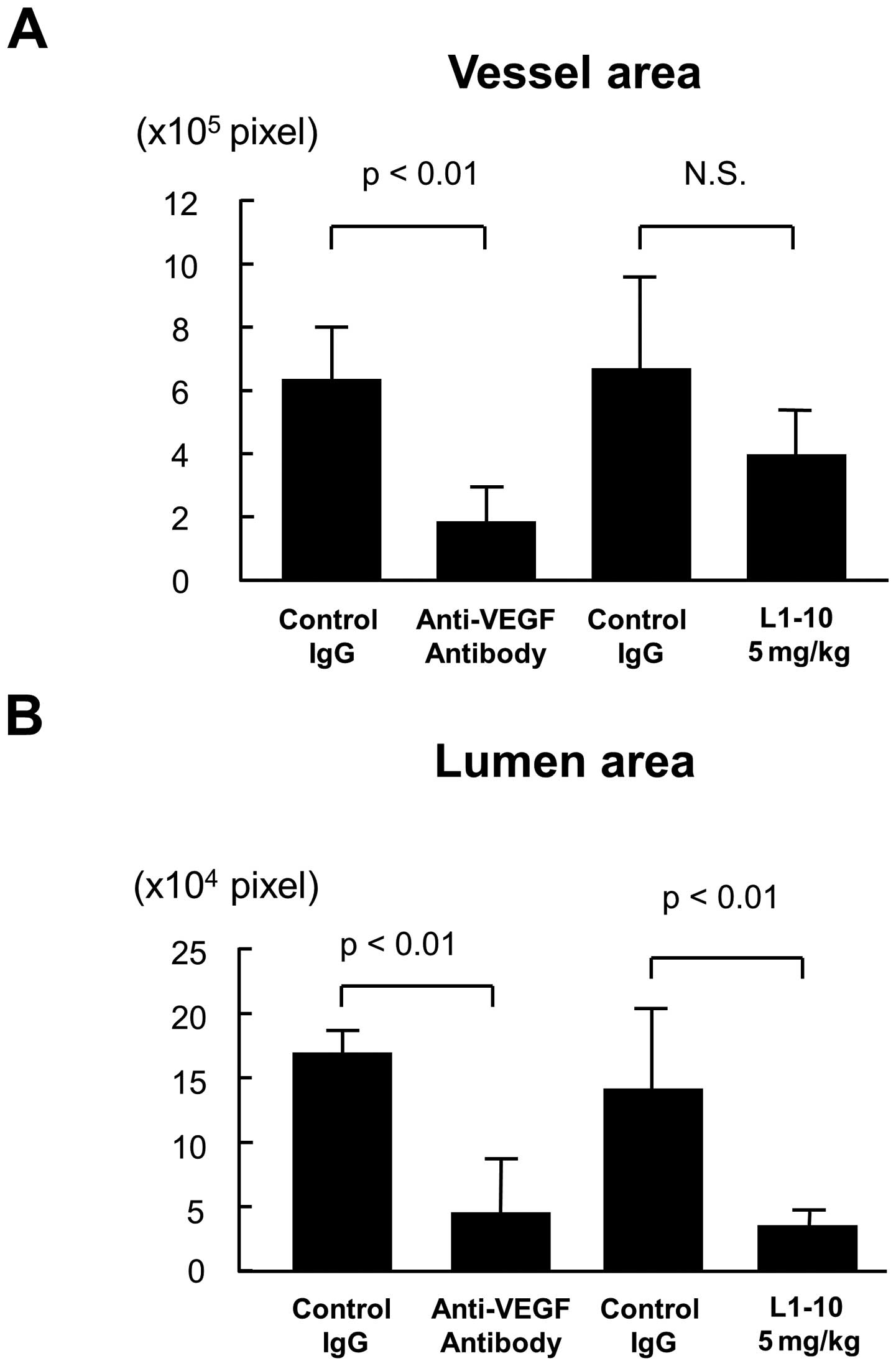
Image analysis for lumen formation
To evaluate lumen formation in each treatment, the
contour of vessel or lumen was traced using the image analysis
software, and the vessel area and the lumen area was estimated as
described in Materials and methods. Treatment with anti-VEGF
antibody significantly decreased both vessel area and lumen area
(p<0.01 for both, Fig. 5A and
B). On the other hand, L1-10 treatment at 5 mg/kg did not
decrease vessel area (Fig. 5A),
but it significantly decreased the lumen area (p<0.01, Fig. 5B). When the ratio of lumen area to
vessel area was calculated, there was a significant decrease in
L1-10 treatment group (p<0.01, Fig.
5C).
Combination treatment
Since the histopathological analysis indicated that
inhibition of either Ang2 or VEGF showed different anti-vascular
effect, we examined whether the two treatments would produce
enhanced tumour inhibitory effects. After inoculation of KM12SM
cells into nude mice, control IgG, anti-VEGF antibody, and L1-10
were administered as summarized in Table I.
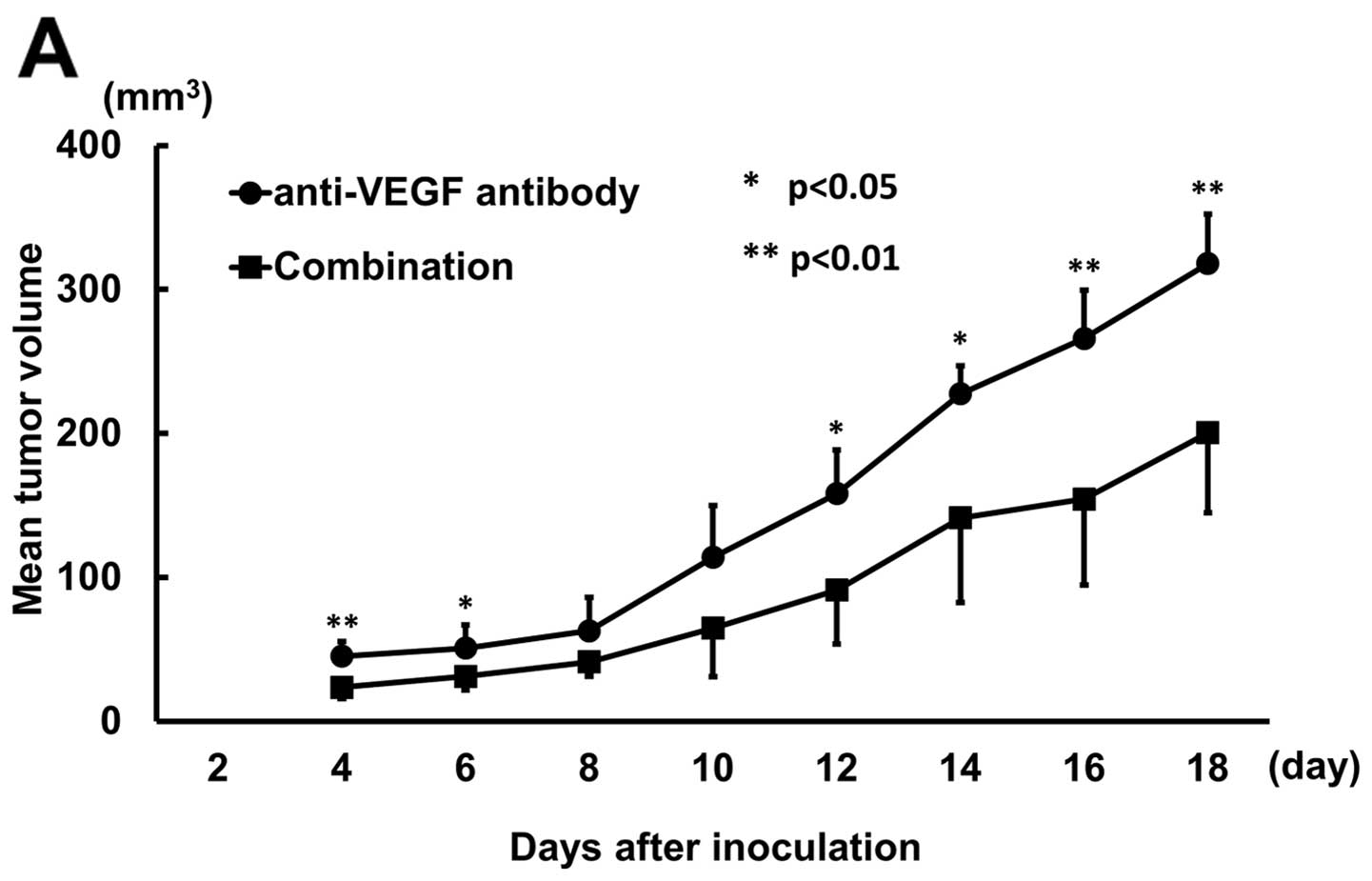
Combination treatment with anti-VEGF and L1-10
administered from the day of inoculation significantly decreased
tumour volume compared to anti-VEGF treatment except days 8 and 10
(Fig. 6A). When treatment started
5 days after inoculation, combination treatment showed no
significant difference compared to anti-VEGF treatment (Fig. 6B).
Discussion
In this study, we found Ang2 inhibition exerts a
superb efficacy especially in vivo. Histological analysis on
xenografts planted in nude mice suggests that Ang2 inhibition could
have disturbed in vivo vascular differentiation, i.e., lumen
formation. There are two important aspects on tumour angiogenesis,
that is, growth of vascular endothelial cells and vascular
differentiation. Compared to regulation of vascular endothelial
cell growth, the underlying mechanism on vascular differentiation
remains largely unknown. About three decades ago, Folkman and
Haudenschild observed vacuoles that penetrate from one endothelial
cell to another one by in vitro system (17). Subsequent in vitro
investigation gradually revealed that pinocytosis occurs via
interaction of integrin-extracellular matrix through CDC42 or
Rac1-dependent manner (18,19).
It was also demonstrated that several vacuoles, generating at 2–4 h
in a single HUVEC, undergo intra-cellular fusion, and later at
24–48 h HUVECs make assembly body, resulting in lumen formation,
raising VE cadherin as a molecular basis (20). By contrast, in vivo lumen
formation has not been assessed for a long time because direct
observation is rather difficult on the process of lumen formation
that occurs profoundly in the animal body. In vivo tube
formation of the vascular endothelial cells was reported for the
first time in 2006, through observation of zebrafish by two-photon
imaging system (21).
In this study, we employed L1-10 peptibody, the Ang2
selective inhibitor that showed 1,000-fold inhibitory selectivity
for Ang2 over Ang1 (7,13,14).
We confirmed by ELISA that L1-10 abolished in vitro binding
affinity between Ang2 and Tie2 in a dose-dependent manner (Fig. 2A).
We further confirmed that phosphorylation at Tyr992
of Tie2 receptor, which had undergone dephosphorylation by
stimulation with the recombinant Ang2, is rescued by the addition
of L1-10. (Fig. 2B). These data
indicate that L1-10 indeed blocks Ang2-Tie2 signal
transduction.
Previous reports on Ang2 inhibition in tumour cells
showed decreased tumour volume. These studies used the Colo205
colon cancer cells (7–10,22,23)
and other colon and breast cancer cells (8), the LuCap23.1 prostatic cancer cells
(13) and U-87 glioma cells
(24). The KM12SM colon cancer
cells employed display high levels of Ang2 and VEGF among several
CRC cells (Fig. 1) and produced
abundant tumour vessels in xenografts (Fig. 4). Moreover, the KM12SM cells were
initially isolated as highly metastatic cells that develop marked
spontaneous metastasis to liver (11) and shown to be highly activated
state in β catenin/TCF oncogenic pathway (25,26).
We considered that this cell type with such aggressive features
could be a suitable material in evaluation of superb efficacy of
Ang2-targeted therapy.
In vitro studies showed that VEGF enhanced
proliferation and tube formation of HUVECs, and caused a clear
increase in Rac1 and CDC42 expression when cultured in the collagen
matrix, whereas neither Ang2 nor L1-10 affected in vitro
behaviour of HUVECs or levels of the proteins (Fig. 3). By in vivo system we found
that Ang2 inhibition with treatment of L1-10 dose dependently
decreased tumour growth. Furthermore, we found that L1-10 led to
extension of the tumour-associated vessels whilst it suppressed
formation of a sound lumen. Ratio of lumen area to vessel area was
significantly decreased by L1-10 treatment compared to that of
VEGF. The double staining of both endothelial cells and pericytes
revealed that the endothelial cells were tightly covered with
abundant pericytes in the tumour-associated vessels of
L1-10-treated mice, when compared to control groups (Fig. 4B). Therefore, the difference in
effects endowed by L1-10 could be the existence of both endothelial
cells and pericytes in vivo, but not in vitro in
which pericytes are lacking. Our data suggest that Ang1/Ang2
balance plays an essential role in in vivo vascular
differentiation. Thus, it is assumed that Ang1/Ang2 balance may be
shifted to Ang1 dominance by L1-10, which should facilitate
recruitment of pericytes along the endothelial cells, considering
established model on angiogenesis by Angs-Tie2 signaling (1,27).
Indeed, several recent studies provided evidence that Ang2
inhibition causes an increase in pericyte-coverage over the
endothelial cells (9,10,22).
We postulate that lack of pericytes in the in
vitro system unables the demonstration of the relevance of Ang2
in vascular differentiation.
Several agents against Ang2, Ab536 (7) or anti-Ang2 monoclonal antibody 3.19.3
(8), or peptide antibody fusions
including 2×Con4, L1-7, L1-10, CovX-Bodies (7,9,10,13,22,23)
generally suppressed in vivo tumour growth implanted in nude
mice. These studies provided histological features of the tumours
such as deceased vessel density, increased apoptosis and pericyte
coverage. We first report here that vascular endothelial cells were
extended, but lacked lumen formation by L1-10 mediated Ang2
inhibition. One may suppose that such characterized phenotype is
rather specific to the KM12SM cells. However, we consider that our
finding is not unique to just the cell type because Chae et
al reported that forced expression of Ang2 caused enlargement
of vascular lumen in xenografts of U87 glioma cells (24), in which coverage of the endothelial
cells with pericytes was rather diminished by over-expression of
Ang2. Therefore, it is likely that in vivo vascular
differentiation is highly dependent on endothelial cell-pericyte
interaction through Ang2 mediated mechanism.
We found that combination of the anti-VEGF antibody
and L1-10 enhanced tumour inhibitory effects as compared to
anti-VEGF alone in nude mice. This is consistent with a recent
report by Hashizume et al (22). Since histological analysis revealed
that anti-VEGF or anti-Ang2 treatment caused a distinct inhibitory
effect on formation of tumour-associated vessels, it is probable
that the suppressive effects on KM12SM tumour could be through a
tumour angiogenesis-mediated mechanism. VEGF inhibition is already
clinically feasible when applied with chemotherapy including
5-FU/leucovorin, oxaliplatin and irinotecan. One of its mechanisms
is thought to be normalization of tumour-associated vessels which
facilitate the entry of chemoagents to the tumour cells (28). With a view of pericyte-endothelial
cell interaction, Ang2 inhibition would contribute to intense
coverage of vascular endothelial cells with pericytes and this is
in the line with anti-VEGF for vascular normalization in tumour
tissues. In conclusion, we propose that Ang2 is essential to in
vivo vascular differentiation.
Abbreviations:
|
Ang2
|
angiopoietin 2;
|
|
CRC
|
colorectal cancer;
|
|
EC
|
endothelial cell;
|
|
VEGF
|
vascular endothelial growth
factor;
|
|
PBS
|
phosphate-buffered saline;
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
References
|
1.
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar
|
|
2.
|
Kabbinavar F, Hurwitz HI, Fehrenbacher L,
et al: Phase II, randomized trial comparing bevacizumab plus
fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with
metastatic colorectal cancer. J Clin Oncol. 21:60–65. 2003.
View Article : Google Scholar
|
|
3.
|
Ogawa M, Yamamoto H, Nagano H, et al:
Hepatic expression of ANG2 RNA in metastatic colorectal cancer.
Hepatology. 39:528–539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Maisonpierre PC, Suri C, Jones PF, et al:
Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo
angiogenesis. Science. 277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Suri C, Jones PF, Patan S, et al:
Requisite role of angiopoietin-1, a ligand for the TIE2 receptor,
during embryonic angiogenesis. Cell. 87:1171–1180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Oliner J, Min H, Leal J, et al:
Suppression of angiogenesis and tumor growth by selective
inhibition of angiopoietin-2. Cancer Cell. 6:507–516. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Brown JL, Cao ZA, Pinzon-Ortiz M, et al: A
human monoclonal anti-ANG2 antibody leads to broad antitumor
activity in combination with VEGF inhibitors and chemotherapy
agents in preclinical models. Mol Cancer Ther. 9:145–156. 2010.
View Article : Google Scholar
|
|
9.
|
Coxon A, Bready J, Min H, et al:
Context-dependent role of angiopoietin-1 inhibition in the
suppression of angiogenesis and tumor growth: implications for AMG
386, an angiopoietin-1/2-neutralizing peptibody. Mol Cancer Ther.
9:2641–2651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Falcon BL, Hashizume H, Koumoutsakos P, et
al: Contrasting actions of selective inhibitors of angiopoietin-1
and angiopoietin-2 on the normalization of tumor blood vessels. Am
J Pathol. 175:2159–2170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Morikawa K, Walker SM, Nakajima M, Pathak
S, Jessup JM and Fidler IJ: Influence of organ environment on the
growth, selection, and metastasis of human colon carcinoma cells in
nude mice. Cancer Res. 48:6863–6871. 1988.PubMed/NCBI
|
|
12.
|
Yamamoto H, Kondo M, Nakamori S, et al:
JTE-522, a cyclooxygenase-2 inhibitor, is an effective
chemopreventive agent against rat experimental liver fibrosis1.
Gastroenterology. 125:556–571. 2003.PubMed/NCBI
|
|
13.
|
Morrissey C, Dowell A, Koreckij TD, et al:
Inhibition of angiopoietin-2 in LuCaP 23.1 prostate cancer tumors
decreases tumor growth and viability. Prostate. 70:1799–1808.
2010.PubMed/NCBI
|
|
14.
|
Tressel SL, Kim H, Ni CW, et al:
Angiopoietin-2 stimulates blood flow recovery after femoral artery
occlusion by inducing inflammation and arteriogenesis. Arterioscler
Thromb Vasc Biol. 28:1989–1995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bayless KJ and Davis GE: The Cdc42 and
Rac1 GTPases are required for capillary lumen formation in
three-dimensional extracellular matrices. J Cell Sci.
115:1123–1136. 2002.PubMed/NCBI
|
|
16.
|
Davis GE, Bayless KJ and Mavila A:
Molecular basis of endothelial cell morphogenesis in
three-dimensional extracellular matrices. Anat Rec. 268:252–275.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Folkman J and Haudenschild C: Angiogenesis
in vitro. Nature. 288:551–556. 1980. View Article : Google Scholar
|
|
18.
|
Bayless KJ, Salazar R and Davis GE:
RGD-dependent vacuolation and lumen formation observed during
endothelial cell morphogenesis in three-dimensional fibrin matrices
involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J
Pathol. 156:1673–1683. 2000. View Article : Google Scholar
|
|
19.
|
Davis GE and Bayless KJ: An integrin and
Rho GTPase-dependent pinocytic vacuole mechanism controls capillary
lumen formation in collagen and fibrin matrices. Microcirculation.
10:27–44. 2003. View Article : Google Scholar
|
|
20.
|
Yang S, Graham J, Kahn JW, Schwartz EA and
Gerritsen ME: Functional roles for PECAM-1 (CD31) and VE-cadherin
(CD144) in tube assembly and lumen formation in three-dimensional
collagen gels. Am J Pathol. 155:887–895. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kamei M, Saunders WB, Bayless KJ, Dye L,
Davis GE and Weinstein BM: Endothelial tubes assemble from
intracellular vacuoles in vivo. Nature. 442:453–456. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hashizume H, Falcon BL, Kuroda T, et al:
Complementary actions of inhibitors of angiopoietin-2 and VEGF on
tumor angiogenesis and growth. Cancer Res. 70:2213–2223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Huang H, Lai JY, Do J, et al: Specifically
targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing
monocyte infiltration, and tumor growth. Clin Cancer Res.
17:1001–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chae SS, Kamoun WS, Farrar CT, et al:
Angiopoietin-2 interferes with anti-VEGFR2-induced vessel
normalization and survival benefit in mice bearing gliomas. Clin
Cancer Res. 16:3618–3627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Herynk MH, Tsan R, Radinsky R and Gallick
GE: Activation of c-Met in colorectal carcinoma cells leads to
constitutive association of tyrosine-phosphorylated beta-catenin.
Clin Exp Metastasis. 20:291–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kim Y, Park H, Lim Y, et al: Decreased
syndecan-2 expression correlates with trichostatin-A
induced-morphological changes and reduced tumorigenic activity in
colon carcinoma cells. Oncogene. 22:826–830. 2003. View Article : Google Scholar
|
|
27.
|
Shim WS, Ho IA and Wong PE: Angiopoietin:
a TIE(d) balance in tumor angiogenesis. Mol Cancer Res. 5:655–665.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|