Introduction
Programmed cell death (PCD) plays an essential role
in regulating various biological processes, including
morphogenesis, maintaining tissue homeostasis, and eliminating
damaged and infected cells. Two major forms of PCD have been
classified as apoptosis and autophagy (1–3).
Apoptotic cell death (type I PCD) is characterized by membrane
blebbing, chromosomal DNA fragmentation, cell shrinkage and the
formation of apoptotic bodies (4–6).
Autophagic cell death (type II PCD) is a catabolic process in which
cytosolic macromolecules and damaged organelles are sequestered in
double-membrane autophagosomes, which subsequently fuse with
lysosomes for degradation by forming acidic autophagolysosomes
(7,8). Different from apoptosis, autophagy
may contribute to cell survival or death. Although apoptosis and
autophagy are two distinct biological processes, crosstalk exists
between them (9–11).
Lung cancer is the most common cause of
cancer-related morbidity and mortality in men and women around the
world, accounting for approximately 30% of all cancer deaths
(12). Lung cancers are generally
classified into two histological types, small cell lung cancer and
non-small cell lung cancer (NSCLC). NSCLC accounts for
approximately 85% of all lung cancers. Most lung cancers are
closely related to an advanced stage at diagnosis with a poor
prognosis. Over the last few decades, chemotherapy has improved the
outcome for patients with late stage NSCLC, but only slightly
(13,14). Therefore, novel and more effective
antitumor agents must be explored and developed.
Dendropanax morbifera Leveille has been used
in traditional medicine to treat several diseases, such as
headache, infectious diseases and skin diseases. Previous studies
have shown that the components of this plant have many
pharmacological activities, including anti-complement,
anti-diabetic and anti-atherogenic properties (15–17).
Recently, oleifolioside B (OB), a cycloartane-type glycoside, was
isolated from the lower stem parts of D. morbifera, which
has anti-plasmodial activity in vitro (18). However, little is known regarding
the anticancer activity of OB or its signal molecular mechanisms in
cancer cells. In this present study, we investigated for the first
time the anticancer mechanisms by which OB induces apoptosis and
autophagy in A549 NSCLC cells.
Materials and methods
Materials
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), bafilomycin A1, 4,6-diamidino-2-phenylindole (DAPI),
monodansylcadaverine (MDC) and doxorubicin were purchased from the
Sigma-Aldrich Chemical Co. (St. Louis, MO). Antibodies specific for
actin, Bad, Bax, Bcl-2, Bcl-xL, Bid, caspase-3, caspase-8,
caspase-9, cIAP-1, cIAP-2, death receptor (DR) 4, DR5, Fas, Fas
legend (FasL), Fas-associated death domain (FADD), cellular
FLICE-like inhibitory protein (c-FLIP), nuclear factor erythroid
2-related factor 2 (Nrf2), poly(ADP-ribose) polymerase (PARP),
survivin, XIAP, heme oxygenase 1 (HO-1) and goat anti-rabbit
IgG-FITC were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA). Antibodies specific for Atg3, Atg5, Atg7, Atg12, Beclin-1 and
microtubule-associated protein 1 light chain 3 (LC3) were purchased
from Cell Signaling (Beverly, MA). The antibody specific for
phospho (p)-Nrf2 was purchased from Epitomics (Burlingame, CA).
Peroxidase-labeled donkey anti-rabbit and sheep anti-mouse
immunoglobulins and an enhanced chemiluminescence (ECL) kit were
purchased from Amersham (Arlington Heights, IL). Caspase activity
assay kits were purchased from R&D Systems (Minneapolis, MN).
z-VAD-fmk was purchased from Calbiochem (San Diego, CA). OB was
kindly provided by Professor Hyung-In Moon of Dong-A University
(Busan, Republic of Korea) (18)
and dissolved in dimethyl sulfoxide (DMSO) as a stock solution at
30 mM concentration. Dilutions were made in Dulbecco’s modified
Eagle’s medium (DMEM, Gibco-BRL, Gaithersburg, MD).
Cell culture and cell viability
assay
The A549 cell line was obtained from the American
Type Culture Collection (Rockville, MD) and cultured in DMEM
supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2
mM glutamine, 100 U/ml penicillin, and 100 μg/ml
streptomycin (Gibco-BRL). Cell viability was measured based on
formation of blue formazan metabolized from colorless MTT by
mitochondrial dehydrogenases, which are active only in live
cells.
Flow cytometric analysis
To analyze the percentage of apoptotic cells, cells
were collected, washed with cold phosphate-buffered saline (PBS),
and fixed in 75% ethanol at 4°C for 30 min. The DNA content of the
cells was measured using a DNA staining kit (CycleTEST PLUS Kit,
Becton-Dickinson, San Jose, CA). Propidium iodide (PI)-stained
nuclear fractions were obtained by following the kit protocol. The
cells were then filtered through 35-mm mesh, and DNA content
fluorescence was determined using a FACSCalibur flow cytometer
within 1 h. The cellular DNA content was analyzed with CellQuest
software (Becton-Dickinson).
DNA fragmentation assay
After OB treatment, the cells were lysed in a buffer
containing 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA and 0.5%
Triton X-100 for 1 h at room temperature. The lysates were vortexed
and cleared by centrifugation at 15,000 rpm for 10 min at 4°C. The
DNA in the supernatant was extracted using a 25:24:1 (v/v/v) equal
volume of neutral phenol:chloroform:isoamyl alcohol. To assay the
DNA fragmentation pattern, samples were loaded onto 1.0% agarose
gel containing 0.1 μg/ml ethidium bromide (EtBr) and
electrophoresis was carried out.
Caspase activity assay
The enzymatic activity of the caspases was assayed
using a colorimetric assay kit according to the manufacturer’s
protocol. The cells were incubated in the absence and presence of
OB for the indicated times. The cells were harvested and lysed in a
lysis buffer for 30 min on an ice bath. Lysed cells were
centrifuged at 14,000 rpm for 20 min, and equal amounts of protein
(100 μg per 50 μl) were incubated with 50 μl
reaction buffer and 5 μl colorimetric tetrapeptides,
Asp-Glu-Val-Asp (DEVD)-p-nitroaniline (pNA) for caspase-3,
Ile-Glu-Thr-Asp (IETD)-pNA for caspase-8, and Leu-Glu-His-Asp
(LEHD)-pNA for caspase-9, at 37°C for 2 h in the dark. Caspase
activity was determined by measuring the changes in absorbance at
405 nm using an ELISA reader.
MDC staining
To observe autophagy formation, A549 cells were
grown on glass coverslips for 24 h. The cells were incubated in the
absence and presence of OB for the indicated times, and then the
cells were treated with 0.05 mM MDC at 37°C in 5% CO2
for 1 h. The cells were then fixed with 4% paraformaldehyde in PBS
for 10 min. The cellular changes were analyzed with a fluorescence
microscope.
Protein extraction and western blot
analysis
Whole-cell protein extracts from A549 cells were
prepared with cell lysis buffer (20 mM sucrose, 1 mM EDTA, 20
μM Tris-HCl, pH 7.2, 1 mM DTT, 10 mM KCl, 1.5 mM
MgCl2 and 5 μg/ml aprotinin) for 30 min. Cells
were disrupted by sonication and extracted at 4°C for 30 min. The
protein extracts were quantified using the Bio-Rad kit (Pierce
Biotechnology, Rockford, IL). For western blot analysis, lysate
proteins (30–50 μg) were resolved over sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis and transferred
onto nitrocellulose transfer membranes (Schleicher & Schuell,
Keene, NH). Specific proteins were detected with an ECL Western
blotting kit according to the recommended procedure. In a parallel
experiment, cells were washed with ice-cold PBS and collected. Then
cytoplasmic and nuclear proteins were prepared using NE-PER Nuclear
and Cytoplasmic Extraction Reagents (Pierce Biotechnology).
Immunocytochemistry
Cells were grown on coverslips and treated as
indicated. Cells were washed twice in PBS, fixed with 4%
paraformaldehyde in PBS at room temperature for 30 min, and then
permabilized with 0.25% Triton X-100 solution for 10 min. The cells
were subsequently incubated in a blocking solution of 1% bovine
serum albumin (BSA) and incubated with primary antibodies at room
temperature for 1 h. After the incubation period, the samples were
rinsed four times with PBS and then incubated with the secondary
anti-rabbit-FITC diluted 1:200 in buffer for 1 h at room
temperature. Nuclei were stained with 1 μg/ml DAPI, and then
captured using a Zeiss LSM 510 laser scanning confocal device (Carl
Zeiss).
Statistical analysis
All data are expressed as mean ± SD. The significant
differences between the groups were determined using an unpaired
Student’s t-test. A value of p<0.05 was considered significant.
All figures shown represent results from at least two independent
experiments with a similar pattern.
Results
Induction of apoptosis by OB in A549
cells
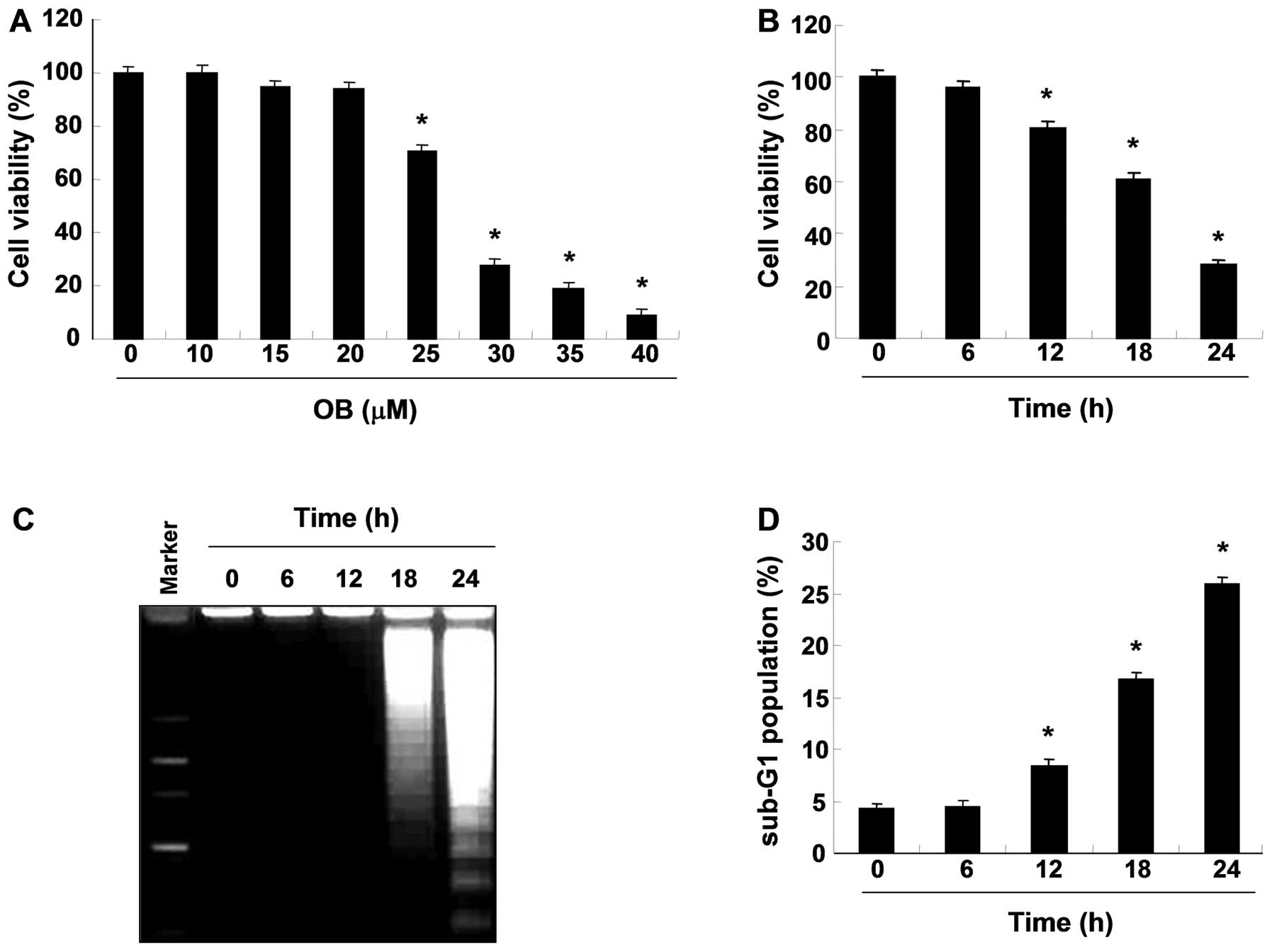
To investigate the effects of OB on cell viability
in A549 cells, cells were treated with OB and subjected to an MTT
assay. As shown in Fig. 1A and B,
treatment with OB significantly reduced cell viability in a
concentration- and time-dependent manner. Subsequently, to examine
whether OB inhibits the proliferation of A549 cells by inducing
apoptosis, genomic DNA was extracted from cells and agarose gel
electrophoresis were assessed. As indicated Fig. 1C, treatment with OB
time-dependently induced DNA fragmentation, a hallmark of
apoptosis, in a time-dependent manner. In addition, flow cytometric
analysis also revealed that treatment with OB increased the
accumulation of cells at the apoptotic sub-G1 phase in a
time-dependent manner (Fig. 1D).
Taken together, these results indicate that the cytotoxic effects
observed in response to OB are associated with the induction of
apoptotic cell death in A549 cells.
Modulation of apoptosis regulatory
proteins by OB in A549 cells
To determine which pathway was involved in the
apoptosis induction of OB-treated A549 cells, the expression levels
of death receptor-related proteins and the Bcl-2 and IAP family of
proteins were determined with western blot analysis to measure the
expression of the proteins. As shown in Fig. 2, exposure to OB led to a
significant reduction in the anti-apoptotic protein cIAP-2,
survivin and c-FLIP in a time-dependent fashion. However, OB
treatment resulted in a time-dependent increase in the level of the
pro-apoptotic FasL proteins. Under these conditions, although we
did not detect the truncated form of the pro-apoptotic protein Bid,
a BH3-only pro-apoptotic member of the Bcl-2 family, our results
indicate that OB treatment caused time-dependent downregulation of
the whole form of the Bid proteins, which reflects Bid cleavage and
activation.
Caspase activation by OB in A549
cells
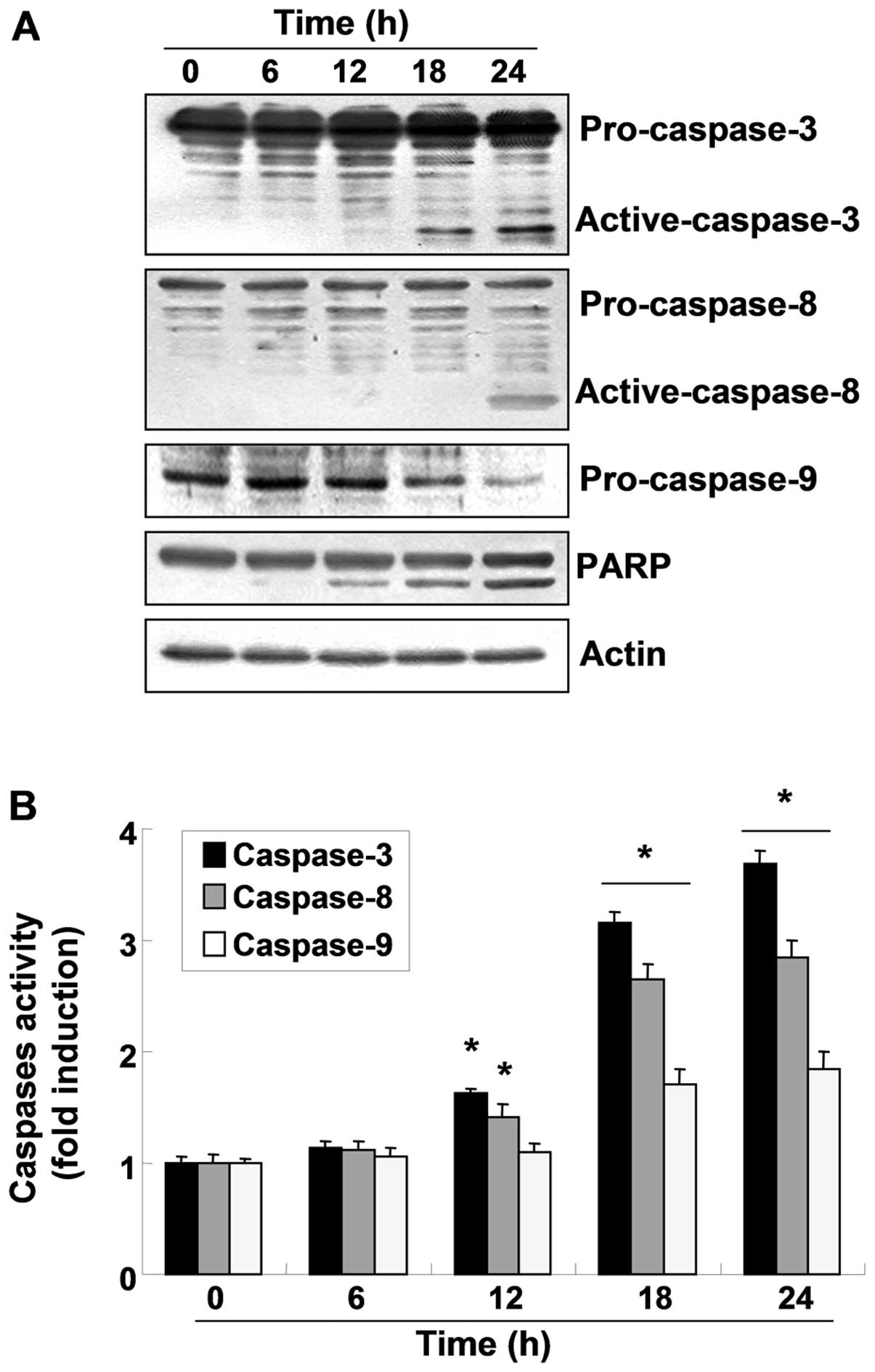
To investigate whether OB-induced apoptosis in A549
cells involves the caspase cascade pathway, the caspase expression
levels and activity were determined. As shown in Fig. 3A, western blot analyses indicated
that the active forms of caspase-3 and -8 increased and the
expression of pro-caspase-9 decreased in a time-dependent manner
following OB treatment. For further quantification of the
proteolytic activation of caspases, protein in the lysates of cells
treated with OB was normalized and then assayed for in vitro
activities using fluorogenic substrates. As indicated in Fig. 3B, treatment with OB resulted in a
time-dependent increase in caspase activity (−3, −8 and −9)
compared with the control cells, which was associated with the
progressive proteolytic cleavage products of PARP, an activated
caspase-3 substrate protein. To further investigate the
significance of caspase activation in OB-induced apoptosis, A549
cells were pretreated with z-VAD-fmk, a broad-spectrum caspase
inhibitor, for 1 h, followed by treatment with 30 μM OB for
24 h. Interestingly, pretreatment with z-VAD-fmk did not restore
cell viability compared with control (Fig. 4A), and failed to suppress the
OB-induced apoptosis (Fig. 4B).
However, under the same condition, z-VAD-fmk significantly
suppressed doxorubicin-induced growth inhibition and apoptosis
(Fig. 4). Taken together, these
results suggest that OB-induced apoptosis was independent of
caspase activation in A549 cells.
Induction of autophagy by OB in A549
cells
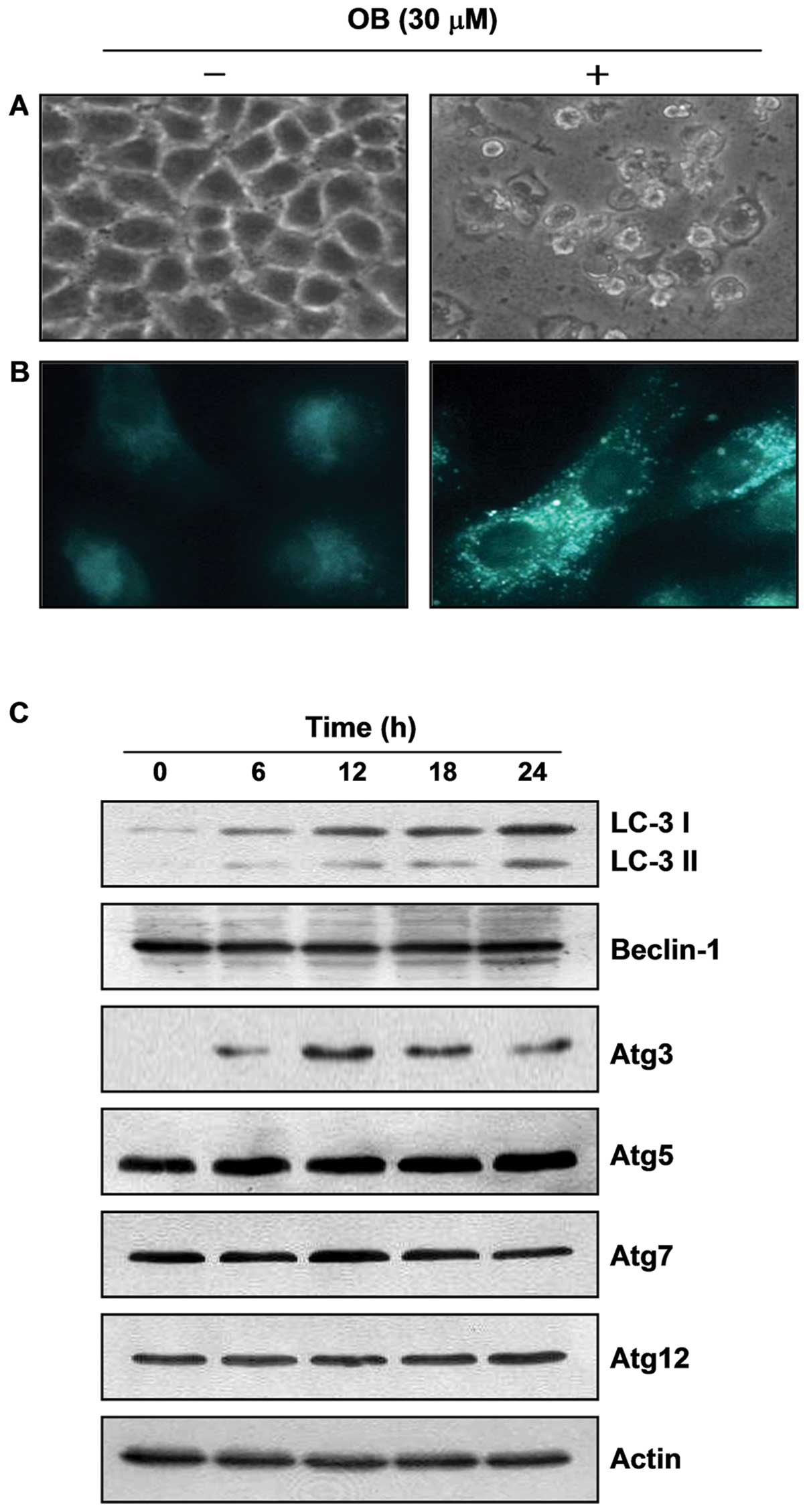
As shown in Fig.
5A, based on our finding that extensive cytoplasm vacuolization
compared with control cells appeared in cytosol after OB treatment,
we hypothesized that the vacuoles might be associated with
autophagy. For this study, we used MDC, a commonly used autophagic
dye, as a more specific marker for autophagy. The results indicated
that untreated control cells presented diffused staining, but OB
treatment clearly resulted in an extensive punctuate MDC staining
pattern (Fig. 5B). However, LC3,
another typical marker of autophagy, exists in the cytosol and is
called LC3-I, after its C-terminal region, which is cleaved through
post-translational modification. When autophagosomes are formed,
LC3-I changes to LC3-II through conjugation with
phosphatidylethanolamine and is recruited into the autophagosome
membrane. That is why LC3-II accumulation is a critical marker of
autophagy (19). As shown in
Fig. 6C, OB treatment induced an
increase in LC3-II expression. In addition, treatment with OB
strongly increased the essential autophagosome-regulatory gene
expression of Atg3, and slightly increased the gene expression of
Atg5, but not Atg7 and Atg12. We also found an accumulation of LC3
puncta following OB treatment through fluorescence microscopic
observation (Fig. 7A). Taken
together, these results indicate that OB-induced cytoplasmic
vacuoles are related to autophagy induction.
Modulation of Nrf2 in OB-treated A549
cells
Because many recent studies have reported that Nrf2
is involved in cancer cell survival and in the acquisition of drug
resistance against anticancer therapies, we investigated whether
the Nrf2 signaling pathway is involved in OB-caused cell death. As
shown in Fig. 6, although exposure
of cells to OB led to dephosphorylation of Nrf2 proteins without
altering their total levels, Nrf2 and p-Nrf2, the active form of
Nrf2, were translocated to the nucleus in response to OB treatment,
which was associated with decreased expression of HO-1 whose
transcription is regulated by Nrf2. Therefore, OB-induced cell
death appeared to be responsible for nuclear translocation of
p-Nrf2.
OB-induced autophagy as a death mechanism
in A549 cells
To elucidate the molecular mechanism controlling the
relationship between OB-induced apoptosis and autophagy, we next
investigated whether OB-induced autophagy functioned as a survival
mechanism or a death mechanism. As shown in Fig. 7A, pretreatment with bafilomycin A1,
a well-known inhibitor of autophagosomal lysosome degradation,
blocked the formation of OB-induced LC3 puncta, representing
recruited LC3-II in the cytosol. Western blot analysis also showed
that PARP cleavage, caspase-3 activation, and p-Nrf2 translocation
were restored to levels seen in the control cells in response to
bafilomycin A1 pretreatment, which was connected with significant
blockage of OB-induced apoptosis and growth inhibition (Fig. 7C and D). Taken together, these
results clearly show that OB treatment induces autophagy as a cell
death mechanism in A549 cells.
Discussion
In the present study, we demonstrated that the
growth inhibitory effects of OB, a cycloartane-type triterpene
glycoside isolated from D. morbifera, were associated with
induction of apoptosis and autophagy in A549 NSCLC cells. Our data
also suggested that OB-induced autophagy functioned as a death
mechanism, which was connected to the translocation of p-Nrf2 from
cytoplasm to the nucleus.
Apoptotic cell death, type I PCD, is an important
process that allows a cell to self-degrade to eliminate damaged
cells (4,5). There are two main apoptotic pathways:
the intrinsic mitochondrial pathway and the extrinsic death
receptor pathway. The intrinsic pathway, which is triggered by
various extracellular and intracellular stresses, produces
mitochondrial-mediated signals, causing the mitochondrial
permeability transition pore and the release of pro-apoptotic
proteins such as cytochrome c. The release of cytochrome
c is essential for caspase-9 activation. Activated caspase-9
in turn activates the downstream effector caspase-3 and -7, which
rapidly cleave intracellular substrates (4–6). The
extrinsic signaling pathway initiates apoptosis through
transmembrane receptor-mediated interactions. The best
characterized cytoplasmic death receptors are Fas, DR4 and DR5.
After the respective ligands bind to the receptors, an adapter
protein called Fas-associated death domain (FADD) or
TRAIL-associated death domain (TRADD) is recruited to the death
receptor, forming DISC to activate pro-caspase-8. The activation of
caspase-8 is antagonized by c-FLIP, an enzymatically-inactive
relative of caspase-8 that binds to DISC. Therefore, the knockdown
of c-FLIP augments DISC recruitment, activation and processing of
caspase-8, thus enhancing effector-caspase stimulation and
apoptosis (20,21). Several pro-apoptotic and
anti-apoptotic proteins are involved in the upstream and downstream
of this process, such as IAP and Bcl-2 member proteins, as well as
the expression of death receptors (22,23).
In our study, among the Bcl-2 family proteins, OB treatment
markedly downregulated the whole form of the Bid proteins in a
time-dependent manner reflecting Bid cleavage and activation. OB
also significantly inhibited the expression of c-FLIP without
altering the DR4, DR4, FADD, Fas and FasL levels. Caspases are
integral components of the apoptotic pathway. Although many reports
have shown that chemotherapeutic agents induced apoptosis through
the caspase-dependent pathway in cancer cells, apoptotic cell death
can occur in a caspase-independent manner (24–26).
In our case, OB induced activation of initiator caspases (caspase-8
and -9) and an effector caspase (caspase-3), but did not restore
cell viability and failed to suppress the OB-induced apoptotic cell
deaths by treatment with z-VAD-fmk, a broad-spectrum caspase
inhibitor. The results indicate that treatment with OB-induced
apoptosis was caspase-independent of the death receptor signaling
and mitochondria pathways in A549 cells.
In addition to caspase-dependent cell death, some
cell death processes occur in a caspase-independent manner.
Autophagy (type II PCD) is a cellular process characterized by
sequestration of part of the cell cytoplasm, including long-lived
protein organelles, in autophagic vesicles and their delivery to
and subsequent degradation following fusion with the cellular
lysosomes throughout the lifecycle of an organism in a
caspase-independent manner (8,27).
Autophagy thus maintains cellular homeostasis throughout the
lifecycle of an organism. Recently, many reports have shown that
chemo-therapeutic agent-induced autophagy was associated with
upregulation and processing of LC3/Beclin-1 coupled with induction
of Atgs, and its recruitment to the autophagosomes (28,29).
In the present study, OB did not alter the expression level of
Beclin-1; however, the Atg3 and Atg5 levels, and the autophagic
form of LC3 were increased after OB treatment indicating that OB
also induced autophagy in A549 cells.
Nrf2, an essential member of basic leucine zipper
transcription factors, by binding to antioxidant response element
(ARE) plays a key physiological role in regulating oxidative
stress. Previous studies have reported that activation of the
Nrf2-ARE/HO-1 pathway by chemotherapeutic agents has been
correlated with preventing inflammatory diseases and cancer
(30,31). Recently, Ansari et al
(32) also reported that
activation of the Nrf2-ARE/HO-1 pathway by chemotherapeutic agents
caused protection against hydrogen peroxide-induced cell death.
Interestingly, our results show that treatment with OB led to a
significant reduction in the phosphorylation levels of Nrf2
proteins and expression of HO-1 proteins, but did not affect the
total levels of Nrf2 expression. Furthermore, treatment with OB
resulted in the translocation of p-Nrf2 from cytosol to the nucleus
in A549 cells. Although further studies are needed, these results
indicate that OB-induced cell death is involved in the
translocation of p-Nrf2 into the nucleus in A549 cells.
Apoptosis and autophagy are two distinct forms of
programmed cell death; however, apoptosis is always associated with
cell death, while autophagy normally contributes either to cell
survival or to cell death. In some cases, apoptosis and autophagy
are interconnected positively or negatively (11,33–35).
Thus, the molecular mechanism controlling the relationship between
apoptosis and autophagy during cancer cell death by certain
chemotherapeutic agents must be elucidated. Interestingly, in our
study, OB-induced autophagy and OB-induced cell death and growth
inhibition were significantly attenuated by pretreatment with an
autophagy inhibitor bafilomycin A1. Furthermore, pretreatment with
bafilomycin A1 had an impact in suppressing OB-induced
downregulation of p-Nrf2. Taken together, the present results
suggest that OB-induced autophagy functioned as a death mechanism
and the Nrf2 signaling pathway is involved in this process in A549
cells.
In conclusion, the present results demonstrated that
OB-induced cell death occurs through induction of autophagy as cell
death mechanisms and caspase-dependent apoptosis. Although the role
of the Nrf2-ARE/HO-1 pathway in OB-induced autophagy remains
unknown, promoting autophagy could be an effective strategy for
enhancing the antitumor activity of OB in A549 NSCLC cells.
Acknowledgements
This study was supported by the
National Research Foundation of Korea (NRF) grant funded by the
Korea government (2012-0000476 and 2012046358).
References
|
1.
|
Corcelle EA, Puustinen P and Jäättelä M:
Apoptosis and autophagy: Targeting autophagy signalling in cancer
cells - ‘trick or treats’? FEBS J. 276:6084–6096. 2009.
|
|
2.
|
Levine B: Cell biology: autophagy and
cancer. Nature. 446:745–747. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Okada H and Mak TW: Pathways of apoptotic
and non-apoptotic death in tumour cells. Nat Rev Cancer. 4:592–603.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
|
|
7.
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Gozuacik D and Kimchi A: Autophagy and
cell death. Curr Top Dev Biol. 78:217–245. 2007. View Article : Google Scholar
|
|
9.
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cheng Y, Qiu F, Ye YC, Guo ZM, Tashiro S,
Onodera S and Ikejima T: Autophagy inhibits reactive oxygen
species-mediated apoptosis via activating p38-nuclear factor-kappa
B survival pathways in oridonin-treated murine fibrosarcoma L929
cells. FEBS J. 276:1291–1306. 2009. View Article : Google Scholar
|
|
11.
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
13.
|
Gridelli C, Maione P, Ferrara ML and Rossi
A: Cetuximab and other anti-epidermal growth factor receptor
monoclonal antibodies in the treatment of non-small cell lung
cancer. Oncologist. 14:601–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kapp FG, Sommer A, Kiefer T, Dölken G and
Haendler B: 5-alpha-reductase type I (SRD5A1) is up-regulated in
non-small cell lung cancer but does not impact proliferation, cell
cycle distribution or apoptosis. Cancer Cell Int. 12:12012.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Park BY, Min BS, Oh SR, Kim JH, Kim TJ,
Kim DH, Bae KH and Lee HK: Isolation and anticomplement activity of
compounds from Dendropanax morbifera. J Ethnopharmacol.
90:403–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Chung IM, Kim MY, Park WH and Moon HI:
Antiatherogenic activity of Dendropanax morbifera essential
oil in rats. Pharmazie. 64:547–549. 2009.
|
|
17.
|
Moon HI: Antidiabetic effects of
dendropanoxide from leaves of Dendropanax morbifera Leveille
in normal and streptozotocin-induced diabetic rats. Hum Exp
Toxicol. 30:870–875. 2011. View Article : Google Scholar
|
|
18.
|
Chung IM, Kim MY, Park SD, Park WH and
Moon HI: In vitro evaluation of the antiplasmodial activity of
Dendropanax morbifera against chloroquine-sensitive strains
of Plasmodium falciparum. Phytother Res. 23:1634–1637.
2009.PubMed/NCBI
|
|
19.
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Riccioni R, Pasquini L, Mariani G, Saulle
E, Rossini A, Diverio D, Pelosi E, Vitale A, Chierichini A, Cedrone
M, Foà R, Lo Coco F, Peschle C and Testa U: TRAIL decoy receptors
mediate resistance of acute myeloid leukemia cells to TRAIL.
Haematologica. 90:612–624. 2005.PubMed/NCBI
|
|
21.
|
Lee EW, Seo J, Jeong M, Lee S and Song J:
The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep.
45:496–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Deveraux QL and Reed JC: IAP family
proteins-suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar
|
|
24.
|
Constantinou C, Papas KA and Constantinou
AI: Caspase-independent pathways of programmed cell death: the
unraveling of new targets of cancer therapy? Curr Cancer Drug
Targets. 9:717–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wu XX and Kakehi Y: Enhancement of
lexatumumab-induced apoptosis in human solid cancer cells by
cisplatin in caspase-dependent manner. Clin Cancer Res.
15:2039–2047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Takeda S, Matsuo K, Yaji K,
Okajima-Miyazaki S, Harada M, Miyoshi H, Okamoto Y, Amamoto T,
Shindo M, Omiecinski CJ and Aramaki H: (-)-Xanthatin selectively
induces GADD45γ and stimulates caspase-independent cell death in
human breast cancer MDA-MB-231 cells. Chem Res Toxicol. 24:855–865.
2011.PubMed/NCBI
|
|
27.
|
Bröker LE, Kruyt FA and Giaccone G: Cell
death independent of caspases: a review. Clin Cancer Res.
11:3155–3162. 2005.
|
|
28.
|
Pattingre S, Espert L, Biard-Piechaczyk M
and Codogno P: Regulation of macroautophagy by mTOR and Beclin 1
complexes. Biochimie. 90:313–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Marquez RT and Xu L: Bcl-2: Beclin 1
complex: multiple, mechanisms regulating autophagy/apoptosis toggle
switch. Am J Cancer Res. 2:214–221. 2012.PubMed/NCBI
|
|
30.
|
Kang KW, Lee SJ and Kim SG: Molecular
mechanism of nrf2 activation by oxidative stress. Antioxid Redox
Signal. 7:1664–1673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Khan NM, Sandur SK, Checker R, Sharma D,
Poduval TB and Sainis KB: Pro-oxidants ameliorate radiation-induced
apoptosis through activation of the calcium-ERK1/2-Nrf2 pathway.
Free Radic Biol Med. 51:115–128. 2011. View Article : Google Scholar
|
|
32.
|
Ansari N, Khodagholi F and Amini M:
2-Ethoxy-4,5-diphenyl-1,3-oxazine-6- one activates the Nrf2/HO-1
axis and protects against oxidative stress-induced neuronal death.
Eur J Pharmacol. 658:84–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Choi KS: Autophagy and cancer. Exp Mol
Med. 44:109–120. 2012. View Article : Google Scholar
|
|
34.
|
Singh P, Godbole M, Rao G, Annarao S,
Mitra K, Roy R, Ingle A, Agarwal G and Tiwari S: Inhibition of
autophagy stimulates molecular iodine-induced apoptosis in hormone
independent breast tumors. Biochem Biophys Res Commun. 415:181–186.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Lee Y and Hong Y, Lee SR, Chang KT and
Hong Y: Autophagy contributes to retardation of cardiac growth in
diabetic rats. Lab Anim Res. 28:99–107. 2012. View Article : Google Scholar : PubMed/NCBI
|