Introduction
Nasopharyngeal carcinoma (NPC) is a widely prevalent
head and neck cancer in Southern China (1). It is closely associated with
Epstein-Barr virus (EBV) and shows highly invasive and metastatic
features. At the time of diagnosis, ∼75% of patients present with
regional lymph node metastasis and 10% present with distant
metastasis (2,3). Radiotherapy with or without
concurrent chemotherapy remains the standard treatment for NPC
patients (4). Although
improvements both in radiotherapy and chemotherapy have been
achieved, distant metastasis still occurs and remains the major
patterns of failure in patients with NPC (1,5).
Therefore, a better understanding of the molecular mechanisms of
NPC invasion and metastasis is helpful for improving the survival
of NPC patients.
It is now generally accepted that inflammatory
microenvironment plays critical roles in tumor development
including the tumor initiation, promotion, invasion and metastasis
(6,7). Interleukin-6 (IL-6), a
multifunctional cytokine, has emerged as an important contributor
to the tumor microenvironment. Many studies have shown that IL-6 is
widely expressed and profoundly linked to poor prognosis in a large
variety of malignant tumors (8–11).
The biological effects of IL-6 are mediated through a membrane
receptor complex that contains an IL-6-specific binding receptor
(IL-6R) and a signal transducing receptor (glycoprotein-130,
gp130). Briefly, IL-6 binds to IL-6R and triggers the dimerization
and phosphorylation of gp130, leading to the activation of Janus
tyrosine kinase (JAK). Then the subsequent signal-transduction
pathways are activated, including the JAK/signal transducer and
transcription activators (JAK/STATs), Ras/mitogen activated protein
kinase (Ras/MAPK), and phosphoinositol-3 kinase/Akt (PI3K/ Akt)
pathways (12,13). IL-6 and its related signaling
pathways have been identified to contribute to proliferation,
migration and invasion of various tumor cells (14–16).
Tumor invasion and metastasis result from a
multi-step process that includes the degradation of surrounding
extra-cellular matrix (ECM), allowing cancer cell spread to distal
organs. Matrix metalloproteinases (MMPs) are a family of
zinc-dependent proteinases whose enzymatic activity is directed
against components of the extracellular matrix (ECM) (17). Two of these enzymes, MMP-2
(gelatinase A, 72 kDa) and MMP-9 (gelatinase B, 92 kDa) which
selectively degrade type IV collagen, a major component of ECM,
play a key role in the metastatic process. Multiple studies have
suggested that MMP-2 and MMP-9 are implicated in the invasion,
metastasis and poor prognosis of various cancers (18–21).
The expression and activation of MMP-2 and MMP-9 can be regulated
by environmental influences from surrounding stroma, such as the
cytokines (22–25). In addition, IL-6 was found to be
able to upregulate the expression of MMP-2 and MMP-9 in some cancer
cells (26–28). These results indicated that
targeting IL-6 may be an effective strategy to control tumor
invasion and metastasis.
Clinical studies have shown that elevated serum IL-6
is closely associated with the distant metastasis of NPC patients
(29,30). It has also been observed that MMP-2
and MMP-9 are linked to metastasis and poor prognosis of NPC
(31–33). However, the role of IL-6 in
modulating the migration and invasion activity of NPC cells is not
fully understood. It is also not clear whether IL-6 can regulate
the expression of MMP-2 and MMP-9 in NPC cells. So we performed
this study to investigate whether IL-6 modulates NPC migration and
invasion, as well as whether the effect of IL-6 is mediated through
regulating the expression of MMP-2 and MMP-9.
Materials and methods
Reagents
The recombinant human IL-6 was purchased from
PeproTech (Rocky Hill, NJ, USA). Mouse monoclonal anti-human IL-6R
antibody (anti-IL-6R mAb) used for blocking IL-6 activity was
obtained from Invitrogen Corp. (clone BR-6; Carlsbad, CA, USA).
Primary antibodies including rabbit anti-human MMP-2, MMP-9 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal
antibodies were purchased from Epitomics (Burlingame, CA, USA). The
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). The RNAiso Plus and PrimeScript™ 1st Strand cDNA
Synthesis kit were obtained from Takara Biotechnology (Dalian,
China). The 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium
bromide (MTT), ECM gel (E1270) and gelatin were obtained from Sigma
(St. Louis, MO, USA).
Cell lines culture
The human NPC cell lines, including HNE1, HONE1,
CNE1, CNE1-LMP1 and 5–8F, were obtained from the Cancer Research
Institute of Central South University (Changsha, China). HNE1 and
HONE1 were both derived from a poorly differentiated NPC and lost
the EBV genome as cells were passaged (34,35).
CNE1 was an EBV negative poorly differentiated cell line and
CNE1-LMP1 was established by introducing the gene of latent
membrane protein-1 (LMP1) into CNE1 cells (36). 5–8F was constructed from the NPC
cell line SUNE-1 and stably expresses LMP1 (37,38).
HNE1, HONE1 and CNE1-LMP1 were maintained in Roswell Park Memorial
Institute (RPMI)-1640 medium (Gibco, Grand Island, NY, USA) with
10% newborn calf serum (Gibco), while CNE1 and 5–8F were cultured
in RPMI-1640 medium containing 10% fetal bovine serum (Gibco). All
cell lines were incubated at 37°C in a humidified atmosphere of 5%
CO2.
RNA extraction, cDNA synthesis and
reverse transcription-polymerase chain reaction (RT-PCR)
Cultured cells were harvested and washed with
phosphate-buffered saline (PBS). Then total RNA was extracted using
RNAiso Plus according to the manufacturer’s instructions. The RNA
pellets were reconstituted in 20 μl of RNase-free water and stored
at −80°C. RNA concentration and purity were assessed prior to cDNA
synthesis. The first strand cDNA was synthesized using the
PrimeScript 1st Strand cDNA Synthesis kit according to the
manufacturer’s protocol. Polymerase chain reactions (PCR) were
performed in a total volume of 20 μl, containing 10
μl 2X Taq PCR Master Mix, 1 μl of each primer, 1
μl cDNA template and 7 μl of sterile water. The
amplification protocol consisted of an initial denaturation at 94°C
for 5 min, followed by 35 cycles of denaturation for 30 sec at
94°C, annealing for 45 sec at 54°C and extension for 1 min at 72°C,
followed by a final extension at 72°C for 10 min. The PCR products
were verified by 1.0% agarose gel electrophoresis and analyzed
using the Gel Doc™ XR Imaging System (Bio-Rad, Foster City, CA,
USA). Primers for PCR were designed by Primer Premier 5.0 software
(Premier Biosoft International, Palo Alto, CA, USA) and listed in
Table I.
 | Table I.Primer sequences used for RT-PCR. |
Table I.
Primer sequences used for RT-PCR.
| Gene | Sense primer
(5′-3′) | Antisense primer
(5′-3′) | Product (bp) |
|---|
| IL-6 |
AATGAGGAGACTTGCCTGGTGAA |
ACAATCTGAGGTGCCCATGCTAC | 340 |
| IL-6R |
CAGTATTCCCAGGAGTCCCAGAAG |
CATCCATGTTGTGAATGTCTTTG | 312 |
| gp130 |
GCAACATTCTTACATTCGGACAGC |
ATCCCTTACCATCTTCCTTCATACAGC | 618 |
| GAPDH |
GGTCGGAGTCAACGGATTTG |
GGAAGATGGTGATGGGATTTC | 218 |
Enzyme-linked immunosorbent assay
(ELISA)
Fresh medium (5 ml) was added to NPC cell lines when
60–70% confluent in 25 cm2 flasks. After 24 h cell
culture supernatants were collected, aliquoted, and frozen at −80°C
until assayed. Cells were trypsinized and counted, and all results
were standardized for amount of IL-6 secreted as
pg/ml/106 cells. The quantitation of IL-6 was performed
using the human IL-6 ELISA kit according to the manufacturer’s
instructions (Boster, Wuhan, China). Complete medium was used as a
blank.
Cell proliferation assay
The effects of IL-6 with or without anti-IL-6R mAb
on NPC cell proliferation were assessed using an MTT assay. Cells
were seeded in 96-well plates at a density of 3×103
cells/well and allowed to attach for 12 h. Then the cells were
treated by different concentrations of IL-6 (0, 10, 20 and 50
ng/ml) with or without anti-IL-6R mAb (1 μg/ml) for 24 h.
After the incubation, the effects of IL-6 with or without
anti-IL-6R mAb on cell proliferation were determined by a
colorimetric assay using MTT.
Wound-healing migration assay
For the wound-healing migration assay, cells were
seeded in 24-well plates and grown to confluent monolayer
overnight. The monolayer was scratched straight with a fine 10
μl pipette tip, and cellular debris was removed by washing
with PBS. Then the cells were incubated in serum-free medium
containing different concentrations of IL-6 (0, 10 and 50 ng/ml)
with or without anti-IL-6R mAb (1 μg/ml). Migration was
visualized at the indicated times (0, 12, and 24 h) under a
microscope (TE2000, Nikon). The migration distances were measured
by ImageJ analysis software (National Institutes of Health,
Bethesda, MD, USA).
Transwell migration and invasion
assays
Migration and invasion assays were performed using
Transwell 24-well plates with 8-μm diameter filters
(Corning, NY, USA). For invasion assay, filters were precoated with
40 μl of diluted ECM gel for 4 h. The following procedures
were the same for both migration and invasion assays. Approximately
1×105 cells in 200 μl of serum-free medium
containing different concentrations of IL-6 (0, 10 and 50 ng/ml)
with or without anti-IL-6R mAb (1 μg/ml) were placed in the
upper chamber and 500 μl 10% newborn calf serum was placed
in the lower chamber. The plates were incubated for 20–24 h, and
then cells were fixed in methanol for 15 min and stained with 0.1%
crystal violet for 15 min. Cells on the upper side of the filters
were removed with a cotton swab, and the filters were washed with
PBS. Cells on the under side of the filters were examined and
counted under a microscope at ×200 magnification. Each experiment
was repeated at least three times.
Western blot analysis
Following treatment, cells were washed with ice-cold
PBS and lysed in lysis buffer (20 mmol/l Tris-HCl pH 7.5, 1%
Na-deoxycholate, 1% Triton X-100, 150 mmol/l NaCl, and 1 mmol/l
EDTA) on ice. The protein concentration was measured using
bicinchoninic acid (BCA) protein assay kit according to the
manufacturer’s instructions (Beyotime, Shanghai, China). The
proteins were separated on SDS-PAGE and transferred to a
polyvinylidene difluoride membrane (PVDF; Millipore, MA, USA).
Immunoblots were performed by incubating PVDF membranes with 5%
non-fat milk in TBST (Tris-buffered saline and 2.5% Tween-20) for 1
h at room temperature. Then each membrane was incubated with
primary antibodies at 4°C for 20 h. After repeating washing with
TBST, the membrane was incubated with secondary antibodies.
Immunoblots were developed using enhanced chemiluminescence (ECL)
detecting substrate (Pierce, Rockford, IL, USA). Images were
captured with Micro-Chemi (DNR Bio-Imaging Systems, Israel), and
the optical density of the bands was determined using ImageJ
software.
Gelatin zymography analysis
The expression of activated MMPs in conditioned
medium was detected by gelatin zymography. Cells were seeded in
culture flasks and grown to 70–80% confluency. Then the cells were
cultured in serum-free medium containing different concentrations
of IL-6 (0, 10 and 50 ng/ml) with or without anti-IL-6R mAb (1
μg/ml) for 24 h. Afterwards the supernatants were collected
and mixed with 4X SDS sample buffer without reducing agent or
heating. The samples were loaded onto an SDS-polyacrylamide gel
containing 0.1% (w/v) gelatin and subjected to electrophoresis.
Following electrophoresis, the gel was washed with 2.5% Triton
X-100 to remove SDS, and incubated with developing buffer (50 mM
Tris-HCl buffer, pH 7.4, and 10 mM CaCl2) at 37°C for
40–48 h. Then gels were stained with 0.1% Coomassie Brilliant Blue
R-250 (Invitrogen Corp.) for 4–6 h and washed with destaining
solution until clear bands against an intensely stained background
appeared.
Statistical analysis
SPSS software (version 19.0; SPSS, Inc., Chicago,
IL, USA) was used to carry out the statistical analyses. All data
are presented as mean ± standard deviations (SD). Statistical
comparison was performed using Student’s t-test and P<0.05 was
considered statistically significant.
Results
IL-6 and its receptors are expressed
broadly in various NPC cell lines
Receptors involved in the recognition of IL-6 can be
subdivided into two subunits, the IL-6R and gp130. While gp130 is
ubiquitously expressed by cells, the expression of IL-6R is
restricted in selected cells (12). As a consequence, the number of
cells that respond to IL-6 is limited. Considering the relatively
few studies which reported the IL-6 responsiveness of NPC cell
lines, firstly we investigated NPC cell lines for the expression of
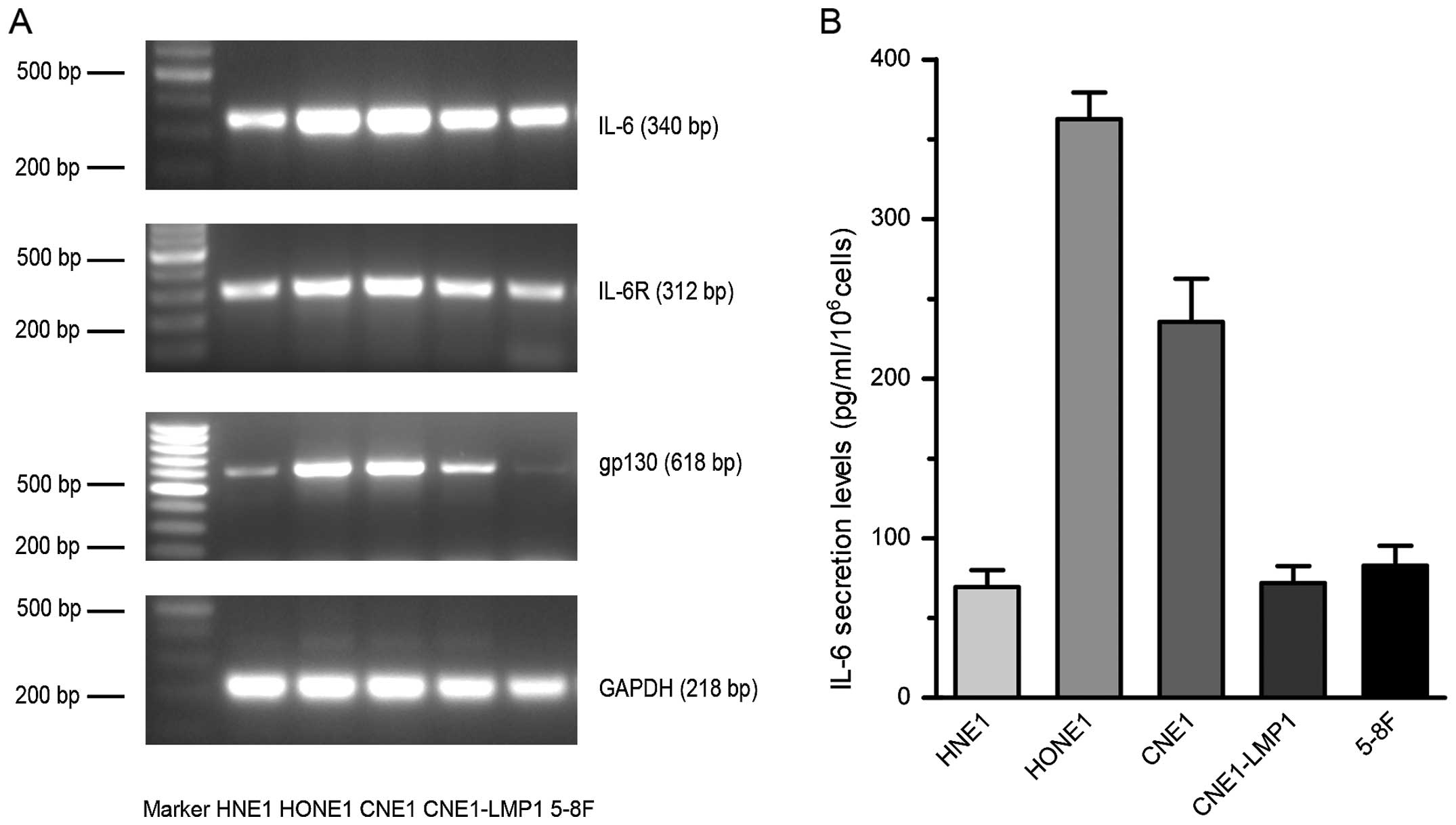
IL-6 and its receptors. All tested NPC cell lines expressed mRNA
for both IL-6 and its receptors though a few bands were faint in
some cell lines (Fig. 1A). The
IL-6 levels in NPC cell lines were also examined and they varied
from 69.3 pg/ml/106 to 362.7 pg/ml/106 cells (Fig. 1B). As all tested cell lines
expressed IL-6 and its receptors broadly, they were suitable to
receive IL-6 stimulation. Given that HNE1 and CNE1-LMP1 had lower
IL-6 levels and might be more sensitive to IL-6 exposure than other
cell lines, we selected them for our subsequent experiments.
IL-6 promotes the proliferation of NPC
cells
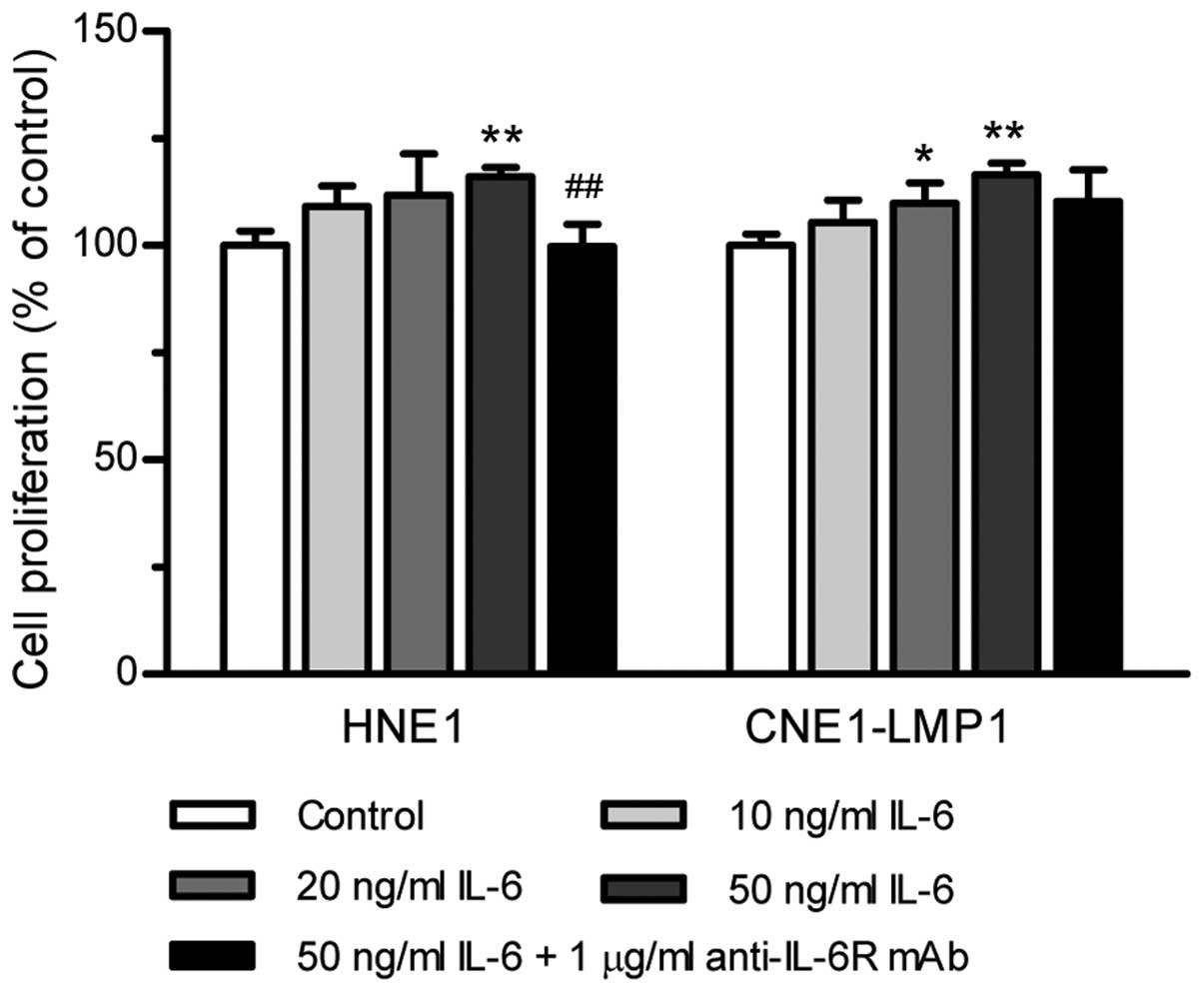
Cell proliferation experiments were performed with
IL-6 added exogenously to NPC cell lines. When HNE1 treated with
increasing concentrations of IL-6 (10, 20 and 50 ng/ml), the
proliferation rate increased slightly with the level of 109, 112
and 116% of untreated cells, respectively (Fig. 2). As shown, the enhancing effect of
IL-6 on CNE1-LMP1 proliferation was also weak. The most pronounced
proliferation rate exerted by IL-6 (50 ng/ml) was only 117%. To
test the specificity of IL-6 stimulation, we added the IL-6
receptor antagonist, anti-IL-6R mAb, to the culture medium to block
IL-6-induced proliferation. The activity of IL-6 on cell
proliferation in both HNE1 and CNE1-LMP1 cells was reversed by
anti-IL-6R mAb (Fig. 2), which
demonstrated that IL-6 elicits its effect upon interaction with its
receptors. All these results suggested that IL-6 can promote the
proliferation of NPC cells although its effect is weak.
IL-6 promotes the migration activity of
NPC cells
The wound-healing migration assay and transwell
migration assay were used to examine the effect of IL-6 on NPC
migration. As shown in Fig. 3A,
IL-6 increased wound-healing migration activity of NPC cells
significantly. When HNE1 and CNE1-LMP1 were incubated with IL-6 (50
ng/ml) for 24 h, the migration rate was increased to 90.4 and
91.0%, respectively (Fig. 3B). We
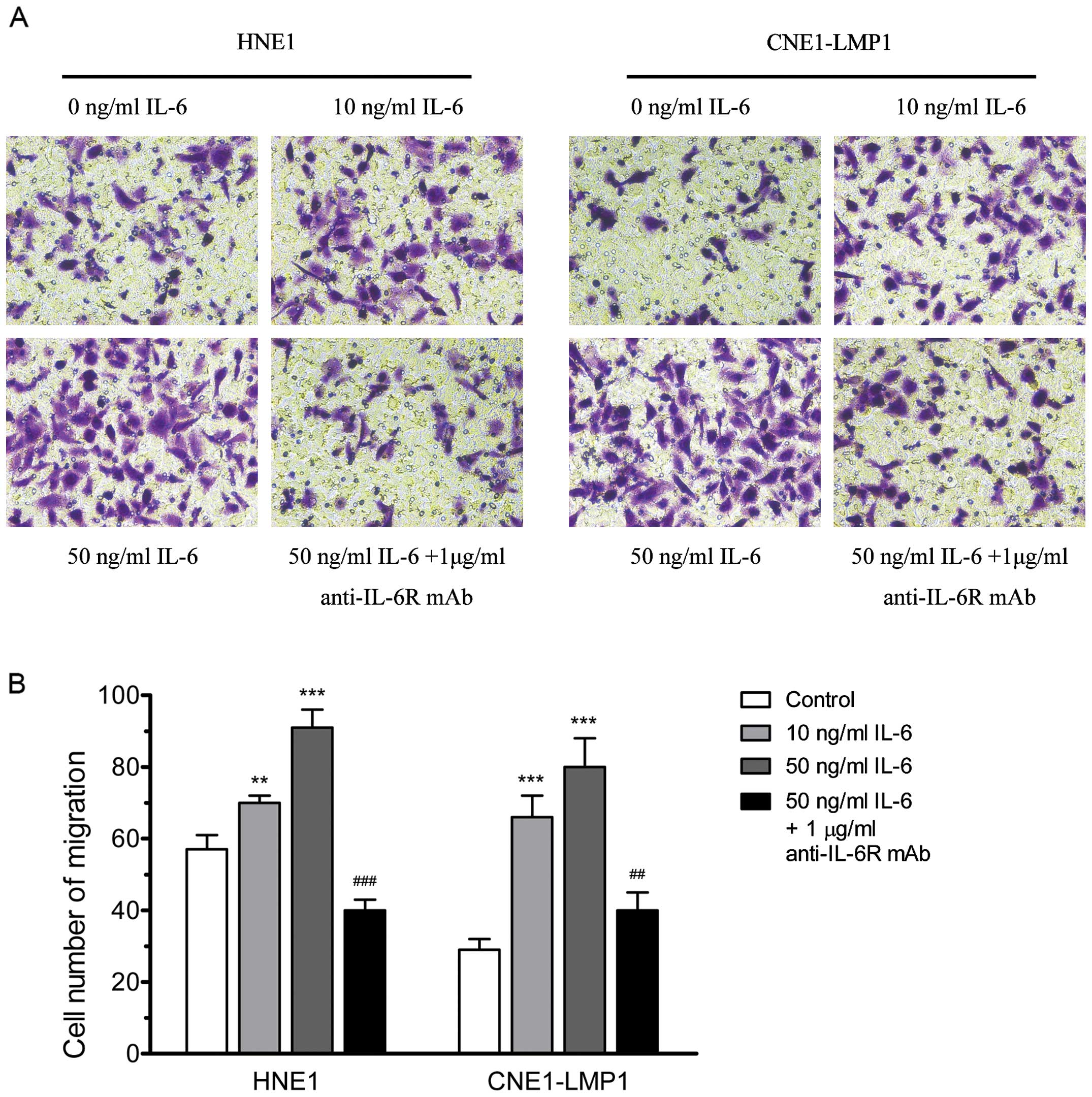
also found that IL-6 can improve the transwell migration activity
of NPC cells. When IL-6 (10 and 50 ng/ml) was added to the upper
compartment of the transwell plates, the number of cells that
migrated to the lower filters increased significantly (Fig. 4). In addition, when HNE1 and
CNE1-LMP1 cells were stimulated by IL-6 (50 ng/ ml) in combination
with anti-IL-6R mAb (1 μg/ml), the IL-6-induced cell
migration was markedly inhibited (Figs. 3 and 4). These data implied that IL-6 could
clearly accelerate the migration ability of NPC cells.
IL-6 promotes the invasion activity of
NPC cells
To examine the effect of IL-6 on NPC invasion,
artificial ECM was precoated to the upper side of all filters.
Treatment with IL-6 (50 ng/ml) accelerated significantly the
invasion ability of HNE1 and CNE1-LMP1 cells. As shown in Fig. 5, the number of cells that invaded
through ECM raised markedly upon IL-6 stimulation. On the contrary,
the IL-6-stimulated invasive ability could be blocked by IL-6R
antibody. The number of cells that penetrated into the lower
chamber decreased upon anti-IL-6R mAb (1 μg/ml) treatment.
These results demonstrated IL-6 can augment the invasiveness of NPC
cells in vitro.
IL-6 upregulates the expression of MMP-2
and MMP-9
We next assessed the IL-6-stimulated NPC cells in
terms of the expression of invasion-linked MMP-2 and MMP-9. As
shown in Figs. 6 and 7, the expression of MMP-2 and MMP-9 was
relatively low in the original HNE1 and CNE1-LMP1 cells, but
increased dose-dependently upon addition of IL-6. On the other
hand, this increase in MMP-2 and MMP-9 expression by IL-6
stimulation could be suppressed by anti-IL-6R mAb. These results
indicated that IL-6-induced invasion of NPC cells might result from
upregulating the expression of MMP-2 and MMP-9, and IL-6R antibody
could suppress the expression of MMP-2 and MMP-9.
Discussion
NPC is highly radiosensitive and, as a consequence,
radiotherapy is the backbone of treatments for this disease. With
the improvement in local control achieved by more precise
radiotherapies, such as the intensity-modulated radiotherapy
(IMRT), local control has been substantially improved and distant
metastasis become the main cause of treatment failure (39). Thus, identifying factors related to
NPC metastasis may provide a potential drug target for preventing
and inhibiting NPC metastasis. In this study, we provided evidence
that IL-6 can promote the migration and invasion of NPC cell lines
and upregulate the expression of MMP-2 and MMP-9.
As with other human solid tumors, NPC is a tissue
and systemic disease. The nasopharyngeal epithelial cells are
continuously exposed to the environmental challenges and NPC stroma
is rich in inflammatory cells (40). Moreover, NPC cells can directly
maintain and amplify the local inflammation process recruiting and
activating additional immune cells in the nasopharyngeal path and
promoting tumor progression (41).
The evidence indicated that inflammation may play a role in NPC
initiation and progression. IL-6 is a pro-inflammatory cytokine
produced primarily by the cells which constitute the tumor
microenvironment. Several studies have shown that serum level of
IL-6 in NPC patients is elevated and correlated with the advanced
stages (29,30,42).
In addition, IL-6 is related to EBV infection which is commonly
observed in NPC. EBV infection can activate the STAT3 and NF-κB
signal cascades in nasopharyngeal epithelial cells, and then
upregulates the expression of their downstream targets, including
IL-6 (43,44). In turn, IL-6 increases
phosphorylated STAT3 levels which permit the EBV LMP1 expression.
This would establish a positive feedback loop of IL-6 induction,
STAT3 phosphorylation, and reinforced LMP1 expression (45). Therefore, targeting of IL-6 might
be considered as a rational therapeutic strategy for treatment of
NPC.
IL-6 has been found to play an important role in
cell proliferation, survival, migration, invasion and angiogenesis
(46). However, the effect of IL-6
on tumor behavior may depend on the tumor cell types (47). In colorectal cancer, IL-6 can only
stimulate proliferation of selected cell lines, as different cells
possess different IL-6 and IL-6R expression capabilities (48). Thus, we tested the expression of
IL-6 and its receptors in NPC cell lines at the start of this
study. In our test, all NPC cell lines expressed IL-6 and its
receptors, indicating that they were suitable to receive IL-6
stimulation. In the subsequent experiments, we observed that IL-6
was able to promote the proliferation of NPC. The amounts of cells
in IL-6 stimulation were increased, although only slightly higher
than that of control group. The weak effect of IL-6 on NPC cell
proliferation may result from the cell type-specificity. We further
examined the effect of IL-6 on the migration and invasion activity
of NPC cells. Our results indicated that IL-6 could promote cell
migration and invasion significantly. Upon IL-6 stimulation, both
the wound-healing rate and cell number of migration and invasion
were clearly accelerated. Based on these findings, we considered
that IL-6 is able to promote malignant behavior especially the
migration and invasion of NPC cell lines.
MMPs, especially MMP-2 and MMP-9, play crucial roles
in tumor invasion and metastasis. A variety of cytokines and growth
factors, such as interleukin 1β (IL-1β), tumor necrosis factor-α
(TNF-α) and epidermal growth factor (EGF), could influence their
expressions (22–25). Some previous studies have indicated
that IL-6 induced the expression of MMP-2 and MMP-9 (26–28).
Thus, we hypothesized that IL-6 may promote the migration and
invasion activity of NPC cells via upregulating the expression of
MMP-2 and MMP-9. The IL-6 stimulation of the expression of MMP-2
and MMP-9 in NPC cells identified in this study was in agreement
with the above studies. MMP-2 and MMP-9 protein levels in both HNE1
and CNE1-LMP1 cells ascended upon IL-6 stimulation. In addition,
the elevation of activated MMP-2 and MMP-9 in conditioned media was
detected by zymography. Our results confirmed that IL-6 could
upregulate the expression of MMP-2 and MMP-9 in NPC cell lines.
Given the importance of IL-6 in various cancers,
IL-6 blockade using monoclonal antibodies directed against IL-6 and
IL-6R have been tested preclinically and clinically (46,49).
Siltuximab (CNTO-328), a human-murine anti-IL-6 monoclonal
antibody, has been applied to a number of preclinical and clinical
trials targeting malignancies ranging from ovarian and renal cancer
to prostate cancer and multiple myeloma (50–55).
Results from these trials showed that siltuximab could inhibit
tumor growth either alone or in combination with cytotoxic
chemotherapies. Tocilizumab, a humanized anti-IL-6R monoclonal
antibody, also shows its effectiveness in treating malignant
diseases including oral cancer, glioma and multiple myeloma
(56–58). In the present study, we
demonstrated that targeting IL-6 with an anti-IL-6R monoclonal
antibody was able to reverse the IL-6-stimulated proliferation,
migration and invasion of NPC cell lines. Moreover, the addition of
anti-IL-6R antibody to IL-6-stimulated NPC cells diminished the
expression of both MMP-2 and MMP-9. The inhibition effect of
anti-IL-6R mAb identified in this study was consistent with the
results of a previous study (59).
In conclusion, we have demonstrated that IL-6 is
able to promote malignant behavior, especially the migration and
invasion of NPC cell lines. The effect of IL-6 on NPC migration and
invasion may result from the upregulation of the expression of
MMP-2 and MMP-9. Targeting IL-6 signaling with anti-IL-6R antibody
was sufficient to reverse the effect of IL-6. Thus, IL-6 or its
related signaling pathway may be a promising target for preventing
and inhibiting NPC metastasis.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (81272491).
References
|
1.
|
Chan AT: Nasopharyngeal carcinoma. Ann
Oncol. 21(Suppl 7): vii308–vii312. 2010.PubMed/NCBI
|
|
2.
|
Wei WI and Mok VW: The management of neck
metastases in nasopharyngeal cancer. Curr Opin Otolaryngol Head
Neck Surg. 15:99–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Huang CJ, Leung SW, Lian SL, Wang CJ, Fang
FM and Ho YH: Patterns of distant metastases in nasopharyngeal
carcinoma. Kaohsiung J Med Sci. 12:229–234. 1996.PubMed/NCBI
|
|
4.
|
Lee AW, Lin JC and Ng WT: Current
management of nasopharyngeal cancer. Semin Radiat Oncol.
22:233–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wang J, Shi M, Hsia Y, et al: Failure
patterns and survival in patients with nasopharyngeal carcinoma
treated with intensity modulated radiation in Northwest China: a
pilot study. Radiat Oncol. 7:22012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar
|
|
7.
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chang CH, Hsiao CF, Yeh YM, et al:
Circulating interleukin-6 level is a prognostic marker for survival
in advanced nonsmall cell lung cancer patients treated with
chemotherapy. Int J Cancer. 132:1977–1985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ravishankaran P and Karunanithi R:
Clinical significance of preoperative serum interleukin-6 and
C-reactive protein level in breast cancer patients. World J Surg
Oncol. 9:182011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Schafer ZT and Brugge JS: IL-6 involvement
in epithelial cancers. J Clin Invest. 117:3660–3663. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yanaihara N, Anglesio MS, Ochiai K, et al:
Cytokine gene expression signature in ovarian clear cell carcinoma.
Int J Oncol. 41:1094–1100. 2012.PubMed/NCBI
|
|
12.
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Muller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Grivennikov S and Karin M: Autocrine IL-6
signaling: a key event in tumorigenesis? Cancer Cell. 13:7–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yi H, Cho HJ, Cho SM, et al: Blockade of
interleukin-6 receptor suppresses the proliferation of H460 lung
cancer stem cells. Int J Oncol. 41:310–316. 2012.PubMed/NCBI
|
|
15.
|
Xie G, Yao Q, Liu Y, et al: IL-6-induced
epithelial-mesenchymal transition promotes the generation of breast
cancer stem-like cells analogous to mammosphere cultures. Int J
Oncol. 40:1171–1179. 2012.
|
|
16.
|
Ara T and Declerck YA: Interleukin-6 in
bone metastasis and cancer progression. Eur J Cancer. 46:1223–1231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43(Suppl): S42–S51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
19.
|
Libra M, Scalisi A, Vella N, et al:
Uterine cervical carcinoma: role of matrix metalloproteinases
(review). Int J Oncol. 34:897–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kurahara S, Shinohara M, Ikebe T, et al:
Expression of MMPs, MT-MMP, and TIMPs in squamous cell carcinoma of
the oral cavity: correlations with tumor invasion and metastasis.
Head Neck. 21:627–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kataoka M, Yamagata S, Takagi H, et al:
Matrix metalloproteinase 2 and 9 in esophageal cancer. Int J Oncol.
8:773–779. 1996.PubMed/NCBI
|
|
22.
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Patterns of MMP-2 and MMP-9 expression in
human cancer cell lines. Oncol Rep. 21:1323–1333. 2009.PubMed/NCBI
|
|
23.
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9
in human cervical and ovarian cancer cell lines by cytokines,
inducers and inhibitors. Oncol Rep. 23:605–614. 2010.
|
|
24.
|
Roomi MW, Monterrey JC, Kalinovsky T,
Niedzwiecki A and Rath M: Modulation of MMP-2 and MMP-9 by
cytokines, mitogens and inhibitors in lung cancer and malignant
mesothelioma cell lines. Oncol Rep. 22:1283–1291. 2009.PubMed/NCBI
|
|
25.
|
Roomi MW, Kalinovsky T, Monterrey J, Rath
M and Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9
in adult human sarcoma cell lines by cytokines, inducers and
inhibitors. Int J Oncol. 43:1787–1798. 2013.
|
|
26.
|
Kossakowska AE, Edwards DR, Prusinkiewicz
C, et al: Interleukin-6 regulation of matrix metalloproteinase
(MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase
(TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood.
94:2080–2089. 1999.PubMed/NCBI
|
|
27.
|
Wang Y, Li L, Guo X, et al: Interleukin-6
signaling regulates anchorage-independent growth, proliferation,
adhesion and invasion in human ovarian cancer cells. Cytokine.
59:228–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wang X, Lee SO, Xia S, et al: Endothelial
cells enhance prostate cancer metastasis via IL-6→androgen
receptor→TGF-β→MMP-9 signals. Mol Cancer Ther. 12:1026–1037.
2013.PubMed/NCBI
|
|
29.
|
Chow KC, Chiou SH, Ho SP, et al: The
elevated serum interleukin-6 correlates with the increased serum
butyrate level in patients with nasopharyngeal carcinoma. Oncol
Rep. 10:813–819. 2003.PubMed/NCBI
|
|
30.
|
Tan EL, Selvaratnam G, Kananathan R and
Sam CK: Quantification of Epstein-Barr virus DNA load,
interleukin-6, interleukin-10, transforming growth factor-beta1 and
stem cell factor in plasma of patients with nasopharyngeal
carcinoma. BMC Cancer. 6:2272006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Horikawa T, Yoshizaki T, Sheen TS, Lee SY
and Furukawa M: Association of latent membrane protein 1 and matrix
metalloproteinase 9 with metastasis in nasopharyngeal carcinoma.
Cancer. 89:715–723. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wong TS, Kwong DL, Sham JS, Wei WI, Kwong
YL and Yuen AP: Clinicopathologic significance of plasma matrix
metalloproteinase-2 and -9 levels in patients with undifferentiated
nasopharyngeal carcinoma. Eur J Surg Oncol. 30:560–564. 2004.
View Article : Google Scholar
|
|
33.
|
Liu Z, Li L, Yang Z, et al: Increased
expression of MMP9 is correlated with poor prognosis of
nasopharyngeal carcinoma. BMC Cancer. 10:2702010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Glaser R, Zhang HY, Yao KT, et al: Two
epithelial tumor cell lines (HNE-1 and HONE-1) latently infected
with Epstein-Barr virus that were derived from nasopharyngeal
carcinomas. Proc Natl Acad Sci USA. 86:9524–9528. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Du CW, Wen BG, Li DR, et al: Latent
membrane protein-1 of Epstein-Barr virus increases sensitivity to
arsenic trioxide-induced apoptosis in nasopharyngeal carcinoma
cell. Exp Oncol. 27:267–272. 2005.PubMed/NCBI
|
|
36.
|
Yan Z, Yong-Guang T, Fei-Jun L, Fa-Qing T,
Min T and Ya C: Interference effect of epigallocatechin-3-gallate
on targets of nuclear factor kappaB signal transduction pathways
activated by EB virus encoded latent membrane protein 1. Int J
Biochem Cell Biol. 36:1473–1481. 2004.
|
|
37.
|
Song LB, Yan J, Jian SW, et al: Molecular
mechanisms of tumorgenesis and metastasis in nasopharyngeal
carcinoma cell sublines. Ai Zheng. 21:158–162. 2002.(In
Chinese).
|
|
38.
|
Li J, Fan Y, Chen J, Yao KT and Huang ZX:
Microarray analysis of differentially expressed genes between
nasopharyngeal carcinoma cell lines 5-8F and 6-10B. Cancer Genet
Cytogenet. 196:23–30. 2010.
|
|
39.
|
Chan AT: Current treatment of
nasopharyngeal carcinoma. Eur J Cancer. 47(Suppl 3): S302–S303.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Gourzones C, Barjon C and Busson P:
Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer
Biol. 22:127–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Liao Q, Guo X, Li X, et al: Analysis of
the contribution of nasopharyngeal epithelial cancer cells to the
induction of a local inflammatory response. J Cancer Res Clin
Oncol. 138:57–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Chang KP, Chang YT, Wu CC, et al:
Multiplexed immuno-bead-based profiling of cytokine markers for
detection of nasopharyngeal carcinoma and prognosis of patient
survival. Head Neck. 33:886–897. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Eliopoulos AG, Stack M, Dawson CW, et al:
Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in
epithelial cells via an NF-kappaB pathway involving TNF
receptor-associated factors. Oncogene. 14:2899–2916. 1997.
View Article : Google Scholar
|
|
44.
|
Lo AK, Lo KW, Tsao SW, et al: Epstein-Barr
virus infection alters cellular signal cascades in human
nasopharyngeal epithelial cells. Neoplasia. 8:173–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Chen H, Hutt-Fletcher L, Cao L and Hayward
SD: A positive autoregulatory loop of LMP1 expression and STAT
activation in epithelial cells latently infected with Epstein-Barr
virus. J Virol. 77:4139–4148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Ataie-Kachoie P, Pourgholami MH and Morris
DL: Inhibition of the IL-6 signaling pathway: a strategy to combat
chronic inflammatory diseases and cancer. Cytokine Growth Factor
Rev. 24:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Weidle UH, Klostermann S, Eggle D and
Kruger A: Interleukin 6/interleukin 6 receptor interaction and its
role as a therapeutic target for treatment of cachexia and cancer.
Cancer Genomics Proteomics. 7:287–302. 2010.PubMed/NCBI
|
|
48.
|
Hsu CP and Chung YC: Influence of
interleukin-6 on the invasiveness of human colorectal carcinoma.
Anticancer Res. 26:4607–4614. 2006.PubMed/NCBI
|
|
49.
|
Sansone P and Bromberg J: Targeting the
interleukin-6/Jak/Stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Hudes G, Tagawa ST, Whang YE, et al: A
phase 1 study of a chimeric monoclonal antibody against
interleukin-6, siltuximab, combined with docetaxel in patients with
metastatic castration-resistant prostate cancer. Invest New Drugs.
31:669–676. 2013. View Article : Google Scholar
|
|
51.
|
Guo Y, Nemeth J, O’Brien C, et al: Effects
of siltuximab on the IL-6-induced signaling pathway in ovarian
cancer. Clin Cancer Res. 16:5759–5769. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Rossi JF, Negrier S, James ND, et al: A
phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6
monoclonal antibody, in metastatic renal cell cancer. Br J Cancer.
103:1154–1162. 2010. View Article : Google Scholar
|
|
53.
|
Hunsucker SA, Magarotto V, Kuhn DJ, et al:
Blockade of interleukin-6 signalling with siltuximab enhances
melphalan cytotoxicity in preclinical models of multiple myeloma.
Br J Haematol. 152:579–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Voorhees PM, Chen Q, Small GW, et al:
Targeted inhibition of interleukin-6 with CNTO 328 sensitizes
pre-clinical models of multiple myeloma to dexamethasone-mediated
cell death. Br J Haematol. 145:481–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Voorhees PM, Manges RF, Sonneveld P, et
al: A phase 2 multicentre study of siltuximab, an
anti-interleukin-6 monoclonal antibody, in patients with relapsed
or refractory multiple myeloma. Br J Haematol. 161:357–366. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Shinriki S, Jono H, Ota K, et al:
Humanized anti-interleukin-6 receptor antibody suppresses tumor
angiogenesis and in vivo growth of human oral squamous cell
carcinoma. Clin Cancer Res. 15:5426–5434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Kudo M, Jono H, Shinriki S, et al:
Antitumor effect of humanized anti-interleukin-6 receptor antibody
(tocilizumab) on glioma cell proliferation. Laboratory
investigation. J Neurosurg. 111:219–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Yoshio-Hoshino N, Adachi Y, Aoki C,
Pereboev A, Curiel DT and Nishimoto N: Establishment of a new
interleukin-6 (IL-6) receptor inhibitor applicable to the gene
therapy for IL-6-dependent tumor. Cancer Res. 67:871–875. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Hsu CP, Chen YL, Huang CC, et al:
Anti-interleukin-6 receptor antibody inhibits the progression in
human colon carcinoma cells. Eur J Clin Invest. 41:277–284. 2011.
View Article : Google Scholar : PubMed/NCBI
|