Introduction
Although several therapeutically useful compounds
have been identified, their pharmacological properties have been
reported using crude extracts (1,2).
Phloroglucinol is a multipurpose compound with diverse application
as an anti-inflammatory with anticarcinogenic properties that has
attracted recent biomedical research interest (3,4). The
etiology of cancer involves the suppression of apoptotic processes
and dysregulation of cellular proliferation, leading to tumor
development (5). The majority of
cancer treatment approaches, such as chemotherapy and radiation
therapy, destroy cancer cells by inducing apoptosis (6,7).
However, cancer cells often develop resistance and many therapies
indirectly activate apoptosis by damaging DNA (8,9).
Insulin-like growth factor-1 (IGF-1) signaling
promotes cell growth and is used as a crucial proliferative marker
in cancer cells (10,11). The IGF-1 receptor (IGF-1R) has a
dominant role in cancer cell survival via autocrine and paracrine
mechanisms to promote oncogenesis (12,13).
Recruitment of these molecules activates
phosphatidylinositol-3-kinase (PI3K)/Akt and Ras/extracellular
signal-regulated kinase (ERK)-mitogen-activated protein kinase
(MAPK) signaling pathways (14).
Activation of PI3K converts phosphatidylinositol
4,5-biphosphate (PIP2) into phosphatidylinositol 3,4,5 phosphate
(PIP3), which leads to plasma membrane localization of
phosphoinositol-dependent kinase-1 (PDK1) via its pleckstrin
homology (PH) domain (15). Akt is
the major mediator of PI3K signaling with a large number of
downstream substrates, including mammalian target of rapamycin
(mTOR) (16).
Furthermore, cross-talk between the PI3K/Akt and the
Ras/ERK-MAPK pathways is involved in protein translation (17). mTOR and p70S6 kinase (p70S6K) are
the primary downstream effectors that activate translation
initiation factors and inactivate regulatory proteins targeting
translation (18). During
apoptosis, Cyclin D activates the cyclin-dependent kinases (Cdks),
such as Cdk4 and Cdk6, which mediates oncogenic actions and
provides an attractive therapeutic target (19). The current study aimed to
investigate the role of phloroglucinol on apoptosis and IGF-1R
signaling pathways in HT-29 colon cancer cells as a potential
therapeutic target in cancer therapy.
Materials and methods
Cell culture
HT-29 colon cancer cells (ATCC HTB-38; ATCC,
Manassas, VA, USA) were grown in 100-mm culture dishes, containing
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS)
(HyClone, Logan, UT, USA) and penicillin/streptomycin (P/S). The
cells were maintained in a humidified environment under a 5%
CO2, 95% air at 37°C. The medium was replaced every 2
days and trypsinized when the cells reached 80% confluence.
Western blot analysis
HT-29 cells were cultured to 60% confluence and then
incubated in serum-free medium (SFM) for 6 h. Phloroglucinol (0–50
μg/ml) was added to SFM for an additional 24 h. Cell extracts were
prepared by washing cultures with phosphate-buffered saline (PBS)
and suspending in extraction lysis buffer [20 mM Tris-HCl (pH 7.4),
150 mM NaCl, 1% NP-40, 1 mM EGTA, 1 mM EDTA, 0.25% Na-deoxycholate,
2.5 mM sodium pyrophosphate] containing protease inhibitors (1 mM
sodium orthovanadate, 1 μg/ml aprotinin, 1 μg/ml pepstatin, 1 μg/ml
leupeptin, 1 mM NaF, 1 mM PMSF) on ice. Cell extracts were
centrifuged at 12,000 rpm for 10 min and the supernatant was used
for western blotting. Sample buffer was added to the total cell
lysate, and the mixed samples were boiled for 10 min at 100°C.
Proteins were separated in 5–15% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).
Membranes were blocked for 90 min at room temperature in blocking
buffer [1% bovine serum albumin (BSA) in TBS-T], followed by
incubation with primary antibodies (1:1,000 in 1% BSA/TBS-T)
overnight at 4°C or for 150 min at room temperature. The membranes
were then washed three times for 10 min in TBS-T and incubated with
horseradish peroxidase-conjugated goat, mouse, or rabbit secondary
antibodies (1:10,000 in 1% BSA/TBS-T). Immunoreactive bands were
detected using an enhanced chemiluminescence western blotting kit
(Amersham Biosciences, Piscataway, NJ, USA).
Cell cycle analysis
The effect of phloroglucinol on cell cycle
progression was determined using a Muse™ cell cycle analyzer from
Millipore (EMD Millipore Co., Hayward, CA, USA). The cells were
cultured in 6-well plates grown to 60% confluence and incubated in
SFM for 6 h, followed by incubation with phloroglucinol (0–50
μg/ml) for 24 h. Cells were collected in 1% FBS-RPMI-1640 medium,
and test reagent was added to each respective tube. Cells were
mixed by vortexing and the reaction was incubated for 30 min at
room temperature in the dark. Following treatment, cells were
stained to evaluate the G0/G1, S and G2/M phase rates.
mRNA expression analysis
The mRNA expression levels of specific genes were
measured by reverse transcription-polymerase chain reaction
(RT-PCR). HT-29 cells were seeded onto 6-well plates at
2×104 cells/well in 2 ml of medium. Cells were cultured
for 2 days and the medium was replaced with SFM for 6 h, followed
by treatment with phloroglucinol (0–50 μg/ml) for 24 h. Cells were
treated with 1 ml TRIzol reagent (Invitrogen, Carlsbad, CA, USA),
and cDNA was synthesized using the oligo(dt) primer (iNtRON
Biotechnology, Seongnam, Korea). The converted cDNA was added to 2X
TOPsimple™ DyeMIX-nTaq (Enzynomics, Daejeon, Korea) and the primer
(Table I) was dissolved in 0.1%
diethylpyrocarbonate (DEPC) water before adding to the reaction
mixture. The amplified products were separated on a 1% agarose gel
stained with Redsafe™ nucleic acid staining solution (iNtRON
Biotechnology).
 | Table IOligonucleotide sequences of the
primer used in RT-PCR analyses. |
Table I
Oligonucleotide sequences of the
primer used in RT-PCR analyses.
| Gene name | Primer sequence |
|---|
| PI3K |
| F | 5′-AGG AGC GGT ACA
GCA AAG AA-3′ |
| R | 5′-GCC GAA CAC CTT
TTT GAG TC-3′ |
| Akt |
| F | 5′-CAA CTT CTC TGT
GGC GCA GTG-3′ |
| R | 5′-GAC AGG TGG AAG
AAC AGC TCG-3′ |
| PTEN |
| F | 5′-TGG AAA GGG ACG
AAC TGG TG-3′ |
| R | 5′-CAT AGC GCC TCT
GAC TGG GA-3′ |
| PDK1 |
| F | 5′-AAG GGT ACG GGC
CTC TCA AA-3′ |
| R | 5′-CCC ACG TGA TGG
ACT CAA AGA-3′ |
| mTOR |
| F | 5′-CGC TGT CAT CCC
TTT ATC G-3′ |
| R | 5′-ATG CTC AAA CAC
CTC CAC C-3′ |
| p70S6K |
| F | 5′-TAC TTC GGG TAC
TTG GTA A-3′ |
| R | 5′-GAT GAA GGG ATG
CTT TAC T-3′ |
| RPS6 |
| F | 5′-AAG GAG AGA AGG
ATA TTC CTG GAC-3′ |
| R | 5′-AGA GAG ATT GAA
AAG TTT GCG GAT-3′ |
| Ras |
| F | 5′-CCC GTC CTC ATG
TAC TGG TC-3′ |
| R | 5′-ATC TTG GAT ACG
GCA GGT CA-3′ |
| Raf |
| F | 5′-GAT GAT GGC AAA
CTC ACG GAT TC-3′ |
| R | 5′-AAG GCA GTC GTG
CAA GCT CA-3′ |
| MEK |
| F |
5′-CGATGGATCCCCCAAGAAGAAGCCGAC G-3′ |
| R |
5′-CGATCTCGAGTTAGACGCCAGCAGCAT G-3′ |
| GAPDH |
| F | 5′-CAG CCG AGC CAC
ATC G-3′ |
| R | 5′-TGA GGC TGT TGT
CAT ACT TCT C-3′ |
4′,6-Diamidino-2-phenylindole (DAPI)
staining assay
The cells were grown to subconfluency on 12-mm
coverslips and exposed to phloroglucinol for 24 h. The monolayer of
cells was washed with PBS and fixed with 1% paraformaldehyde for 10
min at room temperature. Fixed cells were permeabilized with 0.2%
Triton X-100 in PBS for 10 min at room temperature and incubated
with 1 μg/ml of DAPI for 5 min. The condensed chromatin and
fragmented nuclei in DAPI-stained apoptotic cells were viewed under
a confocal microscope.
Statistical analysis
Study results were analyzed using SPSS software
(ver. 18.0; SPSS, Inc., Chicago, IL, USA). Data are presented as
means ± standard deviation. Duncan’s multiple range test was used
to calculate differences between values. Statistical significance
was indicated at P<0.05.
Results
Phloroglucinol induces nuclear hallmarks
of apoptosis in HT-29 cells
To view apoptotic body formation and nuclear
morphological changes characteristic of apoptosis, HT-29 cells were
treated with phloroglucinol (0, 12.5, 25 and 50 μg/ml) for 24 h and
stained with DAPI. Chromatin was visualized by confocal microscopy.
Untreated cells exhibited swelling and increased homogenous
chromatin morphology, while phloroglucinol-treated cells exhibited
fragmented nuclei with densely stained granular condensed chromatin
(Fig. 1).
Phloroglucinol inhibits HT-29 cell growth
by suppressing IGF-1R signaling
Activation of IGF-1R signaling includes PI3K and
MAPK pathways (20), which can be
regulated by IGF binding proteins. To examine the potential role of
PI3K and Akt signaling in mediating the effects of phloroglucinol,
we performed western blots (Fig.
2A) and RT-PCR (Fig. 2B)
analysis. A 24-h treatment with phloroglucinol dose-dependently
suppressed expression of IGF-1R protein and downstream signaling
proteins such as insulin receptor substrate-1 (IRS-1), PI3K, Akt
and PDK1. These results indicate that phloroglucinol could inhibit
IGF-1R signaling related molecules, as well as activate the PI3K
and Akt pathway.
Caspase activation inhibits effects of
phloroglucinol on IGF-1R-regulated proteins
Previous studies have shown that phloroglucinol
induced apoptosis via Fas-induced signaling pathways, and results
from this study confirmed that phloroglucinol downregulated
IGF-1R-related proteins such as PI3K and Akt. To determine whether
Fas-mediated apoptosis inhibited IGF-1R signaling pathways, HT-29
cells were treated with a pan-caspase inhibitor, which diminished
caspase activation and suppressed PI3K and Akt expression levels
(Fig. 3). Thus, IGF-1R signaling
is a target of phloroglucinol-induced apoptosis.
Phloroglucinol inhibits HT-29 cell
survival by suppressing mTOR signaling pathways
Phloroglucinol decreased Akt levels, which has been
reported to be phosphatase and tensin homolog (PTEN)-dependent.
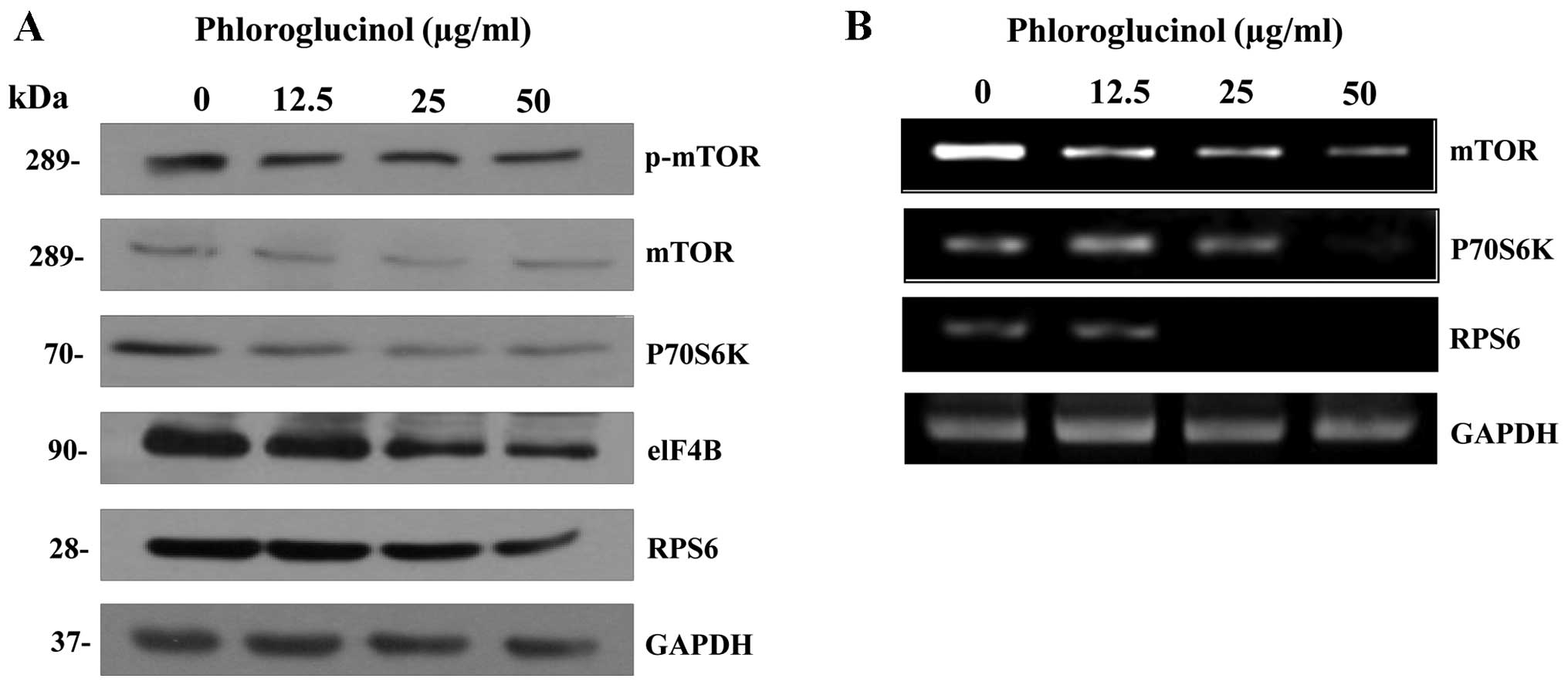
Therefore, we examined the effect of phloroglucinol on mTOR/p70S6K
signaling pathways that regulate cell growth. Phloroglucinol
suppressed protein (Fig. 4A) and
mRNA (Fig. 4B) expression of mTOR
and p70S6K, as well as the translation initiation factors elF4B and
RPS6. Collectively, these data indicate that phloroglucinol
effectively decreases mTOR/p70S6K growth signaling pathways in
colon cancer HT-29 cells.
Phloroglucinol inhibits HT-29 cell growth
by suppressing ERK-MAPK signaling pathways
IGF-1R protein and mRNA expression decreased
significantly after 24 h of phloroglucinol treatment in HT-29 colon
cancer cells. IGF-1R signaling involves activation of Ras, Raf, MEK
and ERK (21), and we determined
that protein (Fig. 5A) and mRNA
(Fig. 5B) expression levels of
Ras, Raf, MEK and phosphorylated ERK were suppressed in
phloroglucinol-treated HT-29 compared with control cells. These
results demonstrate that phloroglucinol inhibits Ras/ERK-MAPK
growth signaling pathways in HT-29 cells.
Effects of phloroglucinol on cell cycle
progression in HT-29 cells
Phloroglucinol-induced apoptosis was sustained via
cell cycle arrest (Fig. 6).
Phloroglucinol increased the ratio of cells in the G0 and G1 phase,
while decreasing the ratio of cells in the G2 and M phases,
suggesting that phloroglucinol inhibits cell cycle progression to
stimulate apoptosis.
Effects of phloroglucinol on cell
cycle-related proteins
To identify the mechanisms mediating
phloroglucinol-induced cell cycle arrest, we examined the
expression of cell cycle regulatory proteins. Western blot analysis
results indicated that the expression of Cdk4, Cdk6, pRb and Cyclin
D, which regulate the G0 and G1 phases, decreased with
phloroglucinol treatment. However, the levels of p21 and p27 were
dose-dependently increased following treatment with phloroglucinol
(Fig. 7). Taken collectively,
these results demonstrate that phloroglucinol inhibits HT-29 cell
proliferation by modulating cell cycle-related proteins.
Discussion
Previous results suggested that phloroglucinol
inhibited growth of HT-29 cells via IGF-1R-mediated signaling
pathways. In this study, we confirmed that phloroglucinol induced
apoptosis and inhibited cell cycle progression by regulating
PI3K/Akt/mTOR and Ras/ERK-MAPK signaling pathways. IGF-1R and
apoptotic pathways play important roles in cancer progression
(19). Reduced expression of
IGF-1R and downstream regulatory proteins inhibits the development
of cancer, and the results presented here demonstrated that
phloroglucinol inhibited cancer by regulating IGF-1R pathways
(Fig. 2). As shown in Fig. 3, a pan-caspase inhibitor suppressed
caspase activation and affected the level of IGF-1R-mediated
PI3K/Akt signaling proteins. Furthermore, we demonstrated that
apoptosis-mediated Fas signaling activity was inhibited by
IGF-1R.
IGF and IGF-1 receptors represent a group of
biological growth transducers involved in diverse pathological
processes (20). IGF-1R is
auto-phosphorylated by its intrinsic tyrosine kinase activity to
facilitate activation of downstream signaling molecules. Activated
lGF-1R and phosphorylated adaptor proteins, such as IRS-1, are
coupled to the PI3K/Akt pathway (21,22).
PI3K/Akt signaling, along with mTOR/p70S6K, are the primary
downstream effectors that activate translation initiation factors
and inactivate their regulators (23). In addition, these pathways
participate in cross-talk with Ras/ERK-MAPK pathways (24). The present results indicated that
phloroglucinol inhibited IGF-1R signaling via mTOR, p70S6K, as well
as Ras, Raf, MEK and phosphorylated ERK molecules (Figs. 4 and 5). The study findings also revealed an
increase in the percentage of cells in the G0 and G1 phase from
26.2 to 44.2%, while the percentage of cells in the remaining
phases decreased following treatment with phloroglucinol from 17.9
to 12.3%, suggesting that phloroglucinol inhibited cell cycle
progression (Fig. 6).
Phloroglucinol-mediated cell cycle arrest was the result of
apoptosis and related to the expression of cyclins and Cdk cell
cycle regulatory proteins (Fig.
7). Thus, phloroglucinol-induced cell cycle arrest resulted in
cell death in proliferative HT-29 colon cancer cells.
The extracellular domain of the IGF receptor is
responsible for ligand binding, inducing the formation of receptor
dimers, and the phosphorylation of tyrosine residues in the
cytoplasmic domain of the receptor through activation of an
intrinsic kinase. The phosphorylated tyrosine residues play a
critical role in intracellular signaling. In cancer cells, IGF-1R
activates IRS-1 by stimulating several intracellular signaling
proteins such as PI3K and Akt kinase (25–27).
Our results provide mechanistic evidence regarding IGF-1R regulated
signaling that includes the PI3K/Akt/mTOR and Ras/ERK-MAPK
pathways. mTOR is a serine/threonine protein kinase that integrates
nutrient availability with growth factor signaling used to modulate
cell proliferation and function (28). Interpretation of these results is
complicated by the common regulatory molecules involved in
apoptosis, such as caspase and the PI3K/Akt/mTOR signaling pathway.
The PI3K/Akt/mTOR signaling pathway is known to play a key role in
cell survival, differentiation and metabolism. Videlicet, an
inhibitor of the PI3K/Akt/mTOR signaling pathway, caused cell death
associated with apoptosis (29,30).
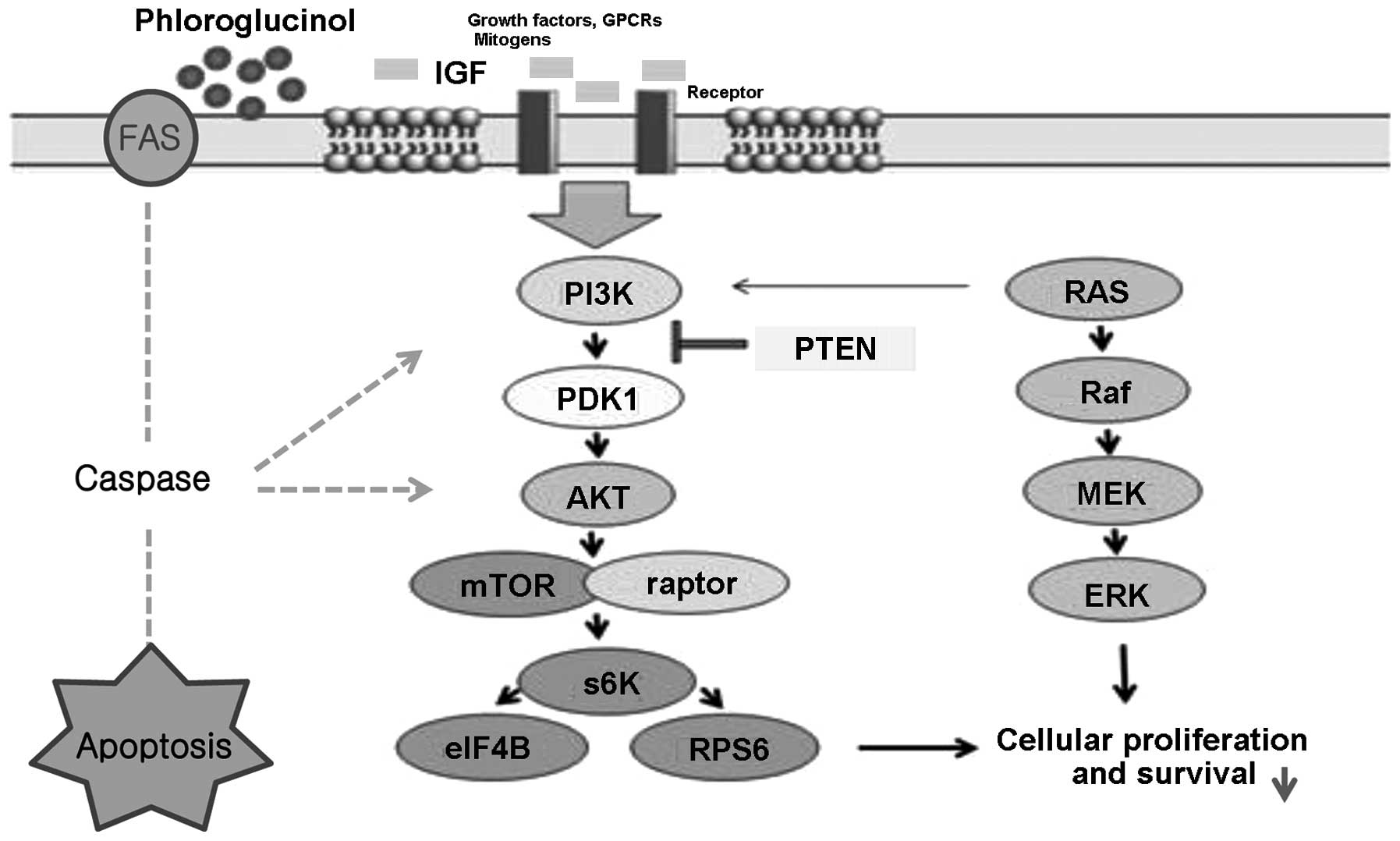
The current results indicate that IGF-1 receptor regulated Akt and
mTOR, as well as other important molecules such as PTEN, PDK-1 and
p70S6K involved in PI3K/Akt/mTOR signaling (Fig. 8).
Likewise, Ras signaling is enhanced due to upstream
events, such as the activation of IGF-1R (31). Regardless of the mechanism,
augmented Ras signaling contributes to a molecular signature for
colon cancers. Ras is a significant oncogene product involved in
the pathogenesis of cancers, and expression of activated Ras is
sufficient to induce oncogenesis in both normal and cancer cells,
supporting the use of common downstream pathways (32,33).
Ras transmits a signal to the serine/threonine kinase Raf, which
subsequently activates MAPK, resulting in cell growth via the
transcriptional activation of multiplex targets (34). Our results demonstrated that IGR-1R
modulated Ras, Raf, MEK and phosphorylated ERK to suppress the
survival of HT-29 colon cancer cells (Fig. 8).
In conclusion, we have demonstrated that
phloroglucinol induced apoptosis via an apoptotic pathway involving
growth factors, accounting for the effect of phloroglucinol on
IGF-1R regulated signaling pathways in HT-29 colon cancer cells.
These findings suggest a vital role for IGF-1R in colon cancer
tumorigenesis, as well as the potential value of phloroglucinol as
a therapeutic agent with anticancer effects on human colon
cancer.
Acknowledgements
This research was supported by Fishery
Commercialization Technology Development Program through iPET
(Korea Institute of Planning and Evaluation for Technology in Food,
Agriculture, Forestry and Fisheries) funded by Ministry of Oceans
and Fisheries (MOF) (111090-03-3-HD110).
References
|
1
|
Matsui T, Omura K, Kawakami K, Morita S
and Sakamoto J: Genotype of thymidylate synthase likely to affect
efficacy of adjuvant 5-FU based chemotherapy in colon cancer. Oncol
Rep. 16:1111–1115. 2006.PubMed/NCBI
|
|
2
|
Oehler C and Ciernik IF: Radiation therapy
and combined modality treatment of gastrointestinal carcinomas.
Cancer Treat Rev. 32:119–138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagayama K, lwamura K, Shibata T, Hirayama
I and Nakamura T: Bactericidal activity of phlorotannins from the
brown alga Ecklonia kurom. J Antimicrob Chemother.
50:889–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park EJ and Pezzuto JM: Botanicals in
cancer chemoprevention. Cancer Metast Rev. 21:231–255. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stan SD, Kar S, Stoner GD and Singh SV:
Bioactive food components and cancer risk reduction. J Cell
Biochem. 104:339–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei A, Zhou D, Xiong C, Cai Y and Ruan J:
A novel non-aromatic β-ring flavonoid: isolation, structure
elucidation and its induction of apoptosis in human colon HT-29
tumor cell via the reactive oxygen species-mitochondrial
dysfunction and MAPK activation. Food Chem Toxicol. 49:2445–2452.
2011.
|
|
7
|
Hsu HF, Houng JY, Kuo CF, Tsao N and Wua
YC: Glossogin, a novel phenylpropanoid from Glossogyne
tenuifolia, induced apoptosis in A549 lung cancer cells. Food
Chem Toxicol. 46:3785–3791. 2008.PubMed/NCBI
|
|
8
|
Kang HS, Chung HY, Kim JY, Son BW, Jung HA
and Choi JS: Inhibitory phlorotannins from the edible brown alga
Ecklonia stolonifera on total reactive oxygen species (ROS)
generation. Arch Pharm Res. 27:194–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MM, Ta QV, Mendis E, Rajapakse N, Jung
WK, Byun HG, Jeon YJ and Kim SK: Phlorotannins in Ecklonia
cava extract inhibit matrix metalloproteinase activity. Life
Sci. 79:1436–1443. 2006.PubMed/NCBI
|
|
10
|
Lin ML, Chen SS, Lu YC, Liang RY, Ho YT,
Yang CY and Chung JG: Rhein induces apoptosis through induction of
endoplasmic reticulum stress and Ca2+-dependent
mitochondrial death pathway in human nasopharyngeal carcinoma
cells. Anticancer Res. 27:3313–3322. 2007.PubMed/NCBI
|
|
11
|
Debatin KM and Krammer PH: Death receptors
in chemotherapy and cancer. Oncogene. 23:2950–2966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu W, Lee SK, Jung MJ, Heo SI, Hur JH and
Wang MH: Induction of cell cycle arrest and apoptosis by the ethyl
acetate fraction of Kalopanax pictus leaves in human colon
cancer cells. Bio Resour Technol. 101:9366–9372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Degterev A and Yuan J: Expansion and
evolution of cell death programmes. Nat Rev Mol Cell Biol.
9:378–390. 2008. View
Article : Google Scholar
|
|
14
|
Tan ML, Ooi JP, Ismail N, Moad Al and
Muhammad TS: Programmed cell death pathways and current antitumor
targets. Pharm Res. 26:1547–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maddika S, Ande SR, Wiechec E, Hansen LL,
Wesselborg S and Los M: Akt-mediated phosphorylation of CDK2
regulates its dual role in cell cycle progression and apoptosis. J
Cell Sci. 121:979–988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grimm S and Brdiczka D: The permeability
transition pore in cell death. Apoptosis. 12:841–855. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cryns V and Yuan J: Proteases to die for.
Genes Dev. 12:1551–1570. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sachdev D, Li SL, Hartell JS,
Fujita-Yamaguchi Y, Miller JS and Yee D: A chimeric humanized
single chain antibody against the type I insulin-like growth factor
(IGF) receptor renders breast cancer cells refractory to the
mitogenic effects of IGF-1. Cancer Res. 63:627–635. 2003.
|
|
21
|
Segrelles C, Moral M, Lara MF, Ruiz S,
Santos M, Leis H, Garcia-Escudero R, Martinez-Cruz AB,
Martinez-Palacio J, Hernandez P, Ballestin C and Paramio JM:
Molecular determinants of Akt-induced keratinocyte transformation.
Oncogene. 25:1174–1185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH,
Kim BW, Choi HY, Jeong MY and Cho SG: Interplay between PI3K/Akt
and MAPK signaling pathways in DNA-damaging drug-induced apoptosis.
Biochim Biophys Acta. 1763:958–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signaling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhandari BK, Feliers D, Duraisamy S,
Stewart JL, Gingras AC, Abboud HE, Choudhury GG, Sonenberg N and
Kasinath BS: Insulin regulation of protein translation repressor
4E-BP1, an elF4E-binding protein, in renal epithelial cells. Kidney
Int. 59:866–875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oldham S and Hafen E: Insulin/IGF and
target of rapamycin signaling: ATOR de force in growth control.
Trends Cell Biol. 13:79–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bjornsti MA and Houghton PJ: The TOR
pathway: a target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
White MF: The IRS-signaling system: a
network of docking proteins that mediate insulin and cytokine
action. Recent Prog Horm Res. 53:119–138. 1998.
|
|
28
|
Mori H, Inoki K, Opland D, Munzberg H,
Villanueva EC, Faouzi M, Guan KL, et al: Critical roles for the
TSC-mTOR pathway in β-cell function. Am J Physiol Endocrinol Metab.
297:E1013–E1022. 2009.
|
|
29
|
Wan X, Harkavy B, Shen N, Grohar P and
Helman L: Rapamycin induces feedback activation of Akt signaling
through an IGF-1R dependent mechanism. Oncogene. 26:1932–1940.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O’Reilly KE, Rojo F, She QB, Solit D,
Mills GB, Smith D, Lane H, Hofmann F, et al: mTOR inhibition
induces upstream receptor tyrosine kinase signaling and activates
Akt. Cancer Res. 66:1500–1508. 2006.PubMed/NCBI
|
|
31
|
Quevedo C, Salinas M and Alcazar A:
Regulation of cap-dependent translation by insulin-like growth
factor-1 in neuronal cells. Biochem Biophys Res Commun.
291:560–566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roux PP, Shahbazian D, Vu H, Holz MK,
Cohen MS, Taunton J, Sonenberg N and Blenis J: RAS/ERK signaling
promotes site-specific ribosomal protein S6 phosphorylation via RSK
and stimulates cap-dependent translation. J Biol Chem.
282:14056–14064. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kiaris H and Spandidos D: Mutations of ras
genes in human tumors (review). Int J Oncol. 7:413–421.
1995.PubMed/NCBI
|
|
34
|
Treisman R: Regulation of transcription by
MAP kinase cascades. Curr Opin Cell Biol. 8:205–215. 1996.
View Article : Google Scholar : PubMed/NCBI
|