Tumor microenvironment, a vastly complicated network
composed of various cell populations, soluble factors, signaling
molecules and extracellular matrix components, orchestrates the
behavior of tumor progression (1).
Amid the growing interest in elucidating individual players in the
tumor microenvironment, IL-8 appears markedly important and has
been presented as one of the prominent promoters of tumor
progression. IL-8 is involved in cancer related inflammation. A
typical example is that, Epstein-Barr virus (EBV)-associated,
undifferentiated type of nasopharyngeal carcinoma (NPC) which is
characterized by several inflammation-like features. The
inflammation-like microenvironment is crucial for the development
of NPC progression. Notably, EBV infection as a critical factor for
cancer progression can induce IL-8 secretion. EBV lytic
transactivator Zta, which exerting its effect through bingding to
Zta-responsive elements, resides in the IL-8 promoter (2).
A pivotal step to establish the progression of a
tumor is the obtainment of aggressive characteristics by carcinoma
cells. A primary process triggering tumor invasion is EMT through
which cancer epithelial cells lose their epithelial properties and
trans-differentiate to a migratory mesenchymal phenotype (3). EMT has recently been recognized as a
key player contributing to tumor progression and the mechanisms
regulating this process have been linked to metastasis and cancer
stem cell-like cell formation (4,5).
Various signaling events have been proposed to facilitate EMT in a
variety of human tumors. However, initiating EMT in a tumor is
mainly dependent on multiple soluble mediators in the surrounding
microenvironment (6).
In this review, we aim at elucidating the complex
interactions of IL-8 induction of EMT through direct and indirect
mechanisms. We introduce current research on the cytokines,
pro-inflammatory mediators and enzymes secreted by neutrophils and
TAMs and the mechanism of their induction of EMT. Additionally, we
address the potential therapeutic implications of IL-8 cancer
treatment.
EMT originally takes place during the process of
embryogenesis, but it also occurs in adult tissues going through
wound healing and remodeling (7).
Moreover, in some certain pathological process it is associated
with fibrosis and tumor progression. During the EMT process,
epithelial cancer cells evolve to a mesenchymal phenotype, by
losing their epithelial characteristics and acquiring a
fibroblastoid-like morphology especially at the invasive front.
Cells undergoing EMT reduce cell polarity and adhesion, exhibit
decreased expression of epithelial surface molecules such as
E-cadherin and cytokeratins. In parallel, epithelial tumor cells
acquire enhanced presentation of mesenchymal proteins such as
vimentin and fibronectin as well as increased cell motility,
invasiveness and metastasis (8).
Recent studies have focused on EMT in the tumor
biology context, since acquisition of mesenchymal features is
linked to an improved invasive capacity, that is, could promote
tumor infiltrating growth and metastasis (9). Multiple studies have shown that the
involvement of EMT is related to tumor progression in different
tumor types (10–12). For instance, in adenoid cystic
carcinoma which is characteristed by local infiltration and distant
metastasis, EMT is considered to promote greatly the high rate of
metastasis (13). Consistently,
downregulation of epithelial marker E-cadherin and increased
expression of the mesenchymal markers N-cadherin and vimentin have
noted to positively correlate with the aggressiveness and
metastasis of breast cancer (14).
Additionally, several reports have shown that cancer
cells undergoing EMT present properties of cancer stem cells (CSC)
(5), including chemo- and
radio-resistance and the ability to self-renewal. Fan demonstrated
that hepatocellular carcinoma cells undergoing EMT acquire enhanced
CSC-like traits when co-cultured with TAM. Furthermore, depletion
of TGF-β1 blocked acquisition of the CSC-like properties by
inhibition of TGF-β1-induced EMT (15). Increasing number of studies have
identified distinct signaling pathways regulating this step
(16,17).
IL-8 (alternatively known as CXCL8), a prototype of
the cysteine-X-cysteine (CXC) chemokines, was originally discovered
as a leukocyte chemoattractant (18) and subsequently found to play
multiple roles in cancer development (3). Human genes for IL-8 are located on
chromosome 4 between 4q13 and 4q21 (19). IL-8 is mainly secreted from
leukocytes and endothelial cells under special conditions such as
exposure to IL-1 or TNF-α. Additionally, fibroblasts and malignant
tumor cells can also secrete IL-8 as a result of various
environmental stress including hypoxia, and chemotherapy agents
(20). Since existing in monomer
or dimer forms, IL-8 activates and regulates its two cell surface
receptors respectively (21).
IL-8 exerts its effect by binding to the IL-8Rs,
which are two heterotrimeric G protein-coupled receptors, CXCR1 and
CXCR2. The two receptors are primarily presented in neutrophils,
monocytes as well as endothelial cells. However, they are also
found on the surface of tumor cells and tumor-associated stromal
cells (22). The two receptors
show different binding specificities as a result of differences in
their N-terminal domains (23).
CXCR1 binds IL-6 and IL-8, while CXCR2 has high binding affinity
for IL-1, 2, 3, 5, 6, 7 and 8 (24).
It has been reported that IL-8 is regulated by
microRNA network at post-transcription level (34) and significant correlation between
microRNAs and IL-8 is identified in a variety of studies. miR-302c
was found to inhibit IL-8 expression and restrain tumor invasion
and metastasis. In parallel, IL-8 signaling also exerts a feedback
effect on modulating miR-302c and IL-8 expression (35). Qu et al suggested that IL-8
was a direct target of miR-203 and miR-23a. Reduced expression of
the two miRNAs promoted nasopharyngeal carcinoma radioresistance
through IL-8/AKT signaling and IL-8/Stat3 pathway respectively
(36,37). Additionally, hsa-miR-200c-3p
directly reduced IL-8 expression in inflamed colon of patients with
ulcerative colitis (38).
Moreover, IL-8 can be suppressed by diverse miRNAs such as miR-K9,
miR-K5, miR-17, miR-484, and miR-148a, through indirect manner
(34,39–41).
On the other hand, microRNA network has been found to be very
important in tumor initiation and progression. Several
anti-metastatic miRNAs have been identified in a number of cancers,
such as miR-335, miR-126, and let-7 family. In addition to
anti-metastatic miRNAs, a number of miRNAs are pro-metastatic such
as miR-21, miR-373 and miR-520c (42,43).
Tumor cells passing through EMT have been
indentified to secrete more chemokine IL-8 as well as to enhance
the expression of its receptors. Tumor-derived IL-8 exerts its
effect through an autocrine loop to maintain the mesenchymal traits
of tumor cells. Furthermore, IL-8 recruits neutrophils and TAMs to
the tumor site via a paracrine fashion. In this review, we
highlight the cytokines, pro-inflammatory mediators and enzymes
secreted by neutrophils and TAMs as well as the mechanism of their
induction of EMT.
It is demonstrated that, once through EMT, tumor
cells maintain their mesenchymal state by ongoing autocrine
signaling loops (44). IL-8
stimulates tumor EMT by activation of various signaling pathways
that finally affect the EMT-related transcription factors (Fig. 1). The transcription factors Slug,
Snail, and Twist are known to bind to the E-box regulatory regions
to repress the expression of E-cadherin (7). Recently, a T-box transcription factor
brachyury was identified as a novel trigger of tumor EMT (45). Therefore, the mesenchymal
transition occurs. In return, the induction of EMT via Snail
upregulation is noted to induce IL-8 secretion. Since Snail could
bind to E3/E4 boxes residing in the IL-8 promoter, it directly
regulates the expression of IL-8 (46).
IL-8 has been shown to activate AKT signaling in
prostate cancer, nasopharyngeal carcinoma (NPC) and thyroid cancer
(TC) cell lines (47–49). The serine/threonine kinase AKT, a
downstream target of PI3K, phosphorylated glycogen synthase kinase
3β (GSK3β) which induced the phosphorylation and translocation of
Snail and Slug (50,51). Thereby, inhibiting GSK-3β activity,
AKT activates Snail and Slug indirectly, leading to EMT. In NPC S18
cells, the elevated level of phosphorylated AKT could be suppressed
by knocking down IL-8 expression using short-hairpin RNA. Moreover,
IL-8-promoted EMT could be inhibited by knocking down AKT
expression or applying the PI3K inhibitor LY294002. Besides,
suppression of AKT has been shown to revert EMT and stemness
responses of TC cells.
MAPK/ERK signaling is accepted as one of the most
important regulators in EMT. Via activating small G proteins, IL-8
promotes activation of the MAPK signaling which is characterized by
Raf/MEK/ERK cascade (52). ERK
translocates to the nucleus and upregulates the activity of several
EMT-related transcription factors such as Snail, Slug and Twist.
Therefore, the expression of E-cadherin is suppressed (53,54).
In addition, IL-8 has been found to induce EMT and promote
hepatocellular carcinoma (HCC) cell migration and invasion through
JAK2/STAT3/Snail signaling pathway (55).
Additionally to the E-box transcription factors,
Brachyury, the T-box transcription factor, has been discovered to
promote tumor EMT and cancer cell metastasis in multiple types of
human cancer (45). In breast
cancer cells, IL-8 induces the overexpression of Brachyury and a
mesenchymal-like phenotype. Furthermore, Brachyury displays
increased expression of IL-8 and CXCR1/2, which amplified the
effect of IL-8 on tumor EMT (56).
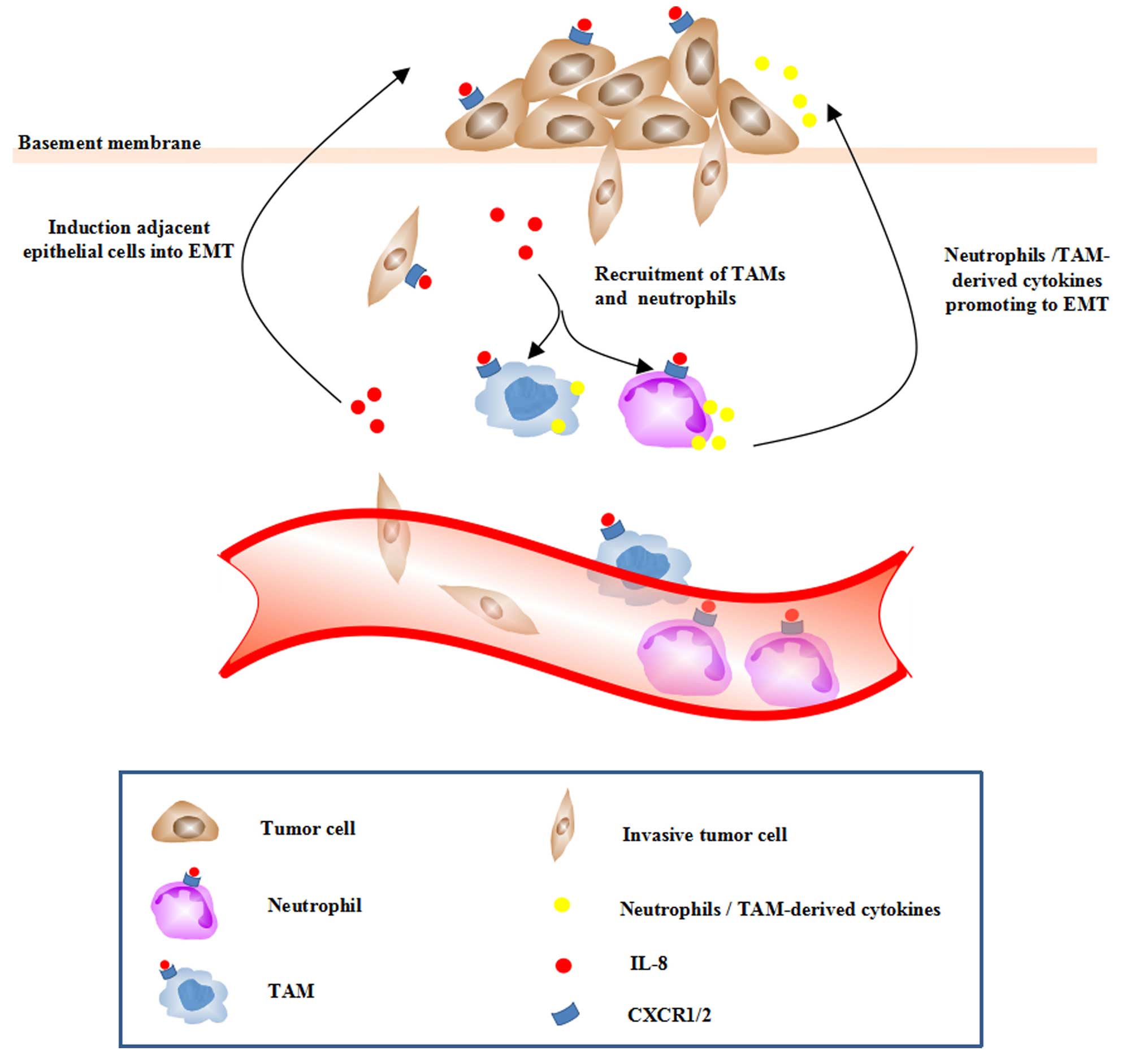
Besides the autocrine loop between IL-8 and tumor
cells that have gone through EMT, IL-8 could potentiate adjacent
epithelial tumor cells into a mesenchymal phenotype via a paracrine
mode (Fig. 2). Tumor cells
undergoing EMT exhibit an elevated level of IL-8 as well as
CXCR1/2, which amplified the effect of IL-8 on tumor EMT. Besides
the effects on tumor cells, IL-8 is identified as an important
regulator of neutrophils and TAMs recruited into the tumor
microenvironment.
Macrophages infiltrated in the tumor sites have been
shown to induce EMT of HCC cells. TAMs can secrete a vast diversity
of cytokines, chemokines and proteases that may influence tumor
cells in various ways. Mast cells induced EMT and stem-like traits
of TC cells (49). The enhanced
intratumoral IL-8 expression could also lead to enhanced
recruitment of neutrophils and TAMs, which, in turn, have been
found to secrete various cytokines, chemokines, enzymes promoting
EMT. Various cytokines (TGF-β1, TNF-α, IL-4, IL-6 and IL-10)
secreted by activated macrophages in the cholangiocarcinoma context
were shown to induce EMT via elevating the expression of
EMT-related genes (57).
Cytokines and chemokines function mainly by binding
to certain transmembrane receptors of tumor cells, which are
members of a large family of G protein-coupled receptors. In
parallel, some enzymes act on the extracellular matrix (ECM),
breakdown connective tissue, inhibit E-cadherin synthesis and
promote the mesenchymal phenotype in tumor cells.
As one of the most important members of the
transforming growth factor family, transforming growth factor-β
(TGF-β) is a potential inducer of EMT in cancer cells (58,59).
TGF-β mediates EMT via two specific pathways, a Smad-dependent
pathway and a Smad-independent pathway (60). After binding to its receptor, TGF-β
phosphorylates Smad2 and Smad3, which collaborate with Smad4, and
then translocate into the nucleus to regulate the transcription of
EMT-associated genes, like Snail (61). Bonde et al found that TAMs
induced intratumoral epithelial cell EMT via TGF-β/Smad signaling.
Data presented in his study identified that macrophage-derived
TGF-β led to decreased expression of the epithelial adhesion,
increased expression of mesenchymal markers and an aggressive
phenotype (62). Additionally,
cancer-associated fibroblasts could also promote EMT of breast
cancer cells through paracrine TGF-β1 (63). Collective studies have shown TGF-β1
induced EMT mainly through Smad, MAPK, PI3K/AKT and ERK
pathway.
Several studies now indicate that EGF activation can
break cell adhesion, enhance cell motility and promote tumor EMT
(64,65). In EGF-treated cholangiocarcinoma
cells, EMT-transcription factors as well as mesenchymal markers
were induced. In addition, the EGF-mediated EMT can be suppressed
by gefitinib, the inhibitor of EGFR (66).
IL-6 has been suggested to induce EMT in breast,
colorectal, prostate and lung cancer cells (67–70)
via aberrant activation of JAK/STAT3 signaling. Additionally, IL-6
boosted the expression of Snail induced by TGF-β/Smad pathway,
contributing greatly to EMT (61,71).
Similarly to IL-6, IL-1β contributes to EMT via
different pathways. IL-1β enhanced binding of Zeb1 to the E-box to
silence E-cadherin expression (72). IL-1β has also been reported to
promote the expression of E-cadherin by upregulaing Snail (73). In addition, cooperated with TGFβ-3,
IL-1β activated matrix metalloproteinase (MMP)-1, MMP-3, and MMP-10
gene expression in A549 lung adenocarcinoma cells through
MAPK-dependent pathways, and both cytokines stimulated EMT and
invasion (74).
Tumor necrosis factor α (TNF-α), which is primarily
derived from by macrophages, is one of the critical
pro-inflammatory cytokines involved in the tumor microenvironment
(75). Several studies suggest
that TNF-α induces EMT via NF-κB or AKT/GSK signaling through
regulating the expression of Twist and Snail in breast, renal,
colon and hypopharyngeal cancer (4,76).
Collectively, the evidence indicates that TNF-α may affect the key
processes of tumor EMT.
TAM with M2 phenotype could produce a chemokine
called chemokine (C-C motif) ligand 18 (CCL18) (77) which exerts its activity mainly by
binding to the transmembrane receptor- PYK2 N-terminal domain
interacting receptor 1 (Nir1). Nir1 is present in human breast
cancer cells (78) and it could
induce EMT by stabilising Snail via the PI3K/AKT/GSK3β signaling
pathway through binding to CCL18 in vitro and in vivo
(79).
Studies have shown that neutrophil-derived elastase
could degradate E-cadherin leading to dyshesion of the pancreatic
ductal adenocarcinoma and HCC cells. Furthermore, the EMT
transcription factor Twist was upregulated, Zeb1 appeared in the
nucleus, β-catenin translocated into the nucleus, and keratins were
downregulated (80). In addition,
the MMPs that exist in the ECM are associated with various EMT
processes. The MMPs have the ability to degrade the functional
components of the ECM and contribute to tumor cell migration.
Therefore, the mesenchymal transition occurs and each EMT case
involves a subset of specific MMPs.
Overexpression of MMP-9 in a prostate cancer model
confirmed the association of MMP-9 with tumor invasiveness
(81). It has also been found in a
gastric carcinoma model that IL-8 upregulates MMP-9 expression and
consequently increased neoangiogenesis (82). Besides, TNF-α induces the
expression of invasion mediators MMP-7, MMP-9, and the
intracellular adhesion molecule-1.
Taken together, various growth factors, cytokines,
chemokines as well as enzymes secreted by TAMs and neutrophils can
facilitate EMT of tumor cells.
As IL-8 is associated with EMT and tumor
progression, it is of interest to speculate that therapy targeting
IL-8 could improve tumor outcome. Blockade of the IL-8 and its
receptors seems a promising therapeutic approach which could
reverse the metastatic phenotype of tumor cells undergoing EMT by
disturbing the autocrine positive loop between IL-8 and tumor
cells. Additionally, it could also reduce the paracrine signals
that IL-8 exerted on other non-metastatic tumor cells by lessening
recruitment of neutrophils and TAMs. CXCR2 is upregulated in some
types of tumors (83–85) and pharmacological inhibition of
CXCR1 and CXCR2 represses neutrophil recruitment into A547 lung
tumor sites resulting in slower tumor growth (86). Small-molecule antagonists for CXCR2
and CXCR1 have been proposed to inhibit IL-8 functions.
Studies show that CXCR1 blockade by either a CXCR1
specific blocking antibody or repertaxin, a small-molecule CXCR1
inhibitor, selectively depleted human breast cancer stem cells
(87). In addition, selectively
targeting CXCR2/CXCR1 with orally active small-molecule inhibitors
is an effective therapeutic approach for repressing melanoma growth
and angiogenesis (88).
CXCR2/CXCR1 antagonists may be a useful therapeutic agent in the
treatment of many other cancers, such as lung carcinomas.
Small-molecule antagonists for CXCR2 and CXCR1 may
represent a promising target for cancer therapy. A better
understanding of the function of IL-8 and further knowledge on the
interaction between IL-8 and tumor microenvironment may open the
way to innovative therapeutic strategies for cancer patients.
EMT plays a central role in tumor invasion and
metastasis and may be induced by local inflammation. In this
review, we focused on IL-8, because it is a major component of the
infiltrates present in the tumor microenvironment and plays a vital
role in the tumor progression and metastasis. We highlight the
cross-link between inflammation and EMT-related tumor development.
IL-8 induction of EMT, despite being sophisticated and requiring
solid experimental investment, opens new horizons for an efficient
tumor therapy.
This study was supported by National Basic Research
Program of China (973 program) no. 2012CB9333004, National Natural
Science Foundation of China (81272360) and National Natural Science
Foundation of China (81472473).
|
1
|
Catalano V, Turdo A, Di Franco S, Dieli F,
Todaro M and Stassi G: Tumor and its microenvironment: A
synergistic interplay. Semin Cancer Biol. 23B:522–532. 2013.
View Article : Google Scholar
|
|
2
|
Hsu M, Wu SY, Chang SS, Su IJ, Tsai CH,
Lai SJ, Shiau AL, Takada K and Chang Y: Epstein-Barr virus lytic
transactivator Zta enhances chemotactic activity through induction
of interleukin-8 in nasopharyngeal carcinoma cells. J Virol.
82:3679–3688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabbah M, Emami S, Redeuilh G, Julien S,
Prévost G, Zimber A, Ouelaa R, Bracke M, De Wever O and Gespach C:
Molecular signature and therapeutic perspective of the
epithelial-to-mesenchymal transitions in epithelial cancers. Drug
Resist Updat. 11:123–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gravdal K, Halvorsen OJ, Haukaas SA and
Akslen LA: A switch from E-cadherin to N-cadherin expression
indicates epithelial to mesenchymal transition and is of strong and
independent importance for the progress of prostate cancer. Clin
Cancer Res. 13:7003–7011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sawada K, Mitra AK, Radjabi AR, Bhaskar V,
Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A,
Kenny HA, et al: Loss of E-cadherin promotes ovarian cancer
metastasis via alpha 5-integrin, which is a therapeutic target.
Cancer Res. 68:2329–2339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao ZL, Ma SR, Wang WM, Huang CF, Yu GT,
Wu TF, Bu LL, Wang YF, Zhao YF, Zhang WF, et al: Notch signaling
induces epithelial-mesenchymal transition to promote invasion and
metastasis in adenoid cystic carcinoma. Am J Transl Res. 7:162–174.
2015.PubMed/NCBI
|
|
14
|
Sarrió D, Rodriguez-Pinilla SM, Hardisson
D, Cano A, Moreno-Bueno G and Palacios J: Epithelial-mesenchymal
transition in breast cancer relates to the basal-like phenotype.
Cancer Res. 68:989–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao
L, Li R, Zhao QD, Yang Y, Lu ZH, et al: Tumor-associated
macrophages promote cancer stem cell-like properties via
transforming growth factor-beta1-induced epithelial-mesenchymal
transition in hepatocellular carcinoma. Cancer Lett. 352:160–168.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Javle MM, Gibbs JF, Iwata KK, Pak Y,
Rutledge P, Yu J, Black JD, Tan D and Khoury T:
Epithelial-mesenchymal transition (EMT) and activated extracellular
signal-regulated kinase (p-Erk) in surgically resected pancreatic
cancer. Ann Surg Oncol. 14:3527–3533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horiguchi K, Sakamoto K, Koinuma D, Semba
K, Inoue A, Inoue S, Fujii H, Yamaguchi A, Miyazawa K, Miyazono K,
et al: TGF-β drives epithelial-mesenchymal transition through
δEF1-mediated downregulation of ESRP. Oncogene. 31:3190–3201. 2012.
View Article : Google Scholar :
|
|
18
|
Matsushima K, Baldwin ET and Mukaida N:
Interleukin-8 and MCAF: Novel leukocyte recruitment and activating
cytokines. Chem Immunol. 51:236–265. 1992.PubMed/NCBI
|
|
19
|
Brat DJ, Bellail AC and Van Meir EG: The
role of interleukin-8 and its receptors in gliomagenesis and
tumoral angiogenesis. Neuro-oncol. 7:122–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie K: Interleukin-8 and human cancer
biology. Cytokine Growth Factor Rev. 12:375–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nasser MW, Raghuwanshi SK, Grant DJ, Jala
VR, Rajarathnam K and Richardson RM: Differential activation and
regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J
Immunol. 183:3425–3432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stillie R, Farooq SM, Gordon JR and
Stadnyk AW: The functional significance behind expressing two IL-8
receptor types on PMN. J Leukoc Biol. 86:529–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy PM: The molecular biology of
leukocyte chemoattractant receptors. Annu Rev Immunol. 12:593–633.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Végran F, Boidot R, Michiels C, Sonveaux P
and Feron O: Lactate influx through the endothelial cell
monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway
that drives tumor angiogenesis. Cancer Res. 71:2550–2560. 2011.
View Article : Google Scholar
|
|
26
|
Araki S, Omori Y, Lyn D, Singh RK,
Meinbach DM, Sandman Y, Lokeshwar VB and Lokeshwar BL:
Interleukin-8 is a molecular determinant of androgen independence
and progression in prostate cancer. Cancer Res. 67:6854–6862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Millar HJ, Nemeth JA, McCabe FL, Pikounis
B and Wickstrom E: Circulating human interleukin-8 as an indicator
of cancer progression in a nude rat orthotopic human non-small cell
lung carcinoma model. Cancer Epidemiol Biomarkers Prev.
17:2180–2187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rofstad EK and Halsør EF: Vascular
endothelial growth factor, interleukin 8, platelet-derived
endothelial cell growth factor, and basic fibroblast growth factor
promote angiogenesis and metastasis in human melanoma xenografts.
Cancer Res. 60:4932–4938. 2000.PubMed/NCBI
|
|
29
|
Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu
C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB,
Bottsford-Miller J, et al: Stress effects on FosB- and
interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J
Biol Chem. 285:35462–35470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmed OI, Adel AM, Diab DR and Gobran NS:
Prognostic value of serum level of interleukin-6 and interleukin-8
in metastatic breast cancer patients. Egypt J Immunol. 13:61–68.
2006.
|
|
31
|
Shahzad A, Knapp M, Lang I and Köhler G:
Interleukin 8 (IL-8) - a universal biomarker? Int Arch Med.
3:112010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pine SR, Mechanic LE, Enewold L,
Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso
NE and Harris CC: Increased levels of circulating interleukin 6,
interleukin 8, C-reactive protein, and risk of lung cancer. J Natl
Cancer Inst. 103:1112–1122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gabellini C, Trisciuoglio D, Desideri M,
Candiloro A, Ragazzoni Y, Orlandi A, Zupi G and Del Bufalo D:
Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human
malignant melanoma progression. Eur J Cancer. 45:2618–2627. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cullen BR: MicroRNAs as mediators of viral
evasion of the immune system. Nat Immunol. 14:205–210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen L, Min L, Wang X, Zhao J, Chen H, Qin
J, Chen W, Shen Z, Tang Z, Gan Q, et al: Loss of RACK1 Promotes
Metastasis of Gastric Cancer by Inducing a miR-302c/IL8 Signaling
Loop. Cancer Res. 75:3832–3841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qu JQ, Yi HM, Ye X, Zhu JF, Yi H, Li LN,
Xiao T, Yuan L, Li JY, Wang YY, et al: MiRNA-203 reduces
nasopharyngeal carcinoma radioresistance by targeting IL-8/AKT
signaling. Mol Cancer Ther. Aug 24–2015.Epub ahead of print.
View Article : Google Scholar
|
|
37
|
Qu JQ, Yi HM, Ye X, Li LN, Zhu JF, Xiao T,
Yuan L, Li JY, Wang YY, Feng J, et al: MiR-23a sensitizes
nasopharyngeal carcinoma to irradiation by targeting IL-8/Stat3
pathway. Oncotarget. 6:28341–28356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van der Goten J, Vanhove W, Lemaire K, Van
Lommel L, Machiels K, Wollants WJ, De Preter V, De Hertogh G,
Ferrante M, Van Assche G, et al: Integrated miRNA and mRNA
expression profiling in inflamed colon of patients with ulcerative
colitis. PLoS One. 9:e1161172014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oglesby IK, Vencken SF, Agrawal R, Gaughan
K, Molloy K, Higgins G, McNally P, McElvaney NG, Mall MA and Greene
CM: miR-17 overexpression in cystic fibrosis airway epithelial
cells decreases interleukin-8 production. Eur Respir J
ERJ-01634-2014. 2015.
|
|
40
|
Mei Q, Xue G, Li X, Wu Z, Li X, Yan H, Guo
M, Sun S and Han W: Methylation-induced loss of miR-484 in
microsatellite-unstable colorectal cancer promotes both viability
and IL-8 production via CD137L. J Pathol. 236:165–174. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li L, Liu Y, Guo Y, Liu B, Zhao Y, Li P,
Song F, Zheng H, Yu J, Song T, et al: Regulatory miR-148a-ACVR1/BMP
circuit defines a cancer stem cell-like aggressive subtype of
hepatocellular carcinoma. Hepatology. 61:574–584. 2015. View Article : Google Scholar
|
|
42
|
Ding XM: MicroRNAs: Regulators of cancer
metastasis and epithelial-mesenchymal transition (EMT). Chin J
Cancer. 33:140–147. 2014. View Article : Google Scholar :
|
|
43
|
Sun Y, Guo F, Bagnoli M, Xue FX, Sun BC,
Shmulevich I, Mezzanzanica D, Chen KX, Sood AK, Yang D, et al: Key
nodes of a microRNA network associated with the integrated
mesenchymal subtype of high-grade serous ovarian cancer. Chin J
Cancer. 34:28–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Scheel C, Eaton EN, Li SH, Chaffer CL,
Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, et al:
Paracrine and autocrine signals induce and maintain mesenchymal and
stem cell states in the breast. Cell. 145:926–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fernando RI, Litzinger M, Trono P,
Hamilton DH, Schlom J and Palena C: The T-box transcription factor
Brachyury promotes epithelial-mesenchymal transition in human tumor
cells. J Clin Invest. 120:533–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hwang WL, Yang MH, Tsai ML, Lan HY, Su SH,
Chang SC, Teng HW, Yang SH, Lan YT, Chiou SH, et al: SNAIL
regulates interleukin-8 expression, stem cell-like activity, and
tumorigenicity of human colorectal carcinoma cells.
Gastroenterology. 141:279–291. 2912011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
MacManus CF, Pettigrew J, Seaton A, Wilson
C, Maxwell PJ, Berlingeri S, Purcell C, McGurk M, Johnston PG and
Waugh DJ: Interleukin-8 signaling promotes translational regulation
of cyclin D in androgen-independent prostate cancer cells. Mol
Cancer Res. 5:737–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li XJ, Peng LX, Shao JY, Lu WH, Zhang JX,
Chen S, Chen ZY, Xiang YQ, Bao YN, Zheng FJ, et al: As an
independent unfavorable prognostic factor, IL-8 promotes metastasis
of nasopharyngeal carcinoma through induction of
epithelial-mesenchymal transition and activation of AKT signaling.
Carcinogenesis. 33:1302–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Visciano C, Liotti F, Prevete N, Cali' G,
Franco R, Collina F, de Paulis A, Marone G, Santoro M and Melillo
RM: Mast cells induce epithelial-to-mesenchymal transition and stem
cell features in human thyroid cancer cells through an
IL-8-Akt-Slug pathway. Oncogene. 34:5175–5186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mannoury la Cour C, Salles MJ, Pasteau V
and Millan MJ: Signaling pathways leading to phosphorylation of Akt
and GSK-3β by activation of cloned human and rat cerebral
D2 and D3 receptors. Mol Pharmacol.
79:91–105. 2011. View Article : Google Scholar
|
|
51
|
Kim JY, Kim YM, Yang CH, Cho SK, Lee JW
and Cho M: Functional regulation of Slug/Snail2 is dependent on
GSK-3β-mediated phosphorylation. FEBS J. 279:2929–2939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Knall C, Young S, Nick JA, Buhl AM,
Worthen GS and Johnson GL: Interleukin-8 regulation of the
Ras/Raf/mitogen-activated protein kinase pathway in human
neutrophils. J Biol Chem. 271:2832–2838. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nagarajan D, Melo T, Deng Z, Almeida C and
Zhao W: ERK/GSK3β/Snail signaling mediates radiation-induced
alveolar epithelial-to-mesenchymal transition. Free Radic Biol Med.
52:983–992. 2012. View Article : Google Scholar :
|
|
54
|
Weiss MB, Abel EV, Mayberry MM, Basile KJ,
Berger AC and Aplin AE: TWIST1 is an ERK1/2 effector that promotes
invasion and regulates MMP-1 expression in human melanoma cells.
Cancer Res. 72:6382–6392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ,
Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, et al:
Macrophage-secreted IL-8 induces epithelial-mesenchymal transition
in hepatocellular carcinoma cells by activating the
JAK2/STAT3/Snail pathway. Int J Oncol. 46:587–596. 2015.
|
|
56
|
Fernando RI, Castillo MD, Litzinger M,
Hamilton DH and Palena C: IL-8 signaling plays a critical role in
the epithelial-mesenchymal transition of human carcinoma cells.
Cancer Res. 71:5296–5306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Techasen A, Loilome W, Namwat N, Dokduang
H, Jongthawin J and Yongvanit P: Cytokines released from activated
human macrophages induce epithelial mesenchymal transition markers
of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 13(Suppl):
S115–S118. 2012.
|
|
58
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar
|
|
59
|
Zhang H, Liu L, Wang Y, Zhao G, Xie R, Liu
C, Xiao X, Wu K, Nie Y, Zhang H, et al: KLF8 involves in
TGF-beta-induced EMT and promotes invasion and migration in gastric
cancer cells. J Cancer Res Clin Oncol. 139:1033–1042. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bonde AK, Tischler V, Kumar S, Soltermann
A and Schwendener RA: Intratumoral macrophages contribute to
epithelial-mesenchymal transition in solid tumors. BMC Cancer.
12:352012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ and
Feng YM: Cancer-associated fibroblasts induce
epithelial-mesenchymal transition of breast cancer cells through
paracrine TGF-β signalling. Br J Cancer. 110:724–732. 2014.
View Article : Google Scholar :
|
|
64
|
Henson ES and Gibson SB: Surviving cell
death through epidermal growth factor (EGF) signal transduction
pathways: Implications for cancer therapy. Cell Signal.
18:2089–2097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Barr S, Thomson S, Buck E, Russo S, Petti
F, Sujka-Kwok I, Eyzaguirre A, Rosenfeld-Franklin M, Gibson NW,
Miglarese M, et al: Bypassing cellular EGF receptor dependence
through epithelial-to-mesenchymal-like transitions. Clin Exp
Metastasis. 25:685–693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Clapéron A, Mergey M, Nguyen Ho-Bouldoires
TH, Vignjevic D, Wendum D, Chrétien Y, Merabtene F, Frazao A,
Paradis V, Housset C, et al: EGF/EGFR axis contributes to the
progression of cholangiocarcinoma through the induction of an
epithelial-mesenchymal transition. J Hepatol. 61:325–332. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sullivan NJ, Sasser AK, Axel AE, Vesuna F,
Raman V, Ramirez N, Oberyszyn TM and Hall BM: Interleukin-6 induces
an epithelial-mesenchymal transition phenotype in human breast
cancer cells. Oncogene. 28:2940–2947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rojas A, Liu G, Coleman I, Nelson PS,
Zhang M, Dash R, Fisher PB, Plymate SR and Wu JD: IL-6 promotes
prostate tumorigenesis and progression through autocrine
cross-activation of IGF-IR. Oncogene. 30:2345–2355. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xiong H, Hong J, Du W, Lin YW, Ren LL,
Wang YC, Su WY, Wang JL, Cui Y, Wang ZH, et al: Roles of STAT3 and
ZEB1 proteins in E-cadherin down-regulation and human colorectal
cancer epithelial-mesenchymal transition. J Biol Chem.
287:5819–5832. 2012. View Article : Google Scholar :
|
|
70
|
Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu
Z, Zhao J and Zhang HT: JAK/STAT3 signaling is required for
TGF-β-induced epithelial-mesenchymal transition in lung cancer
cells. Int J Oncol. 44:1643–1651. 2014.PubMed/NCBI
|
|
71
|
Shih JY and Yang PC: The EMT regulator
slug and lung carcinogenesis. Carcinogenesis. 32:1299–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dohadwala M, Wang G, Heinrich E, Luo J,
Lau O, Shih H, Munaim Q, Lee G, Hong L and Lai C: The role of ZEB1
in the inflammation-induced promotion of EMT in HNSCC. Otolaryngol
Head Neck Surg. 142:753–759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
St John MA, Dohadwala M, Luo J, Wang G,
Lee G, Shih H, Heinrich E, Krysan K, Walser T, Hazra S, et al:
Proinflammatory mediators upregulate snail in head and neck
squamous cell carcinoma. Clin Cancer Res. 15:6018–6027. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Petrella BL, Armstrong DA and Vincenti MP:
Interleukin-1 beta and transforming growth factor-beta 3 cooperate
to activate matrix metalloproteinase expression and invasiveness in
A549 lung adenocarcinoma cells. Cancer Lett. 325:220–226. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wu ST, Sun GH, Hsu CY, Huang CS, Wu YH,
Wang HH and Sun KH: Tumor necrosis factor-α induces
epithelial-mesenchymal transition of renal cell carcinoma cells via
a nuclear factor kappa B-independent mechanism. Exp Biol Med
(Maywood). 236:1022–1029. 2011. View Article : Google Scholar
|
|
77
|
Schraufstatter IU, Zhao M, Khaldoyanidi SK
and Discipio RG: The chemokine CCL18 causes maturation of cultured
monocytes to macrophages in the M2 spectrum. Immunology.
135:287–298. 2012. View Article : Google Scholar :
|
|
78
|
Chen P, Li K, Liang Y, Li L and Zhu X:
High NUAK1 expression correlates with poor prognosis and involved
in NSCLC cells migration and invasion. Exp Lung Res. 39:9–17. 2013.
View Article : Google Scholar
|
|
79
|
Zhang B, Yin C, Li H, Shi L, Liu N, Sun Y,
Lu S, Liu Y, Sun L, Li X, et al: Nir1 promotes invasion of breast
cancer cells by binding to chemokine (C-C motif) ligand 18 through
the PI3K/Akt/GSK3β/Snail signalling pathway. Eur J Cancer.
49:3900–3913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Grosse-Steffen T, Giese T, Giese N,
Longerich T, Schirmacher P, Hänsch GM and Gaida MM:
Epithelial-to-mesenchymal transition in pancreatic ductal
adenocarcinoma and pancreatic tumor cell lines: The role of
neutrophils and neutrophil–derived elastase. Clin Dev Immunol.
2012:7207682012. View Article : Google Scholar
|
|
81
|
Inoue K, Slaton JW, Eve BY, Kim SJ,
Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA,
et al: Interleukin 8 expression regulates tumorigenicity and
metastases in androgen-independent prostate cancer. Clin Cancer
Res. 6:2104–2119. 2000.PubMed/NCBI
|
|
82
|
Kitadai Y, Haruma K, Mukaida N, Ohmoto Y,
Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ,
et al: Regulation of disease-progression genes in human gastric
carcinoma cells by interleukin 8. Clin Cancer Res. 6:2735–2740.
2000.PubMed/NCBI
|
|
83
|
Jamieson T, Clarke M, Steele CW, Samuel
MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, et al:
Inhibition of CXCR2 profoundly suppresses inflammation-driven and
spontaneous tumorigenesis. J Clin Invest. 122:3127–3144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lee YS, Choi I, Ning Y, Kim NY,
Khatchadourian V, Yang D, Chung HK, Choi D, LaBonte MJ, Ladner RD,
et al: Interleukin-8 and its receptor CXCR2 in the tumour
microenvironment promote colon cancer growth, progression and
metastasis. Br J Cancer. 106:1833–1841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yang G, Rosen DG, Liu G, Yang F, Guo X,
Xiao X, Xue F, Mercado-Uribe I, Huang J, Lin SH, et al: CXCR2
promotes ovarian cancer growth through dysregulated cell cycle,
diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res.
16:3875–3886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tazzyman S, Barry ST, Ashton S, Wood P,
Blakey D, Lewis CE and Murdoch C: Inhibition of neutrophil
infiltration into A549 lung tumors in vitro and in vivo using a
CXCR2-specific antagonist is associated with reduced tumor growth.
Int J Cancer. 129:847–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar
|
|
88
|
Grund EM, Kagan D, Tran CA, Zeitvogel A,
Starzinski-Powitz A, Nataraja S and Palmer SS: Tumor necrosis
factor-alpha regulates inflammatory and mesenchymal responses via
mitogen-activated protein kinase kinase, p38, and nuclear factor
kappaB in human endometriotic epithelial cells. Mol Pharmacol.
73:1394–1404. 2008. View Article : Google Scholar : PubMed/NCBI
|