It is well known that cancer is currently one of the
leading causes of mortality in the world, and curing it still
remains a great challenge to scientists and doctors. Many
hypotheses and therapies have been suggested to explain and treat
cancer, and these have given hope to physicians and patients
because many satisfactory results have been reported. However,
there is currently no cancer cure and the mystery of cancer remains
unsolved. The mechanism of tumor formation is largely unclear, and
present hypotheses cannot explain all of the cases of tumor
formation seen in medical practice. Even more noteworthy, therapy
seems to be ineffective in some cases, especially in the terminal
stages of cancer (1).
Theories regarding cancer biology can guide
therapeutic strategies. The role of signal transduction pathways in
cancer has been thoroughly investigated. Signaling pathways control
the normal development of tissues and organs (2–5), and
mutations in signaling molecules may lead to disease, even
malignant cancer. A signaling pathway is a group of molecules that
transduce signals intracellularly and intercellularly, affecting
the fate of cells. The site of signal transduction is important in
cancer development, and recent advances in signaling pathway
research has provided the means to target therapy to specific sites
(5). In addition, better
understanding of signaling pathways may help solve the difficult
problems of cancer therapy in daily medical practice, such as drug
resistance (6). Shi et al
(7) reported that inhibitor of p38
MAPK would increase drug sensitivity in colon cancer cell lines. A
recent study also found that a decrease in SMAD4 promoter activity
may lead to drug resistance in breast cancer cell lines (8). The data indicate that better
understanding of signaling pathway function may provide insight
into the mechanism of drug resistance and reveal potential target
sites for improving drug sensitivity. Increasing evidence showed
that mutations in signaling pathway components may also be an
indicator of prognosis (9). A
recent report showed that high Notch1 expression is associated with
low survival rate in esophageal squamous cell cancer (10). Furthermore, Chu et al
(11) reported that nuclear
factor-kappa B also influences the prognosis of oral squamous cell
carcinoma. In addition, Wnt5a positivity was reported as a sign of
short survival period in NSCLC (12). We can conclude from these data that
signaling pathways may be unique prognosis markers for certain
cancer types, and may also provide clues for further research on
mechanism of cancer prognosis. Taken together, the view of
signaling pathways could be a promising theory to explain cancer
formation and may help guide therapy for cancer treatment in
future.
Many cancer-related signaling pathways have been
reported, such as Notch, Wnt, NF-κB, Ras, JNK, ERK (3,13–16).
These pathways have many aspects in common; for example, they all
have a role in cellular proliferation, differentiation and
survival. Notably, some signal pathways may play double roles in
cancer, acting as both oncogene and suppressor, indicating that
approach to therapy, based on signaling pathway sites, should be
altered according the cancer type. Abnormal expression of signaling
pathway components is observed in many cancers, which serves as a
reminder that these components have a strong relationship with
cancer. In the present review, we mainly focus on the Notch and Wnt
signaling pathways to summarize their mechanism of activation,
their role in tumorigenesis and their potential as targets for
cancer therapy.
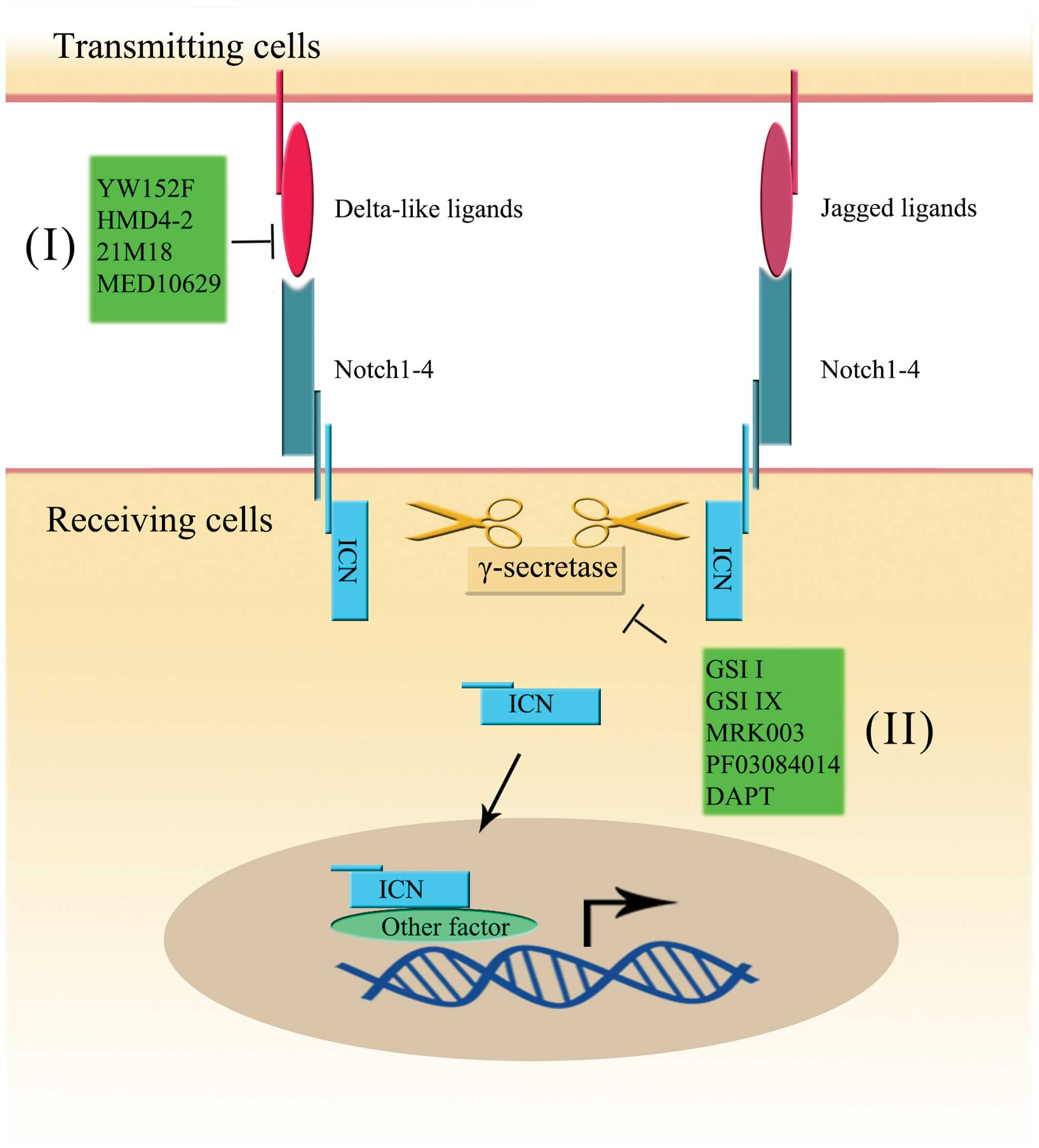
The Notch signaling pathway is a conserved
ligand-receptor signaling pathway in mammals that contains four
Notch receptors and five ligands (17). The four receptors, which are named
Notch 1, Notch 2, Notch 3 and Notch 4, share a similar structure.
Each Notch receptor, Notch 1–4, has 36, 36, 34 and 29 epidermal
growth factor (EGF)-like repeats, respectively (18,19).
The ligands can be divided into two major groups: the Delta-like
ligands (DLL1, DLL3 and DLL4) and Jagged ligands (Jag1 and Jag2)
(17). The four Notch receptors
and five ligands are transmembrane molecules, meaning that
activation of the Notch signaling pathway controls cell fates by
interaction of receptors and ligands on the surface of adjacent
cells (20). Furthermore, the
activation was shown to be regulated via proteolysis by
metalloprotease, tumor necrosis factor-α-converting enzyme (TACE)
and γ-secretase (21,22). When intercellular activation
occurs, the extracellular and intracellular domains of the Notch
receptor (ICN) are released by TACE and γ-secretase, respectively.
Then, the extracellular domain binds to the ligand on the surface
of an adjacent cell, and ICN translocates into the nucleus. ICN
contains ankyrin repeats, a RAM domain, a trans-activation domain
(TAD), a nuclear localization signal (NLS) and a PEST domain
(23). Each domain is necessary
for Notch signaling pathway activity. The ICN forms an active
transcriptional complex and plays a direct role in regulating the
gene expression (23,24) (Fig.
1). From recent study data, the target genes of the Notch
signaling pathway, to name a few, are the Hes family, Hey, NF-κB,
VEGF and c-myc. All of these Notch target genes were found to be
associated with tumorigenesis (25–27).
It has also been shown that the Notch signaling
pathway plays an important role in cell differentiation,
proliferation and apoptosis (20).
Increasing evidence showed that the Notch signaling pathway can
affect the expression of inflammatory cytokines (28,29),
vasculogenesis (30,31) and drug resistance (22). Thus, it is reasonable to speculate
that disruptions in the Notch signaling pathway may lead to
tumorigenesis, as many studies have reported. There is no doubt
that a better understanding of the Notch signaling pathway may lead
to new insight into cancer therapy and can bring hope to those who
treat and suffer from Notch-related tumors.
As previously discussed, the Notch signal pathway
controls many cellular functions, such as differentiation,
proliferation and apoptosis. When these functions are disrupted or
altered, the outcome may be detrimental. Interestingly, the
function of the Notch signaling pathway strongly depends on the
cellular context (32). Notch can
act both as an oncogene and suppressor gene (32). We mainly summarize the latest
studies of the Notch signaling pathway in different tumors, from
both oncogene and antioncogene points of view.
Studies have demonstrated that the Notch 1 receptor
played an important role in T-cell development and cell fate
(37,38), and therefore, Notch 1 receptor
dysfunction negatively impacts T-cell function. There are three
theories to explain how Notch 1 receptor mutation contributes to
T-ALL. The most popular theory suggests that changes in the amino
acid sequence (such as substitution, insertion and deletion) that
encodes the heterodimerization domain lead to the abnormal
sensitivity to the ligand (17,32).
Another viewpoint suggests that a nonsense or frameshift mutation
in the PEST domain, which was detected in 20–25% of T-ALLs, causes
T-ALL (17,32). The PEST domain controls the
stability of intercellular Notch 1 (ICN1) and this mutation leads
to an increase in the concentration of ICN1 (17,36).
The third theory implicates a mutation in the juxtamembrane
expansion, which increases the activation of the Notch 1 receptor
(39). Interestingly and
unexpectedly, Notch 1 receptor mutations in T-ALL were associated
with a good prognosis in recent studies (40–42).
These results indicate that the Notch signaling pathway may be a
novel target for the treatment of T-ALL and its mechanism of action
in T-ALL should be further studied in detail.
In addition to T-ALL, the Notch signaling pathway is
also dysregulated in other hematological malignancies. Chronic
lymphocytic leukemia (CLL) is an incurable neoplasm with abnormal B
cells. Increasing evidence from recent studies showed that the
Notch signaling pathway plays a role in CLL, with the Notch 1
receptor mutant expressed in ~10–20% patients (17,32,43).
Genetic studies reveal that the majority of mutations of the Notch
1 receptor are in the PEST domain (44,45).
In contrast to what is observed in T-ALL, Notch 1 receptor
mutations indicate a poor prognosis in patients with CLL (46,47),
and higher frequency of mutation is also observed in
chemorefractory CLL (43). In
addition to the Notch 1 receptor, other Notch family members also
play an important role in hematological malignancy. The Notch 2
receptor is involved in B cell development, which means that
mutations in the Notch 2 receptor will likely lead to abnormal
formation of B cells and result in further pathologic changes
(48). Increasing evidence shows
that a Notch 2 receptor mutation can be detected in tumors of B
cell origin (49,50).
In addition to its important role in the
hematological malignancy, the Notch signaling pathway also has been
shown to be involved in solid tumors, such as breast, lung, gastric
and liver cancer. In these solid tumors, the dual role of Notch as
an oncogene and suppressor gene is evident. We mainly focus below
on the Notch signaling pathway in breast, lung and gastric cancer,
aiming at summarizing the latest research advances on the Notch
signaling pathway in these diseases.
Research into the various roles of the Notch
signaling pathway has been ongoing for some time. It is known that
the Notch signaling pathway plays a crucial role in breast
development (20). Evidence showed
that the Notch 4 receptor and Notch 3 receptor control normal
breast epithelial cells and luminal cells, respectively (51–53).
Alteration of the Notch signaling pathway has the potential to
cause breast cancer. Of special interest is that the four receptors
of the Notch signaling pathway have different roles in breast
cancer. The Notch 1 receptor, Notch 3 receptor and Notch 4 receptor
have a negative signaling role in breast cancer (54–56).
In recent studies, crosstalk of the Notch 1 receptor and other
genes (such as Ras, c-myc and JAG1) was proven to contribute the
formation of breast cancer (35,54,57).
Both the Notch 3 and Notch 4 receptors were found to have the
potential to promote transformation (55,56),
and inhibition of Notch 3 receptor expression can reduce metastasis
of breast cancer to the bone (58). Therefore, the Notch 3 receptor may
be a drug target for the treatment of breast cancer. The Notch 1
receptor was associated with poor prognostic outcomes, while the
Notch 2 receptor was associated with a good survival rate, as
reported by Parr et al (59). They found that overexpression of
the Notch 2 receptor can inhibit the tumor growth and promote tumor
cell death, indicating that the Notch 2 receptor can be a promising
target for further therapy.
Lung cancer, which is the leading cause of cancer
death in the world, can be divided into two types: small cell lung
carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC).
Research of the Notch signaling pathway has been ongoing for a long
time, but its role in lung cancer remains a subject of debate. As
we previously mentioned, the role of the Notch signaling pathway in
cancer depends on the cellular context, thus, the study of the
Notch signaling pathway in lung cancer should be carried out in
both SCLC and NSCLC.
In a KrasG12D-driven endogenous NSCLC mouse model,
Notch 1 deletion led to a reduction in tumor formation, while Notch
2 receptor deletion led to increased carcinogenesis (60). It was also found that Notch 2
receptor expression was weak in human NSCLC samples, indicating
that Notch 2 may play the role of a tumor suppressor in NSCLC
(60). Furthermore, in the study
by Yang et al (61), it was
found that Notch 1 activation may protect the A549 cell line
against the anti-tumor effect of pterostilbene. Licciulli et
al (62) also reported that
the Notch 1 receptor is essential for tumor formation via
suppression of p53. In addition, Notch 1 also seemed to be required
for resistance to chemotherapy (63,64)
and radiotherapy in lung cancer (65). However, Wael et al (66) found that the Notch 1 receptor can
significantly induce apoptosis in SCLC and the A549 adenocarcinoma
cell line of NSCLC, while its tumor inhibitory function fails in
SCC cells of NSCLC. A recent study demonstrated that the Notch 1
receptor can predict prognosis in lung adenocarcinomas: lower Notch
1 receptor expression in a lung adenocarcinoma cell line and
patients with positive Notch 1 receptor expression have a longer
survival time and a lower rate of recurrence (67). Taken together, before the role of
the Notch 1 receptor in lung cancer can be confirmed, further
studies are required. Compared with the Notch 1 receptor, the Notch
3 receptor has received less attention, but its role cannot be
ignored. Zhou et al (68)
detected Notch 3 receptor expression in different types of lung
cancer, and they found that Notch 3 expression was high in lung
squamous cell carcinoma and adenocarcinoma, but low in small cell
carcinoma, compared with the corresponding non-tumor tissue. In
addition, the Notch 3 receptor showed its potential ability of
predicting the prognosis of patients with NSCLC (69). In this recent report, high levels
of the Notch 3 receptor were detected in ~51.1% of cases and were
associated with a short survival rate.
Thus, taking all the evidence above into account, we
conclude that the Notch signaling pathway is important in lung
cancer genesis, progression and prognosis, and it is a promising
target for therapy for lung cancer, though its role varies in
different types of lung cancer.
Gastric cancer (GC) is one of the most common
cancers in the world and it is also the leading cause of
cancer-related death (70),
despite the fact that surgical resection and lymph node dissections
are performed. Among the variety of factors that cause gastric
cancer, the Notch signaling pathway seems to play an important
role. The Notch 1 signaling pathway, in particular, draws great
attention in the research of the Notch signaling pathway in GC. At
present, Notch 1 seems to function as an oncoprotein in GC, as
evidenced by a recent report that Notch 1 contributed to GC
progression by inducing COX2 expression (71). Furthermore, Yao et al
(72) reported that activation of
the Notch 1 receptor can reduce TNFα-induced apoptosis in BGC-823
cells. Interestingly, though their structures are similar, Notch 1
and Notch 2 receptors have different roles in vivo (73). Sun et al (74) reported that expression of Notch 1
in different types of GC varies, indicating that Notch 1 may be a
sign of gastric lesions with intestinal-like phenotypes, while the
expression of Notch 2 showed a strong relationship with GC
formation. However, the role of Notch 2 in GC remains indistinct.
With evidence of an oncogenic role (75) and a suppressor role (76), Notch 2 should be further studied to
confirm its function in GC.
In addition to the Notch receptors, ligands of the
Notch signaling pathway have also received attention in GC.
Recently, Piazzi et al (77) reported that the Delta-Like1 (DLL1)
controlled the activation of the Notch 1 receptor in GC. Moreover,
evidence also showed that DLL1 was the most important ligand for
Notch 1 (78), indicating that
DLL1 may be a potential target for Notch 1 receptor. Furthermore,
another Notch ligand, Delta-Like4 (DLL4), was also reported to be
involved in GC progression. Li et al (79) showed that activation of DLL4 may
promote tumor proliferation, migration, invasion and tumorigenicity
in SGC7901 via overexpression of MMP-2 proenzyme. Sun et al
(80) also showed that DLL4 and
Jagged1 siRNA gene therapy may greatly reduce the proliferation and
invasion of the SGC7901 cell line.
The Wnt signaling pathway has been a subject of
research during the last several decades, and has been shown to
function in cell proliferation, growth, cell fate and
differentiation (81,82). Mutation of Wnt signaling pathway
components causes many diseases, including cancer (82). It is important to understand the
Wnt signaling pathway also offers potential benefits for genetic
therapy.
The components of the Wnt signaling pathway can be
divided into Wnt ligands and Wnt receptors (83). There are 19 Wnt ligands, which all
have a cysteine-rich domain, and may activate different types of
Wnt signaling pathway by binding specific Wnt signaling receptors
(84). In addition, some Wnt
ligands are also involved in cancer formation and progression. Wnt1
encodes a number of glycoproteins and was reported as a sign of
advanced metastasis for patients with tumors (85). Wnt3a was found to be overexpressed
and associated with the level of MMP-9 in colorectal tumor tissue
(86). Moreover, a recent report
also showed that Wnt3a can promote the proliferation of MCF-7 cells
by downregulating β-catenin acetylation (87).
Normally, the Wnt signaling pathway can be
categorized as either the canonical Wnt pathway or non-canonical
Wnt pathway (88). In the
canonical Wnt pathway, β-catenin is the central molecule that
controls the on/off ‘switch’ of the Wnt signaling pathway. The Wnt
pathway is in the ‘off’ state when Wnt ligands do not bind to any
receptors, and β-catenin is released from the cytomembrane.
β-catenin is then captured by a protein complex, which is composed
of adenomatous polyposis coli (APC), the scaffolding protein Axin,
glycogen synthase kinase 3β (GSK3β) and casein kinase 1 (CK1)
(88,89). β-catenin is phosphorylated by CK1
and GSK3β and targeted for proteasomal degradation (89), leading to decreased β-catenin
concentration, inhibition of nuclear translocation of β-catenin and
thus, inhibition of target gene activation (88–90).
When the switch is on, a different mechanism unfolds. Wnt ligands
bind to the transmembrane Fz receptor and low-density lipoprotein
receptor-related proteins (LRP5/6). Then, CK1 and GSK3β are
attracted and function as a phosphorylase to LRP5/6. This leads to
inactivation of the protein complex, and β-catenin is able to
escape degradation, making it possible for it to enter into the
nucleus and promote the transcription of target genes (88,91)
(Fig. 2).
The non-canonical pathway, which is β-catenin
independent, has two modes of activation: the Wnt/Ca2+
pathway and the planar cell polarity (PCP) pathway (92,93).
In the Wnt/Ca2+ pathway, Wnt ligands bind to the
Frizzled (Fzd) transmembrane receptor and activate a series of
proteins that increase the intracellular calcium level, which
activates other signal pathways (89,94).
The PCP pathway leads to alterations in the cytoskeletal
organization, which may influence cellular movement, metastasis and
invasion (88,93,95).
It has been demonstrated that the Wnt signaling
pathway is involved in deciding cellfate, and mutation of Wnt
signaling pathway components also showed a strong association with
different types of human cancer, such as lung, breast and ovarian
cancer (83,96–98. Among these cancers, the Wnt signaling pathway is
most involved in hepatocellular carcinoma (HCC) and colorectal
cancer (CRC) (99,100). In this section, we mainly
discussed the recent research on the Wnt signaling pathway in HCC
and CRC, summarizing the effect and potential drug targets of the
Wnt signaling pathway in the two cancer types.
From these data, we may preliminarily conclude that
activation of the Wnt signaling pathway plays an important role in
liver fibrosis, hepatocellular tumorigenesis and tumor development,
and inhibition of the Wnt signaling pathway could strongly inhibit
carcinogenesis.
Colorectal cancer (CRC) is one of the most common
cancers and one of the leading causes of cancer-related mortality
in the world (111,112). Gaining insight into the genetic
changes of CRC is important for both treatment and diagnosis. Among
the various genes involved in CRC, the Wnt signaling pathway has
attracted much attention (113),
as more than half of CRC cases have a β-catenin mutation (114). A recent report showed that the
activation of different parts of Wnt signaling pathway may lead to
development of one of two types of colorectal neoplasia: serrated
or conventional adenoma/polyp (115). Accumulated research also showed
that the application of Wnt signaling pathway inhibitors could
greatly promote apoptosis and reduce proliferation of CRC cells
(116,117). Tumova et al (118) also reported that the use of
monensin could inhibit expression of β-catenin in human colorectal
carcinoma cells and decrease cell proliferation, indicating that
Wnt/β-catenin could be used as gene target for CRC and monensin is
a potential drug for therapy. In addition, evidence from clinical
practice also showed that CRC patients have a high rate of
overexpression of Wnt signaling pathway proteins (119). Voorham et al (120) reported observation of methylated
Wnt pathway antagonists from clinical specimens. It is possible
that the Wnt signaling pathway could also be used as a potential
diagnostic and prognostic biomarker in CRC patients (119,121). Thus, both experimental and
clinical data showed that the over activation of the Wnt signaling
pathway may be a potential target for CRC. In addition to these
studies of the oncogenic role of Wnt in CRC, Abdelmaksoud-Dammak
et al (122) found that
Wnt5a expression was lower in tumor compared with normal tissue,
indicating that Wnt5a may play a role as an antioncogene in CRC.
Interestingly, Bauer and colleagues found that the Wnt5a gene could
result in proteins of different length, Wnt5a-long and Wnt5a-short.
These two genes have inverse functions, with the Wnt5a-long
functioning as a suppressor and Wnt5a-short as an oncogene
(123).
From these data, we may conclude that Wnt/β-catenin
is an oncogenic pathway in CRC, though some Wnt ligands may act as
suppressor, as some studies showed. Compounds that could inactivate
Wnt/β-catenin could be used as potential drugs for CRC.
Reports have shown that ~95% esophageal cancer is
esophageal squamous cell cancer (ESCC), with 15% 5-year survival
rate (124). At present, studies
have found that Wnt signal pathway has played an indispensable role
in the development of ESCC, indicating that the components of Wnt
signal pathway could be the potential targets for treating
ESCC.
Wnt inhibitory factor-1 (WIF1) is one of the most
important Wnt inhibitors. A recent study has shown that WIF1
promoter is methylated in ESCC tissues, and re-expression of WIF1
could decrease the transcription activity of β-catenin/TCF and
inhibit the cell proliferation and migration (127). Interestingly, Ge et al
(128) found that the low
expression of WIF1 in ESCC is due to the high expression of Hotair,
which is a well-known long non-coding RNA. They reported that
Hotair could directly promote histone H3K27 methylation in WIF1
promoter region and activated the Wnt signal pathway. In addition,
Liu et al (129) reported
that LKB1, a tumor suppressor, could inhibit the Wnt signal pathway
through increasing GSK3β activity, causing low expression of
β-catenin. Another tumor suppressor, SOX10, was also reported to
inhibit the epithelial to mesenchymal transition (EMT) and stemness
ability in ESCC cells, by competing with TCF4 to bind β-catenin
(130).
From these studies, we may conclude that Wnt signal
pathway plays an important role in ESCC. Abnormal expression of Wnt
signal pathway may be the initial factor and the stimulative factor
in ESCC. Moreover, studies have shown that targeting the components
of Wnt signal pathway could inhibit the malignant activity of ESCC
cell lines, indicating it is a potential treatment of ESCC.
As we have discussed above, both the Notch and Wnt
signaling pathways have been proven to have a strong relationship
with different types of tumors, playing dual roles of oncogene and
suppressor. This means that the Notch and Wnt signaling pathway can
be used as promising targets for therapy. In our opinion, the
genetic therapy can be designed in three aspects: process or
substance that actives genes, expression of the gene itself and
mimic of the function of the genes.
The clinical approach to targeting the Notch signal
pathway is divided into the use of antibodies against the Notch
receptor or ligand, and the inhibition of γ-secretase (5) (Fig.
1).
DLL4 plays an important role in Notch-related stem
cell self-renewal, and its overexpression has been found in various
tumors (79,131,132). Thus, inhibition of DLL4 seems to
be a promising treatment to cure cancer. The humanized phage
antibody YW152F, could specifically bind to DLL4 receptor and was
proven to be able to inhibit tumor growth by deregulating
angiogenesis (133). The use of
monoclonal antibodies against murine DLL4 (HMD4-2) in mice was also
proven to be able to inhibit both tumor growth and angiogenesis
(134). Monoclonal anti-DLL4
antibody, also known as 21M18, has drawn much attention. It has
been reported that it could have anticancer stem cell,
anti-angiogenesis and antitumor growth functions (135,136). Recently, MED10629, an
investigational human therapeutic antibody, was also reported in
anti-interaction of DLL4 and Notch1, inhibiting angiogenesis in
vivo (137). In addition,
studies also showed that the combined use of DLL4 antibody and
other therapies, such as radiation treatment and γ-secretase
inhibitor, could improve the overall therapeutic effect (138,139). Besides DLL4, other components of
the Notch signaling pathway are targeted by antibodies.
Aste-Amézaga et al (140)
reported on an antibody that could target Notch1 and inhibit the
expression of Notch target genes. Sharma et al (141) also showed the antibody against
Notch1 could decrease cell proliferation and induce apoptotic cell
death. From the data analyzed in the current study, we conclude
that the use of an antibody against Notch seems to be a promising
anticancer therapeutic strategy. However, antibodies against the
Notch receptor should be used cautiously, for it has been reported
that the chronic use of these antibodies could lead to vascular
neoplasms (142).
In the Wnt/β-catenin signal pathway, β-catenin has
been proven to play a central role in cancer. Thus, inhibitors of
β-catenin could be used for cancer treatment (102). Dahmani et al (150) reported in their review that
inhibitors of β-catenin could be divided into three types: those
affecting the interaction between Wnt ligands and Fzd receptors,
those that destroy complex stability and those affecting the
activity of β-catenin in the nucleus.
In investigating the interaction between Wnt ligands
and receptors, specific therapeutic antibodies have been widely
used (150). Evidence has shown
that antibodies that bind to Wnt ligands and receptors could
inhibit the Wnt/β-catenin signaling pathway (Table II). Fontenot et al
(151) reported in their recent
research that SFRP2 monoclonal antibody could induce the antitumor
effect and inhibit the Wnt/β-catenin signaling pathway in breast
models. Wang and colleagues also found a dose-dependent effect of
anti-cadherin-17 antibody in suppressing β-catenin in a HCC model
(152). Similarly, Gao et
al (153) reported that HS20,
a human monoclonal antibody against glypican-3, could disrupt the
stability of Wnt3a and glypican-3 and inhibit the Wnt/β-catenin
signaling pathway in HCC cells. A recent study reported that a
monoclonal antibody, which could target Fzd receptors and prevent
their integration with Wnt ligands, has been widely used in
treating cancer (89). Recent
studies also showed that LRP5/6, closely related membrane receptors
for the Wnt signaling pathway, can also be targeted by antibodies
for further treatment in cancer (154,155). In addition to antibodies, a
number of molecules could also function as Wnt inhibitors. Wnt
inhibitory proteins and secreted Fzd-related proteins were the most
commonly studied molecules that bind to their targets, and as a
result, they inhibit Wnt/β-catenin activity (150). Fzd receptors were also reported
to be potential targets for cancer treatment, as soluble Fzd-7 and
Fzd-8 were reported to have antitumor effect (150,156,157). IWP2 and Wnt-C59 could inhibit Wnt
protein secretion and thus, prevent Wnt signal activation (101). Other molecules, such as
flavonoids, monensin and resveratrol, were also shown to have
potential antitumor ability via inhibition of the Wnt/β-catenin
signaling pathway (118,158,159).
When targeting β-catenin to the destruction complex
stability in cytoplasm, the situation seems to be complex. There
are two approaches to targeting β-catenin in cytoplasm: the
destruction complex stability and β-catenin itself. Axin, as one of
the most important components in the destruction complex, has
attracted a lot attention recently. Small peptides, such as IWR2
and IW55, were reported to prevent Axin from degradation and
inhibit β-catenin activity (101). Other approaches to increase Axin
stability could use a tankyrase inhibitor, such as XAV939, which
could prevent the interaction of tankyrases and Axin (160). Parp poly-(ADP-ribose) polymerase
(PARP) could promote the ribosylation of Axin, which would cause
the degradation of Axin and increase β-catenin levels. The use of a
PARP inhibitor could improve the level and stability of Axin, and
thus reduce the activation of β-catenin (161). In addition to Axin, CK1α, another
member of the destruction complex, is also a potential target for
inhibiting β-catenin activity. In a recent study, Park et al
(162) found that calotropin
could inhibit the Wnt signaling pathway by increasing CK1α protein
levels. Their finding is the first to discover a small molecule
that could increase CK1α protein, indicating that calotropin could
be used as a potential drug for cancer therapy. Additionally, the
use of pyrvinium to treat familial adenomatous polyposis by
inhibiting the Wnt signaling pathway via activation of CK1α has
also been reported (163).
Another approach to targeting the Wnt/β-catenin
signal pathway is nuclear, β-catenin and its co-activators are the
targets. In the nucleus, the activation of Wnt/β-catenin signaling
is mediated by formation of a β-catenin/Lef-Tcf complex (150). Thus, a molecule that can disrupt
this complex could be ideal for treating Wnt-related cancer. Wei
et al (164)reported that
they found three small molecules that could decrease Tcf4/β-catenin
binding capability and transcriptional activity in HCC cell lines,
indicating that these three molecules could be used as anti-HCC
drugs. Recently, a 2,3,6-trisubstituted quinoxaline derivative
(GDK-100017) was also reported as an inhibitor of the
β-catenin/Lef-Tcf complex, which also enhances radio-sensitivity
and reduces cell proliferation (165). In addition, lycopene was also
reported to inhibit Wnt-Tcf signaling in breast cancer cells
(166). Earlier, streponigrin was
reported to inhibit the β-catenin/TCF complex binding to DNA,
leading to a proliferation inhibitory effect (167). Furthermore, ICG-001 inhibited
β-catenin activity in nucleus by disrupting β-catenin/CBP
interaction (168) (Fig. 2).
In the present review, we mainly focused on the
Notch signaling and Wnt signaling pathways, aiming to briefly
describe the role of these two signaling pathway in different types
of cancer and discussing inhibitors that may be potential
anticancer therapies. Both the Notch and Wnt signaling pathways
play an important role in maintaining normal cell fate, and these
signaling pathways are also involved in a number of cancers. Better
understanding of these signaling mechanisms could lead to better
cancer therapies. Mutational and abnormal expression of Notch and
Wnt signaling pathway components are observed in different types of
cancer, as we have discussed in this review. Targeting these
disrupted genes potentially reverse cancer damage, in as yet
unknown manner. In recent studies, evidence has shown that the
combination of signaling inhibitors and traditional therapy (such
as radiotherapy and chemotherapy) can treat cancer more effectively
compared with single therapy (145,169). However, the biosafety of
signaling pathway inhibitors should be closely monitored, as recent
studies reported toxicity associated with chronic antibody
application in clinical practice (142). Signaling pathways play an
important role in tumorigenesis, tumor development and prognosis,
and targeting signaling pathways has the potential to be effective
for treating cancer.
|
1
|
Ramdass B, Duggal R, Minev B, Chowdhary A,
Ramdass B, Duggal R, Minev B, Chowdhary A and Koka P: Functional
role of solid tumor stem cells in disease etiology and
susceptibility to therapeutic interventions. J Stem Cells.
8:189–231. 2013.
|
|
2
|
Chung E and Kondo M: Role of
Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and
leukemia development. Immunol Res. 49:248–268. 2011. View Article : Google Scholar
|
|
3
|
Knight T and Irving JA: Ras/Raf/MEK/ERK
pathway activation in childhood acute lymphoblastic leukemia and
its therapeutic targeting. Front Oncol. 4:1602014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monga SP: Role and regulation of β-catenin
signaling during physiological liver growth. Gene Expr. 16:51–62.
2014. View Article : Google Scholar
|
|
5
|
Andersson ER and Lendahl U: Therapeutic
modulation of Notch signalling: are we there yet? Nat Rev Drug
Discov. 13:357–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasillas MP, Shields S, Reilly R, Strnadel
J, Behl C, Park R, Yates JR III, Klemke R, Gonias SL and Coppinger
JA: Proteomic analysis reveals a role for Bcl2-associated
athanogene 3 and major vault protein in resistance to apoptosis in
senescent cells by regulating ERK1/2 activation. Mol Cell
Proteomics. 14:1–14. 2015. View Article : Google Scholar :
|
|
7
|
Shi X, Wu S, Yang Y, Tang L, Wang Y, Dong
J, Lü B, Jiang G and Zhao W: AQP5 silencing suppresses p38 MAPK
signaling and improves drug resistance in colon cancer cells.
Tumour Biol. 35:7035–7045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu SL, Lee DC, Son JW, Park CG, Lee HY and
Kang J: Histone deacetylase 4 mediates SMAD family member 4
deacetylation and induces 5-fluorouracil resistance in breast
cancer cells. Oncol Rep. 30:1293–1300. 2013.PubMed/NCBI
|
|
9
|
Jiang AG, Yu H and Huang JA: Expression
and clinical significance of the phosphatidylinositol
3-kinase/protein kinase B signal transduction pathway in non-small
cell lung carcinoma. Oncol Lett. 8:601–607. 2014.PubMed/NCBI
|
|
10
|
Ogawa R, Ishiguro H, Kimura M, Funahashi
H, Wakasugi T, Ando T, Shiozaki M and Takeyama H: NOTCH1 expression
predicts patient prognosis in esophageal squamous cell cancer. Eur
Surg Ress. 51:101–107. 2013. View Article : Google Scholar
|
|
11
|
Chu W, Song X, Yang X, Ma L, Zhu J, He M,
Wang Z and Wu Y: Neuropilin-1 promotes epithelial-to-mesenchymal
transition by stimulating nuclear factor-kappa B and is associated
with poor prognosis in human oral squamous cell carcinoma. PLoS
One. 9:e1019312014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao L, Sun B, Zhao X, Zhao X, Gu Q, Dong
X, Zheng Y, Sun J, Cheng R, Qi H, et al: Overexpression of Wnt5a
promotes angiogenesis in NSCLC. BioMed Res Int. 2014:8325622014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carvalho FL, Simons BW, Eberhart CG and
Berman DM: Notch signaling in prostate cancer: A moving target.
Prostate. 74:933–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jamieson C, Sharma M and Henderson BR:
Targeting the β-catenin nuclear transport pathway in cancer. Semin
Cancer Biol. 27:20–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gasparini C, Celeghini C, Monasta L and
Zauli G: NF-kappaB pathways in hematological malignancies. Cellular
and molecular life sciences. Cell Mol Life Sci. 71:2083–2102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tournier C: The 2 Faces of JNK Signaling
in Cancer. Genes Cancer. 4:397–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ntziachristos P, Lim JS, Sage J and
Aifantis I: From fly wings to targeted cancer therapies: A
centennial for notch signaling. Cancer Cell. 25:318–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okajima T and Irvine KD: Regulation of
notch signaling by o-linked fucose. Cell. 111:893–904. 2002.
View Article : Google Scholar
|
|
19
|
Haines N and Irvine KD: Glycosylation
regulates Notch signalling. Nat Rev Mol Cell Biol. 4:786–797. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo S, Liu M and Gonzalez-Perez RR: Role
of Notch and its oncogenic signaling crosstalk in breast cancer.
Biochim Biophys Acta. 1815:197–213. 2011.PubMed/NCBI
|
|
21
|
Lai EC: Notch signaling: Control of cell
communication and cell fate. Development. 131:965–973. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee
S, Kong D and Sarkar FH: Targeting Notch signaling pathway to
overcome drug resistance for cancer therapy. Biochim Biophys Acta.
1806:258–267. 2010.PubMed/NCBI
|
|
23
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Louvi A and Artavanis-Tsakonas S: Notch
and disease: A growing field. Semin Cell Dev Biol. 23:473–480.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rizzo P, Osipo C, Foreman K, Golde T,
Osborne B and Miele L: Rational targeting of Notch signaling in
cancer. Oncogene. 27:5124–5131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang
Y and Sarkar FH: Down-regulation of notch-1 inhibits invasion by
inactivation of nuclear factor-kappaB, vascular endothelial growth
factor, and matrix metalloproteinase-9 in pancreatic cancer cells.
Cancer Res. 66:2778–2784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J
and Sarkar FH: Down-regulation of Notch-1 contributes to cell
growth inhibition and apoptosis in pancreatic cancer cells. Mol
Cancer Ther. 5:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zavadil J, Cermak L, Soto-Nieves N and
Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sansone P, Storci G, Tavolari S, Guarnieri
T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P,
Marcu KB, et al: IL-6 triggers malignant features in mammospheres
from human ductal breast carcinoma and normal mammary gland. J Clin
Invest. 117:3988–4002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel NS, Li JL, Generali D, Poulsom R,
Cranston DW and Harris AL: Up-regulation of delta-like 4 ligand in
human tumor vasculature and the role of basal expression in
endothelial cell function. Cancer Res. 65:8690–8697. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawson ND, Vogel AM and Weinstein BM:
sonic hedgehog and vascular endothelial growth factor act upstream
of the Notch pathway during arterial endothelial differentiation.
Dev Cell. 3:127–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
South AP, Cho RJ and Aster JC: The
double-edged sword of Notch signaling in cancer. Semin Cell Dev
Biol. 23:458–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ellisen LW, Bird J, West DC, Soreng AL,
Reynolds TC, Smith SD and Sklar J: TAN-1, the human homolog of the
Drosophila notch gene, is broken by chromosomal translocations in T
lymphoblastic neoplasms. Cell. 66:649–661. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Capobianco AJ, Zagouras P, Blaumueller CM,
Artavanis-Tsakonas S and Bishop JM: Neoplastic transformation by
truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol Cell Biol.
17:6265–6273. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Girard L, Hanna Z, Beaulieu N, Hoemann CD,
Simard C, Kozak CA and Jolicoeur P: Frequent provirus insertional
mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice
suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes
Dev. 10:1930–1944. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weng AP, Ferrando AA, Lee W, Morris JP IV,
Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT and Aster
JC: Activating mutations of NOTCH1 in human T cell acute
lymphoblastic leukemia. Science. 306:269–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Radtke F, Wilson A, Mancini SJ and
MacDonald HR: Notch regulation of lymphocyte development and
function. Nat Immunol. 5:247–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoyne GF: Notch signaling in the immune
system. J Leukoc Biol. 74:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sulis ML, Williams O, Palomero T, Tosello
V, Pallikuppam S, Real PJ, Barnes K, Zuurbier L, Meijerink JP and
Ferrando AA: NOTCH1 extracellular juxtamembrane expansion mutations
in T-ALL. Blood. 112:733–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Breit S, Stanulla M, Flohr T, Schrappe M,
Ludwig WD, Tolle G, Happich M, Muckenthaler MU and Kulozik AE:
Activating NOTCH1 mutations predict favorable early treatment
response and long-term outcome in childhood precursor T-cell
lymphoblastic leukemia. Blood. 108:1151–1157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park MJ, Taki T, Oda M, Watanabe T,
Yumura-Yagi K, Kobayashi R, Suzuki N, Hara J, Horibe K and Hayashi
Y: FBXW7 and NOTCH1 mutations in childhood T cell acute
lymphoblastic leukaemia and T cell non-Hodgkin lymphoma. Br J
Haematol. 145:198–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Clappier E, Collette S, Grardel N, Girard
S, Suarez L, Brunie G, Kaltenbach S, Yakouben K, Mazingue F, Robert
A, et al; EORTC-CLG. NOTCH1 and FBXW7 mutations have a favorable
impact on early response to treatment, but not on outcome, in
children with T-cell acute lymphoblastic leukemia (T-ALL) treated
on EORTC trials 58881 and 58951. Leukemia. 24:2023–2031. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weissmann S, Roller A, Jeromin S,
Hernández M, Abáigar M, Hernández-Rivas JM, Grossmann V, Haferlach
C, Kern W, Haferlach T, et al: Prognostic impact and landscape of
NOTCH1 mutations in chronic lymphocytic leukemia (CLL): A study on
852 patients. Leukemia. 27:2393–2396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Di Ianni M, Baldoni S, Rosati E, Ciurnelli
R, Cavalli L, Martelli MF, Marconi P, Screpanti I and Falzetti F: A
new genetic lesion in B-CLL: A NOTCH1 PEST domain mutation. Br J
Haematol. 146:689–691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sportoletti P, Baldoni S, Cavalli L, Del
Papa B, Bonifacio E, Ciurnelli R, Bell AS, Di Tommaso A, Rosati E,
Crescenzi B, et al: NOTCH1 PEST domain mutation is an adverse
prognostic factor in B-CLL. Br J Haematol. 151:404–406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wickremasinghe RG, Prentice AG and Steele
AJ: p53 and Notch signaling in chronic lymphocytic leukemia: Clues
to identifying novel therapeutic strategies. Leukemia.
25:1400–1407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rossi D, Rasi S, Fabbri G, Spina V,
Fangazio M, Forconi F, Marasca R, Laurenti L, Bruscaggin A, Cerri
M, et al: Mutations of NOTCH1 are an independent predictor of
survival in chronic lymphocytic leukemia. Blood. 119:521–529. 2012.
View Article : Google Scholar :
|
|
48
|
Kiel MJ, Velusamy T, Betz BL, Zhao L,
Weigelin HG, Chiang MY, Huebner-Chan DR, Bailey NG, Yang DT, Bhagat
G, et al: Whole-genome sequencing identifies recurrent somatic
NOTCH2 mutations in splenic marginal zone lymphoma. J Exp Med.
209:1553–1565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee SY, Kumano K, Nakazaki K, Sanada M,
Matsumoto A, Yamamoto G, Nannya Y, Suzuki R, Ota S, Ota Y, et al:
Gainof-function mutations and copy number increases of Notch2 in
diffuse large B-cell lymphoma. Cancer Sci. 100:920–926. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rossi D, Trifonov V, Fangazio M,
Bruscaggin A, Rasi S, Spina V, Monti S, Vaisitti T, Arruga F, Famà
R, et al: The coding genome of splenic marginal zone lymphoma:
Activation of NOTCH2 and other pathways regulating marginal zone
development. J Exp Med. 209:1537–1551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Uyttendaele H, Soriano JV, Montesano R and
Kitajewski J: Notch4 and Wnt-1 proteins function to regulate
branching morphogenesis of mammary epithelial cells in an opposing
fashion. Dev Biol. 196:204–217. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bellavia D, Checquolo S, Campese AF, Felli
MP, Gulino A and Screpanti I: Notch3: From subtle structural
differences to functional diversity. Oncogene. 27:5092–5098. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Melchor L and Smalley MJ: Highway to
heaven: Mammary gland development and differentiation. Breast
Cancer Res. 10:3052008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Weijzen S, Rizzo P, Braid M, Vaishnav R,
Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC,
et al: Activation of Notch-1 signaling maintains the neoplastic
phenotype in human Ras-transformed cells. Nat Med. 8:979–986. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hu C, Diévart A, Lupien M, Calvo E,
Tremblay G and Jolicoeur P: Overexpression of activated murine
Notch1 and Notch3 in transgenic mice blocks mammary gland
development and induces mammary tumors. Am J Pathol. 168:973–990.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Imatani A and Callahan R: Identification
of a novel NOTCH-4/INT-3 RNA species encoding an activated gene
product in certain human tumor cell lines. Oncogene. 19:223–231.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Reedijk M, Odorcic S, Chang L, Zhang H,
Miller N, McCready DR, Lockwood G and Egan SE: High-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survival. Cancer Res.
65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Z, Wang H, Ikeda S, Fahey F,
Bielenberg D, Smits P and Hauschka PV: Notch3 in human breast
cancer cell lines regulates osteoblast-cancer cell interactions and
osteolytic bone metastasis. Am J Pathol. 177:1459–1469. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Parr C, Watkins G and Jiang WG: The
possible correlation of Notch-1 and Notch-2 with clinical outcome
and tumour clinicopathological parameters in human breast cancer.
Int J Mol Med. 14:779–786. 2004.PubMed/NCBI
|
|
60
|
Baumgart A, Mazur PK, Anton M, Rudelius M,
Schwamborn K, Feuchtinger A, Behnke K, Walch A, Braren R, Peschel
C, et al: Opposing role of Notch1 and Notch2 in a Kras(G12D)-driven
murine non-small cell lung cancer model. Oncogene. 34:578–588.
2015. View Article : Google Scholar
|
|
61
|
Yang Y, Yan X, Duan W, Yan J, Yi W, Liang
Z, Wang N, Li Y, Chen W, Yu S, et al: Pterostilbene exerts
antitumor activity via the Notch1 signaling pathway in human lung
adenocarcinoma cells. PLoS One. 8:e626522013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Licciulli S, Avila JL, Hanlon L, Troutman
S, Cesaroni M, Kota S, Keith B, Simon MC, Puré E, Radtke F, et al:
Notch1 is required for Kras-induced lung adenocarcinoma and
controls tumor cell survival via p53. Cancer Res. 73:5974–5984.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xie M, He CS, Wei SH and Zhang L: Notch-1
contributes to epidermal growth factor receptor tyrosine kinase
inhibitor acquired resistance in non-small cell lung cancer in
vitro and in vivo. Eur J Cancer. 49:3559–3572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hassan KA, Wang L, Korkaya H, Chen G,
Maillard I, Beer DG, Kalemkerian GP and Wicha M: Notch pathway
activity identifies cells with cancer stem cell-like properties and
correlates with worse survival in lung adenocarcinoma. Clin Cancer
Res. 19:1972–1980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Theys J, Yahyanejad S, Habets R, Span P,
Dubois L, Paesmans K, Kattenbeld B, Cleutjens J, Groot AJ,
Schuurbiers OC, et al: High NOTCH activity induces radiation
resistance in non small cell lung cancer. Radiother Oncol.
108:440–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wael H, Yoshida R, Kudoh S, Hasegawa K,
Niimori-Kita K and Ito T: Notch1 signaling controls cell
proliferation, apoptosis and differentiation in lung carcinoma.
Lung Cancer. 85:131–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang J, Song H, Liu B, Yu B, Wang R and
Chen L: Expression of Notch-1 and its clinical significance in
different histological subtypes of human lung adenocarcinoma. J Exp
Clin Cancer Res. 32:842013. View Article : Google Scholar :
|
|
68
|
Zhou M, Jin WY, Fan ZW and Han RC:
Analysis of the expression of the Notch3 receptor protein in adult
lung cancer. Oncol Lett. 5:499–504. 2013.PubMed/NCBI
|
|
69
|
Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML,
Chang BW and Zhang YB: Notch3 overexpression associates with poor
prognosis in human non-small-cell lung cancer. Med Oncol.
30:5952013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch1 signal
pathway is associated with gastric cancer progression through
cyclooxygenase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yao J and Qian C: Over-activated Notch-1
protects gastric carcinoma BGC-823 cells from TNFalpha-induced
apoptosis. Dig Liver Dis. 41:867–874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Carson C, Murdoch B and Roskams AJ: Notch
2 and Notch 1/3 segregate to neuronal and glial lineages of the
developing olfactory epithelium. Dev Dyn. 235:1678–1688. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sun Y, Gao X, Liu J, Kong QY, Wang XW,
Chen XY, Wang Q, Cheng YF, Qu XX and Li H: Differential Notch1 and
Notch2 expression and frequent activation of Notch signaling in
gastric cancers. Arch Pathol Lab Med. 135:451–458. 2011.PubMed/NCBI
|
|
75
|
Tseng YC, Tsai YH, Tseng MJ, Hsu KW, Yang
MC, Huang KH, Li AF, Chi CW, Hsieh RH, Ku HH, et al: Notch2-induced
COX-2 expression enhancing gastric cancer progression. Mol
Carcinog. 51:939–951. 2012. View Article : Google Scholar
|
|
76
|
Guo LY, Li YM, Qiao L, Liu T, Du YY, Zhang
JQ, He WT, Zhao YX and He DQ: Notch2 regulates matrix
metallopeptidase 9 via PI3K/AKT signaling in human gastric
carcinoma cell MKN-45. World J Gastroenterol. 18:7262–7270. 2012.
View Article : Google Scholar
|
|
77
|
Piazzi G, Fini L, Selgrad M, Garcia M,
Daoud Y, Wex T, Malfertheiner P, Gasbarrini A, Romano M, Meyer RL,
et al: Epigenetic regulation of Delta-Like1 controls Notch1
activation in gastric cancer. Oncotarget. 2:1291–1301. 2011.
View Article : Google Scholar
|
|
78
|
Pellegrinet L, Rodilla V, Liu Z, Chen S,
Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J and Radtke F:
Dll1- and dll4-mediated notch signaling are required for
homeostasis of intestinal stem cells. Gastroenterology.
140:1230–1240.e7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li GG, Li L, Li C, Ye LY, Li XW, Liu DR,
Bao Q, Zheng YX, Xiang DP, Chen L, et al: Influence of
up-regulation of Notch ligand DLL4 on biological behaviors of human
gastric cancer cells. World J Gastroenterol. 19:4486–4494. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sun HW, Wu C, Tan HY and Wang QS:
Combination DLL4 with Jagged1-siRNA can enhance inhibition of the
proliferation and invasiveness activity of human gastric carcinoma
by Notch1/VEGF pathway. Hepatogastroenterology. 59:924–929.
2012.
|
|
81
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rosenbluh J, Wang X and Hahn WC: Genomic
insights into WNT/β-catenin signaling. Trends Pharmacol Sci.
35:103–109. 2014. View Article : Google Scholar :
|
|
83
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Niehrs C: The complex world of WNT
receptor signalling. Nat Rev Mol Cell Biol. 13:767–779. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang JM, Huang FC, Kuo MH, Wang ZF, Tseng
TY, Chang LC, Yen SJ, Chang TC and Lin JJ: Inhibition of cancer
cell migration and invasion through suppressing the Wnt1-mediating
signal pathway by G-quadruplex structure stabilizers. J Biol Chem.
289:14612–14623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee MA, Park JH, Rhyu SY, Oh ST, Kang WK
and Kim HN: Wnt3a expression is associated with MMP-9 expression in
primary tumor and metastatic site in recurrent or stage IV
colorectal cancer. BMC Cancer. 14:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang SH, Li N, Wei Y, Li QR and Yu ZP:
β-catenin deacetylation is essential for WNT-induced proliferation
of breast cancer cells. Mol Med Rep. 9:973–978. 2014.PubMed/NCBI
|
|
88
|
Miao CG, Yang YY, He X, Huang C, Huang Y,
Zhang L, Lv XW, Jin Y and Li J: Wnt signaling in liver fibrosis:
Progress, challenges and potential directions. Biochimie.
95:2326–2335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Barbolina MV, Burkhalter RJ and Stack MS:
Diverse mechanisms for activation of Wnt signalling in the ovarian
tumour microenvironment. Biochem J. 437:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Andersen P, Uosaki H, Shenje LT and Kwon
C: Non-canonical Notch signaling: Emerging role and mechanism.
Trends Cell Biol. 22:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Clark CE, Nourse CC and Cooper HM: The
tangled web of non-canonical Wnt signalling in neural migration.
Neurosignals. 20:202–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
González-Sancho JM, Brennan KR,
Castelo-Soccio LA and Brown AM: Wnt proteins induce dishevelled
phosphorylation via an LRP5/6-independent mechanism, irrespective
of their ability to stabilize beta-catenin. Mol Cell Biol.
24:4757–4768. 2004. View Article : Google Scholar
|
|
95
|
Beier F and Loeser RF: Biology and
pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling
pathways in chondrocytes. J Cell Biochem. 110:573–580. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Asad M, Wong MK, Tan TZ, Choolani M, Low
J, Mori S, Virshup D, Thiery JP and Huang RY: FZD7 drives in vitro
aggressiveness in Stem-A subtype of ovarian cancer via regulation
of non-canonical Wnt/PCP pathway. Cell Death Dis. 5:e13462014.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bernemann C, Hülsewig C, Ruckert C,
Schäfer S, Blümel L, Hempel G, Götte M, Greve B, Barth PJ, Kiesel
L, et al: Influence of secreted frizzled receptor protein 1 (SFRP1)
on neoadjuvant chemotherapy in triple negative breast cancer does
not rely on WNT signaling. Mol Cancer. 13:1742014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xi Y and Chen Y: Wnt signaling pathway:
Implications for therapy in lung cancer and bone metastasis. Cancer
Lett. 353:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nejak-Bowen KN and Monga SP: Beta-catenin
signaling, liver regeneration and hepatocellular cancer: Sorting
the good from the bad. Semin Cancer Biol. 21:44–58. 2011.
View Article : Google Scholar :
|
|
100
|
Colussi D, Brandi G, Bazzoli F and
Ricciardiello L: Molecular pathways involved in colorectal cancer:
Implications for disease behavior and prevention. Int J Mol Sci.
14:16365–16385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pez F, Lopez A, Kim M, Wands JR, Caron de
Fromentel C and Merle P: Wnt signaling and hepatocarcinogenesis:
Molecular targets for the development of innovative anticancer
drugs. J Hepatol. 59:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Armengol C, Cairo S, Fabre M and Buendia
MA: Wnt signaling and hepatocarcinogenesis: The hepatoblastoma
model. Int J Biochem Cell Biol. 43:265–270. 2011. View Article : Google Scholar
|
|
103
|
Gedaly R, Galuppo R, Daily MF, Shah M,
Maynard E, Chen C, Zhang X, Esser KA, Cohen DA, Evers BM, et al:
Targeting the Wnt/β-catenin signaling pathway in liver cancer stem
cells and hepatocellular carcinoma cell lines with FH535. PLoS One.
9:e992722014. View Article : Google Scholar
|
|
104
|
Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J,
Hu H, Guan W and Ma Y: Inhibitory effect and mechanism of
mesenchymal stem cells on liver cancer cells. Tumour Biol.
35:1239–1250. 2014. View Article : Google Scholar
|
|
105
|
Zucchini-Pascal N, Peyre L and Rahmani R:
Crosstalk between beta-catenin and snail in the induction of
epithelial to mesenchymal transition in hepatocarcinoma: Role of
the ERK1/2 pathway. Int J Mol Sci. 14:20768–20792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bustos VH, Ferrarese A, Venerando A, Marin
O, Allende JE and Pinna LA: The first armadillo repeat is involved
in the recognition and regulation of beta-catenin phosphorylation
by protein kinase CK1. Proc Natl Acad Sci USA. 103:19725–19730.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Singh Y, Port J, Schwarz M and Braeuning
A: Genetic ablation of β-catenin inhibits the proliferative
phenotype of mouse liver adenomas. Br J Cancer. 111:132–138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Calderaro J, Nault JC, Bioulac-Sage P,
Laurent A, Blanc JF, Decaens T and Zucman-Rossi J: ALDH3A1 is
overexpressed in a subset of hepatocellular carcinoma characterised
by activation of the Wnt/ss-catenin pathway. Virchows Arch.
464:53–60. 2014. View Article : Google Scholar
|
|
109
|
Cheng JH, She H, Han YP, Wang J, Xiong S,
Asahina K and Tsukamoto H: Wnt antagonism inhibits hepatic stellate
cell activation and liver fibrosis. Am J Physiol Gastrointest Liver
Physiol. 294:G39–G49. 2008. View Article : Google Scholar
|
|
110
|
Li W, Zhu C, Li Y, Wu Q and Gao R: Mest
attenuates CCl4-induced liver fibrosis in rats by inhibiting the
Wnt/β-catenin signaling pathway. Gut Liver. 8:282–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tenesa A and Dunlop MG: New insights into
the aetiology of colorectal cancer from genome-wide association
studies. Nat Rev Genet. 10:353–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pandurangan AK: Potential targets for
prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt
pathways. Asian Pac J Cancer Prev. 14:2201–2205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Curtin JC: Novel drug discovery
opportunities for colorectal cancer. Expert Opin Drug Discov.
8:1153–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sparks AB, Morin PJ, Vogelstein B and
Kinzler KW: Mutational analysis of the APC/beta-catenin/Tcf pathway
in colorectal cancer. Cancer Res. 58:1130–1134. 1998.PubMed/NCBI
|
|
115
|
Murakami T, Mitomi H, Saito T, Takahashi
M, Sakamoto N, Fukui N, Yao T and Watanabe S: Distinct
WNT/beta-catenin signaling activation in the serrated neoplasia
pathway and the adenoma-carcinoma sequence of the colorectum. Mod
Pathol. 28:146–158. 2015. View Article : Google Scholar
|
|
116
|
Raghu D and Karunagaran D: Plumbagin
downregulates Wnt signaling independent of p53 in human colorectal
cancer cells. J Nat Prod. 77:1130–1134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Tai WP, Hu PJ, Wu J and Lin XC: The
inhibition of Wnt/β-catenin signaling pathway in human colon cancer
cells by sulindac. Tumori. 100:97–101. 2014.PubMed/NCBI
|
|
118
|
Tumova L, Pombinho AR, Vojtechova M,
Stancikova J, Gradl D, Krausova M, Sloncova E, Horazna M, Kriz V,
Machonova O, et al: Monensin inhibits canonical Wnt signaling in
human colorectal cancer cells and suppresses tumor growth in
multiple intestinal neoplasia mice. Mol Cancer Ther. 13:812–822.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bruun J, Kolberg M, Nesland JM, Svindland
A, Nesbakken A and Lothe RA: Prognostic Significance of β-catenin,
E-cadherin, and SOX9 in colorectal cancer: Results from a large
population-representative series. Front Oncol. 4:1182014.
View Article : Google Scholar
|
|
120
|
Voorham QJ, Janssen J, Tijssen M,
Snellenberg S, Mongera S, van Grieken NC, Grabsch H, Kliment M,
Rembacken BJ, Mulder CJ, et al: Promoter methylation of
Wnt-antagonists in polypoid and nonpolypoid colorectal adenomas.
BMC Cancer. 13:6032013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Serafino A, Moroni N, Zonfrillo M,
Andreola F, Mercuri L, Nicotera G, Nunziata J, Ricci R, Antinori A,
Rasi G, et al: WNT-pathway components as predictive markers useful
for diagnosis, prevention and therapy in inflammatory bowel disease
and sporadic colorectal cancer. Oncotarget. 5:978–992. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Abdelmaksoud-Dammak R, Miladi-Abdennadher
I, Saadallah-Kallel A, Khabir A, Sellami-Boudawara T, Frikha M,
Daoud J and Mokdad-Gargouri R: Downregulation of WIF-1 and Wnt5a in
patients with colorectal carcinoma: clinical significance. Tumour
Biol. 35:7975–7982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Bauer M, Bénard J, Gaasterland T, Willert
K and Cappellen D: WNT5A encodes two isoforms with distinct
functions in cancers. PLoS One. 8:e805262013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chai J, Modak C, Ouyang Y, Wu SY and Jamal
MM: CCN1 Induces β-catenin translocation in esophageal squamous
cell carcinoma through integrin α11. ISRN Gastroenterol.
2012:2072352012. View Article : Google Scholar
|
|
125
|
Moyes LH, McEwan H, Radulescu S,
Pawlikowski J, Lamm CG, Nixon C, Sansom OJ, Going JJ, Fullarton GM
and Adams PD: Activation of Wnt signalling promotes development of
dysplasia in Barrett's oesophagus. J Pathol. 228:99–112.
2012.PubMed/NCBI
|
|
126
|
Long A, Giroux V, Whelan KA, Hamilton KE,
Tétreault MP, Tanaka K, Lee JS, Klein-Szanto AJ, Nakagawa H and
Rustgi AK: WNT10A promotes an invasive and self-renewing phenotype
in esophageal squamous cell carcinoma. Carcinogenesis. 36:598–606.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yang SH, Li SL, Dong ZM and Kan QC:
Epigenetic inactivation of Wnt inhibitory factor-1 in human
esophageal squamous cell carcinoma. Oncol Res. 20:123–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY,
Xue WQ, Chen YB, Zhang Y and Jia WH: HOTAIR, a prognostic factor in
esophageal squamous cell carcinoma, inhibits WIF-1 expression and
activates Wnt pathway. Cancer Sci. 104:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu K, Luo Y, Tian H, Yu KZ, He JX and
Shen WY: The tumor suppressor LKB1 antagonizes WNT signaling
pathway through modulating GSK3beta activity in cell growth of
esophageal carcinoma. Tumour Biol. 35:995–1002. 2014. View Article : Google Scholar
|
|
130
|
Tong X, Li L, Li X, Heng L, Zhong L, Su X,
Rong R, Hu S, Liu W, Jia B, et al: SOX10, a novel
HMG-box-containing tumor suppressor, inhibits growth and metastasis
of digestive cancers by suppressing the Wnt/β-catenin pathway.
Oncotarget. 5:10571–10583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kuramoto T, Goto H, Mitsuhashi A, Tabata
S, Ogawa H, Uehara H, Saijo A, Kakiuchi S, Maekawa Y, Yasutomo K,
et al: Dll4-Fc, an inhibitor of Dll4-notch signaling, suppresses
liver metastasis of small cell lung cancer cells through the
downregulation of the NF-κB activity. Mol Cancer Ther.
11:2578–2587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Stewart KS, Zhou Z, Zweidler-McKay P and
Kleinerman ES: Delta-like ligand 4-Notch signaling regulates bone
marrow-derived pericyte/vascular smooth muscle cell formation.
Blood. 117:719–726. 2011. View Article : Google Scholar :
|
|
133
|
Ridgway J, Zhang G, Wu Y, Stawicki S,
Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I,
et al: Inhibition of Dll4 signalling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Oishi H, Sunamura M, Egawa S, Motoi F,
Unno M, Furukawa T, Habib NA and Yagita H: Blockade of delta-like
ligand 4 signaling inhibits both growth and angiogenesis of
pancreatic cancer. Pancreas. 39:897–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gurney A and Hoey T: Anti-DLL4, a cancer
therapeutic with multiple mechanisms of action. Vasc Cell.
3:182011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Fischer M, Yen WC, Kapoun AM, Wang M,
O'Young G, Lewicki J, Gurney A and Hoey T: Anti-DLL4 inhibits
growth and reduces tumor-initiating cell frequency in colorectal
tumors with oncogenic KRAS mutations. Cancer Res. 71:1520–1525.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Jenkins DW, Ross S, Veldman-Jones M, Foltz
IN, Clavette BC, Manchulenko K, Eberlein C, Kendrew J, Petteruti P,
Cho S, et al: MEDI0639: A novel therapeutic antibody targeting Dll4
modulates endothelial cell function and angiogenesis in vivo. Mol
Cancer Ther. 11:1650–1660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Liu SK, Bham SA, Fokas E, Beech J, Im J,
Cho S, Harris AL and Muschel RJ: Delta-like ligand 4-notch blockade
and tumor radiation response. J Natl Cancer Inst. 103:1778–1798.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
El Kaffas A, Nofiele J, Giles A, Cho S,
Liu SK and Czarnota GJ: Dll4-notch signalling blockade synergizes
combined ultrasound-stimulated microbubble and radiation therapy in
human colon cancer xenografts. PLoS One. 9:e938882014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Aste-Amézaga M, Zhang N, Lineberger JE,
Arnold BA, Toner TJ, Gu M, Huang L, Vitelli S, Vo KT, Haytko P, et
al: Characterization of Notch1 antibodies that inhibit signaling of
both normal and mutated Notch1 receptors. PLoS One. 5:e90942010.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Sharma A, Paranjape AN, Rangarajan A and
Dighe RR: A monoclonal antibody against human Notch1 ligand-binding
domain depletes subpopulation of putative breast cancer stem-like
cells. Mol Cancer Ther. 11:77–86. 2012. View Article : Google Scholar
|
|
142
|
Yan M, Callahan CA, Beyer JC, Allamneni
KP, Zhang G, Ridgway JB, Niessen K and Plowman GD: Chronic DLL4
blockade induces vascular neoplasms. Nature. 463:E6–E7. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Rosati E, Sabatini R, De Falco F, Del Papa
B, Falzetti F, Di Ianni M, Cavalli L, Fettucciari K, Bartoli A,
Screpanti I, et al: gamma-Secretase inhibitor I induces apoptosis
in chronic lymphocytic leukemia cells by proteasome inhibition,
endoplasmic reticulum stress increase and notch down-regulation.
Int J Cancer. 132:1940–1953. 2013. View Article : Google Scholar
|
|
144
|
Palagani V, El Khatib M, Kossatz U, Bozko
P, Müller MR, Manns MP, Krech T, Malek NP and Plentz RR: Epithelial
mesenchymal transition and pancreatic tumor initiating
CD44+/EpCAM+ cells are inhibited by
γ-secretase inhibitor IX. PLoS One. 7:e465142012. View Article : Google Scholar
|
|
145
|
Schott AF, Landis MD, Dontu G, Griffith
KA, Layman RM, Krop I, Paskett LA, Wong H, Dobrolecki LE, Lewis MT,
et al: Preclinical and clinical studies of gamma secretase
inhibitors with docetaxel on human breast tumors. Clin Cancer Res.
19:1512–1524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
López-Guerra M, Xargay-Torrent S, Rosich
L, Montraveta A, Roldán J, Matas-Céspedes A, Villamor N, Aymerich
M, López-Otín C, Pérez-Galán P, et al: The γ-secretase inhibitor
PF-03084014 combined with fludarabine antagonizes migration,
invasion and angiogenesis in NOTCH1-mutated CLL cells. Leukemia.
29:96–106. 2015. View Article : Google Scholar
|
|
147
|
Saito N, Fu J, Zheng S, Yao J, Wang S, Liu
DD, Yuan Y, Sulman EP, Lang FF, Colman H, et al: A high Notch
pathway activation predicts response to γ secretase inhibitors in
proneural subtype of glioma tumor-initiating cells. Stem Cells.
32:301–312. 2014. View Article : Google Scholar :
|
|
148
|
Groeneweg JW, Hall TR, Zhang L, Kim M,
Byron VF, Tambouret R, Sathayanrayanan S, Foster R, Rueda BR and
Growdon WB: Inhibition of gamma-secretase activity impedes uterine
serous carcinoma growth in a human xenograft model. Gynecol Oncol.
133:607–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li LC, Peng Y, Liu YM, Wang LL and Wu XL:
Gastric cancer cell growth and epithelial-mesenchymal transition
are inhibited by γ-secretase inhibitor DAPT. Oncol Lett.
7:2160–2164. 2014.PubMed/NCBI
|
|
150
|
Dahmani R, Just PA and Perret C: The
Wnt/β-catenin pathway as a therapeutic target in human
hepatocellular carcinoma. Clin Res Hepatol Gastroenterol.
35:709–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Fontenot E, Rossi E, Mumper R, Snyder S,
Siamakpour-Reihani S, Ma P, Hilliard E, Bone B, Ketelsen D, Santos
C, et al: A novel monoclonal antibody to secreted frizzled-related
protein 2 inhibits tumor growth. Mol Cancer Ther. 12:685–695. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang Y, Shek FH, Wong KF, Liu LX, Zhang
XQ, Yuan Y, Khin E, Hu MY, Wang JH, Poon RT, et al:
Anti-cadherin-17 antibody modulates beta-catenin signaling and
tumorigenicity of hepatocellular carcinoma. PLoS One. 8:e723862013.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Gao W, Kim H, Feng M, Phung Y, Xavier CP,
Rubin JS and Ho M: Inactivation of Wnt signaling by a human
antibody that recognizes the heparan sulfate chains of glypican-3
for liver cancer therapy. Hepatology. 60:576–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ettenberg SA, Charlat O, Daley MP, Liu S,
Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, et
al: Inhibition of tumorigenesis driven by different Wnt proteins
requires blockade of distinct ligand-binding regions by LRP6
antibodies. Proc Natl Acad Sci USA. 107:15473–15478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Gong Y, Bourhis E, Chiu C, Stawicki S,
DeAlmeida VI, Liu BY, Phamluong K, Cao TC, Carano RA, Ernst JA, et
al: Wnt isoform-specific interactions with coreceptor specify
inhibition or potentiation of signaling by LRP6 antibodies. PLoS
One. 5:e126822010. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lavergne E, Hendaoui I, Coulouarn C,
Ribault C, Leseur J, Eliat PA, Mebarki S, Corlu A, Clément B and
Musso O: Blocking Wnt signaling by SFRP-like molecules inhibits in
vivo cell proliferation and tumor growth in cells carrying active
β-catenin. Oncogene. 30:423–433. 2011. View Article : Google Scholar
|
|
157
|
Wei W, Chua MS, Grepper S and So SK:
Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes
hepatocellular carcinoma cells towards doxorubicin. Mol Cancer.
10:162011. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Amado NG, Predes D, Moreno MM, Carvalho
IO, Mendes FA and Abreu JG: Flavonoids and Wnt/β-catenin signaling:
Potential role in colorectal cancer therapies. Int J Mol Sci.
15:12094–12106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J, et al: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. PLoS One. 8:e787002013. View Article : Google Scholar
|
|
160
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Waaler J, Machon O, Tumova L, Dinh H,
Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova
O, et al: A novel tankyrase inhibitor decreases canonical Wnt
signaling in colon carcinoma cells and reduces tumor growth in
conditional APC mutant mice. Cancer Res. 72:2822–2832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Park HY, Toume K, Arai MA, Sadhu SK, Ahmed
F and Ishibashi M: Calotropin: A cardenolide from calotropis
gigantea that inhibits Wnt signaling by increasing casein kinase 1α
in colon cancer cells. Chem Bio Chem. 15:872–878. 2014. View Article : Google Scholar
|
|
163
|
Li B, Flaveny CA, Giambelli C, Fei DL, Han
L, Hang BI, Bai F, Pei XH, Nose V, Burlingame O, et al: Repurposing
the FDA-approved pinworm drug pyrvinium as a novel chemotherapeutic
agent for intestinal polyposis. PLoS One. 9:e1019692014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wei W, Chua MS, Grepper S and So S: Small
molecule antagonists of Tcf4/beta-catenin complex inhibit the
growth of HCC cells in vitro and in vivo. Int J Cancer.
126:2426–2436. 2010.
|
|
165
|
Lee SB, Gong YD, Park YI and Dong MS:
2,3,6-Trisubstituted quinoxaline derivative, a small molecule
inhibitor of the Wnt/beta-catenin signaling pathway, suppresses
cell proliferation and enhances radiosensitivity in A549/Wnt2
cells. Biochem Biophys Res Commun. 431:746–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Preet R, Mohapatra P, Das D, Satapathy SR,
Choudhuri T, Wyatt MD and Kundu CN: Lycopene synergistically
enhances quinacrine action to inhibit Wnt-TCF signaling in breast
cancer cells through APC. Carcinogenesis. 34:277–286. 2013.
View Article : Google Scholar
|
|
167
|
Park S and Chun S: Streptonigrin inhibits
β-catenin/Tcf signaling and shows cytotoxicity in
β-catenin-activated cells. Biochim Biophys Acta. 1810:1340–1345.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Emami KH, Nguyen C, Ma H, Kim DH, Jeong
KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al: A small
molecule inhibitor of beta-catenin/CREB-binding protein
transcription [corrected]. Proc Natl Acad Sci USA. 101:12682–12687.
2004. View Article : Google Scholar
|
|
169
|
Yu SD, Liu FY and Wang QR: Notch
inhibitor: A promising carcinoma radiosensitizer. Asian Pac J
Cancer Prev. 13:5345–5351. 2012. View Article : Google Scholar
|
|
170
|
Wei W, Chua MS, Grepper S and So SK:
Blockade of Wnt-1 signaling leads to anti-tumor effects in
hepatocellular carcinoma cells. Mol Cancer. 8:762009. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Mazieres J, You L, He B, Xu Z, Twogood S,
Lee AY, Reguart N, Batra S, Mikami I and Jablons DM: Wnt2 as a new
therapeutic target in malignant pleural mesothelioma. Int J Cancer.
117:326–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Pode-Shakked N, Harari-Steinberg O,
Haberman-Ziv Y, Rom-Gross E, Bahar S, Omer D, Metsuyanim S, Buzhor
E, Jacob-Hirsch J, Goldstein RS, et al: Resistance or sensitivity
of Wilms' tumor to anti-FZD7 antibody highlights the Wnt pathway as
a possible therapeutic target. Oncogene. 30:1664–1680. 2011.
View Article : Google Scholar : PubMed/NCBI
|