Introduction
The epithelial-mesenchymal transition (EMT) was
associated with tumor progression, especially the initiation of
tumor metastasis. In the EMT process, the epithelial apico-basal
polarity disappears, connections between cells decrease, actin
cytoskeleton reorganizes, and cell morphology changes to
spindle-like cells. These changes are beneficial for tumor cells to
gain the ability to invade and metastasize. The molecular
mechanisms of EMT could be due to the upregulation of EMT inducers
(snail/slug, twist, and ZEB1) and mesenchymal markers (vimentin,
and N-cadherin), and downregulation of epithelial markers
(E-cadherin) (1–5).
ADP-ribosylation, which is catalyzed by
ADP-ribosyltransferases, is a post-translational modification that
is widely used to regulate the function of proteins.
ADP-ribosyltransferases cleave the glycosidic bond between
nicotinamide with the adjacent ribose in the NAD+
molecule and then transfer the ADP-ribose moiety to specific amino
acids of proteins. These enzymes include
mono-ADP-ribosyltransferases (mARTs) and poly-ADP-ribose
polymerases (PARPs), which transfer a single ADP-ribose and
polymers of ADP-ribose to substrates, respectively (6,7).
Many studies have reported that PARPs are closely related to cell
proliferation, apoptosis, migration, invasion, angiogenesis and
inflammation (8–13). Although the modification of
mono-ADP-ribosylation is more common than poly-ADP-ribosylation in
cells, few studies have been performed, especially in tumors
(14). Arginine-specific
mono-ADP-ribosytransferase 1 (ART1) belongs to the
mono-ADP-ribosyltransferase family. It is present in humans, mice
and rats (15,16), and it is involved in the
modification of proteins, such as FGF-2, TNF-α, HNP-1, PDGFBB, and
integrin α7β1. The biological effects of ART1 may be related to
cell proliferation, apoptosis, inflammation, angiogenesis and cell
adhesion in non-tumor cells (17–21).
We previously reported that ART1 could enhance proliferation and
inhibit apoptosis in colon carcinoma (22–24).
However, whether ART1 could influence migration through EMT in
colon carcinoma and what the concrete mechanism is has not been
reported.
In this study, we showed that the change of ART1
expression in CT26 cells could affect the expression of EMT
inducers, mesenchymal markers and epithelial markers and then
influence the invasion and metastasis in vitro and in
vivo. ART1 may be a new regulatory factor for EMT, and it is
expected to become a potential therapeutic target for colorectal
carcinoma.
Materials and methods
Cell lines and animals
CT26 cells, a murine colon adenocarcinoma cell line,
were a gift of Professor Yu-Guan Wei (West China Hospital, Chengdu,
China). Lentivirus that express the mouse ART1 cDNA or a short
hairpin RNA (shRNA) against ART1 were respectively constructed by
Genechem (Shanghai, China) and were effectively transfected into
CT26 cells, as previously described (22,25).
The experimental groups included overexpression of ART1 in CT26
cells (GFP-ART1 group) and silencing of ART1 in CT26 cells
(GFP-shART1 group); untransfected CT26 cells (non-transfection
group) and CT26 cells transfected with empty lentivirus vector
(GFP-vector group) were used as the control groups. BALB/c mice
were obtained from Experimental Animal Center of National
Bio-industry Base in Chongqing.
Cell culture
The CT26 cells were cultured in RPMI-1640 medium
(Hyclone, Logan, UT, USA) with 10% fetal bovine serum (FBS)
(Sijiqing, Hangzhou, China) under appropriate humidity conditions
(5% CO2, 37°C).
Viral infection
Adenovirus (Ad)-si-β-catenin was obtained from
Jia-Yi Huang (Departments of Pathophysiology, Chongqing Medical
University, Chongqing, China). The transfection was performed as
previously described (26). The
efficiency of Ad-si-β-catenin transfection was verified by reverse
transcription-PCR (RT-PCR) and western blot analysis.
Reverse transcription-PCR analysis
Total CT26 cellular mRNA in each group was extracted
with TRIzol reagent (Takara, Dalian, China) and we used an RT-PCR
kit (Takara) for reverse transcription. The primers were as
follows: β-catenin, 5′-caatcaagagagcaagctcatc-3′ (F) and
5′-agtcgctgactt gggtctgt-3′ (R) and β-actin,
5′-cacccgcgagtacaaccttc-3′ (F) and 5′-cccatacccaccacacc-3′ (R)
(Sangon Biotech Co., Shanghai, China). The conditions of reverse
transcription included reaction at 56°C for 45 min and 95°C for 3
min, and the conditions of extension were repeated 30 cycles at
95°C for 30 sec, 60°C for 30 sec and 72°C for 60 sec.
Electrophoresis on 2% agarose gels (Genview, Tallahassee, FL, USA)
was used to detect the PCR amplification products.
Detection of cell morphology by
hematoxylin-eosin staining (H&E staining)
Cells were incubated on cover slips, washed with
phosphate-buffered saline (PBS), fixed with 95% ethanol for 20 min,
and stained with hematoxylin-eosin. Each cover slip was detected by
microscopy (Nikon Corp., Tokyo, Japan).
Detection of actin polymerization
assay
CT26 cells were seeded on cover slips. Each cover
slip was washed three times with PBS and fixed in 4%
paraformaldehyde for 20 min. Then, cells were treated with 0.1%
Triton X-100 for 15 min at room temperature and blocked with 5%
bovine serum albumin (BSA) for 30 min at 37°C. Then, the cells were
incubated with 0.5% rhodamine-phalloidin (Sigma, St. Louis, MO,
USA) 30 min at room temperature. Finally, each cover slip was
sealed with an anti-fluorescence quenching agent (Beyotime,
Shanghai, China) and was observed with confocal microscopy (Leica
TCS SP2, Leica Microsystems GmbH, Germany).
Flow cytometry method (FCM)
Cells (1×106/l) were digested by 0.25%
trypsin, fixed with 4% paraformaldehyde for 40 min, washed with PBS
and blocked with 5% BSA for 30 min. Then, cells were centrifuged at
800 rpm for 5 min. Half a percent of phalloidin-rhodamine (200 μl)
was used to re-suspend the cell precipitate, which was incubated
for 30 min. Finally, cells were washed three times with PBS and we
detected the average fluorescence intensity by flow cytometry
(FACSVantage SE, Becton-Dickinson Co., Franklin Lakes, NJ,
USA).
Wound healing assay
Cells (1×106/l) were cultured in 6-well
plates. When cells proliferated to form a full layer in each well,
the cells in the middle of each well were scraped and washed away
with PBS for creating a channel in the middle of each well. After
being cultured in RPMI-1640 containing 0.5% FBS for 24 h, the
changes in the width of the channel, which presented the migration
ability in each group, were detected by microscopy (Nikon
Corp.).
Invasion assay
The upper surface of the polycarbonate membrane was
paved with Matrigel (Becton-Dickinson Co.) and the chamber was
treated with 0.6 ml medium containing 10% FBS. Cells
(5×105) were grown onto the upper chamber with 0.2 ml
serum-free medium. Cells were fixed with 4% paraformaldehyde after
being incubated for 20 h. Then, a cotton swab was used to wipe the
cells from the upper chamber. Cells on the lower surface of the
chamber were stained with crystal violet. The number of cells that
had migrated into the lower surface of chamber was counted in
random selection, five times magnification, without repetition.
Adhesion assay
A 96-well plate was prepared with 10 mg/l
fibronectin (FN) overnight at 4°C and 1% BSA was used to block the
FN for 2 h. One hundred microliters of 10% FBS medium with cells
(1×105) was added to the plate, which was then incubated
at 5% CO2 and 37°C. The floating cells were washed off
with PBS after 2 h. One hundred microliters of medium containing 10
μl cell counting kit-8 solution (CCK-8, Dojindo, Kumamoto, Japan)
was added to each well and then incubated for 2 h at 37°C. Finally,
the OD value was measured by the absorbance at 450 nm with a
microplate reader (Bio-Rad, CA, USA).
Western blot analysis
The total or nuclear proteins of CT26 cells in each
group and mouse transplantation tumor tissues were extracted by the
total and nuclear protein extraction kit (Beyotime), and the
concentration of protein was detected with a bicinchoninic acid
(BCA) protein assay kit (Beyotime). All of the proteins were
separated in SDS-PAGE gel and transferred to PVDF membranes
(Millipore, MA, USA) by electrophoresis. The membranes were blocked
with 5% non-fat milk for 1 h and incubated with primary antibodies
against ART1 (Abgent Technology, San Diego, CA, USA), β-catenin
(Santa Cruz Biotechnology, Santa Cruz, CA, USA), N-cadherin,
E-cadherin (Bioworld Technology, St. Louis, MO, USA), Histone 2B,
vimentin, ROCK1 (Proteintech Group, Chicago, IL, USA), AKT,
phospho-Akt (Ser473), GSK-3β, phospho-GSK-3β (Ser9) (Cell Signaling
Technology, Boston, MA, USA), β-actin (Bioss, Shanghai, China) at
4°C overnight, respectively. Peroxidase-conjugated goat anti-rabbit
IgG (Bioss) was used to incubate these membranes for 2 h at room
temperature. Finally, the membranes were examined with enhanced
chemiluminescence reagents (ECL) (Beyotime) and analyzed using
Quantity One software.
Tumor transplantation model
BALB/c mice (Mus musculus Linnaeus) (female,
6–8 weeks old, and 18–22 g) were used. CT26 cells
(1×107/ml) were collected from each group. Each mouse,
which was anesthetized by 2% chloral hydrate (0.3 g/kg)
intraperitoneal injection, was injected with 5×105 CT26
cells into the splenic capsule. These mice were executed following
2 weeks of feeding after they were injected CT26 cells.
Furthermore, the number of liver metastasis nodules and the liver
weights were recorded respectively. Tumors from the spleen were
used to extract protein for western blot analysis (28).
Statistical analysis
The above experiments were all repeated three times
each. Data were analyzed using SPSS 18.0 software (SPSS, Chicago,
IL, USA). The values are represented as the means ± standard
deviation (SD) (mean ± SD). Differences between each group were
analyzed with one-way ANOVA or the Student's t-test. P-value
<0.05 was identified as a statistically significant
difference.
Results
Effects of ART1 on the CT26 cell
morphology
Cell morphological changes were observed under
optical microscope with H&E staining. Compared to
non-transfection CT26 cells and GFP-vector CT26 cells, GFP-ART1
CT26 cells showed spindle-like morphology, while most of GFP-shART1
CT26 cells showed epithelioid morphology (Fig. 1A).
Effect of ART1 on the expression of
proteins related to EMT in vitro and in vivo
The expressions of snail1, vimentin and N-cadherin
in the GFP-ART1 group were significantly increased, compared to the
non-transfection and GFP-vector groups in vitro and in
vivo (p<0.05). In contrast, expression of these proteins in
the GFP-shART1 group was significantly deceased, compared to the
control groups in vitro and in vivo. However, the
expression of the epithelial marker E-cadherin was reduced in the
GFP-ART1 groups and increased in the GFP-shART1 groups compared to
other control groups in vitro and in vivo (p<0.05)
(Fig. 1B–E).
Effects of ART1 on the change of actin in
CT26 cells
Using confocal laser scanning microscopy, a
disordered actin network and fewer actin fibers in the cytoplasm
were detected in the GFP-shART1 CT26 cells. However, the GFP-ART1
CT26 cells had an ordered actin network and more actin fibers
compared with the control groups (Fig.
2A). To quantify the effect of ART1 on actin, the fluorescence
intensity of F-actin was detected with FCM. The mean fluorescence
intensity of F-actin in the GFP-ART1 CT26 cells was enhanced and it
in the GFP-shART1 CT26 cells was diminished (p<0.01) (Fig. 2B).
Effects of ART1 on adhesion, movement and
invasion of CT26 cells
The change of ART1 was found to affect the adhesion,
movement and invasion in CT26 cells. Compared with the control
groups, GFP-ART1 CT26 cells showed that the rate of cell adhesion,
migration distance and the numbers of CT26 cells which migrated to
the chamber membrane were all increased (p<0.05), while CT26
cells with silenced ART1 showed an obviously opposite trend
(p<0.01) (Fig. 3).
Effect of ART1 on liver metastases of
CT26 cells in BALB/c mice
To investigate whether ART1 could affect the
metastasis of CT26 cells in vivo, we successfully generated
a liver metastasis model of BALB/c mice through injecting CT26
cells into the splenic capsule, the mice were sacrificed after two
weeks, the liver weighed and the metastatic nodules in the liver
counted. We observed that each GFP-ART1 group had ≥20 white nodules
of metastatic carcinoma, while in each GFP-shART1 group only 1–2
nodules of metastatic carcinoma was observed (p<0.01) (Fig. 4A and B). Compared to the GFP-vector
group and non-transfection group, the liver weight was much heavier
in the GFP-ART1 group and lighter in the GFP-shART1 group
(p<0.01) (Fig. 4C). Therefore,
the change in the ART1 dose appears to play a role in colon
carcinoma metastasis in vivo.
Effect of ART1 on the expression levels
of RhoA, ROCK1, phospho-AktSer473, and
phosphor-GSK-3βSer9 in vitro and in vivo
Significant increases in the expression of RhoA,
ROCK1, phospho-AktSer473, phosphor-GSK-3βSer9
were observed in the GFP-ART1 group compared to the
non-transfection and GFP-vector groups (p<0.05). In contrast,
the expression of these proteins in the GFP-shART1 group was
significantly deceased compared to the control groups (p<0.05).
Non-phosphorylation of AKT and GSK-3β were no significantly
different in these groups (p>0.05) (Fig. 5A–D and G–J).
 | Figure 5Expression of RhoA, ROCK1,
phospho-AktSer473, phospho-GSK-3βSer9, total
and nuclear β-catenin detected by western blotting in vitro
and in vivo. (A–F) Expression of RhoA, ROCK1,
phospho-AktSer473, phospho-GSK-3βSer9, total
and nuclear β-catenin showed a significant increase in the GFP-ART1
CT26 cells and displayed a decrease in the GFP-shART1 CT26 cells
compared with the GFP-vector and non-transfection CT26 cells
(**p<0.01). The expression of non-phosphorylation AKT
and GSK-3β were not significantly different among each group
(p>0.05). (G–L) Protein was extracted from the intrasplenic
transplantation tumor of CT26 cells. The expression of ART1, RhoA,
ROCK1, phospho-AktSer473, phospho-GSK-3βSer9,
total and nuclear β-catenin also had an observable decline in the
GFP-shART1 group and significant increase in the GFP-ART1 group,
compared with GFP-vector and non-transfection groups
(**p<0.01). The expression of non-phosphorylation AKT
and GSK-3β were not observably different among each group
(p>0.05). |
Effect of ART1 on the expression of
β-catenin in vivo and in vitro
The results of western blot analysis showed that
there was an increase in both expression levels of the total
β-catenin and nuclear β-catenin in the GFP-ART1 group (p<0.01).
Expression of the total β-catenin and nuclear β-catenin were
decreased in the GFP-shART1 group (p<0.01) (Fig. 5E, F, K and J).
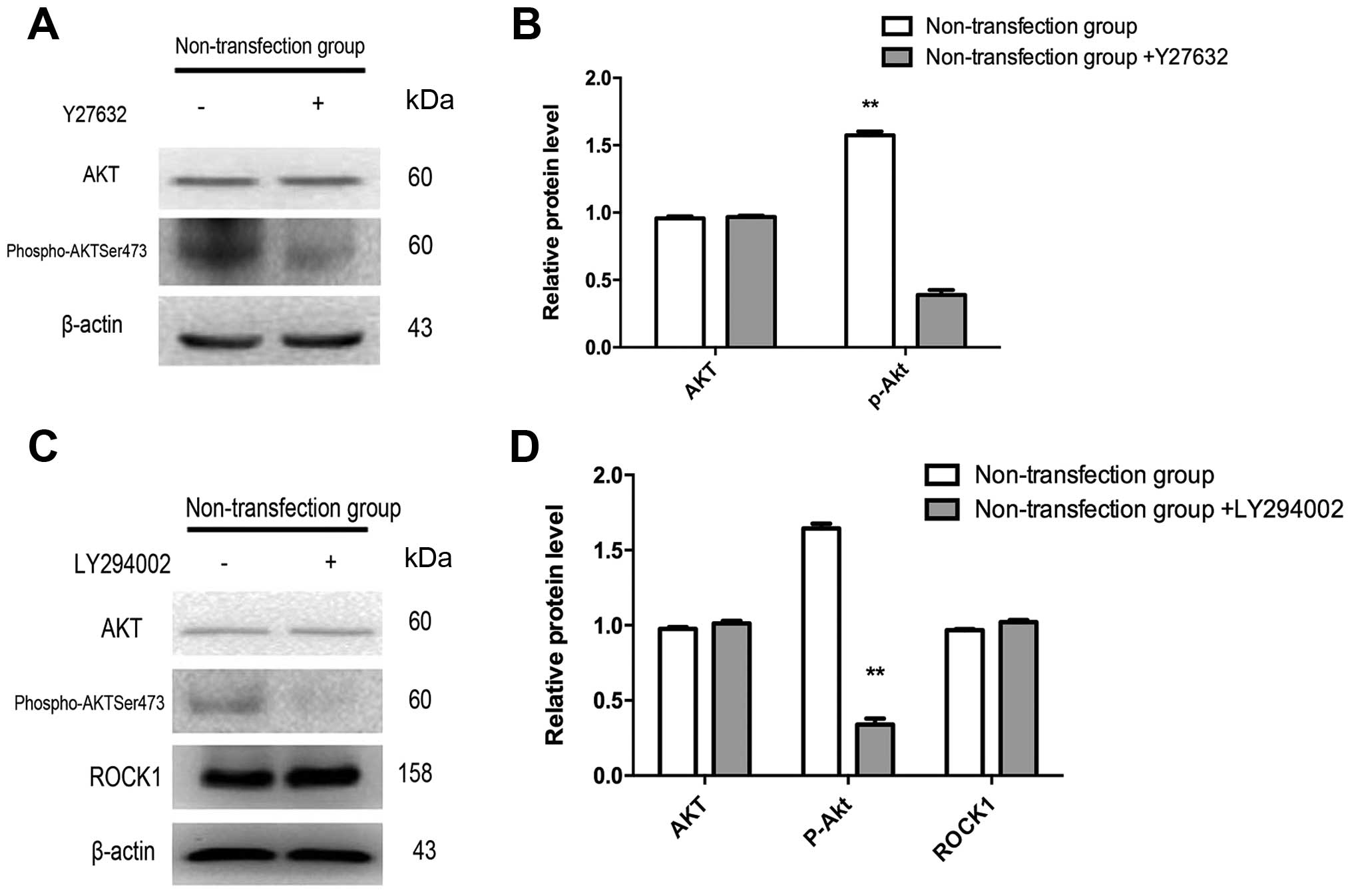
Effects of LY294002 and Y27632 on the
expression of ART1, ROCK1 and phospho-AktSer473 in CT26
cells
To further verify that ART1 could regulate EMT via
the RhoA/ROCK1/AKT pathway, non-transfection CT26 cells were
treated with Y27632 (an inhibitor of ROCK1) and LY294002 (a
specific competitive inhibitor of PI3K), respectively. When the
non-transfection CT26 cells was treated with Y27632, the expression
of phospho-AktSer473 was decreased, compared to the
non-transfection CT26 cells without Y27632 (p<0.01), while the
expressions of non-phosphorylated Akt were not significantly
different between the treated and untreated Y27632 non-transfection
CT26 cells (p>0.05) (Fig. 6A and
B). We also found that the expression of
phospho-AktSer473 was significantly downregulated in the
non-transfection CT26 cells treated with LY294002 compared with the
untreated group (p<0.01), but non-phosphorylation-Akt and ROCK1
were not observably different between the treated and untreated
LY294002 non-transfection CT26 cells (p>0.05) (Fig. 6C and D).
Effects of LY294002 on the expression
levels of phospho-AktSer473 and β-catenin in GFP-ART1
CT26 cells
Western blotting showed that the expression levels
of phospho-AktSer473 and β-catenin in GFP-ART1 CT26
cells treated with LY294002 were lower than it in the GFP-ART1 CT26
cells without LY294002 (p<0.01) (Fig. 7A and B).
Effect of silencing β-catenin on the
expression levels of snail1, N-cadherin, vimentin and E-cadherin in
GFP-ART1 CT26 cells
To further verify that ART1 could be responsible for
EMT through regulating the expression of β-catenin, Ad-si-β-catenin
and Ad-RFP-vectors were successfully infected into GFP-ART1 CT26
cells (Fig. 7C). The efficiency of
adenovirus vector-mediated siRNA interference in suppressing
β-catenin in GFP-ART1 CT26 cells was detected by RT-PCR and western
blot analysis. These methods showed that both of the mRNA and
protein levels of β-catenin in GFP-ART1 CT26 cells transfected with
β-catenin-si RNA were decreased (p<0.01) (Fig. 7D–G). The results of western blot
analysis also showed that there were increases in the expression
levels of snail1, N-cadherin, and vimentin and a decrease in
expression of E-cadherin in the GFP-ART1-Ad-RFP-si-β-catenin
GFP-ART1 CT26 cells compared with the GFP-ART1-Ad-RFP-vector
GFP-ART1 CT26 cells (p<0.01) (Fig.
7F and G).
Effect of the Ad-RFP-si-β-catenin on the
migration and invasion of GFP-ART1 CT26 cells
The migration distance detected by the wound healing
assay was decreased in the GFP-ART1-Ad-RFP-si-β-catenin GFP-ART1
CT26 cells compared with the GFP-ART1-Ad-RFP-vector GFP-ART1 CT26
cells (p<0.01) (Fig. 8A and B).
Concomitantly, the invasive ability of the
GFP-ART1-Ad-RFP-si-β-catenin GFP-ART1 CT26 cells decreased,
compared to the GFP-ART1-Ad-RFP-vector GFP-ART1 CT26 cells
(p<0.01) (Fig. 8C and D).
Discussion
EMT is considered to play an important biological
role in cancer. It could stimulate the transformed cells to lose
the ability for cell-cell adhesion, while gaining increased cell
motility and invasion. The features of EMT have been observed in
many cancers, such as colon, ovarian, breast, and esophageal
cancer. The integrin-extracellular matrix (ECM) interaction is an
important signaling pathway in regulating the change between
transformed cells and metastatic cells during the process of EMT.
Integrins, which include 24 distinct integrin heterodimers that
consist of 18 α-subunits and 8 β-subunits, are heterodimeric
cell-surface receptors (27). In
breast cancer and melanoma cells, integrin α7β1 could interact with
laminin and activate the RhoA signaling cascade to promote tumor
invasion (28–30). Researchers have reported that
mono-ADP-ribosylation of integrin α7β1 catalyzed by ART1 takes
place in differentiated myotubes and this post-translational
modification could activate integrin α7β1 and then increase its
affinity to laminin (31). Hence,
ART1 is likely to be involved in the process of EMT. Our previous
studies have shown that the level of ART1 expression in human colon
adenocarcinoma tissues was higher than that in the adjacent
tissues. Moreover, ART1 promoted CT26 cell proliferation via the
RhoA/c-fos/c-myc pathway and inhibited apoptosis by the
PI3K/Akt/NF-κB pathway in vitro (23,24).
However, there is no report on the role of ART1 in EMT and its
correlation with invasion and metastasis of carcinoma. In this
study, we found that over-expression of ART1 in CT26 cells could
promote the change of the cells from apical-basal polarized
epithelioid cells to spindle-like and mesenchymal-like cells, but
silencing ART1 in CT26 cells showed the opposite phenomenon.
Therefore, we speculate that ART1 may be closely related to EMT in
CT26 cells. The process of EMT is regulated by transcription
inducers, such as snail, mesenchymal markers (vimentin, N-cadherin)
and the epithelial cells marker E-cadherin (32). To further verify the molecular
mechanism of ART1 related to EMT, we found that upregulation of
ART1 simultaneously enhanced expression of snail, vimentin and
N-cadherin and suppressed the expression of E-cadherin in CT26
cells and its xenograft tumor in the spleen. However, GFP-shART1
CT26 cells and its xenograft tumor in spleen showed the opposite
effect. Additionally, we also observed that ART1 overexpression
promoted the invasion, migration and adhesion abilities of CT26
cells. ART1 silencing showed inhibitory effects. To further verify
that ART1 could affect tumor metastasis in vivo, we
constructed a tumor transplantation model through inoculating CT26
cells under the splenic capsule of BALB/c mice and demonstrated
that overexpressing ART1 could led to a significant promotion of
tumor metastasis with a heavier liver weight and more metastasis
nodules in mice. Silencing ART1 had the opposite effect. We presume
that ART1 may affect the invasion, migration and adhesion of
colorectal carcinoma by regulating EMT.
The Rho family belongs to the ras superfamily with
GTP enzyme activity and it is not easy to change its expression in
tumor cells. RhoA, whose mRNA and protein levels are over-expressed
in a series of tumors, is one of the major Rho family members. Many
researchers have suggested that RhoA is closely related to tumor
development (33,34). Rho associated coiled-coil
containing kinase (ROCK) is a direct downstream effect factor of
RhoA. The RhoA/ROCK pathway plays a central role in the
reorganization of the actin cytoskeleton by affecting actin
polymerization named F-actin. F-actin could affect the forming of
microfilaments and stress fibers, and then influences the motility
and morphogenesis of eukaryotic cells. Stable F-actin promoted
actomyosin contractility and detach cells from the extracellular
matrix (35–37). Therefore, the RhoA/ROCK pathway may
regulate cytoskeleton reorganization and actomyosin-based cortical
contractility, affecting mesenchymal migration (38), collectively suggesting that
activation of RhoA promotes cytoskeleton reorganization and the
progression of EMT. Meta-iodobenzyl guaniding (MIBG) is a specific
competitive inhibitor of arginine-specific
mono-ADP-ribosytransferase. It could inhibit vascular smooth muscle
cell proliferation and migration through suppression of
arginine-specific mono-ADP-ribosytransferases, which may be
associated with decreasing the expression of RhoA (39). In these previous studies, we also
showed that silencing ART1 could decrease the expression of RhoA in
CT26 cells (23). Therefore, we
deduced that ART1 might have a close relationship with the
RhoA/ROCK pathway. In this study, ART1 overexpression is associated
with an increase in expression of RhoA, ROCK1 and F-actin. Hence,
ART1 maybe related to the Rho/ROCK pathway in the process of
EMT.
The PI3K/AKT pathway participates in a variety of
oncogenic processes, including cell proliferation, apoptosis, and
EMT (40,41). GSK-3β could be phosphorylated on
Ser-473 by AKT and then inactivated (42). It has been reported that
non-phosphorylated GSK-3β could boost the binding of phosphorylated
APC and Axin with β-catenin, contributing to the degradation of
β-catenin (43,44). Therefore, the increase of
phosphorylated GSK-3β could help β-catenin evade degradation in the
cytoplasm and then translocate into the nucleus. The nuclear
accumulation of β-catenin serves as a cofactor with the
transcription factor LEF/TCF (lymphoid enhancer factor/T cell
factor) to mediate the transcription of EMT inducer snail which
could bind to E-cadherin promoter at E-boxes resulting in the
suppression on the transcription of E-cadherin and the increase of
the expression of mesenchymal marker proteins N-cadherin, and
vimentin (32). In this study,
GFP-ART1 CT26 cells and its intrasplenic tumor of BALB/c mice
showed an increase in the expression of phospho-AKT,
phospho-GSK-3β, total β-catenin and nuclear β-catenin. GFP-shRNA
ART1 CT26 cells and its intrasplenic tumor of BALB/c mice showed
the opposite results. To further confirm whether ART1 could
regulate β-catenin through the AKT pathway, the GFP-ART1 CT26 cells
was treated with LY294002, an inhibitor of AKT phosphorylation. The
results showed that expression of β-catenin in GFP-ART1 CT26 cells
treated with LY294002 was observably decreased, compared to
GFP-ART1 CT26 cells without LY294002. Therefore, ART1 may mediate
the AKT pathway to influence the expression of β-catenin. To
further verify that ART1 could influence EMT related factors and
its downstream factors via β-catenin, the GFP-ART1 CT26 cells were
successfully infected with si-β-catenin adenovirus vectors to
decrease the expression of β-catenin in GFP-ART1 CT26 cells. We
found that silencing β-catenin in the GFP-ART1 CT26 cells
suppressed the invasion and migration abilities of GFP-ART1 CT26
cells. Importantly, expressions of snail1 N-cadherin and vimentin
in GFP-ART1 CT26 cells infected with si-β-catenin adenovirus
vectors were also observably declined, and epithelial marker
protein E-cadherin was increased, compared with GFP-ART1 CT26 cells
without infection with si-β-catenin adenovirus vectors. These
results suggest that the effect of ART1 on EMT may be associated
with the PI3K/AKT/β-catenin pathway.
However, the relationship between RhoA/ROCK and
PI3K/AKT pathways in EMT is not clear. Interestingly, many studies
report a close link between the RhoA/ROCK and PI3K/AKT pathways.
Nonetheless, these ideas on the relationship between two pathways
vary in different cells. The RhoA/ROCK pathway could activate
phosphatidylinositol 3-kinase (PI3k) and AKT in the cardiomyocyte
(45). Myoblast survival is
controlled by the PI3K-Akt signaling pathway, which is induced by
RhoA (46). Plexin-B1, which
mediates RhoA/ROCK to activate the PI3K/Akt pathway, enhancing the
motility of endothelial cells (47). However, some researchers have
suggested that RhoA is downstream of PI3K/AKT in HeLa cells and
that it is derived from mTOR, one of the factors that is downstream
of PI3K/AKT that could stimulate GTP loading of RhoA in starved
HeLa cells (48). Hence, the
relationship between the RhoA/ROCK and PI3K/AKT pathway remains
unclear in CT26 cells. In this study, non-transfection CT26 cells
that were treated with ROCK1 inhibitor and phospho-AKT (ser473)
inhibitor respectively, indicated that the inhibition of ROCK1
could suppress the expression of phospho-AKT (ser473) in CT26
cells. However, inhibition of phospho-AKT could not affect the
expression of ROCK1. These results suggested that ART1 regulates
EMT related factors to influence the progression of tumor via
PI3K/AKT pathways, which may be affected by RhoA/ROCK
signaling.
This study demonstrated that ART1 promoted CT26 cell
invasion and metastasis through EMT in vivo and in
vitro. Also, we showed that ART1 regulated the EMT of CT26
cells through activation of the RhoA-ROCK1-AKT-β-catenin pathway,
which lead to cytoskeleton reorganization, a decrease in the
expression of E-cadherin, an increase in expression of snail1,
MMP-2, MMP-9, vimentin and N-cadherin. In conclusion, this is the
first report on the effect of ART1 on tumor cell invasion and
metastasis by regulating EMT. These new findings most likely
indicate that ART1 could be a novel molecular therapeutic target
for colorectal carcinoma. However, the effect of ART1 on tumor
progression and its other exact molecular mechanisms require
further research.
In conclusion, mono-ADP-ribosylation has been
recognized as a key factor in disease including cancer. ART1 is an
arginine specific mono-ADP-ribosylated transferase. The change of
ART1 could reflect the role of arginine specific
mono-ADP-ribosylation in development of colorectal carcinoma. Our
data showed that the change of ART1 could affect the abilities of
invasion and metastasis, induce EMT in mouse colorectal carcinoma
CT26 cells. In addition, the concrete mechanism may relate to ART1
which regulate the RhoA/ROCK1/AKT/β-catenin pathway and its
downstream factors (snail1, vimentin, N-cadherin and E-cadherin).
ART1 as a new target for therapy in colorectal carcinoma and needs
further in-depth study.
Acknowledgements
We thank Dr Jia-Yi Huang for the generous gift of
adenovirus (Ad)-si-β-catenin. We also thank Professor Ya-Lan Wang
for useful discussion and careful modification. This study was
supported by the Ministry of Education Specialized Research Fund
for the Doctoral Program of Higher Education (grant no.
20105503110009), the Science and Technology Program of Chongqing
Municipal Education Commission (grant no. KJ110322) and the
National Nature Science Foundation of China (NSFC: 30870946).
References
|
1
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brabletz T, Hlubek F, Spaderna S,
Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T: Invasion and
metastasis in colorectal cancer: Epithelial-mesenchymal transition,
mesenchymal-epithelial transition, stem cells and β-catenin. Cells
Tissues Organs. 179:56–65. 2005. View Article : Google Scholar
|
|
3
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagaishi M, Paulus W, Brokinkel B, Vital
A, Tanaka Y, Nakazato Y, Giangaspero F and Ohgaki H:
Transcriptional factors for epithelial-mesenchymal transition are
associated with mesenchymal differentiation in gliosarcoma. Brain
Pathol. 22:670–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kleine H and Lüscher B: Learning how to
read ADP-ribosylation. Cell. 139:17–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zolkiewska A: Ecto-ADP-ribose
transferases: Cell-surface response to local tissue injury.
Physiology (Bethesda). 20:374–381. 2005. View Article : Google Scholar
|
|
8
|
Tuncel H, Tanaka S, Oka S, Nakai S,
Fukutomi R, Okamoto M, Ota T, Kaneko H, Tatsuka M and Shimamoto F:
PARP6, a mono (ADP-ribosyl) transferase and a negative regulator of
cell proliferation, is involved in colorectal cancer development.
Int J Oncol. 41:2079–2086. 2012.PubMed/NCBI
|
|
9
|
Gangopadhyay NN, Luketich JD, Opest A,
Visus C, Meyer EM, Landreneau R and Schuchert MJ: Inhibition of
poly (ADP-ribose) polymerase (PARP) induces apoptosis in lung
cancer cell lines. Cancer Invest. 29:608–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inbar D, Cohen-Armon M and Neumann D:
Erythropoietin-driven signalling and cell migration mediated by
polyADP-ribosylation. Br J Cancer. 107:1317–1326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li M, Threadgill MD, Wang Y, Cai L and Lin
X: Poly (ADP-ribose) polymerase inhibition down-regulates
expression of metastasis-related genes in CT26 colon carcinoma
cells. Pathobiology. 76:108–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caldini R, Fanti E, Magnelli L, Barletta
E, Tanganelli E, Zampieri M and Chevanne M: Low doses of
3-aminobenzamide, a poly (ADP-ribose) polymerase inhibitor,
stimulate angiogenesis by regulating expression of urokinase type
plasminogen activator and matrix metalloprotease 2. Vasc Cell.
3:122011. View Article : Google Scholar
|
|
13
|
Giansanti V, Donà F, Tillhon M and
Scovassi AI: PARP inhibitors: New tools to protect from
inflammation. Biochem Pharmacol. 80:1869–1877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wielckens K, Bredehorst R, Adamietz P and
Hilz H: Protein-bound polymeric and monomeric ADP-ribose residues
in hepatic tissues. Comparative analyses using a new procedure for
the quantification of poly (ADP-ribose). Eur J Biochem. 117:69–74.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laing S, Unger M, Koch-Nolte F and Haag F:
ADP-ribosylation of arginine. Amino Acids. 41:257–269. 2011.
View Article : Google Scholar :
|
|
16
|
Okazaki IJ and Moss J:
Mono-ADP-ribosylation: A reversible posttranslational modification
of proteins. Adv Pharmacol. 35:247–280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jones EM and Baird A: Cell-surface
ADP-ribosylation offibroblast growth factor-2 by an
arginine-specific ADP-ribosyltransferase. Biochem J. 323:173–177.
1997. View Article : Google Scholar
|
|
18
|
Hottiger MO, Boothby M, Koch-Nolte F,
Lüscher B, Martin NM, Plummer R, Wang ZQ and Ziegler M: Progress in
the function and regulation of ADP-Ribosylation. Sci Signal.
4:mr52011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paone G, Wada A, Stevens LA, Matin A,
Hirayama T, Levine RL and Moss J: ADP ribosylation of human
neutrophil peptide-1 regulates its biological properties. Proc Natl
Acad Sci USA. 99:8231–8235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saxty BA, Yadollahi-Farsani M, Upton PD,
Johnstone SR and MacDermot J: Inactivation of platelet-derived
growth factor-BB following modification by ADP-ribosyltransferase.
Br J Pharmacol. 133:1219–1226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zolkiewska A and Moss J: Integrin alpha 7
as substrate for a glycosylphosphatidylinositol-anchored
ADP-ribosyltransferase on the surface of skeletal muscle cells. J
Biol Chem. 268:25273–25276. 1993.PubMed/NCBI
|
|
22
|
Kuang J, Wang Y-L, Xiao M, Tang Y, Chen
WW, Song GL, Yang X and Li M: Synergistic effect of
arginine-specific ADP-ribosyltransferase 1 and poly (ADP-ribose)
polymerase-1 on apoptosis induced by cisplatin in CT26 cells. Oncol
Rep. 31:2335–2343. 2014.
|
|
23
|
Xu J-X, Wang Y-l, Tang Y and Xiong W:
Effect of ART1 gene silencing by RNA interference on the
proliferation of mouse colon carcinoma cells and its possible
mechanism. Tumor. 32:949–954. 2012.
|
|
24
|
Xiao M, Tang Y, Wang Y-L, Yang L, Li X,
Kuang J and Song GL: ART1 silencing enhances apoptosis of mouse
CT26 cells via the PI3K/Akt/NF-κB pathway. Cell Physiol Biochem.
32:1587–1599. 2013.
|
|
25
|
Tang Y, Wang Y-L, Yang L, Xu JX, Xiong W,
Xiao M and Li M: Inhibition of arginine ADP-ribosyltransferase 1
reduces the expression of poly (ADP-ribose) polymerase-1 in colon
carcinoma. Int J Mol Med. 32:130–136. 2013.PubMed/NCBI
|
|
26
|
Shen L, Zhang X, Hu D, Feng T, Li H, Lu Y
and Huang J: Hepatitis B virus X (HBx) play an anti-apoptosis role
in hepatic progenitor cells by activating Wnt/β-catenin pathway.
Mol Cell Biochem. 383:213–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jinka R, Kapoor R, Sistla PG, Raj TA and
Pande G: Alterations in cell-extracellular matrix interactions
during progression of cancers. Int J Cell Biol. 2012:2191962012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vizirianakis IS, Yao C-C, Chen Y, Ziober
BL, Tsiftsoglou AS and Kramer RH: Transfection of MCF-7 carcinoma
cells with human integrin α7 cDNA promotes adhesion to laminin.
Arch Biochem Biophys. 385:108–116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kramer RH, Vu MP, Cheng Y-F, Ramos DM,
Timpl R and Waleh N: Laminin-binding integrin alpha 7 beta 1:
Functional characterization and expression in normal and malignant
melanocytes. Cell Regul. 2:805–817. 1991.PubMed/NCBI
|
|
30
|
Yao C-C, Ziober BL, Squillace RM and
Kramer RH: α7 integrin mediates cell adhesion and migration on
specific laminin isoforms. J Biol Chem. 271:25598–25603. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Z, Gruszczynska-Biegala J and
Zolkiewska A: ADP-ribo-sylation of integrin alpha7 modulates the
binding of integrin alpha7beta1 to laminin. Biochem J. 385:309–317.
2005. View Article : Google Scholar
|
|
32
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar
|
|
33
|
Sahai E and Marshall CJ: RHO-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar
|
|
34
|
Rathinam R, Berrier A and Alahari SK: Role
of Rho GTPases and their regulators in cancer progression. Front
Biosci (Landmark Ed). 16:2561–2571. 2011. View Article : Google Scholar
|
|
35
|
Yamazaki D, Kurisu S and Takenawa T:
Regulation of cancer cell motility through actin reorganization.
Cancer Sci. 96:379–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Watanabe N, Madaule P, Reid T, Ishizaki T,
Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM and Narumiya
S: p140mDia, a mammalian homolog of Drosophila diaphanous, is a
target protein for Rho small GTPase and is a ligand for profilin.
EMBO J. 16:3044–3056. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gomez dPT and Lacal JC: RHOA (ras homolog
gene family, member A). Atlas Genet Cytogenet Oncol Haematol.
11:124–127. 2007.
|
|
38
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yau L, Litchie B, Thomas S, Storie B,
Yurkova N and Zahradka P: Endogenous mono-ADP-ribosylation mediates
smooth muscle cell proliferation and migration via protein kinase
N-dependent induction of c-fos expression. Eur J Biochem.
270:101–110. 2003. View Article : Google Scholar
|
|
40
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar
|
|
41
|
Cho HJ, Baek KE, Saika S, Jeong M-J and
Yoo J: Snail is required for transforming growth factor-β-induced
epithelial-mesenchymal transition by activating PI3 kinase/Akt
signal pathway. Biochem Biophys Res Commun. 353:337–343. 2007.
View Article : Google Scholar
|
|
42
|
Qiao M, Sheng S and Pardee AB: Metastasis
and AKT activation. Cell Cycle. 7:2991–2996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3β to the APC-β-catenin
complex and regulation of complex assembly. Science. 272:1023–1026.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamamoto H, Kishida S, Kishida M, Ikeda S,
Takada S and Kikuchi A: Phosphorylation of axin, a Wnt signal
negative regulator, by glycogen synthase kinase-3β regulates its
stability. J Biol Chem. 274:10681–10684. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Del Re DP, Miyamoto S and Brown JH: Focal
adhesion kinase as a RhoA-activable signaling scaffold mediating
Akt activation and cardiomyocyte protection. J Biol Chem.
283:35622–35629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reuveny M, Heller H and Bengal E: RhoA
controls myoblast survival by inducing the phosphatidylinositol
3-kinase-Akt signaling pathway. FEBS Lett. 569:129–134. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Basile JR, Gavard J and Gutkind JS:
Plexin-B1 utilizes RhoA and Rho kinase to promote the
integrin-dependent activation of Akt and ERK and endothelial cell
motility. J Biol Chem. 282:34888–34895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jacinto E, Loewith R, Schmidt A, Lin S,
Rüegg MA, Hall A and Hall MN: Mammalian TOR complex 2 controls the
actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol.
6:1122–1128. 2004. View Article : Google Scholar : PubMed/NCBI
|