Introduction
Breast cancer is the most common cancer and the
leading cause of cancer death among women worldwide. Global cancer
statistics reported 1.7 million newly-diagnosed cases and 521,900
deaths in 2012 (1). While breast
cancer incidence and mortality rates in most developing countries
have been rapidly increasing (2),
in developed countries, the mortality rates of breast cancer have
been stable or decreasing due to early detection and development of
novel treatment modalities (3–6).
Molecular targeted therapies such as tamoxifen,
aromatase inhibitors, and trastuzumab (Herceptin) have markedly
improved the clinical outcome of breast cancer treatment (7,8).
Tamoxifen and aromatase inhibitors modulate an estrogen-related
signaling pathway and trastuzumab is very effective to breast
cancers with overexpression of human epidermal growth factor 2
(HER2/ERBB2) (7). Although
molecular targeted drugs are available to breast cancer patients
with certain molecular characteristics, a subset of patients
without the dysfunction of these growth pathways is not able to
have the benefit from these treatment options. For example,
triple-negative breast cancers (TNBC), which do not express
estrogen receptor or progesterone receptor, and do not reveal
overexpression of HER2, show poorer prognosis than other subtypes
for which established molecular target drugs are available
(9). While clinical trials are
investigating poly(ADP-ribose) polymerase (PARP) inhibitors as the
most promising agents for TNBC, only a subset of patients with TNBC
who carry either BRCA1 or BRCA2 mutation expects the
treatment benefit (10,11). Therefore, it is required to develop
additional molecular target drugs to be applicable to a wide range
of breast cancer patients.
Genes upregulated specifically in cancer cells have
been considered as potential molecular targets for development of
targeted therapy with high efficacy and minimum risk of adverse
reactions. However, to effectively develop molecular-targeted
drugs, it is critically essential to characterize and understand
the precise molecular mechanism how the gene products of interest
contribute to development and progression of human cancers.
In this study, we demonstrate that
phosphatidylinositol glycan anchor biosynthesis, class X (PIGX),
which plays a critical role in the biosynthetic pathway of
glycosylphosphatidylinositol (GPI)-anchor motif (12), is upregulated significantly in
breast cancer tissues and involved in cancer cell proliferation. We
also demonstrate that PIGX forms a complex with reticulocalbin 1
(RCN1) and reticulocalbin 2 (RCN2) proteins, which may regulate
calcium-dependent activities in the endoplasmic reticulum (ER)
lumen or post-ER compartment, and that the complex regulates gene
expression of putative tumor suppressors, Zic family member 1
(ZIC1) and EH-domain containing 2 (EHD2), and thereby contribute to
human breast cancer proliferation.
Materials and methods
Cell culture and clinical samples
Human breast cancer cell lines, BT-20, BT-549,
HCC-1937, MCF7, MDA-MB-231, SK-BR3, and T-47D, as well as human
embryonic kidney 293T cells and human cervical cancer HeLa cells
were purchased from the American Type Culture Collection (ATCC,
Rockville, MD, USA). HBC4 and HBC5 breast cancer cell lines were
kindly provided by Dr T. Yamori (Molecular Pharmacology, Cancer
Chemotherapy Center of the Japanese Foundation for Cancer
Research). All cell lines were grown in monolayers in appropriate
media supplemented with 10% fetal bovine serum and 1%
antibiotic/antimycotic solution (Sigma-Aldrich, St. Louis, MO,
USA): Dulbecco’s modified Eagle’s medium (D-MEM) for 293T cells;
Eagle’s minimal essential medium (E-MEM) for BT-20, MCF7, and HeLa
cells; RPMI-1640 for HCC-1937, HBC4, HBC5, T-47D, and BT-549 cells;
Leibovitz’s L-15 for MDM-MB-231 cells; McCoy’s 5A for SK-BR3 cells.
MDA-MB-231 cells were maintained at 37°C in the atmosphere of
humidified air without CO2. Other cells were maintained
at 37°C in humid air with 5% CO2 condition. Cells were
transfected with FuGENE6 or FuGENE HD (Promega, Madison, WI, USA)
following the manufacturer’s protocols. Detailed information of the
clinical samples used for the microarray was described previously
(13). The use of all clinical
materials in this study was approved by Ethics Committees of
Institute of Medical Science in the University of Tokyo.
Reverse transcription and real-time
PCR
RNA was extracted from the breast cancer cell lines
using RNeasy kit (Qiagen, Valencia, CA, USA) according to the
manufacturer’s instructions. RNA extracted from a normal mammary
gland (BioChain, Newark, CA, USA) was used as a control. The
sequences of specific primers are 5′-GCAAATTCCATGGCACCGTC-3′ and
5′-TCGCCCCACTTGATTTTGG-3′ for GAPDH (housekeeping gene),
5′-TTGGCTTGACTCAGGATTTA-3′ and 5′-ATGCTATCACCTCCCCTGTG-3′ for
ACTB (housekeeping gene), 5′-GTGAAGATGGAGAAGCCTCG-3′ and
5′-GCAC AGGATTGTAATGAGCA-3′ for PIGX, 5′-GCAGAGAGC
AAGTGGAGTTTT-3′ and 5′-CACAAGAGGCAGTAAGCA GAG-3′ for
phosphatidylinositol glycan anchor biosynthesis, class M
(PIGM), 5′-ATGATGGGGATGGCTTTGT-3′ and
5′-AACCATAGGTGGCTTGTTTGT-3′ for RCN1, 5′-GAT
GATACTGTGACTTGGGATG-3′ and 5′-ACTCAAACCGG GACCTGAA-3′ for
RCN2, 5′-TGCGAGTTCACGCTTAAC ATC-3′ and
5′-ATCAGCAGCTCCTGCATTTT-3′ for EHD2,
5′-GTCCTACACGCATCCCAGTT-3′ and 5′-GCGATAAG GAGCTTGTGGTC-3′ for
ZIC1, and 5′-GGTGACGTGTCTGATGTTGG-3′ and
5′-AGCTCCCAGCTGTAAGACCA-3′ for histone deacetylase 8
(HDAC8). For semi-quantitative reverse transcription PCR
(RT-PCR), PCR reactions were performed as previously described
(13). The amplification cycle
numbers were 22 cycles for ACTB and 27 cycles for
PIGX. For quantitative RT-PCR (qRT-PCR), PCR reactions were
performed using SYBR Select Master mix and on ViiA 7 real-time PCR
system (Life Technologies, Grand Island, NY, USA) following the
manufacturer’s protocol. mRNA levels were normalized to GAPDH mRNA
expression.
Construction of short hairpin RNA
(shRNA)-expressing vectors and cell viability assay
Plasmids designed to express shRNA were prepared by
cloning of double-stranded oligonucleotides into psiU6BX vector as
described previously (14). The
oligonucleotide sequences of target sequences for PIGX are
5′-GATGGAGAAGCCTCGATTG-3′ for shPIGX#1, 5′-GCCAATGGAACAAGATGAAGT-3′
for shPIGX#2, and 5′-CTACAAGTTCCAGTGGGAC-3′ for shPIGX#3. T-47D
cells, which expressed PIGX at a high level, were seeded on 10-cm
dishes, transfected with psiU6-PIGX or psiU6-siEGFP using FuGENE6
(Promega) according to the manufacturer’s instructions, and then
cultured in RPMI-1640 containing 800 μg/ml of geneticin
(Sigma-Aldrich) for 14 days. The cells were fixed with 100%
methanol, stained with 0.1% of crystal violet-H2O for
colony formation assay. In cell viability assay, cell viability was
measured using Cell Counting Kit-8 (Dojindo Molecular Technologies,
Kumamoto, Japan) 10 days after the transfection. Absorbance was
measured at 450 nm as a reference, with a Microplate Reader iMark
(Bio-Rad, Hercules, CA, USA). Knockdown effects of these shRNA
expression vectors on endogenous PIGX expression were
validated 7 days after transfection by qRT-PCR.
Small interfering RNA transfection
siRNA oligonucleotide duplexes were purchased from
Sigma-Aldrich for targeting PIGX, PIGM, RCN1, and RCN2 transcripts.
siEGFP and siNegative control (siNC, Cosmo Bio, Tokyo, Japan),
which is a mixture of three different oligonucleotide duplexes were
used as control siRNAs. The siRNA sequences are 5′-GGACAU
UCCUGCAGGACUU-3′ for siPIGX, 5′-GUUCCAUCCUGA UUCAAAU-3′ for
siPIGM#1, 5′-GGUUUAUAGGGCAG GCCAU-3′ for siPIGM#2,
5′-GAAGCUAACUAAAGAG GAA-3′ for siRCN1#1, 5′-GAUAGACACUCACCAGAAU-3′
for siRCN1#2, 5′-GAAUGGAUACUUGUUGAGA-3′ for siRCN2#1, and
5′-CGGAAUUUGUCAUUCAAGA-3′ for siRCN2#2.siRNA duplexes were
transfected with Lipofectamine RNAiMAX (Life Technologies).
Immunoprecipitation
293T cells were lysed 48 h after transfection with
Pierce IP lysis buffer (Thermo Scientific, Waltham, MA, USA)
containing a complete protease inhibitor cocktail (Roche Life
Science, Indianapolis, IN, USA). For FLAG-, HA-, or glutathione
S-transferase (GST)-tagged protein, whole-cell extract was
incubated with anti-FLAG M2 antibody conjugated agarose beads
(Sigma-Aldrich), anti-HA antibody conjugated agarose beads
(Sigma-Aldrich), or glutathione sepharose 4b (GE Healthcare,
Pittsburgh, PA, USA) at 4°C overnight. Following three times
washing with IP lysis buffer, FLAG- or HA-tagged protein bound to
the beads was eluted by incubating with FLAG- or HA-peptide at 4°C
for 1 h. GST-tagged protein were eluted by boiling in Lane Marker
Reducing Sample Buffer (Thermo Scientific).
Mass spectrometry analysis
Immunoprecipitated samples from lysate of 293T cells
transfected with mock or PIGX expression vectors were prepared in
triplicate. The immunoprecipitant eluted with 3X FLAG peptide was
desalted and concentrated with 2D clean-up kit (GE Healthcare). The
purified protein samples were lysed in solution of 8 M urea, 50 mM
HEPES-NaOH, pH 8.0 and reduced with 10 mM
tris(2-carboxyethyl)phosphine (Sigma-Aldrich) at 37°C for 30 min,
followed by alkylation with 50 mM iodoacetamide (Sigma-Aldrich) at
25°C in the dark for 45 min. Proteins were digested with
Immobilized trypsin (Thermo Scientific) at 37°C for 6 h. The
resulting peptides were desalted by Oasis HLB μ-elution plate
(Waters, Milford, MA, USA) and analyzed by LTQ-Orbitrap-Velos mass
spectrometer (Thermo Scientific) combined with UltiMate 3000 RSLC
nano-flow HPLC system (Thermo Scientific). The MS/MS spectra were
searched against Homo sapiens protein sequence database in
SwissProt using Proteome Discoverer 1.4 software (Thermo
Scientific), in which false discovery rate of 1% was set for both
peptide and protein identification filters.
Expression vector construction
PIGX, RCN1, and RCN2 cDNAs were amplified from total
human cDNA prepared by reverse transcription from qPCR Human
Reference Total RNA (Clontech, Mountainview, CA, USA) using KOD DNA
polymerase (Toyobo, Osaka, Japan) and cloned into pCAGGSn3FC or
pCAGGSnHC vector. For pull down experiment, GST sequence was cloned
together with PIGX.
Antibodies
The following primary antibodies were used: anti-HA
(rat, 3F10; Roche Life Science; dilution used in
immunocytochemistry (ICC): 1:1,000), anti-KDEL ER Marker (mouse,
10C3; Santa Cruz Biotechnology; dilution used in ICC: 1:50),
anti-RCN1 [rabbit, A300-407A-M; Bethyl Laboratories; dilution used
in ICC: 1:100, western blotting (WB): 1:1,000], anti-RCN2 (rabbit,
10193-2-AP; Proteintech; dilution used in ICC: 1:100, WB: 1:3,000),
anti-FLAG (mouse, M2; Sigma-Aldrich; dilution used in WB: 1:1,000),
and anti-HA (rabbit, Y-11; Santa Cruz Biotechnology; dilution used
in WB: 1:1,000).
Immunocytochemistry
HeLa cells were fixed 48 h after transfection in 4%
paraformaldehyde in PBS at 4°C for 1 h, permeabilized in 0.1%
Triton X-100 (Sigma-Aldrich) for 3 min at room temperature and
blocked with 3% BSA for 1 h at room temperature. Fixed cells were
incubated with each primary antibody overnight at 4°C followed by
incubation with Alexa Fluor-conjugated secondary antibody (Life
Technologies) for 1 h at room temperature and observed using Leica
confocal microscopy (SP5 tandem Scanner Spectral 2-Photon
Confocal).
Microarray analysis
Purified RNA was labeled using 3′IVT kit
(Affymetrix, Santa Clara, CA, USA) and hybridized onto Human Gene
Chip U133 Plus 2.0 arrays (Affymetrix) following the manufacturer’s
instructions. Probe signal intensities were normalized by MAS5
method using Expression console software (Affymetrix). Signal
intensities with high detection P-value (P>0.05) were eliminated
from the analysis. The signal intensities derived from the samples
with siRNA treatment targeting PIGX, RCN1, or RCN2 were divided by
the ones derived from control siRNA-treated sample. Each experiment
was duplicated and average values were used for the analysis.
Statistical analysis
All experiments were performed as triplicate and the
data are presented as means ± standard deviation (SD). Statistical
significance was calculated using Student’s t-test and the level of
significance was set at P<0.05.
Results
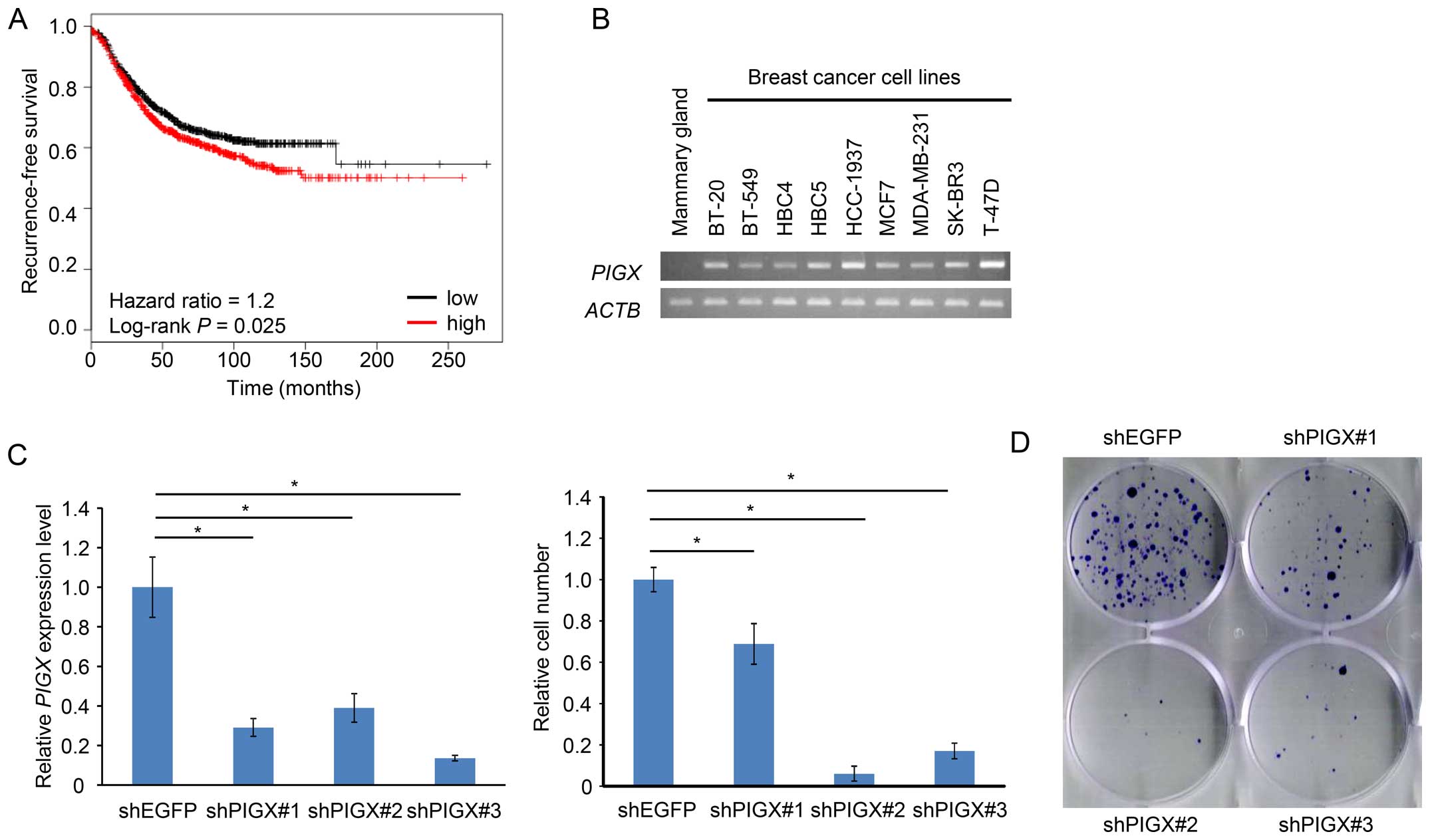
PIGX is overexpressed in breast cancer
and promotes cell proliferation
We conducted cDNA microarray analysis to explore
molecular targets for development of novel drugs for breast cancer
(13), and found that the
expression of PIGX was significantly upregulated in breast
cancer cells compared to normal breast ductal cells. The datasets
from Oncomine database (15)
supported the upregulation of PIGX commonly in various
subtypes of breast cancer. In addition, the dataset from cBioPortal
for Cancer Genomics (16,17) indicated that the amplification of
PIGX gene was found in 3.7% of 963 breast cancer samples
examined, suggesting that PIGX might have a critical role in human
breast carcinogenesis. Furthermore, survival analysis using the KM
Plotter, an online tool that incorporates microarray data with
survival information (18,19), also suggested a significant
association of higher PIGX mRNA expression level with
shorter recurrence-free survival (RFS) of breast cancer patients
(hazard ratio=1.2, P=0.025; Fig.
1A), implying that PIGX may be related to progression or
malignant phenotype of breast cancer. Collectively, these data
suggest that PIGX would be a candidate target for the development
of novel breast cancer therapeutics.
To further assess a possible oncogenic role of PIGX
in human breast cancer, we firstly confirmed that PIGX was
transactivated in various cell lines regardless of molecular
subtypes (BT-20, BT-549, HCC-1937, and MDA-MB-231: TNBC, MCF7,
T-47D, and HBC4: estrogen receptor-positive, SK-BR3 and HBC5: HER-2
type) (Fig. 1B). Since T-47D cells
revealed the highest level of PIGX expression among the cell
lines examined, we chose this cell line for further functional
analysis. We transfected T-47D cells with shRNA-expression vector
targeting PIGX and examined the growth suppressive effect on
these cancer cells. Stable knockdown of PIGX by shRNA
resulted in significant suppression of the cancer cell growth
(Fig. 1C) and colony formation
(Fig. 1D), indicating that PIGX is
essential for the growth of T-47D cells. Because a previous report
suggested PIGX to stabilize PIGM protein involved in the
biosynthetic pathway of GPI-anchor motif (12), we also evaluated knockdown effect
of PIGM on cancer cell growth. Knockdown of PIGM did not result in
any significant growth suppressive effect (Fig. 1E), suggesting that PIGX is likely
to promote cancer cell growth without involvement of PIGM.
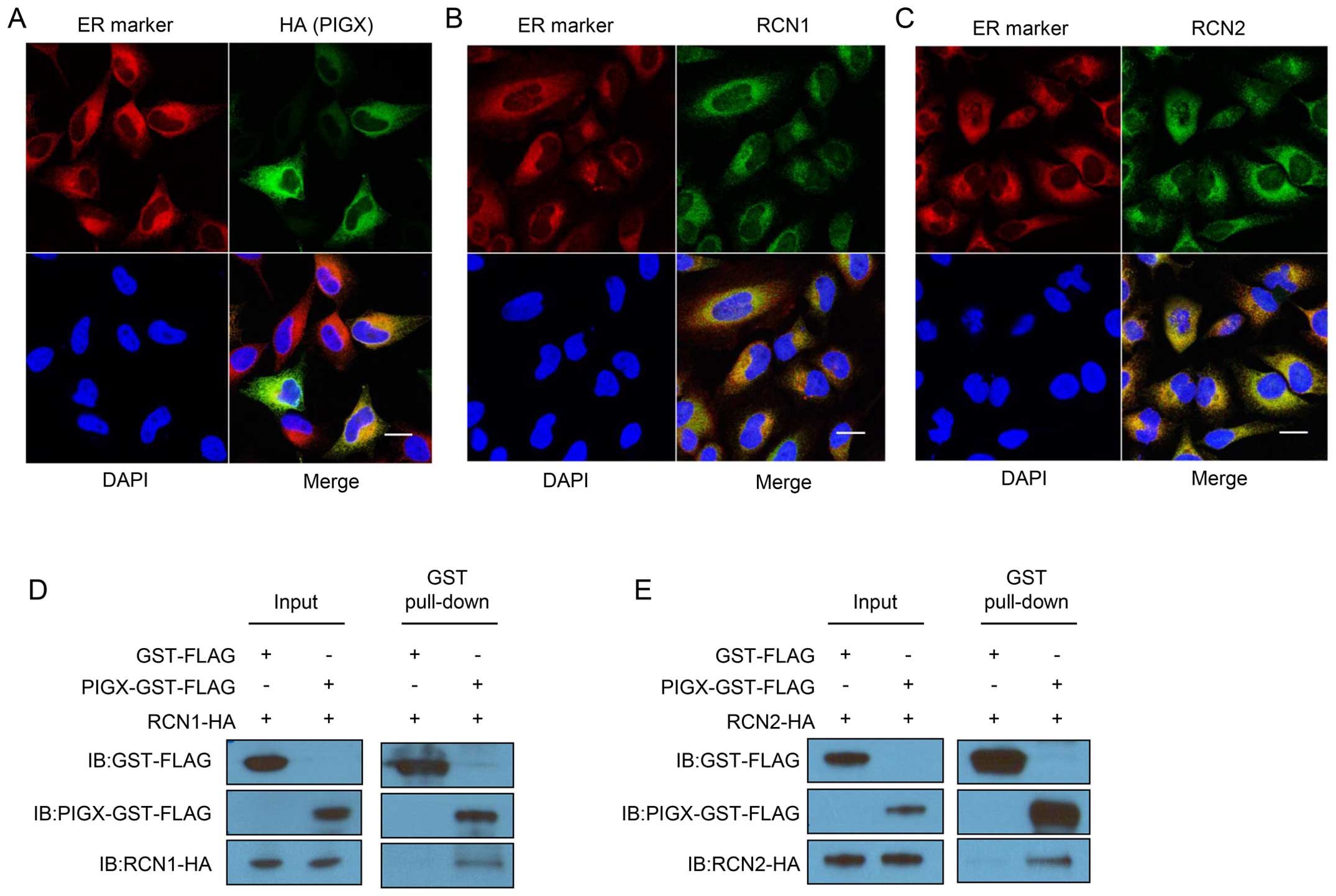
RCN1 and RCN2 form a complex with
PIGX
To elucidate the molecular mechanism of PIGX to
promote cancer cell proliferation, we attempted to find the
functional structural domain using the interproscan program
(20,21), but found no characteristic domain
structure to lead to speculate its biological function. Hence, we
conducted immunoprecipitation followed by mass spectroscopic
analysis to identify an interacting protein(s) with PIGX. We
prepared samples from cell lysate of PIGX overexpressing cells or
control cells, and compared the protein immunoprecipitates. We
identified 51 proteins uniquely in all the triplicate samples from
PIGX overexpressing cell lysate but not in control cells. Among
them, since PIGX is known to be located in ER, we focused on RCN1
and RCN2 as candidate proteins interacting with PIGX. To examine
the subcellular localization of these molecules, we transfected
HeLa cells with each expression vector and performed
immunocytochemical analysis. As shown in Fig. 2A–C, PIGX, RCN1, and RCN2 were
localized in ER. Besides, we confirmed the interaction between
GST-tagged PIGX and HA-tagged RCN1 or RCN2 by GST-pull down
experiment and western blotting (Fig.
2D and E). Importantly, knockdown of RCN1 and
RCN2 also significantly suppressed cancer cell growth
(Fig. 2F and G). Moreover,
survival analysis using the KM Plotter showed that higher
RCN1 and RCN2 expression is significantly associated
with shorter RFS of breast cancer patients (hazard ratio=1.22,
P=0.00072 for RCN1 and hazard ratio=1.33,
P=8.8×10−7 for RCN2) (18,19).
Altogether these results indicate that the RCN1/PIGX/RCN2 complex
has an indispensable role in the growth or survival of cancer
cells.
In addition, since we observed two bands in western
blotting experiments for PIGX in a mild reducing condition (+DTT)
and the molecular weight of a higher band corresponded to the dimer
(58 kDa) of PIGX (Fig. 3A), we
hypothesized that PIGX would make a dimer possibly through
intermolecular disulfide bonds. To address this hypothesis, we
co-overexpressed two different PIGX proteins, one with a HA-tag and
the other with a FLAG-tag, in 293T cells and conducted
immunoprecipitation analysis. As shown in Fig. 3B, HA-tagged PIGX was
co-immunoprecipitated with FLAG-tagged PIGX, indicating that PIGX
form a dimer. Subsequently, we conducted co-immunoprecipitation
analysis with either PIGX, RCN1, or RCN2 knockdown to clarify the
complex structure. The results indicated that the binding between
RCN1 and RCN2 was diminished by knockdown of PIGX (Fig. 3C and D) whereas knockdown of either
RCN1 or RCN2 did not affect the binding of the other RCN protein to
PIGX (Fig. 3E and F). Together,
these results indicate RCN1 and RCN2 bind directly to PIGX and
probably PIGX protein functions as a core protein of this
RCN1/PIGX/RCN2 complex although further biochemical analysis of the
complex is required to determine the precise molecular
stoichiometry in the complex.
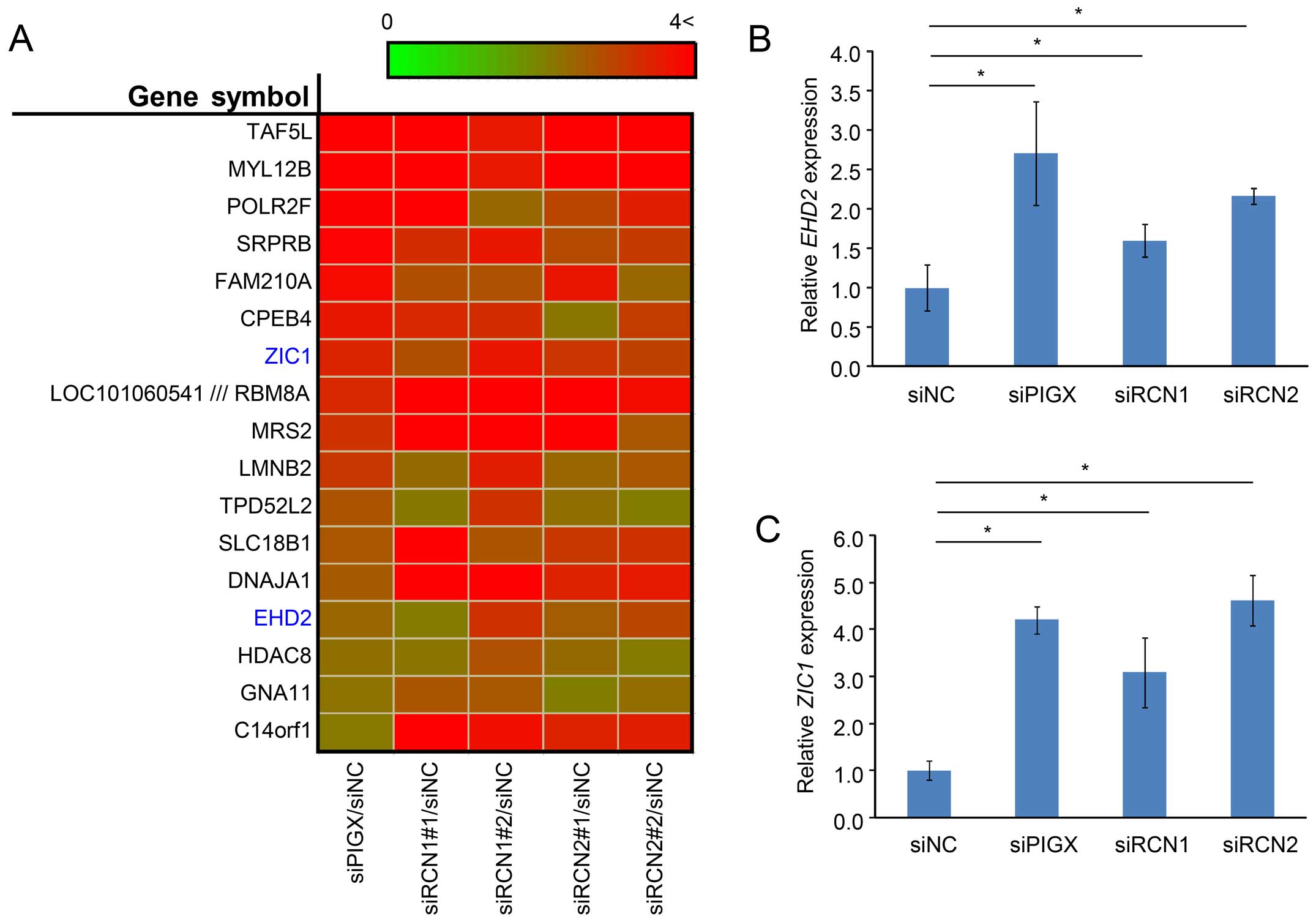
PIGX containing complex regulates EHD2
and ZIC1 transcription
Although previous studies reported that RCN1 and
RCN2 might be involved in human cancer (22–24),
the detailed molecular function of the proteins how to contribute
to cancer remains unclear. Therefore, we conducted cDNA microarray
analysis to assess the transcriptional changes caused by the
knockdown of each of these three genes. We transfected BT-549 cells
with control siRNA or siRNAs targeting PIGX, RCN1, or
RCN2 and subjected RNA sample extraction from cells 48 h
after transfection. Since PIGX, RCN1, and RCN2 are considered to
function as a complex, we explored gene expression changes observed
commonly in all knockdown samples. The result revealed that
significant upregulation of 17 genes by >2-fold was observed by
siRNA treatment targeting PIGX, RCN1, and RCN2 compared to control
samples (Fig. 4A), indicating that
the RCN1/PIGX/RCN2 complex would regulate the expression of these
genes. Among these genes, ZIC1 and EHD2 were recently suggested to
have a function related to human tumorigenesis (25–30).
The upregulation of these potential downstream genes was validated
with qRT-PCR (Fig. 4B and C).
Taken together with previous reports that show ZIC1 and EHD2 would
have functions as putative tumor suppressors (25–28),
our results suggest that the PIGX-containing complex may promote
cancer cell proliferation through, at least in part, the modulation
of expression of these two genes and thereby contribute to human
breast cancer.
Discussion
We have demonstrated that PIGX is
overexpressed in breast cancer and plays a critical role in cancer
cell proliferation. PIGX was identified as a component of a GPI
mannosyltransferase I complex, which is essential to transfer
mannoses to GPI-anchor precursors in the biosynthesis pathway of
GPI-anchor, and its function is suggested to stabilize PIGM, a
catalytic component of the complex (12). GPI-anchoring is known as a
post-translational glycolipid modification that synthesizes through
a multi-step biosynthesis pathway in the ER (31). GPI-anchored proteins are mostly
localized to plasma membrane and involved in many biological
phenomena such as cell-cell interaction, signal transduction and
immune recognition. Abnormalities of GPI-anchoring and elevated
expression levels of GPI-anchored proteins have been found in
cancer (32,33); overexpression of PIGT and GPAA1,
subunits of GPI transamidase that functions in another step of the
biosynthesis pathway, plays important roles in tumorigenesis
(34). These findings implied that
overexpressed PIGX might contribute to development/progression of
breast cancer through the activation of the GPI-anchor biosynthesis
pathway. Indeed, our result suggested that knockdown of PIGX
suppressed breast cancer cell proliferation indicating that PIGX
expression is indispensable for the growth or survival of cancer
cells. However, knockdown of PIGM, the interacting protein of PIGX,
did not cause any growth-suppressive effect on cancer cell growth
(Fig. 1E) in this study,
suggesting that the PIGX-PIGM interaction might not be a key factor
for growth enhancement of breast cancer cells and an interaction
with another binding protein(s) could be important in mammary
tumorigenesis.
Through the LC-MS/MS analysis, we identified two
reticulocalbin (RCN) family proteins, RCN1 and RCN2, which are
ER-located calcium-binding proteins possessing EF-hand motifs
(35,36), as novel binding partners of PIGX.
Although the fundamental roles of RCNs and the functional
difference between RCN1 and RCN2 are still unclear, RCN1 is
reported to be overexpressed in multiple types of human cancer
including breast cancer and was implicated to enhance invasiveness
of breast cancer cells (23,37).
A previous study indicated that RCN2 is a tumor-associated antigen,
whose expression level linearly increased according to the increase
of the breast tumor size. In addition, our cell growth analysis
using siRNAs targeting RCN1/RCN2 and survival analysis employing
public datasets indicate the RCN1/PIGX/RCN2 complex has an
indispensable role relating to cancer cell proliferation.
Furthermore, our gene expression profile analysis after knocking
down of PIGX, RCN1, or RCN2 indicated that the
RCN1/PIGX/RCN2 complex deregulates the expression of ZIC1
and EHD2, which are suggested to function as tumor
suppressors.
ZIC1 is a zinc finger transcription factor and is
known to be involved in vital developmental processes including
neural development and body pattern formation through the
regulation of a variety of signaling pathways (38–41).
ZIC1 was down-regulated in several types of cancer through
hypermethylation in its promoter region (27,42,43).
Furthermore, Zhong et al showed that ectopic expression of
ZIC1 causes the decrease of AKT and ERK1/2 phosphorylation levels,
indicating that ZIC1 would serve a tumor suppressive function
through regulation of the PI3K-MAPK pathway (28). EHD2, another candidate target gene
of the RCN1/PIGX/RCN2 complex, belongs to EH domain-containing
protein family and previously reported to be involved in a variety
of biological processes such as membrane repair (44), endocytic trafficking (45,46),
and myoblast fusion (47). A
recent study showed that the EHD2 was downregulated in
breast cancer cells and possesses important functions in regulating
cancer cell proliferation, migration, and invasion (26). In the Oncomine database (15), downregulation of EHD2 in
breast cancer tissues is also reported. In addition, the expression
levels of both ZIC1 and EHD2 were positively
correlated with that of E-cadherin, downregulation of which is
indicated as a hallmark of epithelial-mesenchymal transition (EMT)
(25,29). Thus, the RCN1/PIGX/RCN2 complex may
contribute to human breast tumorigenesis through not only enhancing
cancer cell proliferation, but also promoting EMT by downregulating
ZIC1 and EHD2 leading to reduction of E-cadherin
expression.
Intriguingly, knockdown of PIGX, RCN1, and RCN2
resulted in upregulation of HDAC8. Given that HDAC8 is
suggested to have an oncogenic function and considered as a
promising therapeutic target for cancer (30), we assume that HDAC8
upregulation was caused as some kind of feedback mechanism to
protect cancer cells from anti-growth inhibitory effect induced by
knockdown of PIGX, RCN1, and RCN2.
In conclusion, we identified that PIGX was
overexpressed in breast cancer clinical samples as well as breast
cancer cell lines, and that PIGX interacted with RCN family member,
RCN1 and RCN2, and regulated the expression of tumor suppressors
ZIC1 and EHD2. Since several studies reported that ZIC1 negatively
regulates the AKT/PI3K pathway, RCN1/PIGX/RCN2 complex may
contribute to mammary tumorigenesis through the suppression of
ZIC1. Although further analysis is required, our data shed light on
the biological role of PIGX in mammary tumorigenesis and suggest
that PIGX may be a good candidate molecule for the development of
novel anticancer drugs for breast cancer.
Acknowledgements
We thank Dr Ryuji Hamamoto for helpful discussion
and critical reading of the manuscript. We also thank the members
of Nakamura laboratory for helpful discussion. This study was
supported partly by the funding from OncoTherapy Science, Inc. Y.
Nakamura is a stock holder and a scientific advisor of OncoTherapy
Science, Inc.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Althuis MD, Dozier JM, Anderson WF, Devesa
SS and Brinton LA: Global trends in breast cancer incidence and
mortality 1973–1997. Int J Epidemiol. 34:405–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Canfell K, Banks E, Moa AM and Beral V:
Decrease in breast cancer incidence following a rapid fall in use
of hormone replacement therapy in Australia. Med J Aust.
188:641–644. 2008.PubMed/NCBI
|
|
5
|
Parkin DM: Is the recent fall in incidence
of post-menopausal breast cancer in UK related to changes in use of
hormone replacement therapy? Eur J Cancer. 45:1649–1653. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Séradour B, Allemand H, Weill A and
Ricordeau P: Changes by age in breast cancer incidence, mammography
screening and hormone therapy use in France from 2000 to 2006. Bull
Cancer. 96:E1–E6. 2009.PubMed/NCBI
|
|
7
|
Schlotter CM, Vogt U, Allgayer H and
Brandt B: Molecular targeted therapies for breast cancer treatment.
Breast Cancer Res. 10:2112008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gasparini G, Longo R, Torino F and
Morabito A: Therapy of breast cancer with molecular targeting
agents. Ann Oncol. 16(Suppl 4): iv28–iv36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, et
al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashida H, Hong Y, Murakami Y, Shishioh N,
Sugimoto N, Kim YU, Maeda Y and Kinoshita T: Mammalian PIG-X and
yeast Pbn1p are the essential components of
glycosylphosphatidylinositol-mannosyltransferase I. Mol Biol Cell.
16:1439–1448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishidate T, Katagiri T, Lin ML, Mano Y,
Miki Y, Kasumi F, Yoshimoto M, Tsunoda T, Hirata K and Nakamura Y:
Genome-wide gene-expression profiles of breast-cancer cells
purified with laser microbeam microdissection: Identification of
genes associated with progression and metastasis. Int J Oncol.
25:797–819. 2004.PubMed/NCBI
|
|
14
|
Tamura K, Furihata M, Tsunoda T, Ashida S,
Takata R, Obara W, Yoshioka H, Daigo Y, Nasu Y, Kumon H, et al:
Molecular features of hormone-refractory prostate cancer cells by
genome-wide gene expression profiles. Cancer Res. 67:5117–5125.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBio-Portal. Sci Signal. 6:pl12013. View Article : Google Scholar
|
|
18
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar
|
|
19
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar
|
|
20
|
Goujon M, McWilliam H, Li W, Valentin F,
Squizzato S, Paern J and Lopez R: A new bioinformatics analysis
tools framework at EMBL-EBI. Nucleic Acids Res. 38(Web Server):
W695–W699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zdobnov EM and Apweiler R: InterProScan -
an integration platform for the signature-recognition methods in
InterPro. Bioinformatics. 17:847–848. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giribaldi G, Barbero G, Mandili G, Daniele
L, Khadjavi A, Notarpietro A, Ulliers D, Prato M, Minero VG,
Battaglia A, et al: Proteomic identification of Reticulocalbin 1 as
potential tumor marker in renal cell carcinoma. J Proteomics.
91:385–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Z, Brattain MG and Appert H:
Differential display of reticulocalbin in the highly invasive cell
line, MDA-MB-435, versus the poorly invasive cell line, MCF-7.
Biochem Biophys Res Commun. 231:283–289. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen JJ, Reid CE, Band V and Androphy EJ:
Interaction of papillomavirus E6 oncoproteins with a putative
calcium-binding protein. Science. 269:529–531. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Y, Liu X, Sun Y, Wu D, Qiu A, Cheng H,
Wu C and Wang X: Decreased expression and prognostic role of EHD2
in human breast carcinoma: Correlation with E-cadherin. J Mol
Histol. 46:221–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang X, Ren H, Yao L, Chen X and He A:
Role of EHD2 in migration and invasion of human breast cancer
cells. Tumour Biol. 36:3717–3726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gan L, Chen S, Zhong J, Wang X, Lam EK,
Liu X, Zhang J, Zhou T, Yu J, Si J, et al: ZIC1 is downregulated
through promoter hypermethylation, and functions as a tumor
suppressor gene in colorectal cancer. PLoS One. 6:e169162011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong J, Chen S, Xue M, Du Q, Cai J, Jin
H, Si J and Wang L: ZIC1 modulates cell-cycle distributions and
cell migration through regulation of sonic hedgehog, PI(3)K and
MAPK signaling pathways in gastric cancer. BMC Cancer. 12:2902012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiang W, Zhao Y, Yang Q, Liu W, Guan H, Lv
S, Ji M, Shi B and Hou P: ZIC1 is a putative tumor suppressor in
thyroid cancer by modulating major signaling pathways and
transcription factor FOXO3a. J Clin Endocrinol Metab.
99:E1163–E1172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chakrabarti A, Oehme I, Witt O, Oliveira
G, Sippl W, Romier C, Pierce RJ and Jung M: HDAC8: A multifaceted
target for therapeutic interventions. Trends Pharmacol Sci.
36:481–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferguson MAJ, Kinoshita T and Hart GW:
Glycosylphosphatidylinositol anchors. Essentials of Glycobiology.
Varki A, Cummings RD and Esko JD: Cold Spring Harbor, NY: 2009
|
|
32
|
Gamage DG and Hendrickson TL: GPI
transamidase and GPI anchored proteins: Oncogenes and biomarkers
for cancer. Crit Rev Biochem Mol Biol. 48:446–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao P, Nairn AV, Hester S, Moremen KW,
O’Regan RM, Oprea G, Wells L, Pierce M and Abbott KL: Proteomic
identification of glycosylphosphatidylinositol anchor-dependent
membrane proteins elevated in breast carcinoma. J Biol Chem.
287:25230–25240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu G, Guo Z, Chatterjee A, Huang X, Rubin
E, Wu F, Mambo E, Chang X, Osada M, Sook Kim M, et al:
Overexpression of glycosylphosphatidylinositol (GPI) transamidase
subunits phosphatidylinositol glycan class T and/or GPI anchor
attachment 1 induces tumorigenesis and contributes to invasion in
human breast cancer. Cancer Res. 66:9829–9836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ozawa M and Muramatsu T: Reticulocalbin, a
novel endoplasmic reticulum resident Ca(2+)-binding protein with
multiple EF-hand motifs and a carboxyl-terminal HDEL sequence. J
Biol Chem. 268:699–705. 1993.PubMed/NCBI
|
|
36
|
Weis K, Griffiths G and Lamond AI: The
endoplasmic reticulum calcium-binding protein of 55 kDa is a novel
EF-hand protein retained in the endoplasmic reticulum by a
carboxyl-terminal His-Asp-Glu-Leu motif. J Biol Chem.
269:19142–19150. 1994.PubMed/NCBI
|
|
37
|
Yu LR, Zeng R, Shao XX, Wang N, Xu YH and
Xia QC: Identification of differentially expressed proteins between
human hepatoma and normal liver cell lines by two-dimensional
electrophoresis and liquid chromatography-ion trap mass
spectrometry. Electrophoresis. 21:3058–3068. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aruga J: The role of Zic genes in neural
development. Mol Cell Neurosci. 26:205–221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagai T, Aruga J, Takada S, Günther T,
Spörle R, Schughart K and Mikoshiba K: The expression of the mouse
Zic1, Zic2, and Zic3 gene suggests an essential role for Zic genes
in body pattern formation. Dev Biol. 182:299–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Merzdorf CS and Sive HL: The zic1 gene is
an activator of Wnt signaling. Int J Dev Biol. 50:611–617. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maurus D and Harris WA: Zic-associated
holoprosencephaly: Zebrafish Zic1 controls midline formation and
forebrain patterning by regulating Nodal, Hedgehog, and retinoic
acid signaling. Genes Dev. 23:1461–1473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang LJ, Jin HC, Wang X, Lam EK, Zhang JB,
Liu X, Chan FK, Si JM and Sung JJ: ZIC1 is downregulated through
promoter hypermethylation in gastric cancer. Biochem Biophys Res
Commun. 379:959–963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang YY, Jiang JX, Ma H, Han J, Sun ZY,
Liu ZM and Xu ZG: Role of ZIC1 methylation in hepatocellular
carcinoma and its clinical significance. Tumour Biol. 35:7429–7433.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marg A, Schoewel V, Timmel T, Schulze A,
Shah C, Daumke O and Spuler S: Sarcolemmal repair is a slow process
and includes EHD2. Traffic. 13:1286–1294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Benjamin S, Weidberg H, Rapaport D, Pekar
O, Nudelman M, Segal D, Hirschberg K, Katzav S, Ehrlich M and
Horowitz M: EHD2 mediates trafficking from the plasma membrane by
modulating Rac1 activity. Biochem J. 439:433–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Naslavsky N and Caplan S: C-terminal
EH-domain-containing proteins: Consensus for a role in endocytic
trafficking, EH? J Cell Sci. 118:4093–4101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Posey AD Jr, Pytel P, Gardikiotes K,
Demonbreun AR, Rainey M, George M, Band H and McNally EM: Endocytic
recycling proteins EHD1 and EHD2 interact with fer-1-like-5
(Fer1L5) and mediate myoblast fusion. J Biol Chem. 286:7379–7388.
2011. View Article : Google Scholar :
|