1. Introduction
The concept of antiangiogenic therapy in cancer
patients started after observations performed by Judah Folkman
approximately 45 years ago. He noticed that in order to grow beyond
1–2 mm3 tumors require blood supply and for that reason
induce the generation of new vessels in the process of
angiogenesis. Based on such observations, it was proposed that
inhibition of tumor vessel formation could suppress tumor growth
and that concept was called antiangiogenic therapy (1,2). The
next step in the field of discovering angiogenesis was the
isolation and characterization of the vascular endothelial growth
factor (VEGF), initially termed the vascular permeability factor
(VPF) by Senger et al (3)
and Ferrara (4). VEGF is the best
characterized angiogenic factor. The function of VEGF is to
modulate vessel permeability, remodeling, endothelial cell (EC)
survival, proliferation and migration (5,6).
VEGF is overexpressed in cancer cells (7,8).
Very high levels of VEGF and other proangiogenic factors result in
the formation of new vessels, but their architecture and function
is abnormal. Tumor vessels are dilated, tortuous, and disorganized
with haphazard patterns (lack microvascular hierarchy) and their
pore sizes are 100 times bigger than is physiologically normal. ECs
forming tumor vessels are loosely connected with each other and
have an irregular morphology. Perivascular cells, i.e. pericytes
and vascular smooth muscle cells that normally stabilize blood
vessels by covering ECs, within tumors are absent or poorly
attached to vessels. The vascular basement membrane is also
abnormal: thick in some tumors or thin or even absent in others.
These structural abnormalities cause functional aberrations. Tumor
vessels are hyperpermeable and hence intravascular fluid and plasma
proteins extravasate, causing an increase in interstitial fluid
pressure (IFP). The blood supply is heterogeneous, some areas are
hyper-whilst other are hypovascularized. As a consequence, hypoxia
and acidosis occur within a tumor. Moreover, hypoxia is one of the
mechanisms regulating VEGF expression; therefore, the formation of
abnormal vasculature intensifies in a self-reinforcing vicious
cycle. The chronic imbalance of the pro- and antiangiogenic factors
in cancer, i.e. excess of pro- and deficiency of antiangiogenic
factors, leads to abnormal angiogenesis (9,10).
Thus, VEGF, as a main agent involved in angiogenesis and signaling
pathway engaged in the regulation of the function of ECs, became a
target in developing antiangiogenic therapies (11).
In this report we discuss current issues related to
the field of antiangiogenic therapy: treatment strategies, vessel
normalization, toxicity, predictive biomarkers and resistance to
antiangiogenic therapy.
2. Treatment strategies
FDA approved antiangiogenic drugs
Currently, there are few main approaches in
targeting angiogenesis which have been tested in clinical trials
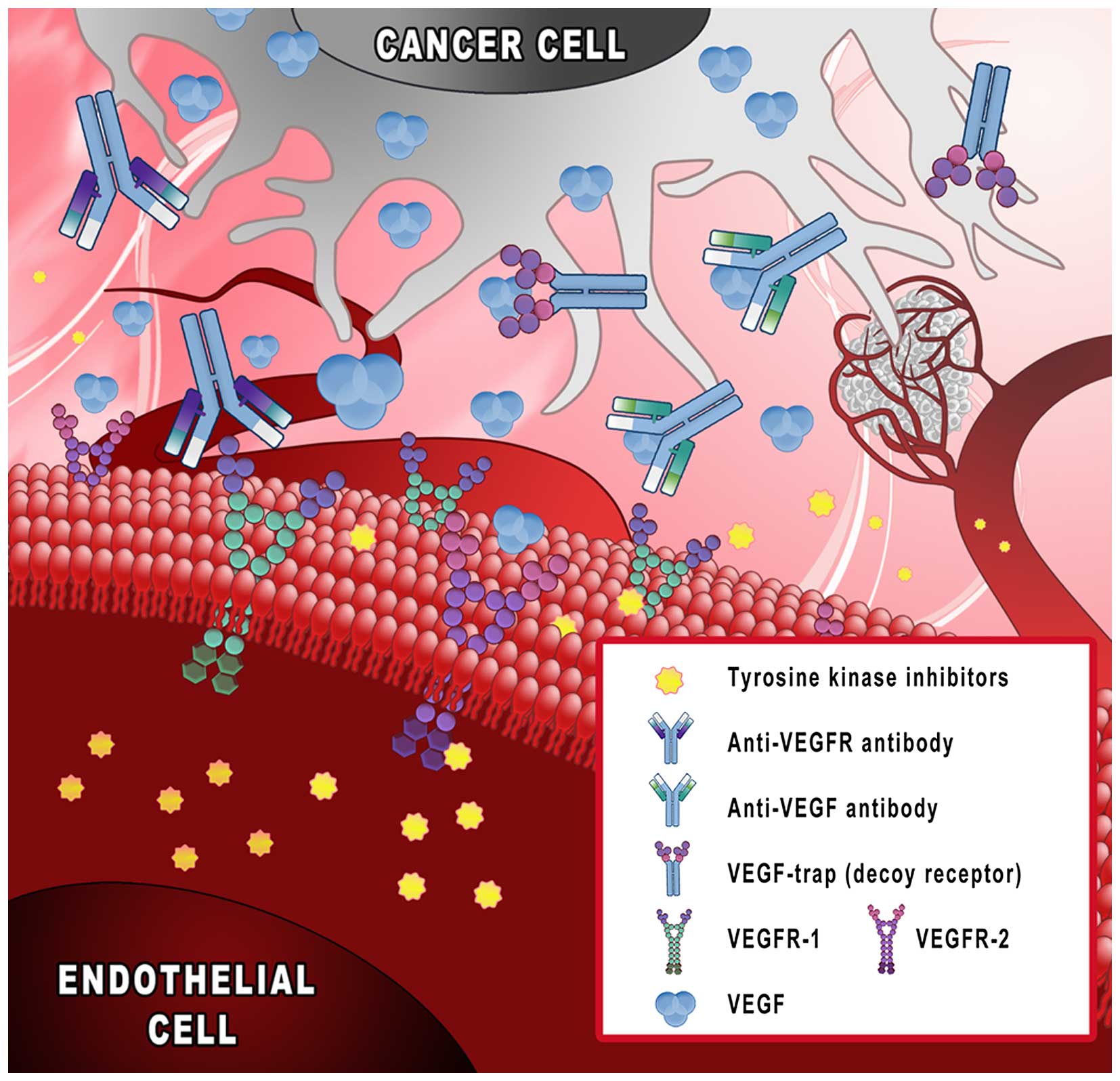
and approved in clinical practice (Fig. 1): i) monoclonal antibodies binding
VEGF (bevacizumab); ii) decoy receptors, ‘VEGF-trap’ (aflibercept);
iii) tyrosine kinase inhibitors (sunitinib and sorafenib); and iv)
monoclonal antibodies targeting VEGF receptors (ramucirumab)
(11–13).
These agents are being used in the treatment of
different cancer types: breast, colorectal, hepatocellular,
gastric, lung and others (14).
One of the first approaches in antiangiogenic therapy was the
monoclonal antibody neutralizing circulating VEGF. In 2004, the
first phase III trial results showed that bevacizumab, a humanized
monoclonal antibody binding specifically to VEGF-A alone, when
combined with chemotherapy in metastatic colorectal cancer improved
progression-free survival PFS (10.6 vs. 6.2 months) and overall
survival OS (23 vs. 15.3 months) compared to chemotherapy arm
(15). An improvement in PFS for
the combination of bevacizumab plus chemotherapy was next shown in
two phase III trials in non-squamous non-small cell lung cancer
(NSCLC) (16–18), but only one study reported an
improvement in OS (16). Within
the next few years, bevacizumab was approved as a monotherapy in
second line treatment of glioblastoma and in combined treatment
with interferon α for renal cell carcinoma. There were some
controversies in cases of using bevacizumab in the treatment of
metastatic breast cancer. The ECOD 2100 trial showed that adding
bevacizumab to paclitaxel improved PFS (11.8 vs. 5.9), as well as
OS rates (36.9 vs. 21.2%) compared to paclitaxel alone. Based on
those results, the US Food and Drug Administration (FDA)
accelerated in 2008 approval of bevacizumab in combination with
paclitaxel in metastatic breast cancer. Further trials, AVADO and
RIBBON-1, confirmed the improvement of PFS by bevacizumab, but
neither demonstrated any improvement of OS. In 2011, FDA withdrew
approval for bevacizumab in metastatic breast cancer (19). In 2014, bevacizumab was approved
for the treatment of patients with platinum-resistant recurrent
epithelial ovarian, fallopian tube, or primary peritoneal cancer in
combination with paclitaxel, pegylated liposomal doxorubicin or
topotecan, based on the results of AURELIA clinical trials
comparing bevacizumab plus chemotherapy with chemotherapy alone
(20,21). Also in 2014, bevacizumab was
approved in combination with paclitaxel and cisplatin or paclitaxel
and topotecan in persistent, recurrent or metastatic cervical
cancer (22,23).
Tyrosine kinase inhibitors (TKIs) are
small-molecular-weight drugs that inhibit the kinase activity of
different receptors. The mechanism of action of TKIs relies on
binding around the ATP binding site of a given receptor and thus
hindering phosphorylation of the tyrosine residue of that receptor
and subsequent transmission of signaling down the intercellular
pathway (2). There are 28
small-molecule kinase inhibitors (tyrosine kinase, serine/threonine
kinase or dual protein kinase inhibitors) approved by the FDA.
Among these, there are some agents that target VEGF receptors
(VEGFR) and these are used to treat different types of cancer, e.g.
sunitinib, sorafenib, axitinib and pazopanib (Table I) (24,25).
Compared to VEGF neutralizing antibodies, TKI do not interfere with
the binding of VEGF to its receptors and they usually target not
only VEGFR but additionally other kinases, such as PDGFR, FGFR and
c-KIT (9).
 | Table IFDA approved tyrosine kinase
inhibitors with known anti-VEGFR activity. |
Table I
FDA approved tyrosine kinase
inhibitors with known anti-VEGFR activity.
| TKI | Activity | Initial US
approval | Indicationsa |
|---|
| Axitinib | VEGFR
1-3 | 2012 | Advanced RCC |
| Cabozantinib | RET, MET, VEGFR
1-3, KIT, TRKB, FLT-3, AXL, TIE-2 | 2012 | Progressive,
metastatic medullary thyroid cancer |
| Lenvatinib | VEGFR 1-3,
FGFR 1-3, PDGFRα, KIT, RET | 2015 | Locally recurrent
or metastatic, progressive, radioactive iodine-refractory thyroid
cancer |
| Nintedanib | FGFR 1-3, PDGFRα/β,
VEGFR 1-3, FLT3 | 2014 | Idiopatic pulmonary
fibrosis |
| Pazopanib | VEGFR 1-3,
PDGFRα/β, FGFR 1/3, KIT, LCK, FMS, Itk | 2009 | Advanced RCC,
advanced soft tissue carcinoma |
| Ponatinib | BCR-ABL, BCR-ABL
T315I, VEGFR, PDGFR, FGFR, EPHR, SRC family kinases, KIT,
RET, TIE2, FLT3 | 2012 | Adult patients with
T3151+ CML (chronic phase, accelerated phase, or blast
phase) or T3151+ Ph+ ALL; adult patients with
chronic phase, accelerated phase, or blast phase CML or
Ph+ ALL for whom no other tyrosine kinase inhibitor
(TKI) therapy is indicated |
| Regorafenib | VEGFR 1-3,
BCR-ABL, B-RAF, B-RAF(V600E), KIT, PDGFRα/β, RET, FGFR1/2, TIE2,
Eph2A | 2012 | Metastatic CRC
treated previously with fluoropyrimidine, oxaliplatin and
irinotecan; locally advanced, unresectable or metastatic GIST
treated previously with imatinib or sunitinib |
| Sorafenib | B/C-RAF,
B-RAF(V600E), KIT, FLT3, RET, VEGFR 1-3, PDGFRβ | 2005 | Unresectable
hepatocellular carcinoma, advanced RCC, locally recurrent or
metastatic, progressive, differentiated TC refractory to
radioactive iodine treatment |
| Sunitinib | PDGFRα/β, VEGFR
1-3, KIT, FLT3, CSF-1R, RET | 2006 | GIST after disease
progression on or intolerance to imatinib mesylate, advanced RCC,
progressive, well-differentiated pNET |
| Vandetanib | EGFRs,
VEGFRs, RET, BRK, TIE2, EPHRs, SRC family kinases | 2011 | Symptomatic or
progressive medullary TC |
Another strategy developed to inhibit angiogenesis
is a human recombinant fusion protein called aflibercept, acting as
a decoy receptor of angiogenic factors. Aflibercept, unlike
bevacizumab, targets not only VEGF-A, but also VEGF-B and placental
growth factor (PlGF). This is a fusion protein of the 2nd
immunoglobulin domain of VEGFR1, 3rd immunoglobulin domain of
VEGFR2 and constant region Fc of human IgG1. In 2012, FDA approved
aflibercept in the treatment of metastatic colorectal cancer (CRC)
with infusional fluorouracil, leucovorin and irinotecan, based on
phase III trial results (26).
Ramucirumab is another human monoclonal antibody
developed to inhibit angiogenesis. It blocks the interaction of
VEGF with its receptor by binding to the extracellular domain of
VEGFR2. Preclinical studies showed that ramucirumab binds
selectively to VEGFR2 with a greater efficacy than its natural
ligand VEGF-A. It is approved in second line treatment in gastric,
NSCLC and colon cancer. Based on the RAISE study, ramucirumab was
approved in combination with FOLFIRI (folinic acid, 5-fluorouracil
and irinotecan) in metastatic CRC patients, if disease progressed
after therapy with bevacizumab, oxaliplatin and fluoropirymidine.
In NSCLC, ramucirumab was approved in combination with docetaxel
after platinum-based chemotherapy. In gastric cancer patients, FDA
approved ramucirumab as a monotherapy in advanced or metastatic
disease or in gastroesophageal junction carcinoma patients for whom
1st line chemotherapy had failed (27,28).
Other strategies in preclinical and
clinical studies
Apart from the above already approved antiangiogenic
agents, additional strategies have been developed aimed at
inhibiting tumor angiogenesis, directly or indirectly, that are
being tested in preclinical and clinical trials. These include
agents that target angiogenesis directly: PlGF, angiopoietin-Tie2
axis, integrins or agents targeting angiogenesis indirectly by
inhibiting oncogenic pathways (e.g. HER2, PI3K/AKT/mTOR and mutated
EGFR) or hormone signaling (9).
Moreover, some known anticancer drugs designed for specified
mechanisms of action may reveal previously unknown antiangiogenic
activity, due to interaction with other signaling pathways that had
not initially been considered. For example, Calero et al
(30) showed that sunitinib, TKI
designed to inhibit VEGF receptor activity, decreased VEGF
secretion from SK-N-BE(2) neuroblastoma cells. The lowered VEGF
expression correlated with both PI3K/AKT signaling pathway
inhibition after sunitinib treatment and increased MYC protein
degradation. In turn, in a lung cancer model, treatment with
imatinib resulted in downregulation of VEGF expression in A549
tumors, which was accompanied by upregulation of p53 expression
(31). Legros et al
(32) showed that imatinib
administration in CML patients resulted in a decrease in plasma
VEGF level and in VEGF secretion in cultured K562 cells as a
consequence of MAPK and PI3K pathway inhibition and VEGF
promoter transcriptional activity inhibition. It was also
surprising that some cytotoxic agents cause ‘antiangiogenic
side-effects’, when applied in low, metronomic doses given more
frequently, e.g. weekly or even daily, and this was more effective
in tumor growth inhibition than the commonly used maximum tolerated
dose (MTD) schedule (33–35). Given in a metronomic schedule,
docetaxel downregulated VEGF expression in gastric cancer BGC-823
cells and VEGF, bFGF, matrix metalloproteinase (MMP)-2 and MMP-9 in
colon adenocarcinoma LS174T cells, while it upregulated TSP-1
expression in HUVEC cells and decreased microvessel density (MVD)
and VEGF and increased TSP-1 in tumor tissue of a BGC-823 model
(36,37).
Another concept in anticancer treatment related to
angiogenesis is the inhibition of metastatic potential through an
influence on the interaction between cancer cells and ECs and
platelets with compounds targeting cyclooxygenase-2/prosta-cyclin
pathways. The use of 1,4-dimethylpyridinium chloride (1,4-DMP) has
been shown to decrease the number of metastases in combination with
cyclophosphamide in a 4T1 breast cancer model (38).
There are also some interesting studies on the
anticancer activity of naturally occurring products and their
potential usefulness as chemopreventive agents. These are also
being tested for their antiangiogenic activity. The antiangiogenic
effect of plant extracted compounds involves: EC proliferation and
migration inhibition, preventing sprout formation, MMP inhibition
and modulation of angiogenic signaling pathways. Plant-based agents
demonstrate synergism when used in combination with chemotherapy
and many of them reveal low levels of undesired side-effects, or
even limit side-effects caused by chemotherapy (14,39,40).
These agents have also been tested in the context of ocular
diseases, where excessive ocular neoangiogenesis is, like cancer
neovascularization, the consequence of an imbalance between pro-
and antiangiogenic factors. It has been shown that different
natural compounds, e.g. curcumin, genistein, luteolin and
resveratrol, suppress retinal neovascularization in different in
vitro and in vivo models (41). One of the plant-derived compounds
extensively studied for anticancer and antiangiogenic activity is
soy isoflavon genistein, first isolated in 1899. The antiangiogenic
activity of genistein was revealed as the ability to decrease
microvessel density, lower VEGF and increase endostatin plasma
level (42,43). However, the exact results of
genistein treatment depend on the doses used: at high and medium
concentrations of soy isoflavones (10–150 μM) antiangiogenic
activity has been observed, while at lower doses (<10 μM)
genistein tended to increase VEGF secretion from breast cancer
cells (44). The antiangiogenic
properties of genistein have also shown its ability to prevent
metastasis or lower blood supply measured in Lewis lung cancer and
B16 melanoma models (45–47). Another natural agent generating
general interest in anticancer research is resveratrol, a
phytochemical of grapes, berries and peanuts. On human ovarian
cancer cells it has been shown that resveratrol attenuates the
induction of HIF-1α and VEGF by lipopolysaccharide LPS (42,48).
Resveratrol also inhibits mediation of tumor necrosis factor α
(TNFα) MMP-9 expression in HepG2 hepatocellular carcinoma cells and
also NF-κB expression and invasion of HepG2 cells (49). The identified mechanisms of
antiangiogenic activity of natural, plant derived agents so far
include: interfering with signaling of VEGF and FGF, decreased
vascular permeability by inhibiting NO release from ECs, modulation
of NF-κB activity, and inhibition of HIF-1α expression and its
downstream targets (VEGF, TNFα, COX2, IL-6 and IL-8) (41).
Another important naturally occurring compound that
is intensively studied in cancer research is vitamin D, a steroid
hormone regulating calcium and phosphate homeostasis (50). Vitamin D is synthesized in the skin
upon UVB exposure from 7-dehydrocholesterol and next hydroxylated
at C-25 and C-1 in the liver and kidney, respectively. Vitamin D
can also be obtained from the diet, especially from food of animal
origin, fish, meat, eggs and milk products, but it has also been
shown that vitamin D can be found in plants (51). Calcitriol, the active form of
vitamin D3, regulates many cellular and tissue processes
involved in carcinogenesis: proliferation, differentiation,
apoptosis, inflammation, invasiveness and metastasis by
transcriptional regulation of gene expression (genomic action) or
by influencing different signaling pathways (rapid, non-genomic
action) (50,52,53).
One of the first pieces of evidence for the antiangiogenic activity
of calcitriol was a study on 4.5-day old chick embryos, where it
was shown that calcitriol and vitamin D3 analog,
22-oxa-1α,25-dihydroxyvitamin D3, inhibited angiogenesis
in chorioallantoic membranes (54). Calcitriol reduces the expression of
proangiogenic factors, e.g. VEGF, and IL-8, and inhibits the
proliferation of ECs derived from tumors through the induction cell
cycle arrest and apoptosis (55–57).
In azoxymethane-induced colon cancer in rats, vitamin D derivative
administration resulted in decreased immunohistochemical staining
of VEGF and microvessel counts (58). In LLC cells, vitamin D derivatives
reduced MMP-2, MMP-9 and VEGF expression and in in vivo
Matrigel assays inhibited angiogenesis induced by bFGF (59). In another study using the LLC
model, it was shown that calcitriol and its analog PRI-2191 inhibit
growth and metastasis of LLC cells transplanted subcutaneously.
Moreover, a tendency to decrease blood vessel diameter, without
influencing their number, was observed. This observation may
suggest the influence of calcitriol and its analog on tumor growth
not only directly, but also through normalization of tumor
vasculature (60). Therefore, a
number of in vitro and in vivo studies have proved
that vitamin D has a significant impact on the process of
angiogenesis (50). Unfortunately,
the biological anticancer activity of calcitriol can only be
obtained when administered in high doses, which limits its use due
to the risk of hypercalcemia. This has inspired many scientists to
synthesize vitamin D analogs in order to dissociate calcemic from
anti-proliferative activity of vitamin D. These analogs were then
tested alone or in combinations in cancer research (53,61–63).
No vitamin D compounds are currently used in clinical practice for
cancer treatment, although in preclinical animal cancer models
several vitamin D analogs have appeared to be potent drugs,
especially in combination with known chemotherapy (31,63–66).
To date, a small number of studies have assessed the influence of
vitamin D and its derivatives on angiogenesis (63). In clinical trials, vitamin D
supplementation has been studied with the aim of reducing the risk
of cancer. In addition, vitamin D has been studied in combination
with chemotherapy, mainly in prostate cancer patients. However,
results are still inconsistent and no clear conclusions have been
made in this field; therefore, more studies are required (67–71).
The uncovered anticancer and antiangiogenic activity
of many natural compounds may offer a great opportunity in cancer
prevention or may strengthen existing anticancer treatment options.
It is believed that the use of such natural health products may be
beneficial, as they may act through many signaling pathways and
reduce the development of resistance by cancer cells and therefore
improve patient outcomes (39,40,42).
3. Vessel disruption or normalization
The rationale behind antiangiogenic therapy was the
concept that blocking blood vessel formation in tumors or its
regression would deprive cancer cells of nutrients and oxygen and
finally starve tumors to death or induce tumor dormancy. One of the
first preclinical studies clearly showed that treatment with an
anti-VEGF monoclonal antibody caused a significant vascular density
reduction and tumor growth delay in mice bearing xenografts of
glioblastoma multiforme, leiomyosarcoma and rhabdomyosarcoma
(72). It turned out, however,
that anti-VEGF monotherapy in clinical trials of human solid tumors
showed only modest objective response rates and lacked noticeable
survival benefits for patients (73). After many years of clinical trials,
anti-VEGF agents appeared to be active as single agents only in a
limited number of cancers, e.g. renal cell carcinoma,
hepatocellular carcinoma, ovarian, neuroendocrine tumors and
glioblastoma. In contrast, in other studied cancers, CRC, NSCLC and
breast cancer, the administration of anti-VEGF drugs was effective
only when combined with chemotherapy, leading to significant
improvements of PFS and OS compared to chemotherapy alone (73,74).
Such clinical results thus generated some confusion.
It is known that the efficacy of chemotherapy depends on efficient
delivery of cytotoxic agents to tumor cells through efficient blood
flow, whilst antiangiogenic therapy, according to the theory,
should destroy blood vessels and thus prevent drug delivery. In
order to elucidate these seemingly counterintuitive observations
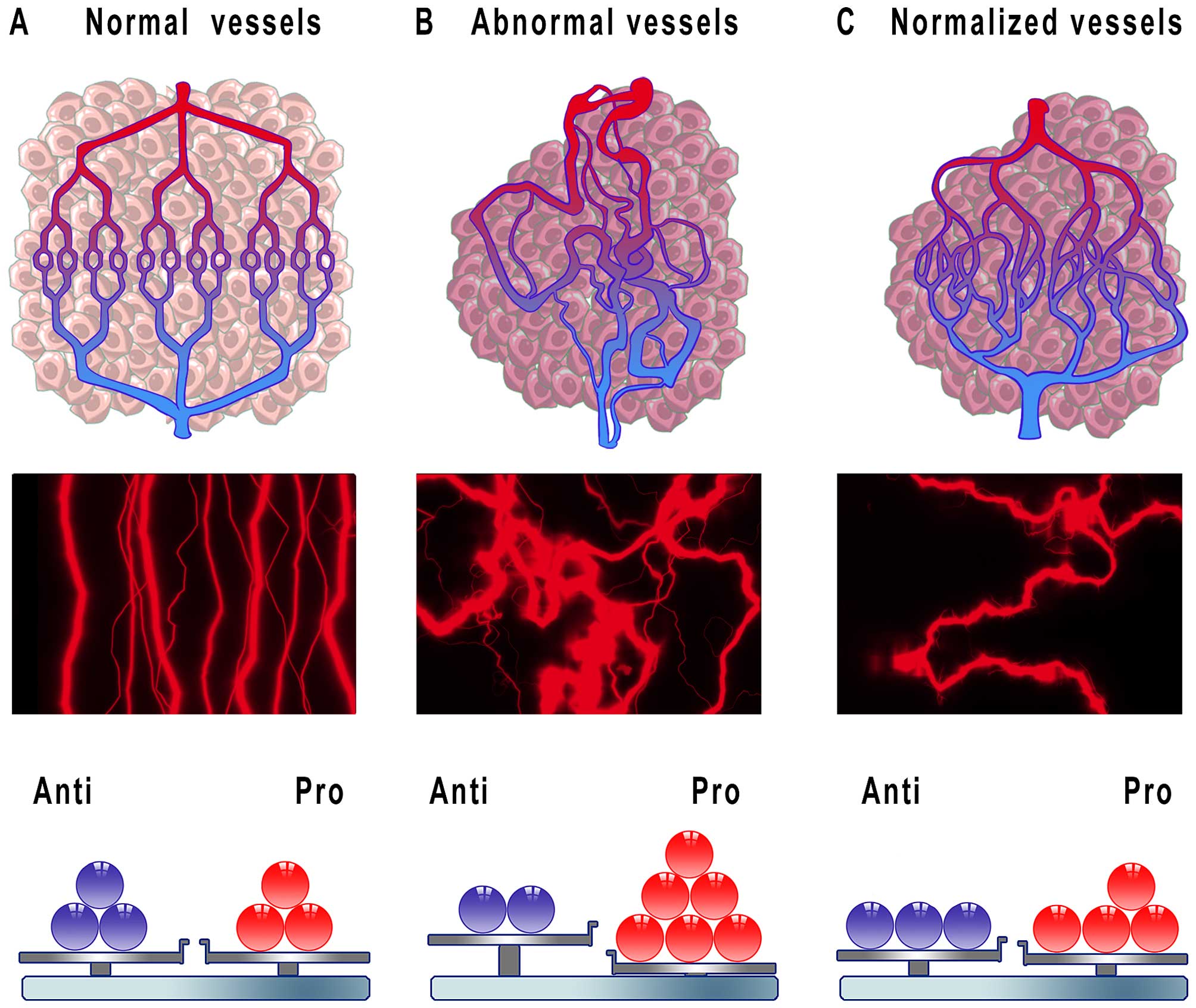
the hypothesis of ‘vessel normalization’ was proposed in 2001, that
is many years after the importance of inhibiting tumor angiogenesis
had been identified (10). It was
assumed that judicious administration of antiangiogenic drugs
reverts the abnormal structure and function of the tumor vessels
towards normal state (Fig. 2). In
this regard, treatment with antiangiogenic agents would correct the
arrangement of vasculature towards a more organized structure
leading to increased homogeneity in blood flow. Furthermore,
improvement in junctions between ECs, increases in pericyte content
around vessels and also better connections between ECs and
pericytes would decrease vascular permeability with a simultaneous
decline in intratumoral fluid pressure. As a result, cytotoxic
drugs would be effectively delivered to cancer cells owing to
increased and efficient blood perfusion within the tumor (75,76).
Indeed, many preclinical and clinical studies have shown that
antiangiogenic therapy results in vascular normalization. For
example, it has been shown in preclinical studies with different
human tumors that administration of an anti-VEGF A4.6.1 antibody
results in a reduction in vessel diameter and tortuosity, a
significant decline in vascular permeability to plasma proteins,
providing evidence that after neutralizing tumor cell-derived VEGF
abnormalities of tumor vasculature could be reversed (77). Similar results have been achieved
in studies with the use of bevacizumab in combination with
chemotherapy or ionizing radiation. After bevacizumab
administration of mice bearing neuroblastoma or rhabdomyosarcoma
xenografts, a substantial decrease in tumor microvessel density and
improved pericyte coverage in tumors has been observed with a
concomitant decrease in vascular permeability, a drop in
intratumoral fluid pressure and an increase in intratumoral oxygen
pressure (78,79). Tumor vessel normalization could
also be observed after using tyrosine kinase inhibitors. In a
murine Lewis lung cancer model, treatment with axitinib resulted in
reduction in microvessel density and vascular sprouting (80). Similarly, the administration of
sunitinib in a human glioma model resulted in a decrease in MVD and
collagen IV density (but no effect on α-SMA density) and an
improvement in tamizolomide penetration into brain tumors (81,82).
Some clinical studies have also demonstrated the occurrence of
vascular normalization in cancer patients after treatment with
antiangiogenic agents. A study in patients with locally advanced
rectal adenocarcinoma receiving bevacizumab 7 weeks before surgical
resection, first dose was given alone and after 2 weeks with
5-fluorouracil, showed a decline in tumor microvessel density, a
reduction of intratumoral fluid pressure, and an increase in the
content of pericytes covering vessels (83). More examples of vessel
normalization as a consequence of antiangiogenic therapy have been
reviewed elsewhere (9).
Hypoxia induced by abnormal tumor vascularity
influences the immune response in cancer. This contributes to
immune tolerance by inhibiting the proliferation and activity of T
lymphocytes and inducing accumulation and polarization of immune
cells towards suppressive phenotypes. It has been proposed that
normalizing tumor vascularity could enhance the adoptive cell
transfer efficacy (84). In 2012,
Huang et al (85)
demonstrated that antiangiogenic treatment influences the
effectiveness of immunotherapy by the modulatory activity of
antiangiogenic agents on tumor microenvironment. In a breast cancer
model, the authors studied the influence of administration of
anti-VEGFR2 antibody on anticancer vaccine therapy in
immune-tolerant and immunogenic mice. The study showed that
treatment with an anti-VEGFR2 antibody enhanced anticancer activity
of whole cancer cell vaccine in a CD8+ T-cell-dependent
manner in both murine models. Additionally, the efficacy of the
tested therapy depended on the dose of the anti-VEGF agent: lower
doses of anti-angiogenic agent were superior to higher doses in
augmenting the infiltration of tumor with CD4+ and
CD8+ T-cells and in polarizing tumor-associated
macrophages, from immunosuppressive M2 towards immunostimulatory M1
phenotypes.
Data obtained in preclinical and clinical studies
have shown that after cessation of the antiangiogenic therapy rapid
revascularization occurs in tumors, which can be followed by rapid
regrowth of the tumor (86–88).
It turned out that vessel normalization obtained as a result of
antiangiogenic therapy is transient. The period when the vessel
normalization is present is called the ‘time window’ or ‘window of
opportunity’. The temporariness of normal features of tumor vessels
may result from discontinuation of therapy or rest periods in
therapy schedules, but also as a consequence of excessive doses or
prolonged administration of antiangiogenic drugs (9,89).
Studies have shown that abnormalities of tumor vessels are reversed
as early as 24–72 h after starting the therapy and are sustained
for different periods, from few to dozen days, or sometimes longer
(78,90,91).
The existence of a time window is important for appropriate
scheduling of combined treatment. Many studies have shown that the
efficacy of combined antiangiogenic and chemotherapy treatment is
schedule-dependent. Since successful activity of cytotoxic drugs
depends on efficient drug delivery to cancer cells, which can be
obtained after vessel normalization, it has been proposed that
chemotherapy or radiotherapy should be applied after administration
of antiangiogenic agents (78,92,93).
In a study of 2 patients with metastatic breast cancer, Chen et
al (89)analyzed the time of
appearance of vessel normalization after bevacizumab administration
by means of three-dimension power Doppler ultrasonography and
observed that the window was open 20–24 h after bevacizumab
injection. Additionally, it was shown that sequential treatment of
bevacizumab: on days 1 and 15, and paclitaxel: on days 2, 9 and 16
resulted in rapid reduction of tumors in brain, as observed in
computed tomography.
The optimal timing of administration of anti-VEGF
agents before cytotoxic agents is required to achieve the highest
anti-cancer response of treatment used. The challenge is the method
of determination of the normalization window in a patient, and what
is more non-invasively. There have been some studies aimed at
probing the time window of vessel normalization. Vangestel et
al (94) used
99mTc-tricarbonyl His-annexin A5, radiotracer of
apoptosis to explore the timing between administration of
bevacizumab and irinotecan in a colon cancer model that would
result in the greatest tumor cell death. Hernandez-Agudo et
al (95) used
18F-misonidazole ((18F)-FMISO) PET as a hypoxia tracer
in order to explore vessel normalization after administration of
divotinib in pancreas and breast cancer models. After a decrease in
hypoxia, and therefore vessel normalization, the delivery of
chemotherapy was improved and so was the cytotoxic effect. Data
obtained in that study suggested that (18F)-FMISO mirrors the
dynamic of hypoxia and changes in vessel normality/abnormality in
response to a short course of antiangiogenic therapy. Other
candidate biomarkers tested to assess the response to anti-VEGF
therapy are: tumor biopsy, measuring plasma protein concentration
e.g. VEGF, or level of circulating ECs and progenitor cells, also
imaging diagnostic methods (CT, PET, MRI) (73).
4. Toxicity
As angiogenesis in adults is a rather rare process,
it was thought that anti-VEGF therapies would be free of toxicity.
However, clinical practice showed that anti-angiogenic therapy is
accompanied with a number of side-effects, including hemorrhage,
hypertension, proteinuria, impaired wound healing, thrombosis and
others (11). Preclinical studies
with non-tumor bearing mice administered with anti-angiogenic
agents have shown that the treatment alters the density and
architecture of vessels in multiple tissues and organs, especially
in endocrine organs that had fenestrated vessels as a result of
antiangiogenic therapy (96). Yang
et al (97) showed that
systemic administration of anti-VEGF and anti-VEGFR neutralizing
antibodies affected the vasculature of multiple organs, with the
greatest vessel regression in endocrine glands, intestine and
uterus. On the other hand, high levels of VEGF produced by cancer
cells correlated with abnormal hepatic sinusoidal blood vessels and
high mortality in a VEGF expressing melanoma model (98). Cancer patients, mostly in the
advanced stage of the disease, experience so-called
cancer-associated systemic syndrome (CASS) or paraneoplastic
syndrome as a result of production and secretion excess amounts of
different peptides and hormones that affect diverse systems, most
frequently the endocrine, gastrointestinal, neurologic,
dermatologic and hematologic systems. Physiologically, these
factors are paracrine hormones, but when overproduced by malignant
cells they enter the circulation and influence distant tissues and
organs deregulating homeostasis (96,99,100). Elevated levels of VEGF expressed
by cancer cells induced CASS in mice, manifesting with severe
anemia, ascites, hepatic dysfunction, and decreases in serum
corticosterone levels, whilst the use of anti-VEGF agents resulted
in vessel normalization in healthy tissues and improved survival of
animals (98,100). These surprising observations show
that antiangiogenic therapy may also have an impact on improving
healthy tissue and organ function in cancer patients.
5. Predictive biomarkers
Efforts are being made to identify some biomarkers
that could predict the clinical benefits of antiangiogenic therapy
for a given patient. Since the monoclonal antibody bevacizumab
specifically targets VEGF, it was assumed that measuring serum VEGF
levels could serve as a predictive marker for patient selection.
Unfortunately, so far it has not been proved that VEGF level, in
blood or in tumor biopsies, could fulfill the requirements of a
predictive biomarker (101,102). Studies on circulating VEGF levels
in cancer patients have shown the importance of VEGF as a
prognostic rather than predictive biomarker (11). On the other hand, it has turned out
that some of the adverse effects related to antiangiogenic agents
appeared to be positively correlated with response to therapy. For
example, it was shown that hypertension associated with the
bevacizumab or TKIs correlated with clinical response in patients
with breast, colorectal and NSCLC, whilst skin rashes correlated
with drug response in patients with colorectal and hepatocellular
carcinoma (96). Another approach
in predicting the response to antiangiogenic therapy was the
imaging of tumor vasculature, with the use of CT, MRI or PET. In a
study of glioblastoma patients treated with cediranib vessel
normalization was shown by means of MRI as a decrease in vessel
diameter and permeability (10).
Some studies have shown a correlation between changes in vascular
features and patient outcome, but there are some limitations and
obstacles that need to be challenged: an understanding of detected
characteristics of vasculature with the biology of tumor, also the
methodologies require standardization (11).
6. Resistance to antiangiogenic therapy
Despite the great success of antiangiogenic therapy,
as for anticancer drugs, resistance to antiangiogenic treatment is
also an important issue. Introduction of anti-VEGF drugs to
anticancer therapy augmented PFS, causing transient disease
stabilization, but improvement in OS can not always be achieved.
What is more, withdrawal of an antiangiogenic drug from a therapy
is followed by rapid regrowth of the tumor. It turned out that some
types of cancer can be intrinsically refractory to antiangiogenic
therapy or during the treatment acquire resistance to anti-VEGF
agents (103,104). The intrinsic resistance may
result e.g. from elevated levels of circulating, soluble VEGFR1
(sVEGFR1) before therapy. sVEGFR1 acts as an intrinsic VEGF decoy
receptor and thus adding an external anti-VEGF drug has no
biological effect. It has been shown that patients with rectal
carcinoma, hepatocellular carcinoma, and metastatic colorectal
carcinoma who had elevated levels of sVEGFR1 did not benefit from
adding bevacizumab to chemotherapy (102). Acquired resistance to
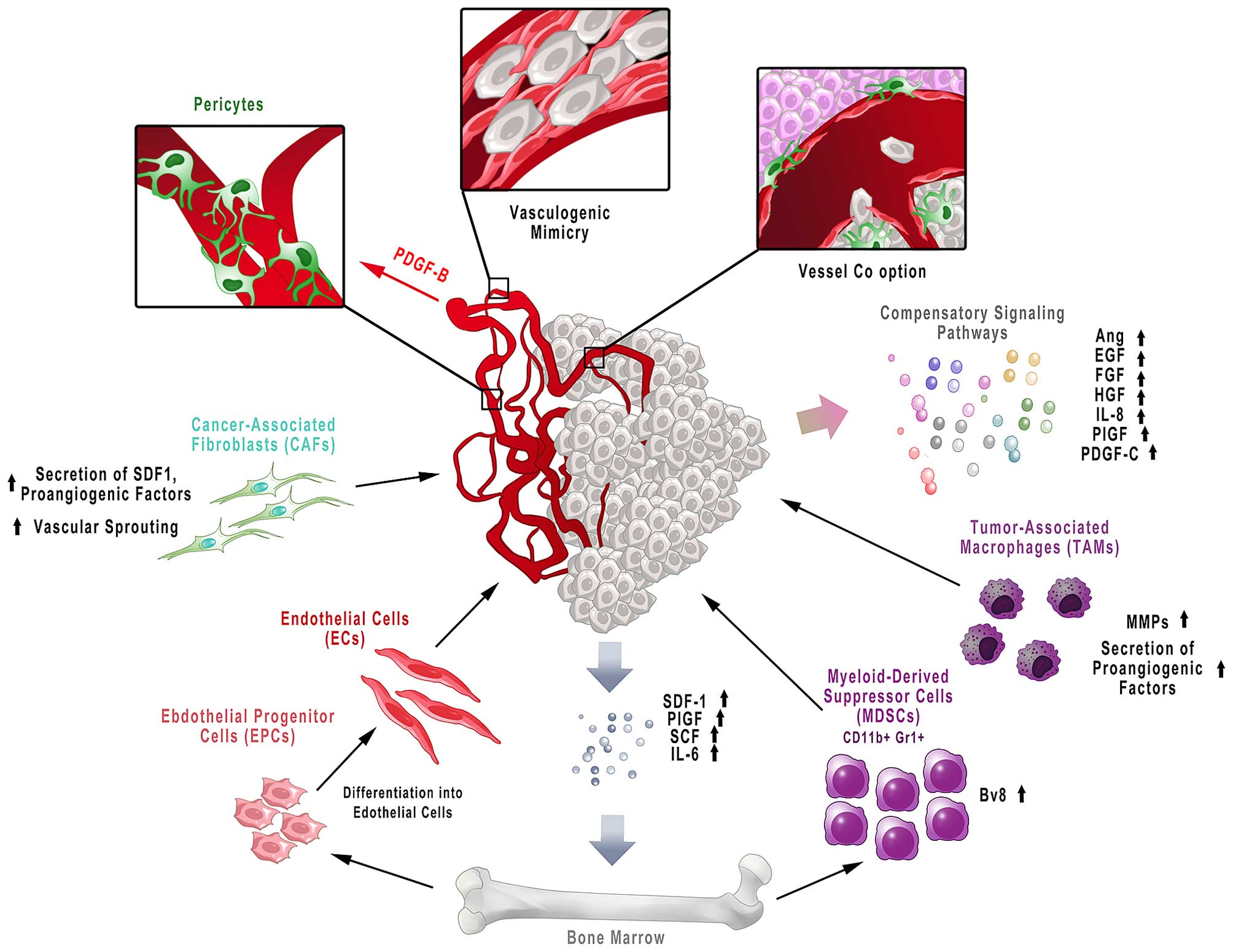
antiangiogenic therapy may result from a few possible mechanisms:
activation of alternative signaling pathways, recruitment of
bone-marrow derived cells, stromal cells of tumor microenvironment,
vessel co-option and vessel mimicry and increased invasiveness and
metastasis (Fig. 3) (105).
There are a number of other pro-angiogenic pathways
and factors that can stimulate blood vessel growth and survival
when the VEGF-mediated pathway is inhibited. Some of these
pro-angiogenic factors are: angiopoietins (Ang), epidermal growth
factor (EGF), fibroblast growth factors (FGF1 and FGF2), hepatocyte
growth factor (HGF), interleukin 8 (IL-8), platelet-derived growth
factor C (PDGF-C) and placental growth factor (PlGF) (11,105). The upregulation of PlGF, FGF2,
IL-8 expression could be observed in colorectal, glioblastoma,
renal cell and hepatocellular cancer patients after anti-VEGF
therapy (106–109). Proteomic analysis performed in
bevacizumab treated breast cancer xenograft showed upregulation of
several compensatory signaling pathways with persistent mTOR
signaling. It was next hypothesized that targeting the PI3K pathway
would increase the efficacy of therapy consisting of bevacizumab
and indeed combining an mTOR inhibitor with bevacizumab increased
the effectiveness of such treatment. Therefore, exploring the
mechanisms activated upon VEGF signaling inhibition and next their
attenuation could avoid failure and simultaneously improve the
efficacy of therapy (110).
Growth factors released by cancer cells also recruit
bone marrow-derived cells into the tumor microenvironment:
monocytes/macrophages, endothelial precursor cells (EPCs),
myeloid-derived suppressor cells (MDSCs) and cancer-associated
fibroblasts (CAFs). These cells contribute to the induction of
resistance to antiangiogenic therapy (105). Anti-VEGF agents have been shown
to induce the expression of factors such as stromal derived
factor-1 (SDF-1), PlGF, stem cell factor (SCF), interleukin 6
(IL-6) and others that are involved in recruitment of bone
marrow-derived cells (111). The
resistance to sunitinib was correlated in studies of metastatic
renal cell carcinoma (mRCC) with infiltration of the tumor tissue
with CD11b+Gr1+ myeloid cells that apart from
sustaining suppression of immune cells produce proangiogenic
factors (112). A study with the
use of an anti-Gr1 antibody and anti-VEGF treatment showed improved
tumor growth inhibition compared to anti-VEGF alone and delayed the
onset of refractoriness (113).
It was found that CD11+Gr1+ cells, upon G-CSF
stimulation, express the Bv8 protein, known as prokineticin-2,
mediator of VEGF-independent angiogenesis. Blocking Bv8 with
neutralizing antibody caused angiogenesis inhibition and tumor
growth and together with anti-VEGF antibodies exhibit an additive
effect (114). Angiogenesis and
the immune system are bidirectionally dependent; therefore,
appropriate knowledge of their relationship may help in developing
new effective treatment strategies (115). Tumor-associated macrophages
(TAMs) contribute to resistance to antiangiogenic therapy by
secretion of a number of proangiogenic factors. Moreover, these
cells secrete MMPs that degrade extracellular matrix with
concomitant release of matrix-sequestered growth factors that
contribute to tumor growth and angiogenesis (116).
Tumors are also infiltrated with stromal cells, such
as CAFs or pericytes, which are also engaged in resistance to
antiangiogenic agents (105).
CAFs contribute to tumor angiogenesis through secretion of
angiogenic factors, whilst via production of SDF-1 they recruit
bone-marrow endothelial progenitor cells (VEGF-independent
mechanism) (117). It has also
been shown that in tumors resistant to anti-VEGF therapy CAFs
expressed pro-angiogenic PDGF-C. Blocking PDGF-C by neutralizing
antibodies inhibited angiogenesis and in combination with anti-VEGF
antibody revealed an additive effect (118). In turn, pericytes are recruited
in response to PDGF-B released by ECs, and are responsible for
vessel stabilization and maturation (119). The role of pericytes is to
protect ECs from antiangiogenic agents, as well as to inhibit EC
proliferation. After the treatment with angiogenesis inhibitors, an
increase in pericyte coverage microvessels could be observed
(105). On the other hand,
enhancing tumor vessel covering by pericytes, the vessel maturation
and resultant decreased leakiness may improve chemotherapy
delivery. Therefore, the role of pericytes and PDGF-B mediated
signaling in resistance to antiangiogenic therapy requires further
study (103).
Tumor vascularization may be a result of a few
different potential mechanisms. Apart from angiogenesis, cancer may
achieve new vasculature by vessel co-option (using existing
vessels), vascular mimicry (the process of forming vessels from
tumor cells) and vasculogenesis (involving bone marrow-derived
progenitor cells) (120). For
example, in glioblastoma multiforme it was shown that VEGF
signaling inhibition caused more invasive tumors and it was
proposed that activation of MET (the cellular receptor for HGF)
after inhibition of VEGF signaling, as well as tumor-derived
EC-induced angiogenesis and vasculogenic mimicry, could be engaged
in anti-VEGF therapy resistance (121).
New possible mechanisms of tumor escape from
antiangiogenic therapy include: EC heterogeneity, antiangiogenic
VEGF, extracellular vesicles, lysosomal sequestration,
glycosylation-dependent resistance and genetic polymorphism
(reviewed in ref. 105).
7. Conclusion
The discovery of tumor angiogenesis and the
subsequent concept of antiangiogenic therapy was a great
breakthrough in anticancer treatment and improved our knowledge of
the biology of cancer. In many cases, antiangiogenic agents when
added to standard chemotherapy offered an improvement in
therapeutic efficacy with different cancers: colorectal, breast,
non-small cell lung cancer and hepatocellular carcinoma. However, a
decade after approval of the first antiangiogenic agents, today all
the above issues and obstacles related to antiangiogenic therapy in
solid tumors have to be reconsidered in order to offer appropriate
treatment for patients. Combining knowledge of the mechanisms of
resistance to antiangiogenic therapy, the relationship between
angiogenesis and immunity in cancer, validation of prognostic and
predictive biomarkers, and targeting multiple signaling molecules,
but with rationally designed schedule, may advance anticancer
therapy and offer new promising results in the future.
Acknowledgements
The present study was supported by a grant from the
National Science Center (Preludium 2013/09/N/NZ4/01720) and the
Wroclaw Center of Biotechnology program The Leading National
Research Center (KNOW) for the years 2014–2018.
References
|
1
|
Folkman J, Bach M, Rowe JW, Davidoff F,
Lambert P, Hirsch C, Goldberg A, Hiatt HH, Glass J and Henshaw E:
Tumor angiogenesis: Therapeutic implications. N Engl J Med.
285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ribatti D: History of research on tumor
angiogenesis. Springer; New York, NY: 2009
|
|
3
|
Senger DR, Galli SJ, Dvorak AM, Perruzzi
CA, Harvey VS and Dvorak HF: Tumor cells secrete a vascular
permeability factor that promotes accumulation of ascites fluid.
Science. 219:983–985. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrara N: Vascular endothelial growth
factor. Arterioscler Thromb Vasc Biol. 29:789–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta K, Kshirsagar S, Li W, Gui L,
Ramakrishnan S, Gupta P, Law PY and Hebbel RP: VEGF prevents
apoptosis of human microvascular endothelial cells via opposing
effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res.
247:495–504. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cross MJ and Claesson-Welsh L: FGF and
VEGF function in angiogenesis: Signalling pathways, biological
responses and therapeutic inhibition. Trends Pharmacol Sci.
22:201–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sennino B, Kuhnert F, Tabruyn SP, Mancuso
MR, Hu-Lowe DD, Kuo CJ and McDonald DM: Cellular source and amount
of vascular endothelial growth factor and platelet-derived growth
factor in tumors determine response to angiogenesis inhibitors.
Cancer Res. 69:4527–4536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: Current progress, unresolved
questions and future directions. Angiogenesis. 17:471–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McIntyre A and Harris AL: Metabolic and
hypoxic adaptation to anti-angiogenic therapy: A target for induced
essentiality. EMBO Mol Med. 7:368–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semin Cancer Biol. 35(Suppl): S224–S243.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, et
al: Phase III trial of cisplatin plus gemcitabine with either
placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAiL. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, et
al; BO17704 Study Group. Overall survival with
cisplatin-gemcitabine and bevacizumab or placebo as first-line
therapy for nonsquamous non-small-cell lung cancer: Results from a
randomised phase III trial (AVAiL). Ann Oncol. 21:1804–1809. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Husein B, Abdalla M, Trepte M, Deremer
DL and Somanath PR: Antiangiogenic therapy for cancer: An update.
Pharmacotherapy. 32:1095–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poveda AM, Selle F, Hilpert F, Reuss A,
Savarese A, Vergote I, Witteveen P, Bamias A, Scotto N, Mitchell L,
et al: Bevacizumab combined with weekly paclitaxel, pegylated
liposomal doxorubicin, or topotecan in platinum-resistant recurrent
ovarian cancer: Analysis by chemotherapy cohort of the randomized
phase III AURELIA trial. J Clin Oncol. 33:3836–3838. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu JF and Matulonis UA: Bevacizumab in
newly diagnosed ovarian cancer. Lancet Oncol. 16:876–878. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krill LS and Tewari KS: Integration of
bevacizumab with chemotherapy doublets for advanced cervical
cancer. Expert Opin Pharmacother. 16:675–683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crafton SM and Salani R: Beyond
chemotherapy: An overview and review of targeted therapy in
cervical cancer. Clin Ther. 38:449–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu P, Nielsen TE and Clausen MH:
FDA-approved small-molecule kinase inhibitors. Trends Pharmacol
Sci. 36:422–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu P, Nielsen TE and Clausen MH:
Small-molecule kinase inhibitors: An analysis of FDA-approved
drugs. Drug Discov Today. 21:5–10. 2016. View Article : Google Scholar
|
|
26
|
Ciombor KK and Berlin J: Aflibercept - a
decoy VEGF receptor. Curr Oncol Rep. 16:3682014. View Article : Google Scholar
|
|
27
|
Aprile G, Rijavec E, Fontanella C, Rihawi
K and Grossi F: Ramucirumab: Preclinical research and clinical
development. Onco Targets Ther. 7:1997–2006. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tiwari P: Ramucirumab: Boon or bane. J
Egypt Natl Canc Inst. 28:133–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
(http://www.fda.gov/).
Accessed 22 Apr 2016
|
|
30
|
Calero R, Morchon E, Johnsen JI and
Serrano R: Sunitinib suppress neuroblastoma growth through
degradation of MYCN and inhibition of angiogenesis. PLoS One.
9:e956282014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maj E, Filip-Psurska B, Świtalska M,
Kutner A, Wietrzyk J and Vitamin D: Vitamin D analogs potentiate
the antitumor effect of imatinib mesylate in a human A549 lung
tumor model. Int J Mol Sci. 16:27191–27207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Legros L, Bourcier C, Jacquel A, Mahon FX,
Cassuto JP, Auberger P and Pagès G: Imatinib mesylate (STI571)
decreases the vascular endothelial growth factor plasma
concentration in patients with chronic myeloid leukemia. Blood.
104:495–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kerbel RS, Viloria-Petit A, Klement G and
Rak J: ‘Accidental’ anti-angiogenic drugs: anti-oncogene directed
signal transduction inhibitors and conventional chemotherapeutic
agents as examples. Eur J Cancer. 36:1248–1257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Klement G, Baruchel S, Rak J, Man S, Clark
K, Hicklin DJ, Bohlen P and Kerbel RS: Continuous low-dose therapy
with vinblastine and VEGF receptor-2 antibody induces sustained
tumor regression without overt toxicity. J Clin Invest.
105:R15–R24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Man S, Bocci G, Francia G, Green SK, Jothy
S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G and Kerbel RS:
Antitumor effects in mice of low-dose (metronomic) cyclophosphamide
administered continuously through the drinking water. Cancer Res.
62:2731–2735. 2002.PubMed/NCBI
|
|
36
|
Wu H, Xin Y, Zhao J, Sun D, Li W, Hu Y and
Wang S: Metronomic docetaxel chemotherapy inhibits angiogenesis and
tumor growth in a gastric cancer model. Cancer Chemother Pharmacol.
68:879–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo XL, Lin GJ, Zhao H, Gao Y, Qian LP, Xu
SR, Fu LN, Xu Q and Wang JJ: Inhibitory effects of docetaxel on
expression of VEGF, bFGF and MMPs of LS174T cell. World J
Gastroenterol. 9:1995–1998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blazejczyk A, Papiernik D, Porshneva K,
Sadowska J and Wietrzyk J: Endothelium and cancer metastasis:
Perspectives for antimetastatic therapy. Pharmacol Rep. 67:711–718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sagar SM, Yance D and Wong RK: Natural
health products that inhibit angiogenesis: A potential source for
investigational new agents to treat cancer-Part 1. Curr Oncol.
13:14–26. 2006.
|
|
40
|
Sagar SM, Yance D and Wong RK: Natural
health products that inhibit angiogenesis: A potential source for
investigational new agents to treat cancer-Part 2. Curr Oncol.
13:99–107. 2006.
|
|
41
|
Sulaiman RS, Basavarajappa HD and Corson
TW: Natural product inhibitors of ocular angiogenesis. Exp Eye Res.
129:161–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh M, Singh P and Shukla Y: New
strategies in cancer chemoprevention by phytochemicals. Front
Biosci (Elite Ed). 4:426–452. 2012. View
Article : Google Scholar
|
|
43
|
Kang X, Jin S and Zhang Q: Antitumor and
antiangiogenic activity of soy phytoestrogen on
7,12-dimethylbenz[alpha] anthracene-induced mammary tumors
following ovariectomy in Sprague-Dawley rats. J Food Sci.
74:H237–H242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Uifălean A, Schneider S, Ionescu C, Lalk M
and Iuga CA: Soy isoflavones and breast cancer cell lines:
molecular mechanisms and future perspectives. Molecules.
21:E132015. View Article : Google Scholar
|
|
45
|
Wietrzyk J, Opolski A, Madej J and
Radzikowski C: Antitumour and antimetastatic effect of genistein
alone or combined with cyclophosphamide in mice transplanted with
various tumours depends on the route of tumour transplantation. In
Vivo. 14:357–362. 2000.PubMed/NCBI
|
|
46
|
Wietrzyk J, Opolski A, Madej J and
Radzikowski C: The antitumor effect of postoperative treatment with
genistein alone or combined with cyclophosphamide in mice bearing
transplantable tumors. Acta Pol Pharm. 57(Suppl): 5–8. 2000.
|
|
47
|
Wietrzyk J, Boratynski J, Grynkiewicz G,
Ryczynski A, Radzikowski C and Opolski A: Antiangiogenic and
antitumour effects in vivo of genistein applied alone or combined
with cyclophosphamide. Anticancer Res. 21:3893–3896. 2001.
|
|
48
|
Park SY, Jeong KJ, Lee J, Yoon DS, Choi
WS, Kim YK, Han JW, Kim YM, Kim BK and Lee HY: Hypoxia enhances
LPA-induced HIF-1alpha and VEGF expression: Their inhibition by
resveratrol. Cancer Lett. 258:63–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu H, Pan C, Zhao S, Wang Z, Zhang H and
Wu W: Resveratrol inhibits tumor necrosis factor-alpha-mediated
matrix metal-loproteinase-9 expression and invasion of human
hepatocellular carcinoma cells. Biomed Pharmacother. 62:366–372.
2008. View Article : Google Scholar
|
|
50
|
Ma Y, Johnson CS and Trump DL: Mechanistic
insights of Vitamin D anticancer effects. Vitam Horm. 100:395–431.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jäpelt RB and Jakobsen J: Vitamin D in
plants: A review of occurrence, analysis, and biosynthesis. Front
Plant Sci. 4:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Haussler MR, Jurutka PW, Mizwicki M and
Norman AW: Vitamin D receptor (VDR)-mediated actions of
1α,25(OH)2vitamin D3: Genomic and non-genomic
mechanisms. Best Pract Res Clin Endocrinol Metab. 25:543–559. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Feldman D, Krishnan AV, Swami S,
Giovannucci E and Feldman BJ: The role of vitamin D in reducing
cancer risk and progression. Nat Rev Cancer. 14:342–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oikawa T, Hirotani K, Ogasawara H,
Katayama T, Nakamura O, Iwaguchi T and Hiragun A: Inhibition of
angiogenesis by vitamin D3 analogues. Eur J Pharmacol. 178:247–250.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bao BY, Yao J and Lee YF: 1alpha,
25-dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate
cancer cell angiogenesis. Carcinogenesis. 27:1883–1893. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ben-Shoshan M, Amir S, Dang DT, Dang LH,
Weisman Y and Mabjeesh NJ: 1alpha,25-dihydroxyvitamin D3
(Calcitriol) inhibits hypoxia-inducible factor-1/vascular
endothelial growth factor pathway in human cancer cells. Mol Cancer
Ther. 6:1433–1439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chung I, Han G, Seshadri M, Gillard BM, Yu
WD, Foster BA, Trump DL and Johnson CS: Role of vitamin D receptor
in the antiproliferative effects of calcitriol in tumor-derived
endothelial cells and tumor angiogenesis in vivo. Cancer Res.
69:967–975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Iseki K, Tatsuta M, Uehara H, Iishi H,
Yano H, Sakai N and Ishiguro S: Inhibition of angiogenesis as a
mechanism for inhibition by 1alpha-hydroxyvitamin D3 and
1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by
azoxymethane in Wistar rats. Int J Cancer. 81:730–733. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nakagawa K, Sasaki Y, Kato S, Kubodera N
and Okano T: 22-Oxa-1alpha,25-dihydroxyvitamin D3
inhibits metastasis and angiogenesis in lung cancer.
Carcinogenesis. 26:1044–1054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wietrzyk J, Filip B, Milczarek M,
Klopotowska D, Maciejewska M, Dabrowska K, Kurzepa A, Dzimira S,
Madej J and Kutner A: The influence of 1,25-dihydroxyvitamin
D3 and 1,24-dihydroxyvitamin D3 on
αvβ3 integrin expression in cancer cell lines. Oncol
Rep. 20:941–952. 2008.PubMed/NCBI
|
|
61
|
Jones G, Strugnell SA and DeLuca HF:
Current understanding of the molecular actions of vitamin D.
Physiol Rev. 78:1193–1231. 1998.PubMed/NCBI
|
|
62
|
Ma Y, Trump DL and Johnson CS: Vitamin D
in combination cancer treatment. J Cancer. 1:101–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Leyssens C, Verlinden L and Verstuyf A:
The future of vitamin D analogs. Front Physiol. 5:1222014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Milczarek M, Psurski M, Kutner A and
Wietrzyk J: Vitamin D analogs enhance the anticancer activity of
5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer.
13:2942013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Milczarek M, Filip-Psurska B, Swiętnicki
W, Kutner A and Wietrzyk J: Vitamin D analogs combined with
5-fluorouracil in human HT-29 colon cancer treatment. Oncol Rep.
32:491–504. 2014.PubMed/NCBI
|
|
66
|
Okamoto R, Delansorne R, Wakimoto N, Doan
NB, Akagi T, Shen M, Ho QH, Said JW and Koeffler HP: Inecalcitol,
an analog of 1α,25(OH)2D3, induces growth
arrest of androgen-dependent prostate cancer cells. Int J Cancer.
130:2464–2473. 2012. View Article : Google Scholar
|
|
67
|
Protiva P, Pendyala S, Nelson C,
Augenlicht LH, Lipkin M and Holt PR: Calcium and
1,25-dihydroxyvitamin D3 modulate genes of immune and inflammatory
pathways in the human colon: A human crossover trial. Am J Clin
Nutr. 103:1224–1231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lappe JM, Travers-Gustafson D, Davies KM,
Recker RR and Heaney RP: Vitamin D and calcium supplementation
reduces cancer risk: Results of a randomized trial. Am J Clin Nutr.
85:1586–1591. 2007.PubMed/NCBI
|
|
69
|
Jacot W, Firmin N, Roca L, Topart D,
Gallet S, Durigova A, Mirr S, Abach L, Pouderoux S, D'Hondt V, et
al: Impact of a tailored oral vitamin D supplementation regimen on
serum 25-hydroxyvitamin D levels in early breast cancer patients: A
randomized phase III study. Ann Oncol. 27:1235–1241. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bjelakovic G, Gluud LL, Nikolova D,
Whitfield K, Krstic G, Wetterslev J and Gluud C: Vitamin D
supplementation for prevention of cancer in adults. Cochrane
Database Syst Rev. (6): CD0074692014.PubMed/NCBI
|
|
71
|
Crew KD: Vitamin D: Are we ready to
supplement for breast cancer prevention and treatment? ISRN
Oncology. 2013:2013.Article ID 483687. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, Phillips HS and Ferrara N: Inhibition of vascular endothelial
growth factor-induced angiogenesis suppresses tumour growth in
vivo. Nature. 362:841–844. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Pract Oncol. 3:24–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jayson GC, Hicklin DJ and Ellis LM:
Antiangiogenic therapy-evolving view based on clinical trial
results. Nat Rev Clin Oncol. 9:297–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yuan F, Chen Y, Dellian M, Safabakhsh N,
Ferrara N and Jain RK: Time-dependent vascular regression and
permeability changes in established human tumor xenografts induced
by an anti-vascular endothelial growth factor/vascular permeability
factor antibody. Proc Natl Acad Sci USA. 93:14765–14770. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Dickson PV, Hamner JB, Sims TL, Fraga CH,
Ng CYC, Rajasekeran S, Hagedorn NL, McCarville MB, Stewart CF and
Davidoff AM: Bevacizumab-induced transient remodeling of the
vasculature in neuroblastoma xenografts results in improved
delivery and efficacy of systemically administered chemotherapy.
Clin Cancer Res. 13:3942–3950. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Myers AL, Williams RF, Ng CY, Hartwich JE
and Davidoff AM: Bevacizumab-induced tumor vessel remodeling in
rhabdomyosarcoma xenografts increases the effectiveness of adjuvant
ionizing radiation. J Pediatr Surg. 45:1080–1085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Inai T, Mancuso M, Hashizume H, Baffert F,
Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G,
Yancopoulos GD, et al: Inhibition of vascular endothelial growth
factor (VEGF) signaling in cancer causes loss of endothelial
fenestrations, regression of tumor vessels, and appearance of
basement membrane ghosts. Am J Pathol. 165:35–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou Q, Guo P and Gallo JM: Impact of
angiogenesis inhibition by sunitinib on tumor distribution of
temozolomide. Clin Cancer Res. 14:1540–1549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhou Q and Gallo JM: Differential effect
of sunitinib on the distribution of temozolomide in an orthotopic
glioma model. Neuro Oncol. 11:301–310. 2009. View Article : Google Scholar :
|
|
83
|
Willett CG, Boucher Y, di Tomaso E, Duda
DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et
al: Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shi S, Chen L and Huang G: Antiangiogenic
therapy improves the antitumor effect of adoptive cell
immunotherapy by normalizing tumor vasculature. Med Oncol.
30:6982013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Huang Y, Yuan J, Righi E, Kamoun WS,
Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR,
Vianello F, et al: Vascular normalizing doses of antiangiogenic
treatment reprogram the immunosuppressive tumor microenvironment
and enhance immunotherapy. Proc Natl Acad Sci USA. 109:17561–17566.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mancuso MR, Davis R, Norberg SM, O'Brien
S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et
al: Rapid vascular regrowth in tumors after reversal of VEGF
inhibition. J Clin Invest. 116:2610–2621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Griffioen AW, Mans LA, de Graaf AMA,
Nowak-Sliwinska P, de Hoog CL, de Jong TAM, Vyth-Dreese FA, van
Beijnum JR, Bex A and Jonasch E: Rapid angiogenesis onset after
discontinuation of sunitinib treatment of renal cell carcinoma
patients. Clin Cancer Res. 18:3961–3971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wolter P, Beuselinck B, Pans S and
Schöffski P: Flare-up: An often unreported phenomenon nevertheless
familiar to oncologists prescribing tyrosine kinase inhibitors.
Acta Oncol. 48:621–624. 2009. View Article : Google Scholar
|
|
89
|
Chen DR, Lin C and Wang YF: Window of
opportunity: A new insight into sequential bevacizumab and
paclitaxel in two cases of metastatic triple-negative breast
cancer. Exp Ther Med. 10:885–888. 2015.PubMed/NCBI
|
|
90
|
Lee CG, Heijn M, di Tomaso E,
Griffon-Etienne G, Ancukiewicz M, Koike C, Park KR, Ferrara N, Jain
RK, Suit HD, et al: Anti-Vascular endothelial growth factor
treatment augments tumor radiation response under normoxic or
hypoxic conditions. Cancer Res. 60:5565–5570. 2000.PubMed/NCBI
|
|
91
|
Zhang L, Takara K, Yamakawa D, Kidoya H
and Takakura N: Apelin as a marker for monitoring the tumor vessel
normalization window during antiangiogenic therapy. Cancer Sci.
107:36–44. 2016. View Article : Google Scholar :
|
|
92
|
McGee MC, Hamner JB, Williams RF, Rosati
SF, Sims TL, Ng CY, Gaber MW, Calabrese C, Wu J, Nathwani AC, et
al: Improved intratumoral oxygenation through vascular
normalization increases glioma sensitivity to ionizing radiation.
Int J Radiat Oncol Biol Phys. 76:1537–1545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Dings RPM, Loren M, Heun H, McNiel E,
Griffioen AW, Mayo KH and Griffin RJ: Scheduling of radiation with
angiogenesis inhibitors anginex and Avastin improves therapeutic
outcome via vessel normalization. Clin Cancer Res. 13:3395–3402.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Vangestel C, Van de Wiele C, Van Damme N,
Staelens S, Pauwels P, Reutelingsperger CPM and Peeters M:
99mTc-(CO)3 His-annexin A5 micro-SPECT
demonstrates increased cell death by irinotecan during the vascular
normalization window caused by bevacizumab. J Nucl Med.
52:1786–1794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hernandez-Agudo E, Mondejar T,
Soto-Montenegro ML, Megias D, Mouron S, Sanchez J, Hidalgo M,
Lopez-Casas PP, Mulero F, Desco M, et al: Monitoring vascular
normalization induced by antiangiogenic treatment with
F-fluoromisonidazole-PET. Mol Oncol. 10:704–718. 2015. View Article : Google Scholar
|
|
96
|
Cao Y: Off-tumor target--beneficial site
for antiangiogenic cancer therapy? Nat Rev Clin Oncol. 7:604–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang Y, Zhang Y, Cao Z, Ji H, Yang X,
Iwamoto H, Wahlberg E, Länne T, Sun B and Cao Y: Anti-VEGF- and
anti-VEGF receptor-induced vascular alteration in mouse healthy
tissues. Proc Natl Acad Sci USA. 110:12018–12023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wong AK, Alfert M, Castrillon DH, Shen Q,
Holash J, Yancopoulos GD and Chin L: Excessive tumor-elaborated
VEGF and its neutralization define a lethal paraneoplastic
syndrome. Proc Natl Acad Sci USA. 98:7481–7486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pelosof LC and Gerber DE: Paraneoplastic
syndromes: An approach to diagnosis and treatment. Mayo Clin Proc.
85:838–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Xue Y, Religa P, Cao R, Hansen AJ,
Lucchini F, Jones B, Wu Y, Zhu Z, Pytowski B, Liang Y, et al:
Anti-VEGF agents confer survival advantages to tumor-bearing mice
by improving cancer-associated systemic syndrome. Proc Natl Acad
Sci USA. 105:18513–18518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Cao Y: Future options of anti-angiogenic
cancer therapy. Chin J Cancer. 35:212016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jain RK: Normalizing tumor
microenvironment to treat cancer: Bench to bedside to biomarkers. J
Clin Oncol. 31:2205–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Loges S, Schmidt T and Carmeliet P:
Mechanisms of resistance to anti-angiogenic therapy and development
of third-generation anti-angiogenic drug candidates. Genes Cancer.
1:12–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
van Beijnum JR, Nowak-Sliwinska P,
Huijbers EJM, Thijssen VL and Griffioen AW: The great escape; the
hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev.
67:441–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Willett CG, Boucher Y, Duda DG, di Tomaso
E, Munn LL, Tong RT, Kozin SV, Petit L, Jain RK, Chung DC, et al:
Surrogate markers for antiangiogenic therapy and dose-limiting
toxicities for bevacizumab with radiation and chemotherapy:
Continued experience of a phase I trial in rectal cancer patients.
J Clin Oncol. 23:8136–8139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kopetz S, Hoff PM, Morris JS, Wolff RA,
Eng C, Glover KY, Adinin R, Overman MJ, Valero V, Wen S, et al:
Phase II trial of infusional fluorouracil, irinotecan, and
bevacizumab for metastatic colorectal cancer: Efficacy and
circulating angiogenic biomarkers associated with therapeutic
resistance. J Clin Oncol. 28:453–459. 2010. View Article : Google Scholar :
|
|
108
|
Huang D, Ding Y, Zhou M, Rini BI, Petillo
D, Qian CN, Kahnoski R, Futreal PA, Furge KA and Teh BT:
Interleukin-8 mediates resistance to antiangiogenic agent sunitinib
in renal cell carcinoma. Cancer Res. 70:1063–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Batchelor TT, Sorensen AG, di Tomaso E,
Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M,
et al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor,
normalizes tumor vasculature and alleviates edema in glioblastoma
patients. Cancer Cell. 11:83–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lindholm EM, Krohn M, Iadevaia S, Kristian
A, Mills GB, Mælandsmo GM and Engebraaten O: Proteomic
characterization of breast cancer xenografts identifies early and
late bevacizumab-induced responses and predicts effective drug
combinations. Clin Cancer Res. 20:404–412. 2014. View Article : Google Scholar
|
|
111
|
Ebos JML, Lee CR, Christensen JG, Mutsaers
AJ and Kerbel RS: Multiple circulating proangiogenic factors
induced by sunitinib malate are tumor-independent and correlate
with antitumor efficacy. Proc Natl Acad Sci USA. 104:17069–17074.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Finke J, Ko J, Rini B, Rayman P, Ireland J
and Cohen P: MDSC as a mechanism of tumor escape from sunitinib
mediated anti-angiogenic therapy. Int Immunopharmacol. 11:856–861.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Shojaei F, Wu X, Malik AK, Zhong C,
Baldwin ME, Schanz S, Fuh G, Gerber HP and Ferrara N: Tumor
refractoriness to anti-VEGF treatment is mediated by
CD11b+Gr1+ myeloid cells. Nat Biotechnol.
25:911–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shojaei F, Wu X, Zhong C, Yu L, Liang XH,
Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al: Bv8
regulates myeloid-cell-dependent tumour angiogenesis. Nature.
450:825–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tartour E, Pere H, Maillere B, Terme M,
Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K,
Karadimou A, et al: Angiogenesis and immunity: A bidirectional link
potentially relevant for the monitoring of antiangiogenic therapy
and the development of novel therapeutic combination with
immunotherapy. Cancer Metastasis Rev. 30:83–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mantovani A, Biswas SK, Galdiero MR, Sica
A and Locati M: Macrophage plasticity and polarization in tissue
repair and remodelling. J Pathol. 229:176–185. 2013. View Article : Google Scholar
|
|
117
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay 0T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Crawford Y, Kasman I, Yu L, Zhong C, Wu X,
Modrusan Z, Kaminker J and Ferrara N: PDGF-C mediates the
angiogenic and tumorigenic properties of fibroblasts associated
with tumors refractory to anti-VEGF treatment. Cancer Cell.
15:21–34. 2009. View Article : Google Scholar
|
|
119
|
Gerhardt H and Betsholtz C:
Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res.
314:15–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Soda Y, Myskiw C, Rommel A and Verma IM:
Mechanisms of neovascularization and resistance to anti-angiogenic
therapies in glioblastoma multiforme. J Mol Med (Berl). 91:439–448.
2013. View Article : Google Scholar
|