Introduction
Human bladder cancer (BCa) is currently one of the
most common cancers worldwide (1).
However, after complex therapies including surgery and
antineoplastic therapy, BCa still frequently recurs and eventually
progresses into muscle-invasive BCa (2). Therefore, new specific molecular
markers and effective therapies are urgently needed.
Our group has collected several human BCa tissues
and normal bladder tissues to conduct a microarray analysis (GEO
accession no. GSE76211) (3,4),
revealing a significantly upregulated gene in BCa tissues, the
lysosomal-associated protein multispanning transmembrane 5
(LAPTM5). LAPMT5 is a lysosomal membrane protein preferentially
expressed in immune cells (5,6) and
hematopoietic cells (7), having a
close interaction with the Nedd4 (8), a member of the E3 ubiquitin ligases
family (8). Nedd4 has been shown
to be specifically upregulated in invasive BCa and be able to
promote the progression of BCa (9). Moreover, some studies demonstrated
that LAPTM5 was highly expressed in malignant B lymphomas and
involved in B cell malignancies (10), involving in negative regulation of
cell surface T and B cell receptor by promoting lysosome
degradation (6). Furthermore,
previous studies suggested that knockdown of LAPTM4B,
another important subtype of the LAPTM family inhibited
proliferation of hepatocellular carcinoma (11), prostate (12) and breast cancer cells (13).
In recent years, epithelial-mesenchymal transition
(EMT) has been suggested to play a key role in the process of
embryonic development, differentiation of tissues and organs,
chronic inflammation and fibrosis, as well as cancer progression
(14). During EMT, cells will
undergo transformation from epithelial phenotype to mesenchymal
phenotype (14) and many
characteristics of cells will change including loss of cell-cell
adhesion and acquisition of aggressive and metastatic ability
(15). Increasing evidence
suggested EMT was involved in cancer invasion, metastasis (16) and the malignancy of tumors
(17), often marked by reduction
of E-cadherin and induction of N-cadherin (18). However, whether LAPTM5 has a
connection with EMT in BCa cells remains largely unknown.
Our transcriptome analysis suggested that
mitogen-activated protein kinase (MAPK) signaling pathway was
linked with bladder cancer by participating in cell cycle
regulation (3,4). In addition, recent studies reported
that LAPTM5 could diminish the activation of MAPK signaling pathway
regulated by tumor necrosis factor (TNF) receptor (19). More importantly, abnormal
regulation of MAPK could contribute to cancer and other human
diseases (20,21), including bladder cancer (3).
The exact role of LAPTM5 in tumorigenesis of human
bladder cancer has not been investigated previously. In the present
study, we first demonstrated that reduction of LAPTM5 had
negative effects on migration, invasion and proliferation of BCa
cells. Furthermore, our results suggested that alteration of MAPK
signaling pathway might participate in regulation of these
processes.
Materials and methods
Ethical statement for human bladder
samples
As described by Cao et al and Wang et
al in 2016 from our group (3,4),
bladder cancer and paracancerous tissue samples (n=13) were
obtained from patients after surgery at Zhongnan Hospital of Wuhan
University, and normal bladder tissue samples (n=3) were from
donors by accidental death. The histology diagnosis was confirmed
pathologically by two pathologists independently. All the tissues
were immediately frozen and stored in liquid nitrogen or fixed in
4% PFA after collection from the operation room. Informed consent
was collected from all subjects. The study using human bladder
tissue samples for RNA isolation and immunohistochemistry staining
analysis was approved by the Ethics Committee at Zhongnan Hospital
of Wuhan University (approval no. 2015029). All methods used for
human bladder tissue samples were performed in accordance with the
approved guidelines and regulations.
Human bladder cancer cell lines
The human BCa cell lines T24 (transitional cell
carcinoma, cat. no. SCSP-536) and 5637 (grade II carcinoma, cat.
no. TCHu 1) were obtained from Chinese Academy of Sciences in
Shanghai. T24 and 5637 cell lines were identified by the China
Centre for Type Culture Collection in Wuhan, China. T24 and 5637
were cultured in RPMI-1640 medium (Gibco, China) containing 10%
fetal bovine serum (FBS) (Gibco, Sydney, Australia) in a humidified
atmosphere with 5% CO2 at 37°C.
RNA expression analyses
Total RNA isolation from bladder
tissues and BCa cells
Total RNA was isolated from BCa cells and bladder
tissues by RNeasy mini kit (cat. no. 74101), combined with
QIAshredder (cat. no. 79654) (both from Qiagen, Hilden, Germany)
using a centrifuge (cat. no. 5424; Eppendorf, Hamburg, Germany),
according to the manufacturer's protocol. In order to remove
genomic DNA, DNase I digestion (cat. no. 79254; Qiagen) was used in
each RNA preparation.
Reverse transcription and quantitative
real-time PCR (qRT-PCR)
For each sample, First-Strand cDNA was synthesized
using 1 µg of total RNA isolated from BCa cells or bladder
tissues by ReverTra Ace qPCR RT kit (Toyobo, Shanghai, China). Each
reaction was conducted with iQ™ SYBR®-Green Supermix
(Bio-Rad, Shanghai, China) using 1 µg of cDNA in a final
volume of 20 µl. All primers were tested for optimal
annealing temperatures and PCR conditions were optimized with
gradient PCRs on an iCycler (cat. no. CFX Connect; Bio-Rad,
Hercules, CA, USA). Primer sequences and annealing temperatures are
summarized in Table I. The cycle
number of threshold (CT) value of LAPTM5 was normalized to
the GAPDH value, and calculated as (22): relative gene expression =
2−ΔΔct, Δct=cttarget gene −
ctGAPDH, for BCa cells ΔΔct=ΔctsiRNA-treated
− ΔctsiRNA-untreated, for bladder tissues
ΔΔct=ΔctBCa tissues − Δctparacancerous
tissues (ct, threshold cycle).
 | Table IThe primers for qRT-PCR. |
Table I
The primers for qRT-PCR.
| Gene | Symbol | Forward primer
(5′–3′) | Reverse primer
(5′–3′) | Annealing
temperature (°C) | Length (bp) |
|---|
|
Lysosomal-associated multispanning
membrane protein 5 | LAPTM5 |
5′-CCTGAGCCTACTGATCGGC-3′ |
5′-CAGGCACAGGAGATAGTCCA-3′ | 60 | 91 |
|
Glyceraldehyde-3-phosphate
dehydrogenase | GAPDH |
5′-TGCACCACCAACTGCTTAG-3′ |
5′-GATGCAGGGATGATGTTC-3′ | 60 | 176 |
Cell culture analyses
Knockdown of LAPTM5 in the BCa
cells
LAPTM5-target specific small interfering RNA
(siRNA) was synthesized by View Solid (Beijing, China). The
sense sequence of LAPTM5-target-specific-siRNA
(si-LAPTM5) is as follows: siRNA1,
5′-CCACCUAUCUCAACUUCAATT-3′; siRNA2, 5′-CCAUCUACCAUGUGAUCAUTT-3′;
siRNA3, 5′-GGUGCUACAGAUUGAUCAATT-3′, and the sense sequence of
si-control is 5′-UUCUCCGAACGUGUCAGGUTT-3′. When cells were
grown to 60%, T24 and 5637 cells were transfected with
si-LAPTM5 and si-control using LipoJet™ (SignaGen,
China). After 48 h transfection, alterations of LAPTM5 mRNA
and protein were evaluated by qRT-PCR, immunofluorescence staining
and western blot analyses.
Transwell chamber migration and
invasion assay
The Transwell migration and invasion assay was
conducted in 24-well plate Transwell chamber system (Corning, Inc.,
NY, USA). For the migration, BCa cells in serum-free medium at a
density of 4–6×104 cells were seeded in the upper
chamber (Corning, Inc.), while the lower chamber was filled with
10% FBS medium. After incubation for 24 h at 37°C, the cells were
removed using cotton swabs in the upper chamber. Then lower side of
the chamber was fixed with 4% PFA and stained with crystal violet,
migrated cell number was counted by phase contrast microscope and
statistically analyzed. To perform invasion assay, Transwell
chambers were percolated with ECM Matrix gel solution
(Sigma-Aldrich, St. Louis, MO, USA). Then solidified at 37°C,
~1×105 cells were seeded as previously described. The
chamber was incubated at 37°C for 48 h. The subsequent staining and
observation procedures were identical to those of the migration
assays.
Wound healing assay
After siRNA-transfection for 24 h, BCa cells were
scratched, and washed with PBS. Adding 0.5% FBS medium to allow
cells to move into the gap, they were photographed at 0 and 12 h in
several pre-marked spots. Migration rate was statistically analyzed
using t-test.
MTT assay
After transfection for 48 h, 3,000–5,000 BCa
cells/200 µl medium were seeded in 96-well plates to grow
for another four days. Then 20 µl MTT was added in each well
and incubated at 37°C for 4 h. After removing the medium, formazan
precipitate was dissolved in DMSO, and absorbance at 490 nm was
measured by a microplate reader (cat. no. SpectraMax M2; Molecular
Devices, Sunnyvale, CA, USA).
Clonogenic survival assay
BCa 1,000–1,500 cells/well were seeded in new 6-well
plates and grew into colonies for ~15 days. Colonies were emerged
and fixed by 4% PFA for 30 min, stained with 0.1% crystal violet
for observation and photographing.
Flow cytometry analysis for cell cycle
arrest and apoptosis
After harvesting and washing by PBS, BCa cells were
fixed with 70% ice cold ethanol (−20°C, overnight), washed again
and incubated with RNaseA (20 µg/ml in PBS), stained by
propidium iodide (50 µg/ml) for 30 min (Sigma-Aldrich) at
37°C in the dark. Cell cycle were assessed on a flow cytometry
(cat. no. FC500; Beckman Coulter, USA). Cell apoptosis analysis was
analyzed by the flow cytometry analysis using Annexin
V-fluorescence isothiocyanate (FITC)/PI apoptosis detection kit (BD
Biosciences, San Jose, CA, USA), according to the manufacturer's
instructions.
Protein analyses
Western blot analyses
Total protein of BCa cells was extracted using RIPA
buffer containing protease inhibitor and phosphatase inhibitor
(Sigma-Aldrich). Bradford protein assay (Bio-Rad, Munich, Germany)
was used to measure protein concentration and Bovine serum albumin
(BSA) as a standard. Protein samples were separated using 10–12.5%
SDS-PAGE and transferred to PVDF membrane (Millipore, Billerica,
MA, USA). PVDF membranes were blocked in 5% non-fat milk, then
incubated with primary antibodies (Table II) and secondary antibodies
(Table III). Bands were
visualized and blots were exposed to Kodak Biomax MR film after
using an enhanced chemiluminescence (ECL) kit (Bio-Rad).
 | Table IIThe primary antibodies. |
Table II
The primary antibodies.
| Antigens | Species antibodies
raised in | Dilution (IF) | Dilution (WB) | Supplier |
|---|
| E-Cadherin,
human | Rabbit,
monoclonal | 1:200 | 1:500 | Cell Signaling
Technology, USA, cat. no. 3195 |
| N-Cadhern,
human | Rabbit,
monoclonal | 1:200 | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 13116 |
| β-Catenin,
human | Rabbit,
monoclonal | – | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 8480 |
| Ki-67, human | Rabbit,
monoclonal | 1:200 | – | Novus Biologicals,
USA, cat. no. NBP2-19012 |
| Slug, human | Rabbit,
monoclonal | – | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 9585 |
| Claudin-1,
human | Rabbit,
monoclonal | – | 1:10,000 | Cell Signaling
Technology, USA, cat. no. 13255 |
| Glyceraldehyde
3-phosphate dehydrogenase (GAPDH), human | Mouse,
monoclonal | – | 1:2,000 | Santa Cruz
Biotechnology Inc., USA, cat. no. sc-365062 |
| Cyclin D1,
human | Rabbit,
monoclonal | – | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 2978 |
| CDK2, human | Rabbit,
monoclonal | – | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 2546 |
| CDK4, human | Rabbit,
monoclonal | – | 1:1,000 | Abcam, UK, cat. no.
ab108357 |
| Cyclin A1/A2 | Rabbit,
monoclonal | – | 1:1,000 | Abcam, UK, cat. no.
ab185619 |
| p-GSK3β, human | Rabbit,
monoclonal | – | 1:10,000 | Cell Signaling
Technology, USA, cat. no. 5558S |
| GSK3β, human | Rabbit,
monoclonal | – | 1:10,000 | Cell Signaling
Technology, USA, cat. no. 12456S |
| LAPTM5, human | Rabbit,
monoclonal | 1:50 | 1:1,000 | Abcam, UK, cat. no.
ab108014 |
| Phospho-p44/42 MAPK
(Erk1/2) (Thr202/Tyr204), human | Rabbit,
monoclonal | – | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 4370 |
| p44/42 MAPK
(Erk1/2), rat | Rabbit,
monoclonal | – | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 4695 |
| Phospho-p38
(Thr180/Tyr182), human | Rabbit,
monoclonal | – | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 4511 |
| p38 MAPK,
human | Rabbit,
monoclonal | – | 1:1,000 | Cell Signaling
Technology, USA, cat. no. 8690 |
 | Table IIIThe secondary antibodies and
counterstaining of nuclei. |
Table III
The secondary antibodies and
counterstaining of nuclei.
| Secondary detection
system used | Host | Method | Dilution | Supplier |
|---|
| Anti-mouse-IgG
(H+L)-HRP | Goat | WB | 1:10,000 | Sungene Biotech,
China, cat. no. LK2003 |
| Anti-rabbit-IgG
(H+L)-HRP | Goat | WB | 1:5,000 | Sungene Biotech,
China, cat. no. LK2001 |
| Anti-rabbit IgG
(H+L), F(ab')2 fragment (Alexa Fluor® 488
Conjugate) | Goat | IF | 1:50 | Cell Signaling
Technology, USA, cat. no. 4412 |
| Hoechst 33342 (1
mg/ml) nucleic acid staining (DAPI) | – | IF | 1:750 | Molecular
Probes/Invitrogen, Carlsbad, CA, USA, cat. no. A11007 |
Immunofluorescence staining for BCa
cells
Coverslips were washed 3 times by cold PBS and fixed
with 4% PFA for 30 min. Then the cells were treated by 0.1% Triton
X-100 and blocked in goat serum for 30 min, incubating with primary
antibody (Table II) at room
temperature for 2 h, washing with PBS and incubating with
Cy3-labeled or FITC-labeled secondary antibody (Table III) for 1 h. Nuclei were labeled
with DAPI (2 µg/ml). Immunofluorescence staining was
analyzed using a fluorescence microscope (cat. no. IX73; Olympus,
Japan).
Immunohistochemistry (IHC) staining
for BCa tissue samples
Briefly, tissues were incubated with citrate buffer
(0.01 M, pH 6.0) for 10 min after hydrated and embedded. After
washing with PBS (pH 7.4) three times, tissue sections were covered
with 3% H2O2 for 15 min at room temperature
and incubated with primary antibody overnight at 4°C. After a
washing procedure, biotinylated secondary antibody was incubated
with the section for 30 min. Then DAB substrate chromogen solution
was added before tissue sections were incubated with HRP substrate
solution for 30 min. Slides were counterstained for 1 min with
hematoxylin, then dehydrated and analyzed by microscopy.
Statistical analyses
All analyses were performed three times and
represent data from three individual experiments. Two-tailed
Student's t-test was used for significance of differences between
subgroups. Statistical analyses were performed with SPSS 16.0.
Statistical significance was set at probability values of
p<0.05.
Results
Upregulation of LAPTM5 in BCa tissues
compared with paracancerous tissues and normal bladder tissues
Oncomine database (www.oncomine.org) showed that LAPTM5 was
significantly upregulated at the transcriptional level in BCa
tissues compared with normal bladder tissues (Fig. 1A), which is consistent with our
microarray data. Furthermore, LAPTM5 also exhibited a
significant upregulation in the BCa tissues compared with the
paired paracancerous tissues (n=13) (Fig. 1B). In addition,
immunohistochemistry staining revealed strong increase of LAPTM5
protein in the BCa tissues, compared with paracancerous bladder
tissues (Fig. 1C).
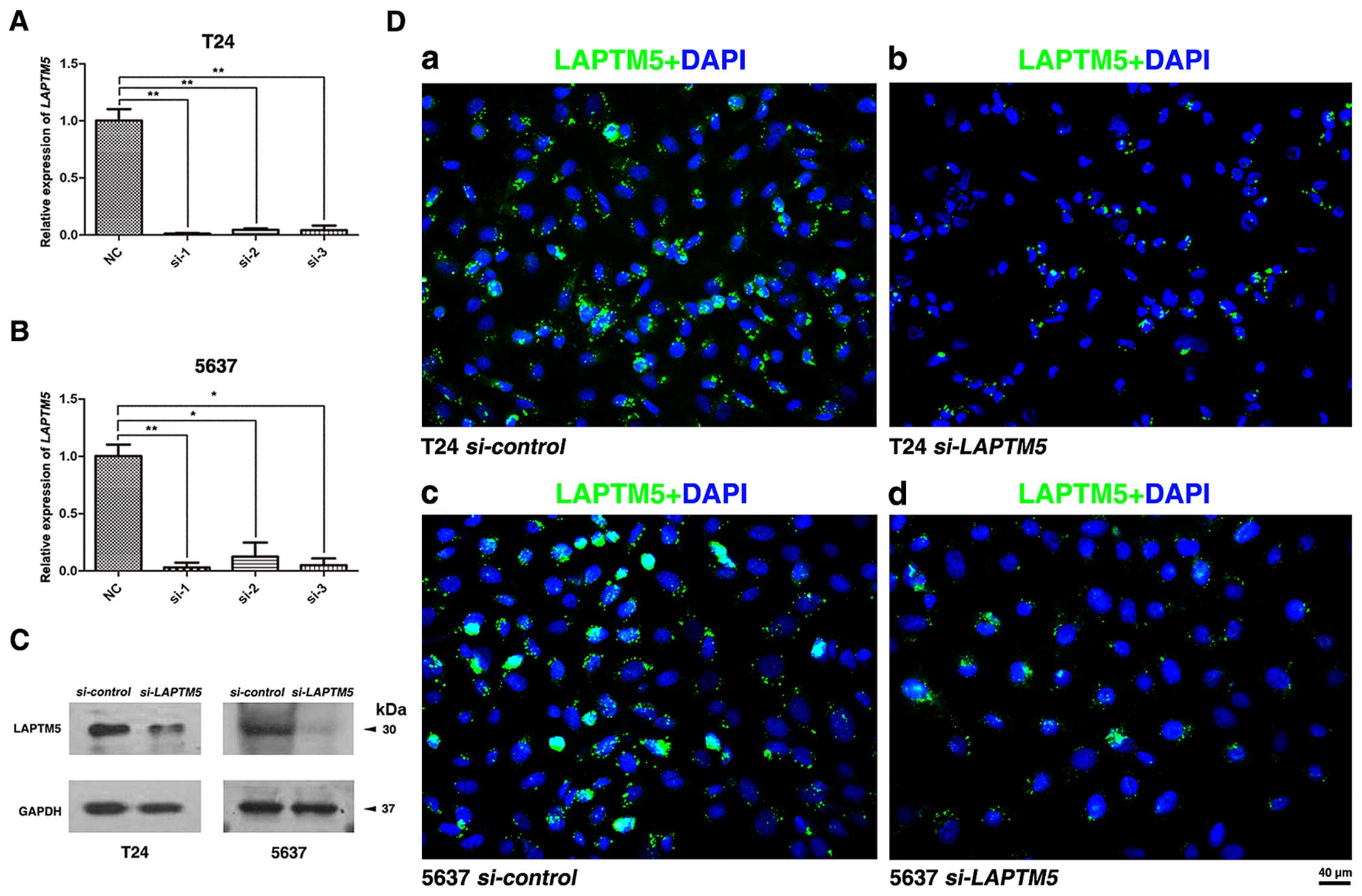
Knockdown of LAPTM5 significantly
inhibits the transcription and protein levels of LAPTM5
To construct a cell model of LAPTM5
deficiency, we used three distinct
LAPTM5-target-specific-siRNA to transfect T24 and 5637.
After 48 h, the knockdown efficiency was validated by qRT-PCR
(Fig. 2A and B) and western blot
analysis (Fig. 2C). Moreover,
immunofluorescence staining also showed the abundance of LAPTM5
protein was strongly downregulated (Fig. 2D). The result showed that LAPTM5
expression at both transcriptional and translational levels was
significantly reduced with LAPTM5-target-specific-siRNA in
the BCa cells.
Downregulation of LAPTM5 restrains
proliferation of BCa cells
To detect the effect of LAPTM5 knockdown on
cell viability in BCa cells, T24 and 5637 were treated by
LAPTM5-siRNA and si-control for 48 h and determined
by MTT assay, suggesting that knockdown of LAPTM5 restrained
BCa cells proliferation drastically (Fig. 3A and B). Clonogenic survival assay
revealed a significant reduction for the colony forming efficiency
in the LAPTM5-siRNA-treated BCa cells T24 and 5637, compared
with the si-control group (Fig.
3C and D). Moreover, immunofluorescence staining showed that
the LAPTM5-siRNA group exhibited considerably less Ki-67
positive cells than the si-control group (Fig. 3E).
Reduced LAPTM5 triggers cell cycle arrest
at G0/G1 phase, but shows no significant changes on apoptosis in
the BCa cells
Flow cytometry analysis was conducted to evaluate
the effect of LAPTM5 knockdown on cell cycle in T24 and 5637
cells (Fig. 4A), indicating a
significant cell cycle arrest at G0/G1 phase (Fig. 4B). Western blot analysis revealed
that proteins involved in G0/G1 phase regulation were strongly
reduced (cyclin A1/2, cyclin D1 and CDK2/4) after
LAPMT5-siRNA treatment (Fig.
4C). However, knockdown of LAPTM5 could not affect
apoptosis in BCa cells significantly (Fig. 4D and E), as revealed by flow
cytometry analysis.
Downregulation of LAPTM5 inhibits
migration and invasion of BCa cells
Transwell migration and invasion assay suggested
that knockdown of LAPTM5 in BCa cells could reduce cell
migration and invasion (Fig. 5A),
which was confirmed by statistical analysis in Fig. 5B. Moreover, wound healing assay
revealed that reduction of LAPTM5 in BCa cells could
suppress the number of migrated cells (Fig. 5A). The gap closure (%) was
statistically analyzed (Fig.
5B).
Proteins involved in MAPK signaling
pathway and EMT regulation are altered after LAPTM5 knockdown
Key members of the MAPK family including ERK1/2 and
p38 were affected in the si-LAPTM5-treated T24 and 5637
cells (Fig. 5C). LAPTM5
knockdown strongly suppressed the expression of phosphorylated
ERK1/2 (p-ERK1/2) and phosphorylated p38 (p-p38) in the BCa cells.
In addition, proteins involved in the EMT process, including
β-catenin, N-cadherin, E-cadherin, claudin-1 and Slug, were
analyzed by western blot analysis (Fig. 5D), showing that the epithelial
marker E-cadherin was upregulated and mesenchymal marker
N-cadherin, β-catenin, Slug, claudin-1 were downregulated after
LAPTM5 knockdown.
Discussion
Our group has established a transcriptome analysis
using bladder cancer tissues versus normal bladder tissues
(3,4). Among thousands of strongly altered
genes involved in development of human bladder cancer (BCa), we
selected the upregulated gene LAPTM5, which is in accordance
with the result from the Oncomine database. LAPTM5 has been
reported to be correlated with NEDD4 (8) which is upregulated in invasive BCa
and could promote progression of BCa (9). Interestingly, our results showed that
the expression of LAPTM5 was strongly enhanced in BCa
tissues at both transcriptional and protein levels compared with
paracancerous tissues. The LAPTM5-siRNA was used for
LAPTM5 knockdown and the efficiency was confirmed by
qRT-PCR, western blot analysis and immunofluorescence staining
analyses. We observed that knockdown of LAPTM5 could reverse
the EMT status, suggesting that deficiency of LAPTM5 could
alleviate malignancy of BCa. Since several studies have reported
that EMT was involved in cancer cell migration and invasion
(16), we also observed that
knockdown of LAPTM5 suppressed migration and invasion of BCa
cells. E-cadherin was found to be associated with epithelial cell
migration and could play a key role in EMT progression (23), often marked by decreased E-cadherin
and increased N-cadherin (18).
After re-localization from membrane to cytoplasm and nucleus,
β-catenin became a transcriptional coactivator to promote EMT
(24). Slug is a zinc-finger
transcription factor and has a functional role in triggering EMT
(25), cancer progression
(26), invasion and migration
(27). Consistently, our results
showed that LAPTM5 knockdown resulted in an increase of
E-cadherin and decrease of N-cadherin, β-catenin and Slug in BCa
cells (Fig. 5).
Another important phenomenon observed was that
reduced LAPTM5 exhibited a negative effect on cell
proliferation. Since cell proliferation was influenced by cell
cycle and apoptosis, we found that BCa cells lacking LAPTM5
significantly induced G0/G1 cell cycle arrest, but apoptotic rate
of BCa cells showed no significant alteration. Proteins involved in
cell cycle regulation, such as cyclin A1/2, cyclin D1 and CDK2/4,
were inhibited by LAPTM5 knockdown.
Our microarray analysis also suggested that MAPK
signaling pathway was linked with bladder cancer through regulating
the cell cycle (3). Recent study
reported that the LAPTM5 protein is a positive regulator of MAPK
signaling pathway in macrophages (19). Similarly, our study also showed
that p-ERK1/2 and p-p38 play important roles in regulating cell
proliferation, survival and apoptosis (28) via connecting extracellular stimuli
from cell membrane to nucleus were substantially downregulated
after LAPTM5 knockdown. It is known that MAPK family members
participate in regulating cell cycle in various manner (29). ERK mainly promotes progression of
G0/G1 to S phase and p38 primarily regulates G2 checkpoint
(30,31). Meloche and Pouysségur reported that
activation of ERK1/2 could regulate the progression of G1 to S
phase by targeting cyclin D1 (29). Our results also revealed that
LAPMT5 knockdown induced cell cycle arrest, which was
confirmed by downregulation of related protein (cyclin D1 and
CDK2/4) and upregulation of their upstream proteins
p-GSK-3β/t-GSK3β (Fig. 4). The
above results suggested that cell cycle arrest induced by
LAPTM5 knockdown may have a connection with MAPK signaling
pathway in bladder cancer.
In conclusion, our results are the first to reveal
that downregulation of LAPTM5 inhibited migration and
invasion by suppressing EMT markers and reduced proliferation in
BCa cells. Moreover, this process may be partially connected with
the alteration of MAPK signal pathway.
Acknowledgments
The excellent technical assistance of Yuan Zhu,
Shanshan Zhang and Danni Shan is gratefully acknowledged. This
study was supported in part by grants from the Natural Sciences
Foundation of Hubei Province (grant no. 2014CFA006), the Medical
Science and Technology Project of Zhejiang Province (grant no.
2016KYB082) and the Fundamental Research Funds for the Central
Universities (grant no. 2042015kf0153). The funders had no role in
study design, data collection and analysis, decision to publish, or
preparation of the manuscript.
References
|
1
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S, et al: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar
|
|
2
|
Rye PD, Nustad K and Stigbrand T: Tumor
marker workshops. Tumour Biol. 24:165–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao R, Meng Z, Liu T, Wang G, Qian G, Cao
T, Guan X, Dan H, Xiao Y and Wang X: Decreased TRPM7 inhibits
activities and induces apoptosis of bladder cancer cells via ERK1/2
pathway. Oncotarget. Sep 20–2016.Epub ahead of print.
|
|
4
|
Wang G, Cao R, Wang Y, Qian G, Dan HC,
Jiang W, Ju L, Wu M, Xiao Y and Wang X: Simvastatin induces cell
cycle arrest and inhibits proliferation of bladder cancer cells via
PPARγ signalling pathway. Sci Rep. 6:357832016. View Article : Google Scholar
|
|
5
|
Ouchida R, Yamasaki S, Hikida M, Masuda K,
Kawamura K, Wada A, Mochizuki S, Tagawa M, Sakamoto A, Hatano M, et
al: A lysosomal protein negatively regulates surface T cell antigen
receptor expression by promoting CD3zeta-chain degradation.
Immunity. 29:33–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seimiya M, O-Wang J, Bahar R, Kawamura K,
Wang Y, Saisho H and Tagawa M: Stage-specific expression of
Clast6/E3/LAPTM5 during B cell differentiation: Elevated expression
in human B lymphomas. Int J Oncol. 22:301–304. 2003.PubMed/NCBI
|
|
7
|
Adra CN, Zhu S, Ko JL, Guillemot JC,
Cuervo AM, Kobayashi H, Horiuchi T, Lelias JM, Rowley JD and Lim B:
LAPTM5: A novel lysosomal-associated multispanning membrane protein
preferentially expressed in hematopoietic cells. Genomics.
35:328–337. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pak Y, Glowacka WK, Bruce MC, Pham N and
Rotin D: Transport of LAPTM5 to lysosomes requires association with
the ubiquitin ligase Nedd4, but not LAPTM5 ubiquitination. J Cell
Biol. 175:631–645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ingham RJ, Gish G and Pawson T: The Nedd4
family of E3 ubiquitin ligases: Functional diversity within a
common modular architecture. Oncogene. 23:1972–1984. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Trotman LC, Koppie T, Alimonti A,
Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo
C, et al: NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN.
Cell. 128:129–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shao GZ, Zhou RL, Zhang QY, Zhang Y, Liu
JJ, Rui JA, Wei X and Ye DX: Molecular cloning and characterization
of LAPTM4B, a novel gene upregulated in hepatocellular carcinoma.
Oncogene. 22:5060–5069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Wei Q, Liu R, Qi S, Liang P, Qi
C, Wang A, Sheng B, Li L and Xu Y: Overexpression of LAPTM4B-35: A
novel marker of poor prognosis of prostate cancer. PLoS One.
9:e910692014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao M, Jia S, Wang H, Wang J, Huang Y and
Li Z: Overexpression of LAPTM4B: An independent prognostic marker
in breast cancer. J Cancer Res Clin Oncol. 139:661–667. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nieto MA: Epithelial plasticity: A common
theme in embryonic and cancer cells. Science. 342:12348502013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei SC, Fattet L and Yang J: The forces
behind EMT and tumor metastasis. Cell Cycle. 14:2387–2388. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Glowacka WK, Alberts P, Ouchida R, Wang JY
and Rotin D: LAPTM5 protein is a positive regulator of
proinflammatory signaling pathways in macrophages. J Biol Chem.
287:27691–27702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JS, Kwon JK, Kim HR, Kim HJ, Kim BS
and Jung JY: Farnesol induces apoptosis of DU145 prostate cancer
cells through the PI3K/Akt and MAPK pathways. Int J Mol Med.
33:1169–1176. 2014.PubMed/NCBI
|
|
21
|
Gupta J, Igea A, Papaioannou M,
Lopez-Casas PP, Llonch E, Hidalgo M, Gorgoulis VG and Nebreda AR:
Pharmacological inhibition of p38 MAPK reduces tumor growth in
patient-derived xenografts from colon tumors. Oncotarget.
6:8539–8551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Wheelock MJ and Johnson KR: Cadherins as
modulators of cellular phenotype. Annu Rev Cell Dev Biol.
19:207–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harris TJ and Peifer M: Decisions,
decisions: Beta-catenin chooses between adhesion and transcription.
Trends Cell Biol. 15:234–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Wu Y, Abbatiello TC, Wu WL, Kim JR,
Sarkissyan M, Sarkissyan S, Chung SS, Elshimali Y and Vadgama JV:
Slug contributes to cancer progression by direct regulation of ERα
signaling pathway. Int J Oncol. 46:1461–1472. 2015.PubMed/NCBI
|
|
27
|
Sun Y, Song GD, Sun N, Chen JQ and Yang
SS: Slug overexpression induces stemness and promotes
hepatocellular carcinoma cell invasion and metastasis. Oncol Lett.
7:1936–1940. 2014.PubMed/NCBI
|
|
28
|
Xiao Y, Karnati S, Qian G, Nenicu A, Fan
W, Tchatalbachev S, Höland A, Hossain H, Guillou F, Lüers GH, et
al: Cre-mediated stress affects sirtuin expression levels,
peroxisome biogenesis and metabolism, antioxidant and
proinflammatory signaling pathways. PLoS One. 7:e410972012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meloche S and Pouysségur J: The ERK1/2
mitogen-activated protein kinase pathway as a master regulator of
the G1- to S-phase transition. Oncogene. 26:3227–3239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacCorkle RA and Tan TH: Mitogen-activated
protein kinases in cell-cycle control. Cell Biochem Biophys.
43:451–461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai SC, Huang WW, Huang WC, Lu CC, Chiang
JH, Peng SF, Chung JG, Lin YH, Hsu YM, Amagaya S, et al:
ERK-modulated intrinsic signaling and G(2)/M phase arrest
contribute to the induction of apoptotic death by allyl
isothiocyanate in MDA-MB-468 human breast adenocarcinoma cells. Int
J Oncol. 41:2065–2072. 2012.PubMed/NCBI
|