Introduction
Hepatocellular carcinoma (HCC) is the most common
form of liver cancer characterized by cell cycle dysregulation,
aberrant angiogenesis, and evasion of apoptosis (1). Liver cancer is considered to be one
the most fatal cancers and is the second leading cause of cancer
death worldwide. According to WHO, Statistics of Korea in liver
cancer death was 22.8% out of 100,000 populations (2nd highest) in
2014, and 745,000 deaths were reportedly in 2012. Administration of
chemotherapeutic drugs like cisplatin, 5-fluorouracil, and
paclitaxel have been used to cure hepatic cancer, but these
preventive measures have raised question due to multidrug
resistance proteins and the decrease of apoptotic proteins
(2). Additionally, these
antineoplastic medications can result in side effects such as
nausea, vomiting, loss of appetite, diarrhea, and hair loss.
Recently there has been a growing interest in medicinal plants as
alternative therapeutic drugs because of their good pharmacological
properties and low cellular toxicity.
Flavonoids are natural polyphenolic compounds which
are known for their biological activities. Numerous laboratory
investigations and human clinical trials revealed cancer
chemoprevention and chemotherapy effects of flavonoids by
carcinogen inactivation, anti-proliferation, cell cycle arrest,
induction of apoptosis and inhibition of angiogenesis (3). Citrus peel has been used as a
traditional medicine from ancient time in several Asian countries.
Citrus peel is rich in vitamin C, and phytochemicals such as
flavonoids, which are quite a remarkable group of phytonutrients
and best known for their biological activities including
anti-oxidant, anti-inflammation, antibacterial, and anticancer
effect (4). Citrus species is now
explored as model of natural products carrying valuable
phytochemicals that is very promising to be developed in cancer
therapy. A large number of research has shown the potential
characteristics of citrus fruits as both chemopreventive and
co-chemotherapeutic agent (5). Our
earlier reported studies have described the mechanism of anticancer
effects by Citrus platymamma Hort.et Tanaka in human gastric
cancer AGS cells (6). C.
platymamma Hort.et Tanaka called as 'Byungkyul' is native to
Jeju Island in South Korea. There are two genetic reports of C.
platymamma which were provided by Japan (7,8).
However, the molecular mechanisms underlying the antitumor effect
of C. platymamma on HCC have not been explored yet.
The process of programmed cell death type I
(apoptosis), is generally characterized by distinct morphological
characteristics and energy-dependent biochemical mechanisms. The
ability to modulate the life or death of a cell is recognized for
its immense therapeutic potential. Various research continue to
focus on the elucidation and analysis of cell cycle machinery and
signaling pathways that control cell cycle arrest and apoptosis.
The sequence of events which defines the extrinsic phase of
apoptosis, are best characterized with the FasL/FasR and
TNF-α/TNFR1 models. The intrinsic signaling pathways that initiate
apoptosis involve a diverse array of non-receptor-mediated stimuli
that produce intracellular signals that act directly on targets
within the cell and are mitochondrial-initiated events (9), which is attributed to caspase cascade
activation. The biochemical pathways that restrain cell cycle
transition and/or induce cell death after stress known to be cell
cycle checkpoints, maintain the fidelity of DNA replication,
repair, and division and may also result in activation of pathways
leading to programmed cell death if cellular damage cannot be
properly repaired (10). Key
points that induce apoptotic cell death in cancer cells by herbal
extract rich in polyphenols and flavonoids lead by cell cytotoxic
effect (11), anti-proliferation,
growth inhibitory effect (12),
DNA fragmentation, cell cycle arrest, endoplasmic reticulum stress
(13), following de-activation of
major cell regulatory pathways (14,15)
and suppression of cell migration and matrix-metalloproteinase
(MMPs) activity (16) as MMPs are
the key players in the events that underlie tumor dissemination
(17) in cancer cells.
Hence in this study treatment of flavonoids from
Byungkyul (BFs) induced cell death with the involvement of
mitochondria-dependent apoptosis, G2/M cell cycle arrest, and
inhibition of cell migration in Hep3B cells. This finding suggests
that Korean Byungkyul - C. platymamma Hort.et Tanaka might
represent the possibility of developing a new bio-therapeutic agent
for HCC treatment.
Materials and methods
Sample preparation and high-performance
liquid chromatography-tandem mass spectrometry (HPLC-MS/MS)
analysis
C. platymamma Hort.et Tanaka fruits was
obtained from the Citrus Genetic Resources Bank, Jeju National
University, Korea. The flavonoids were isolated, and
high-performance liquid chromatography (HPLC) was performed at the
Department of Chemistry, Gyeongsang National University by
Professor Sung Chul Shin as described previously (18). The BFs powdered samples were stored
at −70°C until further use.
Antibodies and reagents
Antibodies for caspase-3 and -9, cleaved caspase-3
and -9, CDC25C, poly-ADP ribose polymerase (PARP), cleaved-PARP,
JNK, p-JNK, p38, p-p38, ERK1/2 and p-ERK1/2, Fas, FasL, PI3K, and
p-PI3K were purchased from Cell Signaling Technology (Beverly, MA,
USA), and Bax, cyclin B1, CDK1, and β-actin were purchased from
Millipore (Temecula, CA, USA), and Akt, p-Akt, MMP-2, and -9 were
purchased form Bioworld Technology Inc. (Louis Park, MN, USA), and
Bcl-xL was purchased from StressGen Biotechnologies Corp.
(Victoria, BC, Canada). All chemicals used in this experiment were
of the purest grade available.
Cell culture and BFs treatment
Dulbecco's modified Eagle's medium (DMEM), RPMI-1640
medium, fetal bovine serum (FBS), and antibiotics
(streptomycin/penicillin) were purchased from Gibco (Grand Island,
NY, USA). Hep3B and HepG2 cells were cultured in DMEM supplemented
with 10% fetal bovine serum, and 1% penicillin/streptomycin at 37°C
in a humidified atmosphere of 5% CO2. Cells grown to 70
or 80% confluence were untreated (control) or treated with BFs for
24 h in complete cell culture media.
Cell viability assay
Cell viability was measured using MTT assay. Cells
were seeded in 48-well plates and incubated overnight, followed by
treatment with various concentrations of the BFs for 24 h. MTT
solution (5 mg/ml) was added to the cells and incubated for 3 h at
37°C. The formazan precipitate was dissolved in 200 µl of
dimethyl sulfoxide (DMSO) for 30 min and absorbance of converted
dye was measured at a wavelength of 540 nm using a micro-plate
reader. Cell viability was expressed as a percentage of
proliferation versus control.
Analysis of cell cycle distribution and
cell apoptosis
Flow cytometry was used to analyze cell cycle
distribution and cell death. Hep3B cells were treated without or
with 25, 50 and 100 µg/ml of BFs for 24 h at 37°C and cells
were collected, washed with cold PBS (phosphate-buffered saline),
and then centrifuged. For cell cycle assay, harvested cells were
fixed with 70% ethanol for 1 h at 4°C, and fixed cells were washed
in PBS. 1 U/ml of RNase A (DNase-free) and 5 µg/ml of
propidium iodide (PI; Sigma-Aldrich) were then added to the cells
and were incubated for 30 min at 4°C in the dark. Cell cycle
distribution was then analyzed using a FACsCalibur flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). For cell death analysis,
FITC-Annexin V apoptosis detection kit (BD Pharmingen, San Diego,
CA, USA) was used. The harvested cell pellet was suspended in 100
µl of Annexin V binding buffer and incubated with 5
µl of an Annexin V-FITC solution and 5 µl of PI
solution at room temperature for 15 min in the dark. After adding
400 µl of binding buffer the samples were analyzed on flow
cytometer.
DNA fragmentation assay
Hep3B cells were treated with indicated
concentration of BFs (0, 25, 50 and 100 µg/ml) for 24 h and
DNA was isolated as described previously (19). Total DNA solutions were then
subjected to 1.5% agarose gel electrophoresis and DNA bands were
visualized by UV light and documented by photography.
DAPI fluorescent staining
For nuclear morphological analysis, Hep3B cells were
incubated with various concentrations of BFs (0, 25, 50 and 100
µg/ml) at 37°C for 24 h. Hep3B cells were fixed with 3.7%
paraformaldehyde for 15 min followed by three washes (10 min each)
and permeabilized with 1% Triton X-100, later stained with Hoechst
33342 fluorescent dye (20 µg/ml) for 15 min. Nuclear
morphology was observed through Leica DM 6000 B fluorescence
microscope.
TUNEL assay
DNA damage was measured by DNA fragmentation
detection kit (QIA33; Calbiochem, San Diego, CA, USA), according to
the manufacturer's instructions. The cells were fixed with 4%
paraformaldehyde and incubated with TdT enzyme solution for 90 min
at 37°C. After PBS washing, the cells were mounted on DAPI and
fluorescent images were observed under a Leica DM 6000 B
fluorescence microscope.
Wound healing assay
Migration of Hep3B cells was measured by a wound
healing assay. Hep3B cells were cultured on 12-well plates
(8×105 cells/well) overnight. After confluent growth,
the cells were scratched horizontally with a micropipette tip to
obtain a constant straight space devoid of cells. Suspended cells
and media were removed and phenol-free medium containing various
concentrations of BFs (0, 25, 50 and 100 µg/ml) was added
and incubated at 37°C for 24 h. Three randomly selected spaces
along the scratched line were photographed using an inverted
microscope.
Western blot analysis
Total cell lysates were prepared using with lysis
buffer (RIPA) containing protease inhibitor cocktail and EDTA. The
50 µg of lysate protein were separated by 12% polyacrylamide
gel following electrophoretical transfer via polyvinylidene
membrane. After blocking in Tris-buffered saline-Tween containing
5% skimmed milk powder for 30 min, each membrane was incubated
overnight at 4°C with primary antibodies against each target
protein. After washing five times, the membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies and
western blots were developed with ECL kit (GE Healthcare).
Normalization was ensured by β-actin and each band was quantified
using ImageJ software.
Statistical analysis
The obtained results were expressed as the mean ±
standard deviation (SD) of a minimum three replicates in
independent experiments. The data were analyzed by unpaired, two
tailed Student's t-test using SPSS version 10.0 for Windows (SPSS,
Chicago, IL, USA). The statistical significance was accepted as
p<0.05 and p<0.01.
Results
Characterization of BFs
Fig. 1 shows the
obtained chromatograms of Byungkul extract possessing 15 flavonoid
peaks at 280 nm in HPLC. In HPLC-MS/MS derived peaks elaborated as
three flavanones namely neoeriocitrin, naringin, and hesperidin
along with eight flavones such as isosinensetin, sinensetin,
teramethyl-O-isoscutellarein, nobiletin, tetramethoxyflavone,
heptamethoxyflavone, tangeretin, and hytroxypentamethoxyflavone,
and four unidentified peaks were characterized (Table I).
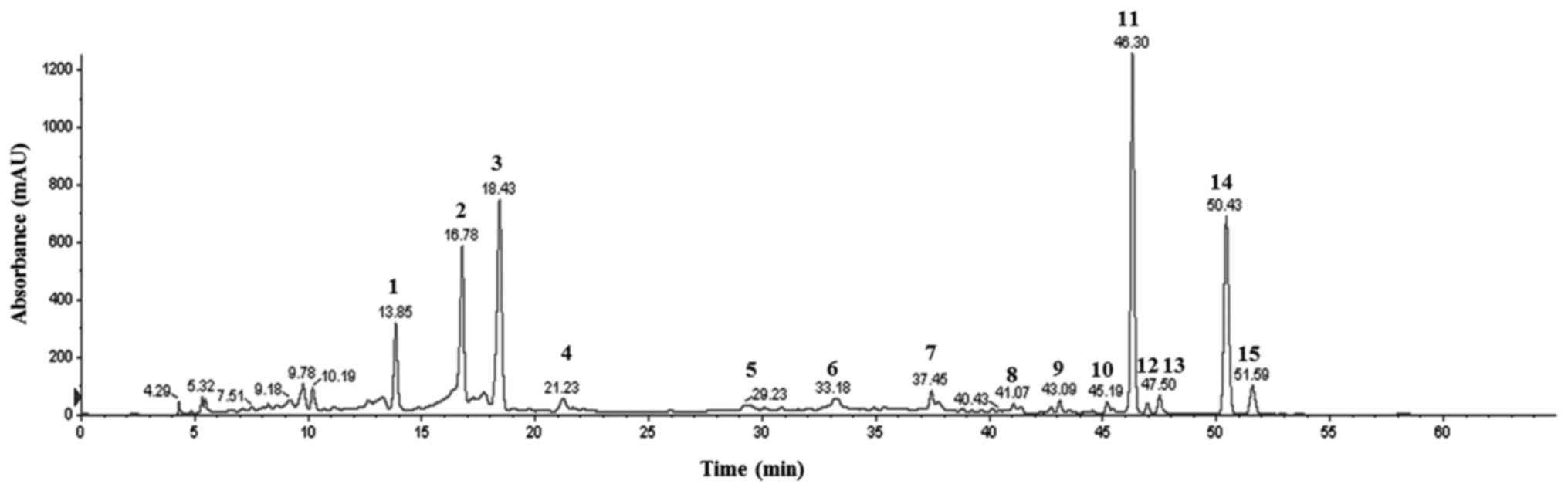 | Figure 1HPLC profile of flavonoid peaks at
280 nm. 1, Neoeriocitrin. 2, Naringin. 3, Hesperidin. 4–7,
Unindentified. 8, Isosinensetin. 9, Sinenseti. 10,
Tetramethyl-O-isoscutellarein. 11, Nobiletin. 12,
Tetramethoxyflavone. 13, Heptamethoxyflavone. 14, Tangeretin. 15,
Hydroxypentamethoxyflavone. |
 | Table ICharacterization of flavonoids
derived from the peel of Byunkyul (C. platymamma). |
Table I
Characterization of flavonoids
derived from the peel of Byunkyul (C. platymamma).
| No. | Compound | RT |
[M+H]+/[M−H]− | MS/MS |
|---|
| 1 | Neoeriocitrin | 13.85 | −/595 | 459, 329, 311, 287,
151, 135, 107 |
| 2 | Naringin | 16.78 | −/579 | 459, 313, 271, 193,
151 |
| 3 | Hesperidin | 18.43 | −/609 | 608, 325, 301 |
| 4 | Unidentified | | | |
| 5 | Unidentified | | | |
| 6 | Unidentified | | | |
| 7 | Unidentified | | | |
| 8 | Isosinensetin | 41.07 | 373 | 358, 357, 343, 329,
181, 165 |
| 9 | Sinensetin | 43.09 | 373 | 358, 357, 343, 340,
329, 312, 162 |
| 10 |
Tetramethyl-O-isoscutellarein | 45.19 | 343 | 328, 313, 299, 285,
240, 152, 133 |
| 11 | Nobiletin | 46.30 | 403 | 388, 373, 355, 327,
241, 211, 165 |
| 12 |
Tetramethoxyflavone | 46.95 | 343 | 327, 313, 282,
150 |
| 13 |
Heptamethoxyflavone | 47.50 | 433 | 418, 403, 385, 211,
165 |
| 14 | Tangeretin | 50.43 | 373 | 358, 343, 325,
297 |
| 15 |
Hydroxypentamethoxyflavone | 51.59 | 389 | 374, 359, 341,
165 |
Effects of BFs on cell viability
The cytotoxicity effect of BFs in HepG2 and Hep3B
cells were determined by MTT assay using various concentrations of
Byungkul extract (0–1,000 µg/ml). BFs treated cells had
different viability effects on HepG2 and Hep3B cells. As observed
in Fig. 2 the growth of Hep3B
cells were reduced significantly from 25 µg/ml followed by a
50% cell growth inhibition at doses between (100–200 µg/ml)
while in HepG2 cells, the cell viability were significantly reduced
from 400 µg/ml of BFs treatment at 24-h duration. These
results suggest that the effect of BFs treatment on cell viability
is more specific to Hep3B than that in HepG2 cells. Thus, Hep3B
cells were used for the following experiments.
BFs induce cell cycle alteration and
apoptosis in Hep3B cells
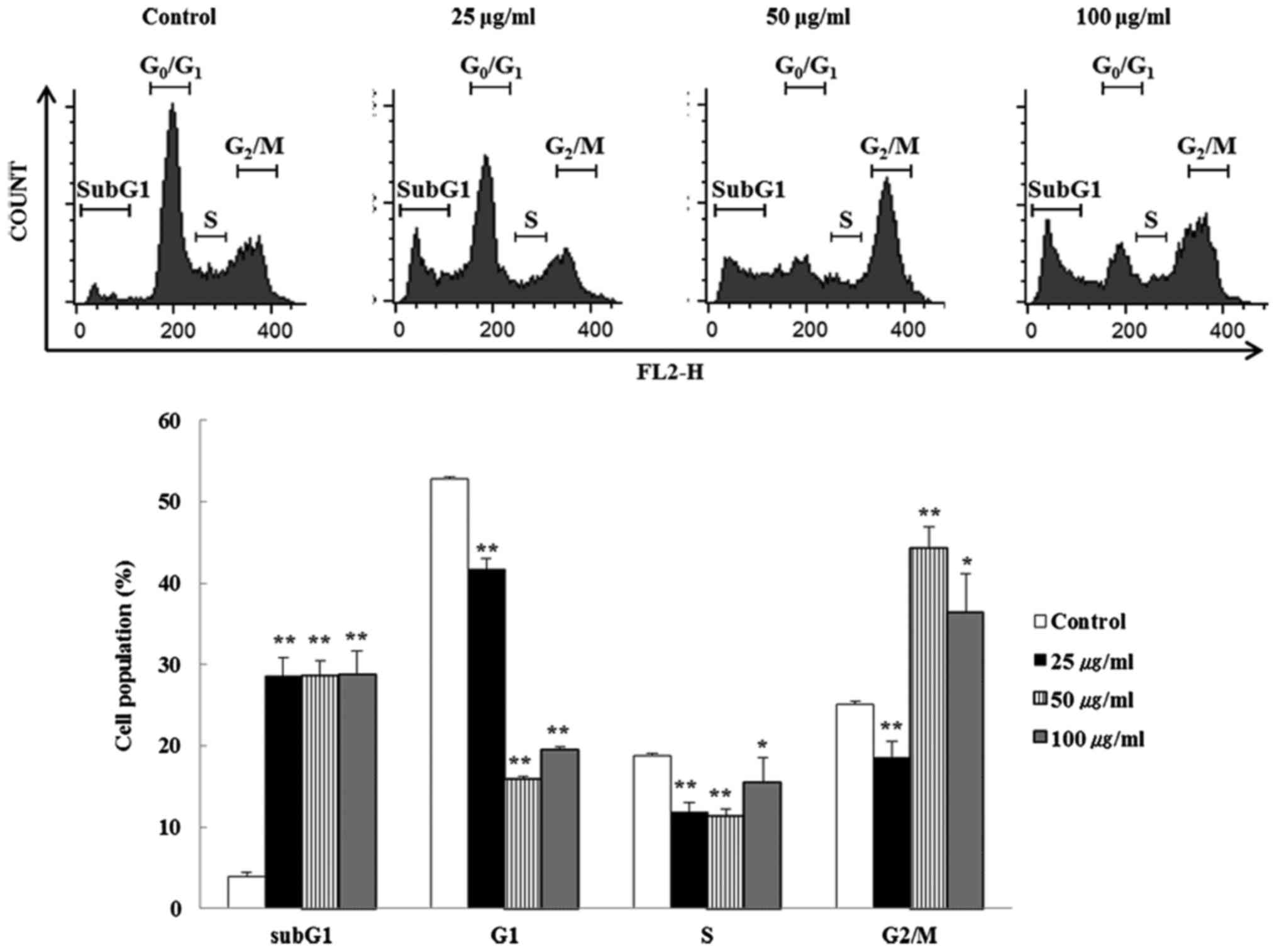
The effect of BFs on cell cycle progress in Hep3B
cells were investigated by flow cytometry. Cells incubated with
different concentrations (0, 25, 50 and 100 µg/ml) of BFs
for 24 h were stained with PI-stain and cell cycle distribution was
analyzed using a flow cytometer. Fig.
3 shows that G2/M phase was significantly increased in
BFs-treated cells when compared with untreated control cells. These
data suggest that BFs arrest Hep3B cells in the G2/M phase. In
addition, subG1 phase cells were enhanced but G1 phase cells were
declined significantly suggesting that BFs induced apoptosis in a
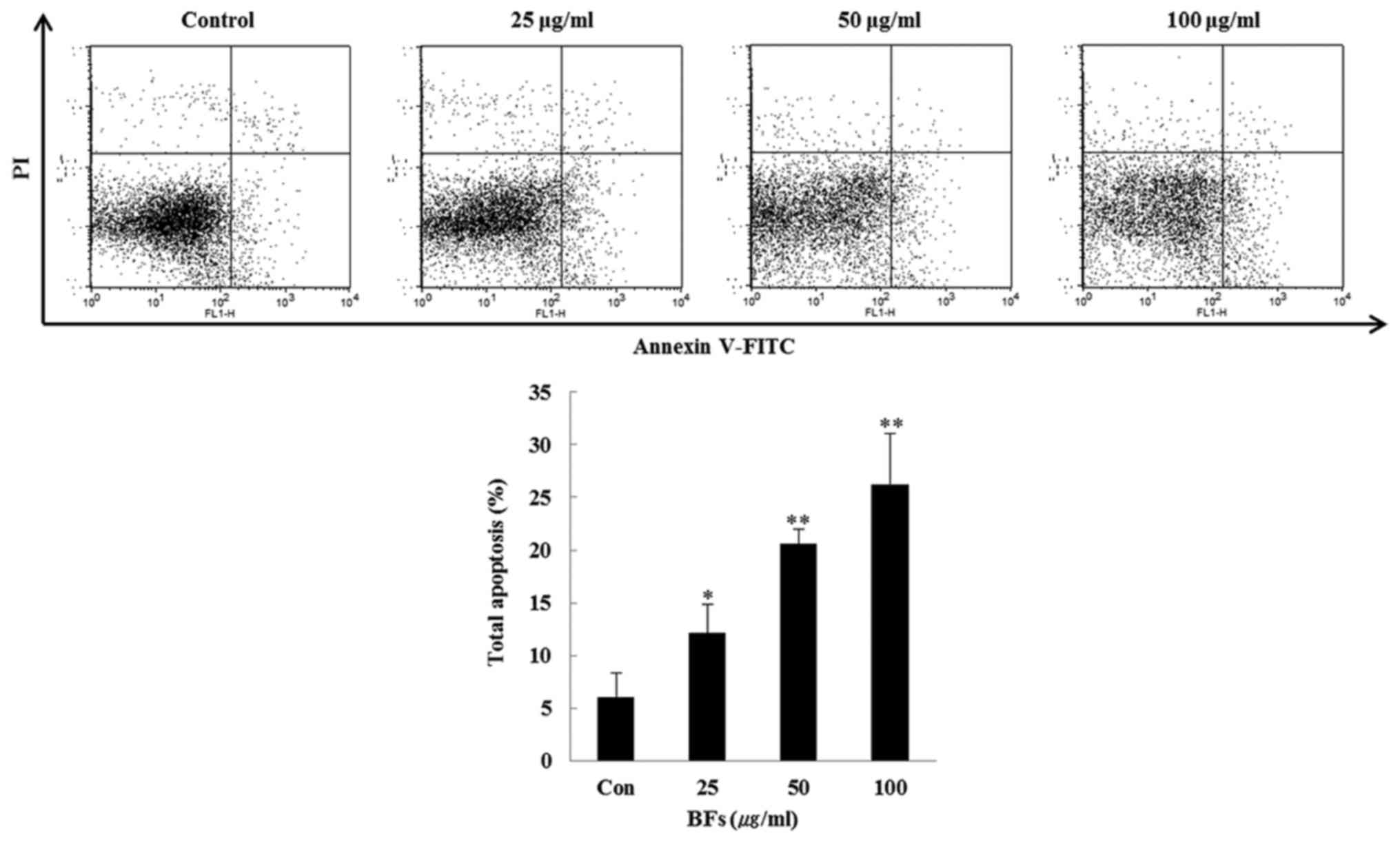
dose-dependent manner. Furthermore, FITC-conjugated Annexin V and
PI double staining was conducted and it was observed that the late
apoptotic cells in BFs treated Hep3B cells were increased
dose-dependently when compared with the untreated control cells
(Fig. 4).
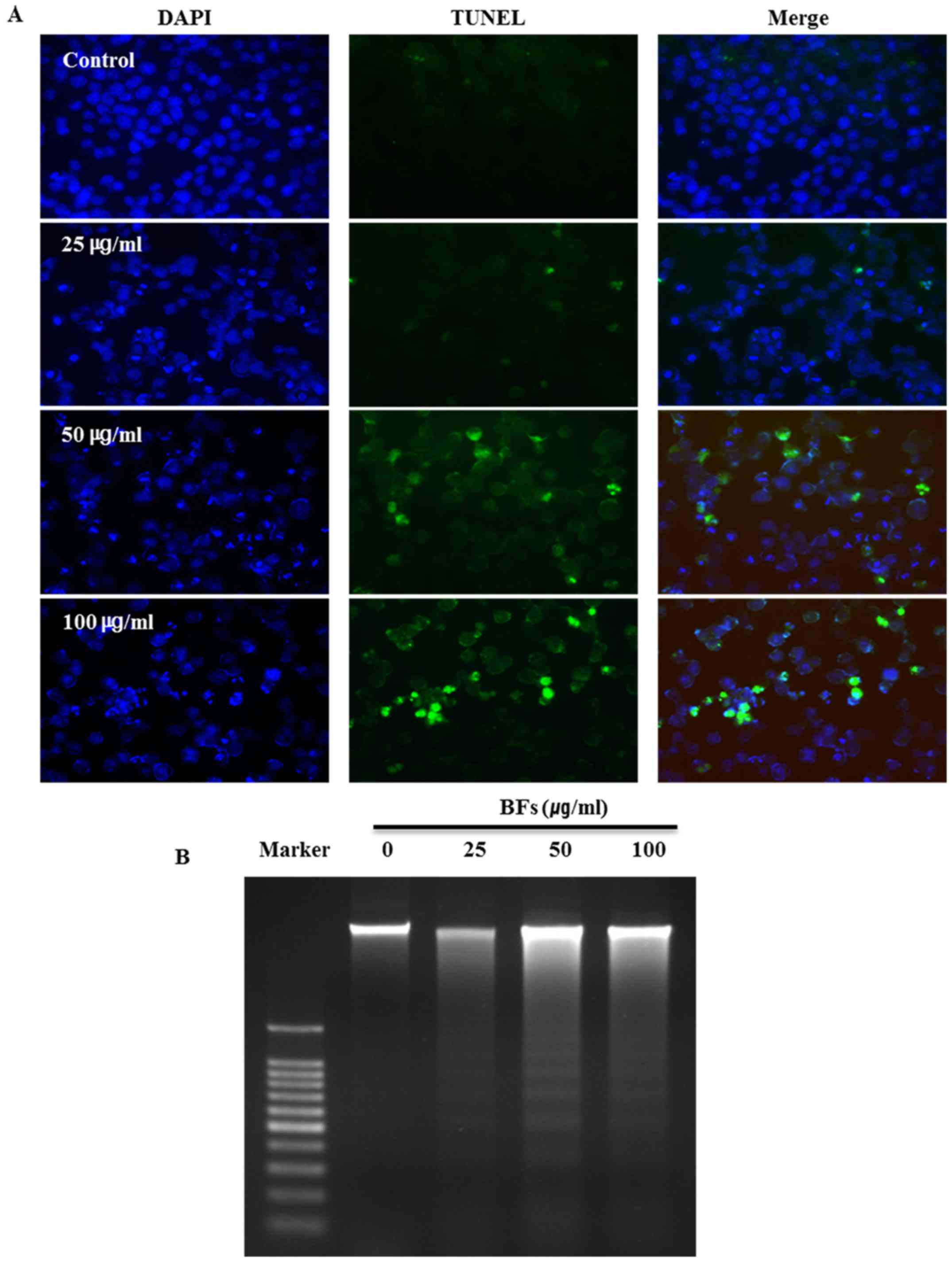
Effects of BFs on nuclear morphology in
Hep3B cells
The influence of BFs on the morphological change and
damage of cell nucleus was examined by DAPI and TUNEL assay. All
nuclei were stained in blue with DAPI and TUNEL-positive cells are
stained in green. DAPI staining shows chromatin pyknosis with
intense blue fluorescence and cleaved nuclei as blue granules by
BFs treatment (0, 25, 50 and 100 µg/ml). Additionally,
TUNEL-positive cells were increased in a dose-dependent manner in
BF-treated cells (Fig. 5A). DNA
fragmentation is one of the main features of apoptosis therefore
DNA ladder assay was conducted by DNA electrophoresis on 1.5%
agarose gel after DNA preparation from BF-untreated or BF-treated
Hep3B cells for 24 DNA ladder pattern was observed in BF-treated
Hep3B cells while there was no ladder formation in untreated
control cells (Fig. 5B). These
results suggest that cell death in BFs treated Hep3B cells is due
to apoptosis.
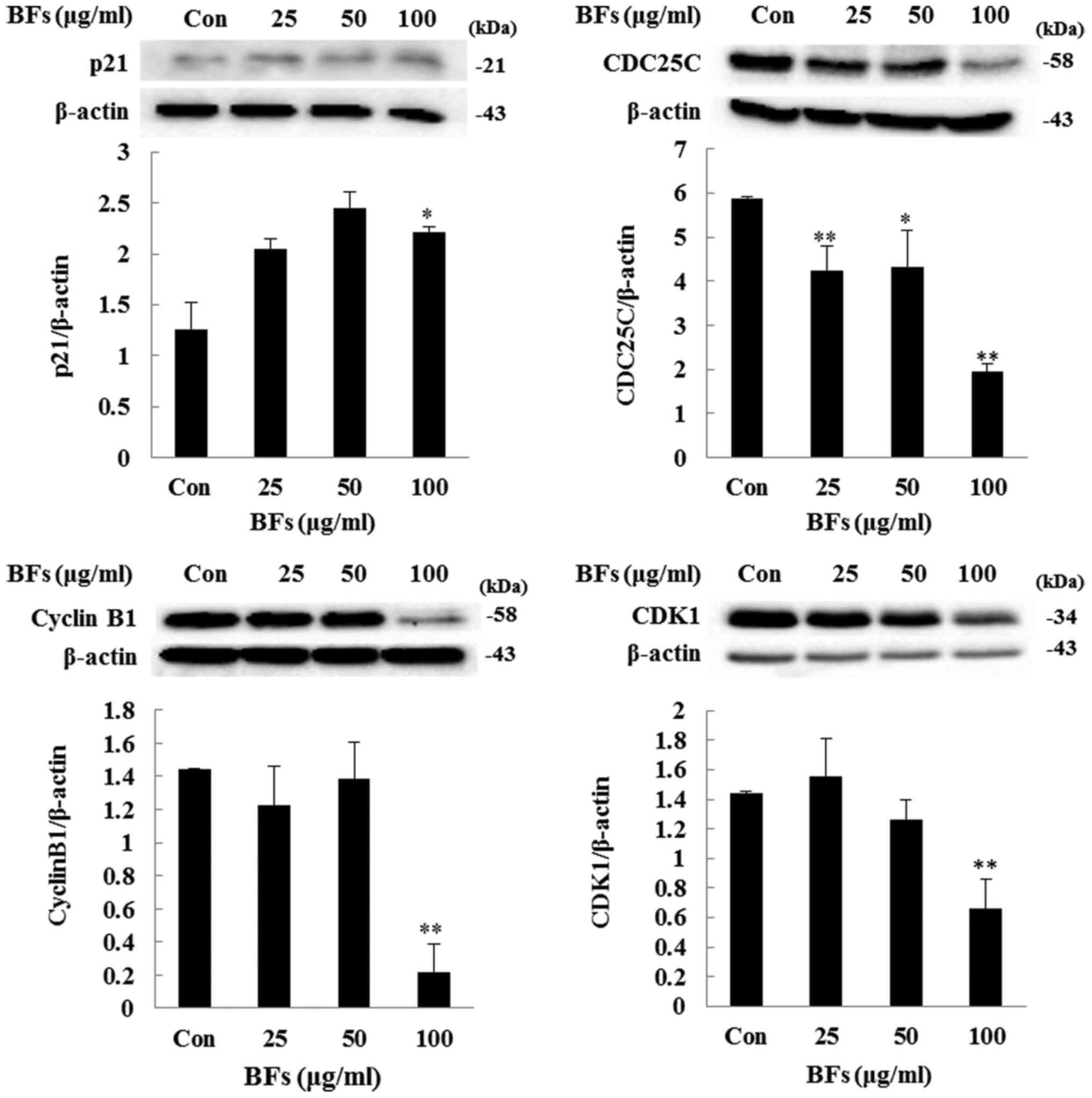
Regulation of CDK1, cyclin B1, CDC25C,
and p21 protein expression in BF-treated Hep3B cells
To further analyze the effect of BFs on cell cycle
arrest, investigation has been done to measure the protein
expression levels of p21, CDK1, cyclin B1, and CDC25C as they play
a major role in cell cycle regulation and cell proliferation.
Immunoblot result revealed that, cells treated with different
concentrations (25, 50 and 100 µg/ml) of BFs have reduced
expression levels of CDK1, cyclin B1, and CDC25C when compared with
the untreated cells. In contrast, the protein level of p21, a
negative regulator of CDK1/CyclineB1, was induced by BFs treatment
in subsequent doses (Fig. 6).
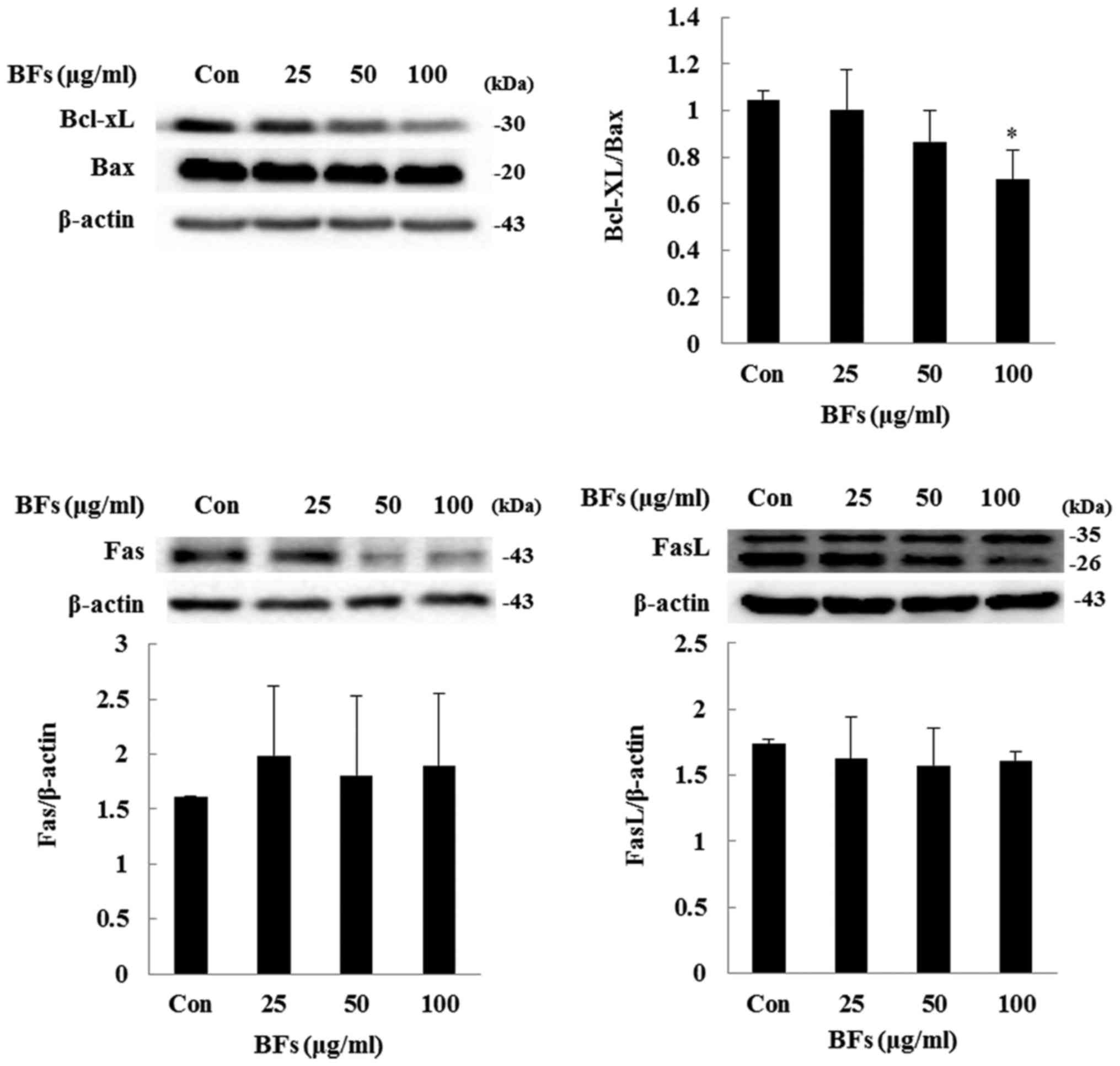
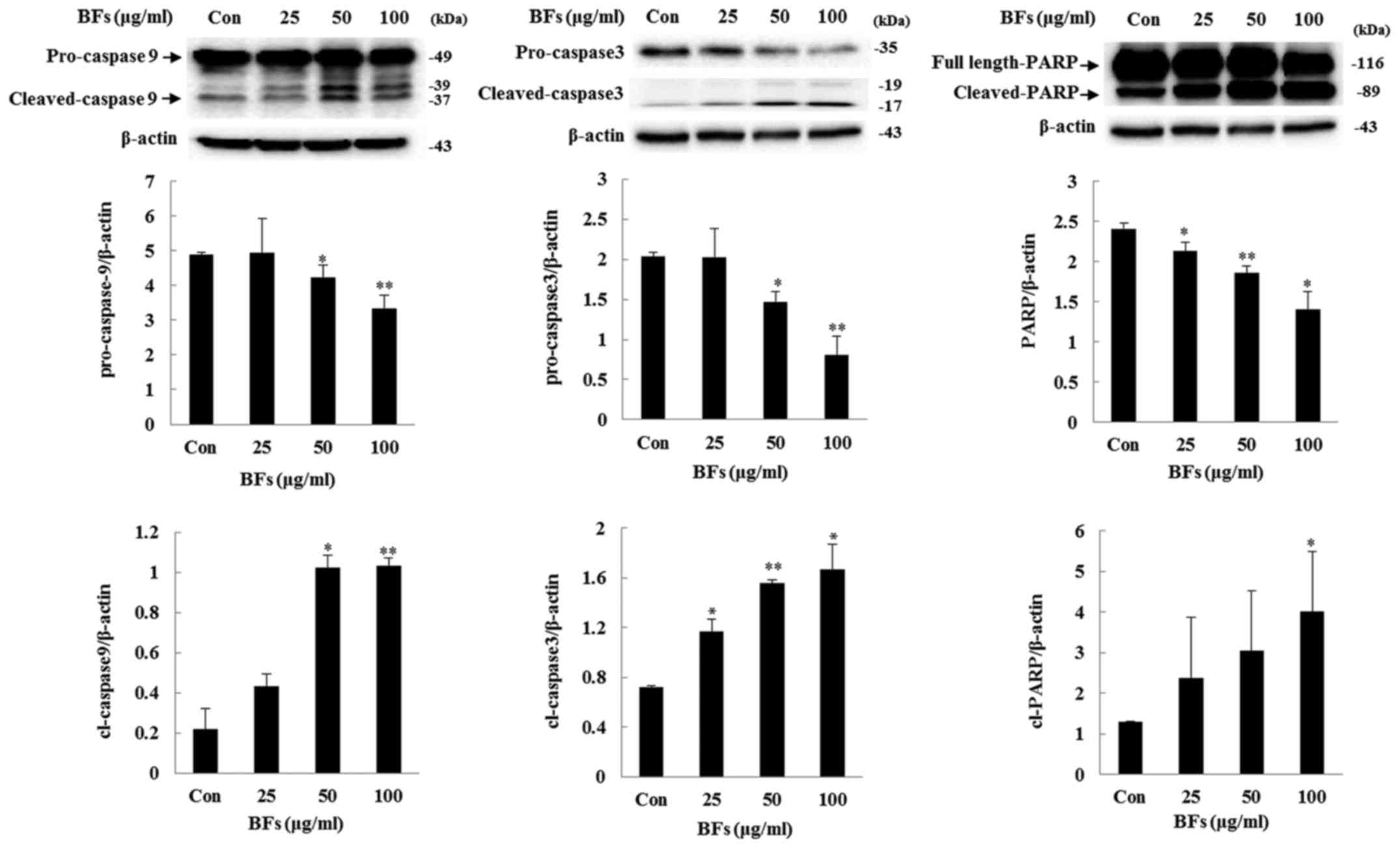
Influence of BFs on the regulators
involved in intrinsic apoptosis
In intrinsic pathway or mitochondrial pathway, the
balance between Bcl-2 family members such as Bcl-xL and Bax is
important while in extrinsic apoptosis pathway Fas and FasL are the
key inducers. In both the pathways cleavage of caspases and PARP
plays a crucial role (20,21). Fig.
7 shows BF-treatment has significantly reduced ratio between
anti-apoptotic proteins Bcl-xL to pro-apoptotic protein Bax at
concentration of 100 µg/ml in Hep3B cells. Also, in order to
confirm extrinsic factor involvement, western blot analysis of
protein expression level of Fas and FasL was performed and it was
observed that BF-treatment at various concentrations (0, 25, 50 and
100 µg/ml) did not significantly changed the protein
expression of Fas and FasL. Furthermore, treatment of BFs led to
significant upregulation of cleaved caspase-3, and -9 as well as
PARP expression levels, confirming the involvement of caspase in
BF-induced cell death (Fig. 8).
These results demonstrated that BFs modulate apoptosis through the
intrinsic apoptotic pathway in Hep3B cells.
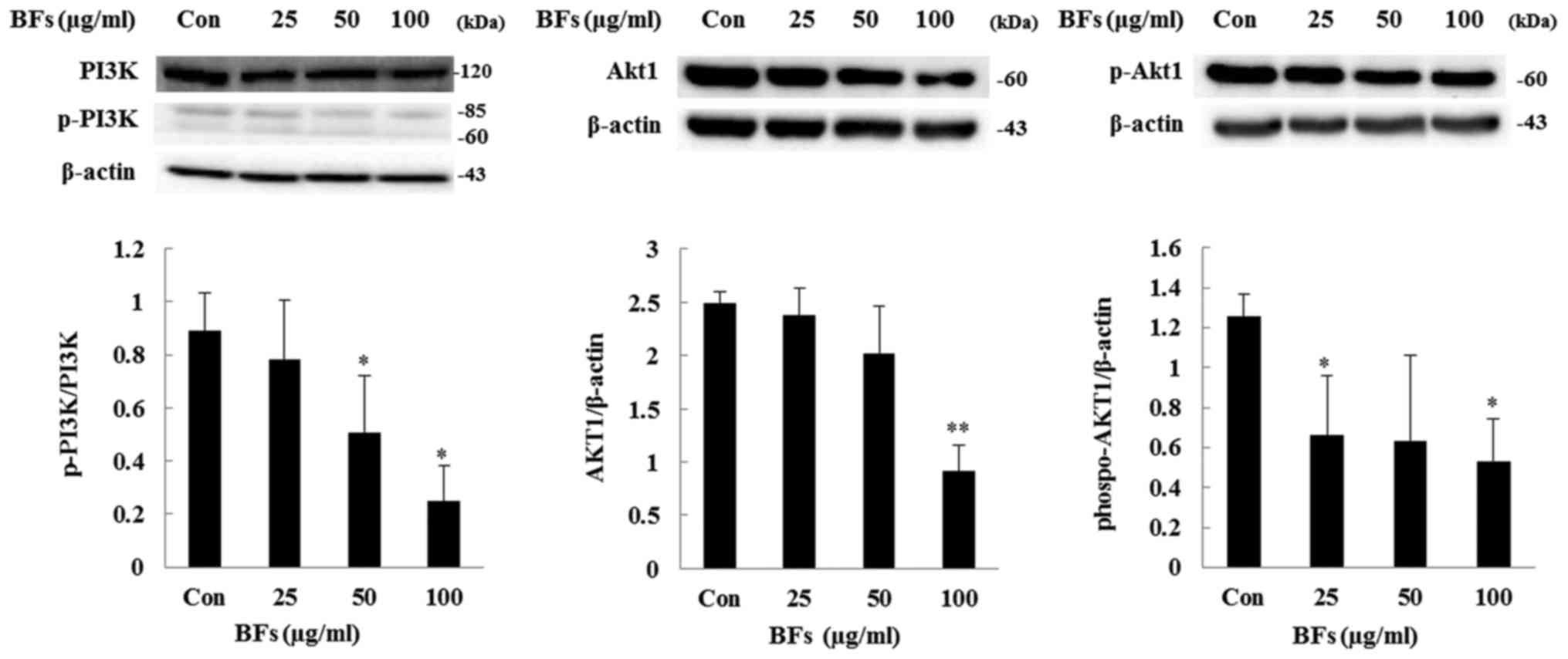
Regulation of PI3K/AKT and MAPK signaling
pathway in Hep3B cells
The PI3K/AKT pathway has been involved in
transmitting oncogenic signals. PI3K plays a role in induction and
progression of several diseases including cancer and AKT regulates
cell growth, cell proliferation, and resistance to apoptosis
(22). In this study, western blot
assay revealed that treatment with BFs (0, 25, 50 and 100
µg/ml) caused inhibition in the phosphorylation of PI3K and
Akt as well as total-Akt protein expression and also
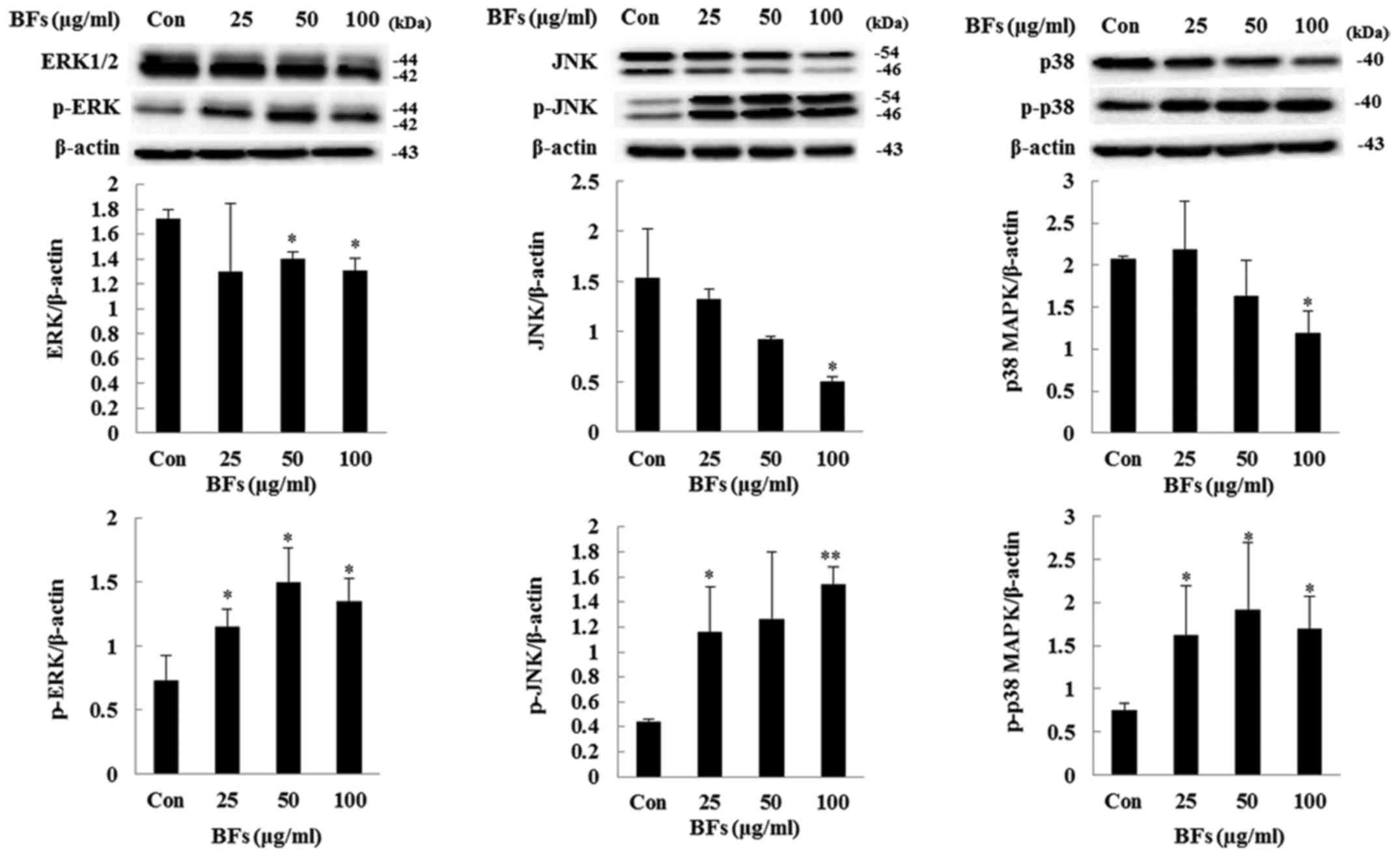
phospho-Akt/total-Akt ratio was reduced by BF-treatment (Fig. 9). Moreover, ERK1/2, JNK, and p38
MAPK pathways which are activated by environmental stimuli play
important roles in apoptosis induction (23). These signals are activated by
phosphorylation. Fig. 10 shows
BF-treated Hep3B cells significantly elevate the phosphoryl ation
level of ERK1/2, JNK and p38 MAPK in a dose-dependent manner. These
above results indicate BFs regulate cell growth and proliferation
leading to apoptosis by inactivation of PI3K/Akt and activation of
MAPKs pathways in Hep3B cells.
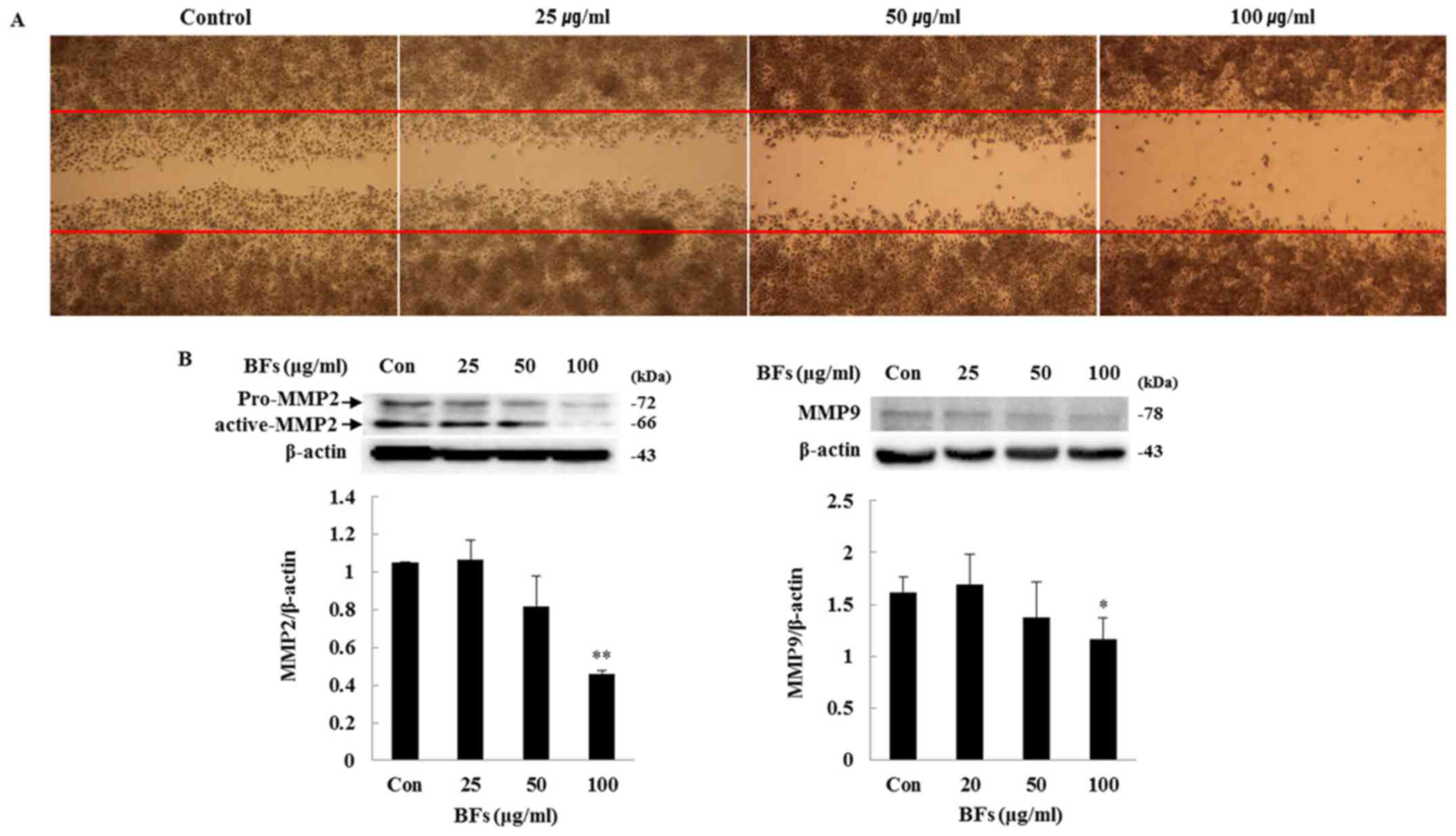
Effect of BFs on cell migration
activity
In order to investigate the influence of BFs on
Hep3B cell migration, scratch wound migration and western blot
assays were conducted. Overnight incubated cells were scratched
horizontally with a micropipette tip followed by removal of any
cellular debris and detached cells. The wounded cell monolayer was
then incubated in fresh complete media with different concentration
of BFs (0, 25, 50 and 100 µg/ml) for 24 h and visualized by
optical microscope. The wound closure of Hep3B cells was suppressed
by different doses of BF-treatment as compared to control cells.
Fig. 11A shows that BFs inhibited
cell mobility of Hep3B in a dose-dependent manner. Interestingly,
western blot analysis also showed that, MMP-2 and MMP-9 protein
expression were reduced significantly at 100 µg/ml of
BF-treatment (Fig. 11B). Taken
together, the data support involvement of BFs in cell migration
inhibition.
Discussion
There is an emerging interest in therapeutic
potential of medicinal plants on several health benefits, due to
their availability with various phytonutrients such as flavonoids
which have shown efficacy and safety in the treatment of
hepatobiliary dysfunction and digestive complaints earlier
(24). In this study, Byungkyul
peel extract was identified with 11 flavonoid peaks
enlisting-hesperidin, naringin, tangeretin, nobiletin,
neoeriocitrin, isosinensetin, sinensetin,
teramethyl-O-isoscutellarein, tetramethoxyflavone,
heptamethoxyflavone, and hytroxypentamethoxyflavone along with 4
unidentified peaks by HPLC. The HPLC-derived flavonoids indicated
that Korean Byungkyul peel contains abundant polymethoxylated
flavones.
Several studies have approved that, hesperidin,
naringin, nobiletin, and tangeretin have health remedial factors,
with roles such as antioxidant, suppressor of carcinogenesis, cell
cycle regulator following apoptotic cell death, angiogenesis,
metastasis, and as co-chemotherapy (5). Tangeretin, one of the
polymethoxylated flavones inhibited cancer cell proliferation,
metastasis and invasion of melanoma B16F10 cells, induced G1 cell
cycle arrest and apoptosis in breast and colon cancer cells, and
suppressed ERK1/2 phosphorylation and growth of human mammary
cancer cells (25). In in
vivo study, nobiletin, another major polymethoxylated flavone,
effectively inhibited skin inflammation and TPA-induced skin tumor
formation, reduced the number of carcinomas in colon tissue, and
showed chemopreventive effect of colon and prostate cancer. In
hepatic cancer, nobiletin blocked cell cycle at G2/M phase,
attenuated Bcl-2 expression and induced Bax and caspase-3 in HCC
cell line, and also significantly inhibited tumor growth (25).
In the present study it was observed that, the
growth of Hep3B cells were reduced significantly from 25
µg/ml in BFs while the cell viability of HepG2 cells were
significantly reduced from 400 µg/ml in BF-treatment
suggesting that BFs have more effective anti-proliferative and
cytotoxic activity in hepatocellular carcinoma Hep3B cells than in
HepG2 cells.
Cancer is a life-threatening disease characterized
by sustained proliferative signaling, evading growth suppressors,
resisting cell death, inducing angiogenesis, and activating
invasion and metastasis (26).
Tangeretin and nobiletin induced G1 arrest by increasing the
expression of p21 in human breast and colon carcinoma cells, and
naringin inhibits proliferation and induced G1 arrest by
upregulation of p21 in human bladder cancer cells (5). In our previous studies we reported
that flavonoids extracted from Korean Citrus aurantium L.
induced A549 cells arrest by downregulation of CDC2, CDC 25C, and
cyclin B1 and the upregulation of p21 resulted in G2/M arrest in a
cell line (27). Similarly, in the
present study, it was observed that BFs induced G2/M cell cycle
arrest by inhibiting the protein expression of CDC25C, cyclin B1,
and CDK1 and induced p21 protein expression.
Flavonoids obtained from C. reticulata peels
induced apoptosis on TMK-1, MKN-45, MKN-74, and KATO-III human
gastric carcinoma cells and showed anti-proliferative effect on
rats liver carcinogenesis model by inducing apoptosis (5). In the present study, Annexin V-PI
double staining showed that BF-treated Hep3B cells upregulated
apoptotic cell death. Moreover, chromatin condensation, granulation
of nucleus and TUNEL positive cells were observed in BF-treated
Hep3B cells. Furthermore, different size of DNA fragments was
confirmed by DNA fragmentation assay in BF-treated Hep3B cells.
These results suggest that the cell death induced by BFs in HEp3B
cells is apoptotic cell death.
Apoptotic programs may be initiated by a variety of
internal and external stimuli. Caspases play a central role in the
execution of the apoptotic process and is activated through either
intrinsic or extrinsic apoptotic pathways. Internal stimuli mainly
initiate apoptosis through the mitochondrial pathway called
intrinsic pathway. In contrast, external stimuli - Fas ligand/Fas
receptors are engaged in extrinsic pathways (28). Apoptosis is regulated by
counterbalancing pro- and anti-apoptotic members of the Bcl-2
family. Bcl-xL suppresses pro-apoptotic triggering protein Bax via
binding to Bcl-xL and Bcl-xL/Bax assembly is embedded in the
mitochondrial outer membrane blocking the transfer of pro-apoptotic
signaling protein cytochrome c from inside mitochondria. Released
cytochrome c activates cascade of caspases and activated
caspase-3 cleaves the PARP protein, which is a marker of the
apoptosis (26). In this study,
Bcl-xL/Bax ratio was reduced in a dose-dependent manner following
induced cleavage of caspase-9, caspase-3, and PARP by BFs treatment
whereas, the protein expression of Fas and FasL did not alter
significantly confirming apoptotic activity of BFs are related to
the intrinsic pathway.
The MAPK signaling has a role in regulating gene
expression, mitosis, proliferation, motility, metabolism, and
programmed cell death (apoptosis) (29). In mammalian, three subfamilies of
MAP kinase have been described; extracellular signal-regulated
kinase (ERK), c-Jun N-terminal kinase (JNK), and p38-MAP kinase
(p38-MAPK) and each MAPK is activated by phosphorylation. In our
previous study, it was reported that increase of JNK, and p38 MAPK
phosphorylation leads to induction of apoptosis and the increase of
ERK1/2 phosphorylation is involved in the G2/M cell cycle arrest
mechanism caused by polyphenolic extract of Lonicera
japonica Thunb. in HepG2 cells (15). Evodiamine derived from herbal
medicine Evodia rutaecarpa results in phosphorylation of JNK
thus inducing apoptotic cell death and G2/M phase arrest in human
colon cancer cells (30). ERK is
characterized as survival signal messenger however, Wang et
al, demonstrated that ERK activation is required for induction
of cisplatin-induced apoptosis in HeLa cells (31). Similarly, BF-stimulated
phosphorylation of ERK, JNK, and p-38 MAPK in Hep3B cell is
associated to apoptotic cell death.
PI3K/AKT signaling is one of the major signaling
pathways activated in human cancer including HCC and is therefore
considered as a suitable molecular target for cancer therapy. Akt
is known to regulate various cellular pathways that promote cell
survival, cell proliferation, angiogenesis, and invasion. Akt is
activated via PI3K pathway which is linked to the development of
HCC (32). The present study
showed that BFs suppressed phosphorylation of PI3K and Akt1, and
also the expression of pan-Akt1. It has been reported that, Akt1
anti-sense oligonucleotide (AS) reduced Akt1 protein expression in
both normal and cancer cells. Akt1 AS treatment significantly
reduced cancer cell growth and induced cancer cell apoptosis.
However, there was no predominant diminution in normal cell growth
and survival due to Akt1 AS treatment (33).
Cancer metastasis is the spread of cancer cells to
tissues and organs resulting in the death of most cancer patients.
Invasion and metastasis of solid tumors require dissolution of the
basement membrane, and matrix metalloproteinases (MMPs) including
MMP-2 and MMP-9 have an ability to degrade extracellular matrix of
the invading surrounding tissues (34). MMP-2 and MMP-9 overexpression is
involved in tumor recurrence and metastasis, and the activation of
MMPs induced HCC invasion in HepG2 and PLC cells (35). Very widely known flavonoids
viz., lutelin and quercetin inhibit cell metastasis in human
epidermoid carcinoma cell line A431 (36) and nobiletin attenuates metastasis
via both ERK and PI3K/Akt pathways in HepG2 cells (37) as earlier reported. In this study,
to characterize the role of C. platymamma in metastasis
wound healing assay and protein expression of MMP-2 and MMP-9 were
studied. It was observed that BFs suppressed the expression of
MMP-2 and MMP-9 and also reduced wound closure of Hep3B cells
suggesting its role as an anti-metastatic agent.
In conclusion, BFs can suppress the propagation of
hepatocellular carcinoma Hep3B cells via MAPKs and PI3K/AKT
pathway. The flavonoids of Byungkyul peel induced apoptosis with
the rise of pro-apoptotic protein caspase-3 and -9 and PARP
cleavage, and diminution of anti-apoptotic protein Bcl-xL, and
induced G2/M phase arrest by suppressing CDK1, cyclin B1, and
CDC25C protein expression and promoting expression of p21.
Moreover, BFs reduced the protein expression of MMP-2 and MMP-9
which are crucial molecular factors in cell metastasis and
invasion. These results indicate the potential beneficial role of
C. platymamma as natural product to be developed as agent
for human HCC treatment. Therefore, further study needs to be
conducted to explore the effectiveness of individual flavonoids in
overcoming cancer both in vitro and in vivo. We
expect further study will validate the effectiveness of C.
platymamma against cancer.
Acknowledgments
This study was suppo2rted by the National Research
Foundation of Korea (NRF) funded by the Ministry of Science, ICT
and Future Planning (no. 2012M3A9B8019303) and the National R&D
Program for Cancer Control, Ministry for Health, Welfare and Family
affairs, Korea (no. 0820050).
References
|
1
|
Schlachterman A, Craft WW Jr, Hilgenfeldt
E, Mitra A and Cabrera R: Current and future treatments for
hepatocellular carcinoma. World J Gastroenterol. 21:8478–8491.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manosroi A, Akazawa H, Kitdamrongtham W,
Akihisa T, Manosroi W and Manosroi J: Potent antiproliferative
effect on liver cancer of medicinal plants selected from the
thai/lanna medicinal plant recipe database 'MANOSROI III'. Evid
Based Complement Alternat Med. 2015:3971812015. View Article : Google Scholar
|
|
3
|
Ren W, Qiao Z, Wang H, Zhu L and Zhang L:
Flavonoids: Promising anticancer agents. Med Res Rev. 23:519–534.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tripoli E, Guardia ML, Giammanco S, Majo
DD and Giammanco M: Citrus flavonoids: Molecular structure,
biological activity and nutritional properties: A review. Food
Chem. 104:466–479. 2007. View Article : Google Scholar
|
|
5
|
Meiyanto E, Hermawan A and Anindyajati:
Natural products for cancer-targeted therapy: Citrus flavonoids as
potent chemopreventive agents. Asian Pac J Cancer Prev. 13:427–436.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HJ, Nagappan A, Park HS, Hong GE,
Yumnam S, Raha S, Saralamma VV, Lee WS, Kim EH and Kim GS:
Flavonoids isolated from Citrus platymamma induce
mitochondrial-dependent apoptosis in AGS cells by modulation of the
PI3K/AKT and MAPK pathways. Oncol Rep. 34:1517–1525.
2015.PubMed/NCBI
|
|
7
|
Penjor T, Yamamoto M, Uehara M, Ide M,
Matsumoto N, Matsumoto R and Nagano Y: Phylogenetic relationships
of citrus and its relatives based on matK gene sequences. PLoS One.
8:e625742013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimizu T and Yano K: A post-labeling
method for multiplexed and multicolored genotyping analysis of SSR,
indel and SNP markers in single tube with bar-coded split tag
(BStag). BMC Res Notes. 4:1612011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182:475–481. 2002. View Article : Google Scholar
|
|
11
|
Hsu SC, Kuo CL, Lin JP, Lee JH, Lin CC, Su
CC, Lin HJ and Chung JG: Crude extracts of Euchresta formosana
radix induce cytotoxicity and apoptosis in human hepatocellular
carcinoma cell line (Hep3B). Anticancer Res. 27B:2415–2425.
2007.
|
|
12
|
Nair SV, Hettihewa M and Rupasinghe HP:
Apoptotic and inhibitory effects on cell proliferation of
hepatocellular carcinoma HepG2 cells by methanol leaf extract of
Costus speciosus. BioMed Res Int. 2014:6370982014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon SB, Lee YJ, Park SK, Kim HC, Bae H,
Kim HM, Ko SG, Choi HY, Oh MS and Park W: Anti-inflammatory effects
of Scutellaria baicalensis water extract on LPS-activated RAW 264.7
macrophages. J Ethnopharmacol. 125:286–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Granado-Serrano AB, Martín MA, Bravo L,
Goya L and Ramos S: Quercetin induces apoptosis via caspase
activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt
and ERK pathways in a human hepatoma cell line (HepG2). J Nutr.
136:2715–2721. 2006.PubMed/NCBI
|
|
15
|
Park HS, Park KI, Lee DH, Kang SR,
Nagappan A, Kim JA, Kim EH, Lee WS, Shin SC, Hah YS, et al:
Polyphenolic extract isolated from Korean Lonicera japonica Thunb.
induce G2/M cell cycle arrest and apoptosis in HepG2 cells:
Involvements of PI3K/Akt and MAPKs. Food Chem Toxicol.
50:2407–2416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park KI, Park HS, Kang SR, Nagappan A, Lee
DH, Kim JA, Han DY and Kim GS: Korean Scutellaria baicalensis water
extract inhibits cell cycle G1/S transition by suppressing cyclin
D1 expression and matrix-metalloproteinase-2 activity in human lung
cancer cells. J Ethnopharmacol. 133:634–641. 2011. View Article : Google Scholar
|
|
17
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar
|
|
18
|
Hong GE, Kim JA, Nagappan A, Yumnam S, Lee
HJ, Kim EH, Lee WS, Shin SC, Park HS and Kim GS: Flavonoids
identified from Korean Scutellaria baicalensis Georgi inhibit
inflammatory signaling by suppressing activation of NF-κB and MAPK
in RAW 264.7 cells. Evid Based Complement Alternat Med.
2013:9120312013. View Article : Google Scholar
|
|
19
|
Saralamma VV, Nagappan A, Hong GE, Lee HJ,
Yumnam S, Raha S, Heo JD, Lee SJ, Lee WS, Kim EH, et al: Poncirin
induces apoptosis in AGS human gastric cancer cells through
extrinsic apoptotic pathway by up-regulation of fas ligand. Int J
Mol Sci. 16:22676–22691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MS, Bak Y, Park YS, Lee DH, Kim JH,
Kang JW, Song HH, Oh SR and Yoon DY: Wogonin induces apoptosis by
suppressing E6 and E7 expressions and activating intrinsic
signaling pathways in HPV-16 cervical cancer cells. Cell Biol
Toxicol. 29:259–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park HY, Kim GY, Moon SK, Kim WJ, Yoo YH
and Choi YH: Fucoidan inhibits the proliferation of human urinary
bladder cancer T24 cells by blocking cell cycle progression and
inducing apoptosis. Molecules. 19:5981–5998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jokinen E, Laurila N and Koivunen JP:
Alternative dosing of dual PI3K and MEK inhibition in cancer
therapy. BMC Cancer. 12:6122012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar S and Pandey AK: Chemistry and
biological activities of flavonoids: An overview. Sci World J.
2013:1627502013. View Article : Google Scholar
|
|
25
|
Rawson NE, Ho C and Li S: Efficacious
anti-cancer property of flavonoids from citrus peels. Food Sci Hum
Wellness. 3:104–109. 2014. View Article : Google Scholar
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park KI, Park HS, Nagappan A, Hong GE, Lee
DH, Kang SR, Kim JA, Zhang J, Kim EH, Lee WS, et al: Induction of
the cell cycle arrest and apoptosis by flavonoids isolated from
Korean Citrus aurantium L. in non-small-cell lung cancer cells.
Food Chem. 135:2728–2735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jain MV, Paczulla AM, Klonisch T, Dimgba
FN, Rao SB, Roberg K, Schweizer F, Lengerke C, Davoodpour P,
Palicharla VR, et al: Interconnections between apoptotic,
autophagic and necrotic pathways: Implications for cancer therapy
development. J Cell Mol Med. 17:12–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chien CC, Wu MS, Shen SC, Ko CH, Chen CH,
Yang LL and Chen YC: Activation of JNK contributes to
evodiamine-induced apoptosis and G2/M arrest in human colorectal
carcinoma cells: A structure-activity study of evodiamine. PLoS
One. 9:e997292014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He X, Zhu Z, Johnson C, Stoops J, Eaker
AE, Bowen W and DeFrances MC: PIK3IP1, a negative regulator of
PI3K, suppresses the development of hepatocellular carcinoma.
Cancer Res. 68:5591–5598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Shi Y, Han EK, Chen Z, Rosenberg
SH, Giranda VL, Luo Y and Ng SC: Downregulation of Akt1 inhibits
anchorage-independent cell growth and induces apoptosis in cancer
cells. Neoplasia. 3:278–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lou L, Ye W, Chen Y, Wu S, Jin L, He J,
Tao X, Zhu J, Chen X, Deng A, et al: Ardipusilloside inhibits
survival, invasion and metastasis of human hepatocellular carcinoma
cells. Phytomedicine. 19:603–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao XL, Sun T, Che N, Sun D, Zhao N, Dong
XY, Gu Q, Yao Z and Sun BC: Promotion of hepatocellular carcinoma
metastasis through matrix metalloproteinase activation by
epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med.
15:691–700. 2011. View Article : Google Scholar
|
|
36
|
Huang YT, Hwang JJ, Lee PP, Ke FC, Huang
JH, Huang CJ, Kandaswami C, Middleton E Jr and Lee MT: Effects of
luteolin and quercetin, inhibitors of tyrosine kinase, on cell
growth and metastasis-associated properties in A431 cells
overexpressing epidermal growth factor receptor. Br J Pharmacol.
128:999–1010. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi MD, Liao YC, Shih YW and Tsai LY:
Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways
in HGF-treated liver cancer HepG2 cells. Phytomedicine. 20:743–752.
2013. View Article : Google Scholar : PubMed/NCBI
|