Introduction
Acute promyelocytic leukemia (APL) accounts for
10–15% of all cases of acute myeloid leukemia (AML) (1) and is characterized by a specific
chromosomal translocation t(15;17) that fuses the promyelocytic
leukemia gene (PML) to the retinoic acid receptor α gene (RARα),
resulting in the translation of fusion proteins PML/RARα and
RARα/PML (2,3). Pharmacological doses of all-trans
retinoic acid (ATRA) produced clinical remission in APL patients by
inducing the maturation of promyelocytes and the degradation of the
PML/RARα protein (4,5). Nevertheless, ATRA does not eliminate
the malignant myeloid clone in APL, and most relapsed APL patients
are resistant to further treatment with ATRA (6). Therefore, we need to evaluate the
combination of ATRA with other agents to work out a solution to
drug resistance and harmful side-effects.
Epigallocatechin-3-gallate (EGCG), a principal
antioxidant derived from green tea, has been shown to block each
stage of carcinogenesis by modulating the signal transduction
pathways involved in cell proliferation, transformation,
differentiation, apoptosis, metastasis and invasion (7–10).
Studies have shown that EGCG has anticancer effects in
hematopoietic malignancy, and several mechanisms have been proposed
for EGCG-induced cell death, including suppression of
anti-apoptosis protein, VEGF receptor and inhibition of radical
oxygen species (ROS) production (11–13).
Recently, it was found that EGCG could suppress the expression of
phosphorylated protein kinase (p-Akt) and phosphorylated
serine/threonine-protein kinase mTOR (pmTOR) via phosphatase and
tensin homolog (PTEN) to regulate the phosphatidylinositol 3-kinase
(PI3K)/Akt/mTOR pathway, reducing proliferation and inducing
apoptosis of cancer cells (14).
Moreover, EGCG effectively induced apoptosis of APL cells through
induction of the intrinsic apoptotic pathway and degradation of
PML/RARα fusion protein (15,16).
PTEN is often lost or inactivated in multiple solid
tumor types consisting of prostate, breast, thyroid, and
endometrial tumors, and others, and is a critical regulator of the
PI3K/Akt signaling pathway (17–19).
Catalyzing the conversion of the membrane lipid second messenger
phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3)
(PIP3) to PIP2, results in the inhibition of PI3K signaling in
mutants lacking functional PTEN, suppressing hyper-proliferation
and releasing differentiation arrest (20–22).
ATRA-mediated differentiation of the APL cell lines NB4 and HL-60
showed that commercial PI3K and Akt inhibitors affect not only
proliferation, but also the differentiative property of leukemia
cells (23). ATRA-induced
differentiation of HL-60 cells increased PTEN expression.
Remarkably, ubiquitinylation of PTEN at specific lysine residues
regulates its nuclear-cytoplasmic partitioning (24–26).
Treatment with ATRA has been shown to trigger PML/RARα degradation
and restores PML-NBs, where PML plays an essential role in the
regulation of the tumor suppressive function of PTEN through
ubiquitin carboxyl-terminal hydrolase 7 (USP7). Through restoration
of nuclear PTEN, Akt has been shown to be antagonized, causing
apoptosis and the production of differentiation stimuli (27,28).
The aforementioned findings prompted us to
investigate whether EGCG could enhance ATRA induced APL cell line
differentiation via PTEN. The results demonstrated that EGCG
induced NB4 cell apoptosis by enhancing the expression of PTEN.
Inhibiting PTEN levels resulted in a lower level of cell
differentiation. Moreover, we found that a combination of ATRA with
EGCG augmented cell differentiation in comparison with treatment
with ATRA only.
Materials and methods
Cell lines and cell culture
The human AML cell lines, HL-60, NB4 and THP-1 were
stored in our own laboratory, and cultured in RPMI-1640 medium
(Gibco-Life Technologies, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco, Melbourne, Australia) in an
environment with 5% CO2 at 37°C.
Cell viability and proliferation
NB4 cells were seeded into 96-well plates with
antibiotics-free RPMI-1640 media complemented with 10% FBS. For
experimental purposes, cells were seeded at a density of
1×104 cells/well and treated with 1 µM/ml ATRA
[dissolved in 0.1% dimethyl sulfoxide (DMSO)] and EGCG (5, 10 and
15 µM, respectively) either alone or in combination for 72
h, and 10 µl Cell Counting kit-8 (CCK-8; 7Sea Cell Counting
kit; Sevenseas Futai Biotechnology, Co., Ltd., Shanghai, China) was
added to each well. After incubating for 2 h, the absorbance of
each well was measured at 450 nm using a spectrophotometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
Cells in each group were washed with ice-cold
phosphate-buffered saline (PBS) three times, the supernatant was
discarded and cells were lysed using ice-cold
radioimmunoprecipitation assay (RIPA) lysis buffer containing
protease inhibitor phenylmethanesulfonyl fluoride (PMSF),
phosphatase inhibitor NaF and Na3VO4. The
protein concentration was measured with the BCA protein assay kit.
PTEN inhibitor SF1670 and PI3K inhibitor were purchased from
Selleck Chemicals (Houston, TX, USA). Primary antibodies: PTEN
(ab32199; 1:1,000; Abcam, Cambridge, UK), PML (EPR1768; 1:1,000;
Abcam), RARα (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA,
USA), Akt (ab32505; 1:1,000; Abcam), p-Akt (1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), p21 (1:1,000; Wanleibio, Co.,
Ltd., Beijing, China), β-actin (1:1,000; Beijing Zhongshan Golden
Bridge Biotechnology, Co., Ltd., Beijing, China).
Cell morphological staining
After 72 h of treatment, cells were collected and
washed with pre-cooled PBS three times and resuspended in fresh
PBS. Cell suspension (10 µl) was daubed onto glass slides,
and then the air dried slides were stained with Wright-Giemsa
staining fluid. For nitro blue tetrazolium (NBT) staining, cells
were collected after 72 h and resuspended in fresh RPMI-1640 medium
supplemented with 10% FBS, and 3×105 cells/well were
seeded on 96-well plates and combined with 200 µl mixture
with 0.2% NBT and 240 µg/ml
12-O-tetradecanoylphorbol-13-acetate (TPA), followed by
incubation for 1 h (37°C, 5% CO2). Samples were
centrifuged at 1000 rpm for 5 min and 200 µl DMSO was added
to each well, followed by shaking for 20 min. Finally, 10 µl
of CCK-8 was added to each well and the absorbance was measured at
570 nm (29,30).
Respiratory burst assay
As a measure of differentiation, the respiratory
burst assay for detecting hydrogen peroxidase was used. Cells were
collected after 72 h, resuspended in fresh RPMI-1640 medium
supplemented with 10% FBS and seeded on 96-well plates. PMA was
added at a final concentration of 200 ng/ml to the cells
(3×105 cells/well). Immediately, 10 µl of CCK-8
was added to each well, with each experimental group paired with
three parallel control groups, and the cells were incubated for 1 h
(37°C, 5% CO2) prior to measuring the absorbance at 412
nm (31).
Analyses of cell differentiation marker
by flow cytometry
For detection of the cell differentiation antigen,
CD11 antigen-like family member B was used (CD11b), after 72 h of
treatment, the cells were collected (1×106/group) and
washed with three times with pre-cooled PBS, then incubated with
phycoerythrin (PE) conjugated CD11b antibody (12011342;
eBioscience, Inc., San Diego, CA, USA) at 4°C for 30 min in the
dark (32). The cells were then
analyzed using flow cytometry (BD FACSVantage; BD Biosciences, San
Jose, CA, USA) and CellQuest Pro software version 5.1 (BD
Biosciences).
Indirect immunofluorescence assay
Cells were fixed with 4% paraformaldehyde for 20
min, subsequently, permeabilized with 0.1% Triton X-100 (in PBS)
for 15 min, and then blocked in 10% goat serum (in PBS) for 30 min
at room temperature. Slides were then incubated overnight with the
indicated primary antibodies. Secondary goat antibody against
rabbit-IgG-TRITC (1:200; Beijing Zhongshan Golden Bridge
Biotechnology) was used to detect rabbit IgG for 1 h at room
temperature. The nuclei were stained using DAPI at room
temperature. Finally, coverslips were immobilized by 70% glycerol
and viewed under a fluorescence microscope (Nikon, Tokyo,
Japan).
Statistical analysis
All data were performed using the SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). Results are represented as the mean
± SD. The Student's t-test was used for statistical analysis.
Results
EGCG in combination with ATRA enhances
NB4 cell differentiation
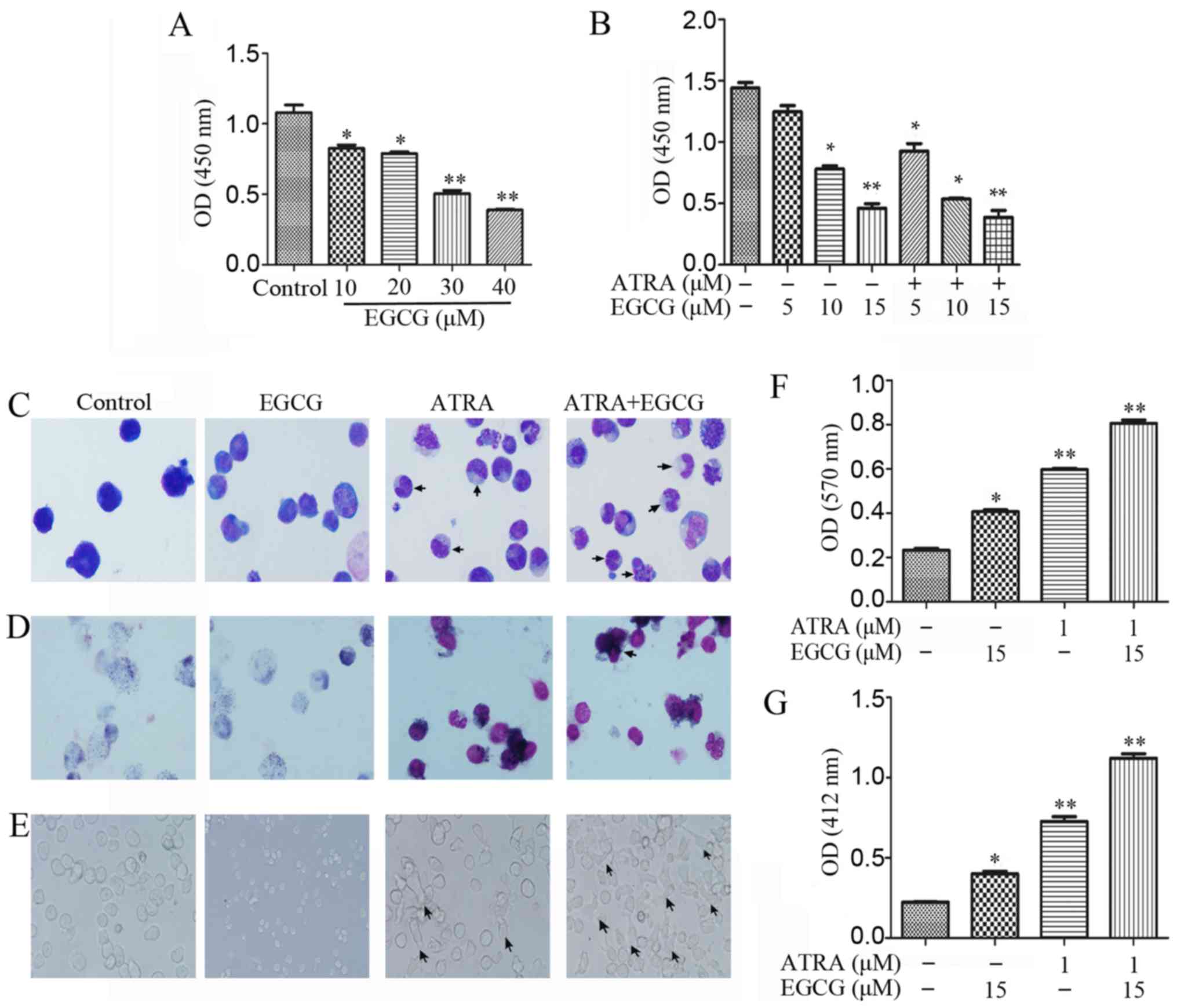
Treatment of NB4 cells with increasing
concentrations (0–40 µM) of EGCG for 48 h resulted in
diminished proliferation (Fig.
1A). To investigate the effects of EGCG in the presence of
ATRA, we measured cell viability following treatment with different
concentrations of EGCG combined with 1 µM ATRA (Fig. 1B). It has been shown previously
that ATRA induced differentiation of APL cells instead of promoting
proliferation. To verify the phenomenon, we investigated the
differentiation of NB4 cells in several ways. Wright-Giemsa
staining was used for morphological analysis, with the results
indicative of augmented differentiation both with ATRA alone and
when combined with EGCG (Fig. 1C).
The NBT reduction assay produced high staining intensities for
these treatments, suggestive of an advanced maturation status
(Fig. 1D and F). Moreover, we
observed the adherent status in a subpopulation of cells following
both treatment with ATRA alone or ATRA and EGCG (Fig. 1E). Respiratory burst activity was
measured to evaluate the oxidation respiratory function of the
differentiated cells. We observed that respiratory burst activity
was higher in the combined treatment than with ATRA alone,
suggesting an advanced cell maturation (Fig. 1G).
Enhancement of ATRA-induced upregulation
of PTEN and its redistribution by EGCG applied to differentiation
in NB4 cells
After 72 h of treatment with EGCG and ATRA, NB4
cells were examined by flow cytometric analysis of the myeloid
differentiation marker CD11b. The combined treatment significantly
increased CD11b level in comparison with ATRA alone (Fig. 2A and B). For the treatment of NB4
cells with 1 µM ATRA for 1, 2 and 3 days, the protein
expression levels of PTEN, CD11b and CCAAT-enhancer-binding protein
beta (C/EBPβ) were increased in a time-dependent manner while the
level of Akt phosphorylation was decreased (Fig. 2C). In addition, we consistently
observed that the increased PTEN expression level was closely
related to CD11b expression with both ATRA alone and the combined
treatment, and the same result was produced in HL-60 and THP-1
cells (Fig. 2E). This suggests
that PML and PML nuclear body (PML-NB) regulation of PTEN
localization may have relevance in APL. PML/RARα inhibits PTEN
expression in NB4 cells. Inhibition of proteasome function using
proteases inhibitor MG132 rescued PTEN from PML/RARα degradation
(Fig. 2D). It is known that
polyubiquitination of PTEN leads to its degradation in the
cytoplasm, while monoubiquitination is essential for important cell
functions, including cell growth, tumor suppression, cell
differentiation and migration (24,26).
Compared to ATRA alone, the combined treatment resulted in
increased PML expression and deubiquitinylation of PTEN was
inhibited, augmenting the level of nuclear PTEN (Fig. 3A). Consistent with previous
results, nuclear extracts had higher concentrations of PTEN than
cytoplasmic extracts (Fig.
3B).
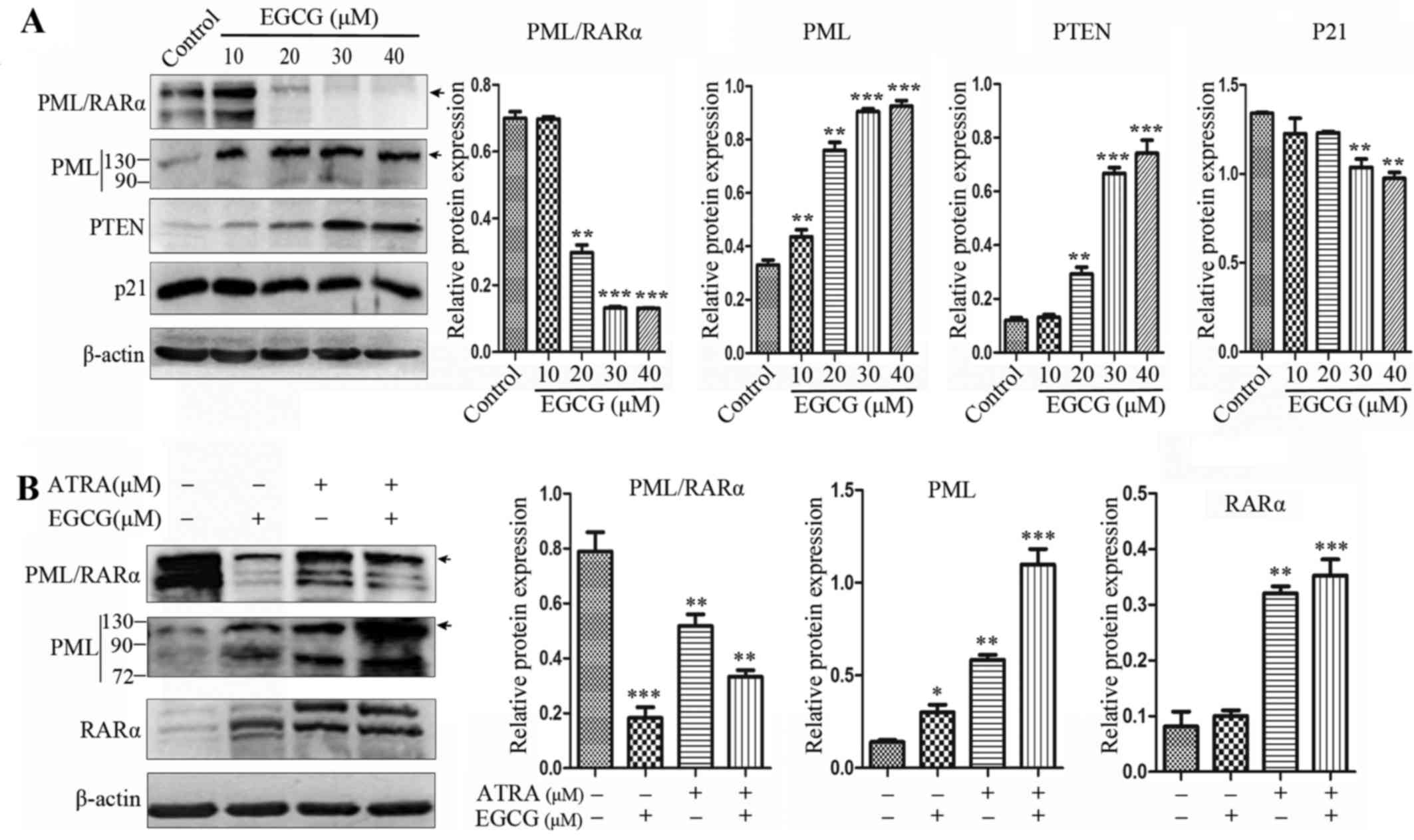
EGCG abrogates PML/RARα expression in NB4
cells
EGCG was shown to trigger PML/RARα degradation and
restore PML function (Fig. 4A).
The expression of PML/RARα and PML at the protein level was
assessed in NB4 cells, where PML/RARα expression was decreased in
cells receiving ATRA alone and the combined treatment, while the
protein expression level of PML increased. However, EGCG treatment
alone greatly abrogated PML/RARα protein expression in whole cell
extracts (Fig. 4B).
PTEN catalyzes the conversion of PIP3 to
PIP2, antagonizing PI3K signaling, inducing cell differentiation
and anti-proliferation
PI3K signaling regulates diverse cellular process,
including cell proliferation and survival, reducing the activity of
PTEN (33). Neutrophil functions,
such as phagocytosis, oxida-tive bursting, polarization, and
chemotaxis were augmented after treatment with PTEN inhibitor
SF1670 (34). In the present
study, we used PTEN inhibitor SF1670 to enhance the PI3K signaling
pathway and repress cell differentiation (Fig. 5A). To further assess the potency of
PTEN inhibition of the PI3K/Akt pathway, PI3K inhibitor LY294002
was used to pretreat NB4 cells, significantly augmenting the cell
differentiation and reducing the expression of p-Akt (Fig. 5B).
Cytoplasmic PTEN is monoubiquitinylated by E3
ubiquitin-protein ligase (NEDD4) and subsequently translocated into
the nucleus. Moreover, PTEN is further ubiquitinated in the
cytoplasm and degraded, by the proteasome. After combination
treatment with ATRA and EGCG, PML/RARα oncoprotein was degraded,
restoring PML levels and, inhibiting HAUSP-mediated
deubiquitinylation and nuclear export of PTEN. Nuclear PTEN can
shuttle back to the cytoplasm, or after deubiquitination, remains
nuclear and protected from cytoplasmic degradation. Importantly,
nuclear PTEN is still able to antagonize the Akt signaling pathway
and induces cell differentiation.
Discussion
APL was successfully treated with ATRA that triggers
PML/RARα fusion protein degradation and induces the maturation of
promyelocytes. However, a large proportion of patients with APL
still face relapse. Therefore, novel agents are essential to
improve the outcomes for APL patients. Previous studies showed that
EGCG induces hematopoietic malignant cell apoptosis by the
production of ROS in vitro. Moreover, EGCG is an
ATP-competitive inhibitor of both PI3K and mTOR, restraining cell
proliferation and Akt phosphorylation at Ser473 in human breast
adenocarcinoma (MDA-MB-231) and lung carcinoma (A549) cell lines
(35). Collectively, the body of
research strongly suggests that EGCG may represent a potential
target for treatment of pancreatic cancer via PTEN activation
regulating the PI3K/Akt/mTOR pathway (36). Research has shown that EGCG in
synergy with ATRA upregulated the expression of some
differentiation markers and differentiation-inducing genes, the
enhancing effects of co-treatment recommended additional mechanism
(15).
PTEN is one of the molecular pathways involved in
the balance between proliferation, differentiation and apoptosis
during hematopoiesis. It inhibits proliferation and promotes
differentiation as a tumor suppressor, including acute
promyelocytic leukemia (37). The
present study established an essential role for PTEN in the balance
between proliferation and differentiation of blood cells. However,
little is known about the molecular mechanism of cell
differentiation regulating by PTEN. In this study, we found that
EGCG potentiated NB4 cell differentiation in combination with ATRA,
at least in part, via the actions of PTEN. We showed that nuclear
PTEN is capable of inducing cell differentiation in leukemia blasts
in response to combination treatment. Thus, it is tempting to
assume a dual function for PTEN as a mediator of cell
differentiation in maturing APL cells. Cytoplasmic PTEN mainly
down-modulates Akt activation via regulation of PIP3 levels. In
many leukemia cell lines, the PTEN expression was suppressed, which
would contribute to activating PI3K/Akt signaling by suppressing
the conversion of PIP3 to PIP2, resulting in hyper-proliferation
and differentiation arrest. However, nuclear PTEN is protected from
degradation, which plays a direct role in the chromosome stability,
DNA repair and cell cycle arrest. Both residues facilitate the PTEN
binding to the membrane, thereby suppressing anchorage-independent
cell proliferation and tumor growth.
The present study highlights a role for PML and
PML-NBs in the regulation of PTEN localization, where disruption of
PTEN localization may have relevance in malignancies where PML and
PML-NBs are compromised, as found in APL. Treatment with ATRA or
arsenic trioxide (ATO) triggers PML/RARα degradation and restores
NBs, acting as part of a PML network to regulate PTEN
deubiquitination. Both mono-and poly-ubiquitinated PTEN exist in
vitro and in vivo, where mono-ubiquitination is
essential for increasing protein stability and nuclear localization
of mutant of PTEN (38). NEDD4 has
both oncogenic (PTEN degradation) and tumor suppressive (PTEN
shuttling) potential (26,39). Consistent with this study, we also
provide evidence for abrogation of PML/RARα expression by EGCG
alone in a different concentration set-up. We first investigated
that the combination treatment can promote degradation of PML/RARα
and restore PML expression. Partial repression of PML/RARα was
observed in the combined treatment with ATRA, but the expression of
PML was increased, and no differentiation blockade was observed in
the combined treatment. Since PML can suppress the function of
HAUSP, inhibiting the deubiquitination of PTEN and increasing the
level of nuclear PTEN, we aim to accentuate that
PML/ubiquitinated-PTEN/Akt signaling pathway is essential for NB4
cell differentiation.
In the present study, we assessed the combined
activity of EGCG and ATRA on NB4 cell differentiation. It was
determined that the two drugs in combination have strong
synergistic effects whereby differentiation is stimulated. We found
that PTEN protein was more strongly expressed in the nucleus than
in the cytoplasm during NB4 cell differentiation and preformed the
effects of PTEN and AKT on differentiation in acute promyelocytic
leukemia NB4 cells. To this end, findings have suggested that PTEN
inhibitor SF1670 and PI3K inhibitor LY294002 inhibited the basal
level and combination treatment level of PTEN and PI3K,
respectively, where the proportion of differentiation NB4 cells was
changed.
Taken together, EGCG may represent a novel effective
and safe drug for APL treatment, and could be used synergistically
with ATRA to promote degradation of PML/RARα and restore PML
expression, inhibiting the deubiquitination of PTEN and increasing
the level of nuclear PTEN. Therefore, we believe that the
PML/ubiquitinated-PTEN/Akt signaling pathway is essential for NB4
cell differentiation. Overall, our results report PTEN as a key
player in both the cell death response and enhancement of
neutrophil differentiation. Our next investigation is aimed at PTEN
and PML to investigate the differentiation of APL cells.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81171658)
and the Natural Science Foundation Project of CQ CSTC (grant no.
2011BA5037).
References
|
1
|
Melnick A and Licht JD: Deconstructing a
disease: RARalpha, its fusion partners, and their roles in the
pathogenesis of acute promyelocytic leukemia. Blood. 93:3167–3215.
1999.PubMed/NCBI
|
|
2
|
Lafage-Pochitaloff M, Alcalay M, Brunel V,
Longo L, Sainty D, Simonetti J, Birg F and Pelicci PG: Acute
promyelocytic leukemia cases with nonreciprocal PML/RARα or
RARα/PML fusion genes. Blood. 85:1169–1174. 1995.PubMed/NCBI
|
|
3
|
Mozziconacci MJ, Liberatore C, Brunel V,
Grignani F, Arnoulet C, Ferrucci PF, Fernandez F, Sainty D, Pelicci
PG, Birg F, et al: In vitro response to all-trans retinoic acid of
acute promyelocytic leukemias with nonreciprocal PML/RARA or
RARA/PML fusion genes. Genes Chromosomes Cancer. 22:241–250. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gianni M, Fratelli M, Bolis M, Kurosaki M,
Zanetti A, Paroni G, Rambaldi A, Borleri G, Rochette-Egly C, Terao
M, et al: RARalpha2 and PML-RAR similarities in the control of
basal and retinoic acid induced myeloid maturation of acute myeloid
leukemia cells. Oncotarget. 8:37041–37060. 2016.
|
|
5
|
Vitaliano-Prunier A, Halftermeyer J,
Ablain J, de Reynies A, Peres L, Le Bras M, Metzger D and de Thé H:
Clearance of PML/RARA-bound promoters suffice to initiate APL
differentiation. Blood. 124:3772–3780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marasca R, Zucchini P, Galimberti S,
Leonardi G, Vaccari P, Donelli A, Luppi M, Petrini M and Torelli G:
Missense mutations in the PML/RARalpha ligand binding domain in
ATRA-resistant As2O3 sensitive relapsed acute
promyelocytic leukemia. Haematologica. 84:963–968. 1999.PubMed/NCBI
|
|
7
|
Fatemi A, Safa M and Kazemi A: MST-312
induces G2/M cell cycle arrest and apoptosis in APL cells through
inhibition of telomerase activity and suppression of NF-kappaB
pathway. Tumour Biol. 36:8425–8437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Y, Kumazoe M, Bae J, Yamada S, Takai
M, Hidaka S, Yamashita S, Kim Y, Won Y, Murata M, et al: Green tea
polyphenol epigallocatechin-O-gallate induces cell death by acid
sphingomyelinase activation in chronic myeloid leukemia cells.
Oncol Rep. 34:1162–1168. 2015.PubMed/NCBI
|
|
9
|
Iwasaki R, Ito K, Ishida T, Hamanoue M,
Adachi S, Watanabe T and Sato Y: Catechin, green tea component,
causes caspase-independent necrosis-like cell death in chronic
myelogenous leukemia. Cancer Sci. 100:349–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vezina A, Chokor R and Annabi B: EGCG
targeting efficacy of NF-kappaB downstream gene products is
dictated by the monocytic/macrophagic differentiation status of
promyelocytic leukemia cells. Cancer Immunol Immunother.
61:2321–2331. 2012. View Article : Google Scholar
|
|
11
|
Lee HS, Jun JH, Jung EH, Koo BA and Kim
YS: Epigalloccatechin-3-gallate inhibits ocular neovascularization
and vascular permeability in human retinal pigment epithelial and
human retinal microvascular endothelial cells via suppression of
MMP-9 and VEGF activation. Molecules. 19:12150–12172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu L, Hou L, Gu S, Zuo X, Meng D, Luo M,
Zhang X, Huang S and Zhao X: Molecular mechanism of
epigallocatechin-3-gallate in human esophageal squamous cell
carcinoma in vitro and in vivo. Oncol Rep. 33:297–303. 2015.
|
|
13
|
Tsukamoto S, Kumazoe M, Huang Y, Lesnick
C, Kay NE, Shanafelt TD and Tachibana H: SphK1 inhibitor
potentiates the anti-cancer effect of EGCG on leukaemia cells. Br J
Haematol. 178:155–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amin AR, Karpowicz PA, Carey TE, Arbiser
J, Nahta R, Chen ZG, Dong JT, Kucuk O, Khan GN, Huang GS, et al:
Evasion of anti-growth signaling: A key step in tumorigenesis and
potential target for treatment and prophylaxis by natural
compounds. Semin Cancer Biol. 35(Suppl): S55–S77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Britschgi A, Simon HU, Tobler A, Fey MF
and Tschan MP: Epigallocatechin-3-gallate induces cell death in
acute myeloid leukaemia cells and supports all-trans retinoic
acid-induced neutrophil differentiation via death-associated
protein kinase 2. Br J Haematol. 149:55–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Chen QS, Xu PP, Qian Y, Wang AH,
Xiao D, Zhao Y, Sheng Y, Wen XQ and Zhao WL: Catechins induced
acute promyelocytic leukemia cell apoptosis and triggered PML-RARα
oncoprotein degradation. J Hematol Oncol. 7:752014. View Article : Google Scholar
|
|
17
|
Bermúdez Brito M, Goulielmaki E and
Papakonstanti EA: Focus on PTEN regulation. Front Oncol. 5:1662015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whiteman DC, Zhou XP, Cummings MC, Pavey
S, Hayward NK and Eng C: Nuclear PTEN expression and
clinicopathologic features in a population-based series of primary
cutaneous melanoma. Int J Cancer. 99:63–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Liu J, Zheng J, Du W, He Y, Liu W
and Huang S: A reappraisal by quantitative flow cytometry analysis
of PTEN expression in acute leukemia. Leukemia. 21:2072–2074. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choorapoikayil S, Kers R, Herbomel P,
Kissa K and den Hertog J: Pivotal role of Pten in the balance
between proliferation and differentiation of hematopoietic stem
cells in zebrafish. Blood. 123:184–190. 2014. View Article : Google Scholar
|
|
21
|
Dragojlovic-Munther M and Martinez-Agosto
JA: Multifaceted roles of PTEN and TSC orchestrate growth and
differentiation of Drosophila blood progenitors. Development.
139:3752–3763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JE, Lim MS, Park JH, Park CH and Koh
HC: PTEN promotes dopaminergic neuronal differentiation through
regulation of ERK-dependent inhibition of S6K signaling in human
neural stem cells. Stem Cells Transl Med. 5:1319–1329. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neri LM, Borgatti P, Tazzari PL, Bortul R,
Cappellini A, Tabellini G, Bellacosa A, Capitani S and Martelli AM:
The phosphoinositide 3-kinase/AKT1 pathway involvement in drug and
all-trans-retinoic acid resistance of leukemia cells. Mol Cancer
Res. 1:234–246. 2003.PubMed/NCBI
|
|
24
|
Huang J, Yan J, Zhang J, Zhu S, Wang Y,
Shi T, Zhu C, Chen C, Liu X, Cheng J, et al: SUMO1 modification of
PTEN regulates tumorigenesis by controlling its association with
the plasma membrane. Nat Commun. 3:9112012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morotti A, Panuzzo C, Crivellaro S, Carrà
G, Guerrasio A and Saglio G: HAUSP compartmentalization in chronic
myeloid leukemia. Eur J Haematol. 94:318–321. 2015. View Article : Google Scholar
|
|
26
|
Trotman LC, Wang X, Alimonti A, Chen Z,
Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo
C, Erdjument-Bromage H, et al: Ubiquitination regulates PTEN
nuclear import and tumor suppression. Cell. 128:141–156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song MS, Salmena L, Carracedo A, Egia A,
Lo-Coco F, Teruya-Feldstein J and Pandolfi PP: The
deubiquitinylation and localization of PTEN are regulated by a
HAUSP-PML network. Nature. 455:813–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trotman LC, Alimonti A, Scaglioni PP,
Koutcher JA, Cordon-Cardo C and Pandolfi PP: Identification of a
tumour suppressor network opposing nuclear Akt function. Nature.
441:523–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferruzzi L, Turrini E, Burattini S,
Falcieri E, Poli F, Mandrone M, Sacchetti G, Tacchini M, Guerrini
A, Gotti R, et al: Hemidesmus indicus induces apoptosis as well as
differentiation in a human promyelocytic leukemic cell line. J
Ethnopharmacol. 147:84–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SH, Danilenko M and Kim TS:
Differential enhancement of leukaemia cell differentiation without
elevation of intracellular calcium by plant-derived sesquiterpene
lactone compounds. Br J Pharmacol. 155:814–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Misra S, Selvam AK, Wallenberg M, Ambati
A, Matolcsy A, Magalhaes I, Lauter G and Björnstedt M: Selenite
promotes all-trans retinoic acid-induced maturation of acute
promyelocytic leukemia cells. Oncotarget. 7:74686–74700.
2016.PubMed/NCBI
|
|
32
|
Song H, Li L, Zhong L, Yang R, Jiang K,
Yang X and Liu B: NLS-RARα modulates acute promyelocytic leukemia
NB4 cell proliferation and differentiation via the PI3K/AKT
pathway. Mol Med Rep. 14:5495–5500. 2016.PubMed/NCBI
|
|
33
|
Goebbels S, Wieser GL, Pieper A, Spitzer
S, Weege B, Yan K, Edgar JM, Yagensky O, Wichert SP, Agarwal A, et
al: A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte
precursor recruitment and myelination. Nat Neurosci. 20:10–15.
2017. View
Article : Google Scholar
|
|
34
|
Li Y, Prasad A, Jia Y, Roy SG, Loison F,
Mondal S, Kocjan P, Silberstein LE, Ding S and Luo HR: Pretreatment
with phosphatase and tensin homolog deleted on chromosome 10 (PTEN)
inhibitor SF1670 augments the efficacy of granulocyte transfusion
in a clinically relevant mouse model. Blood. 117:6702–6713. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Aller GS, Carson JD, Tang W, Peng H,
Zhao L, Copeland RA, Tummino PJ and Luo L: Epigallocatechin gallate
(EGCG), a major component of green tea, is a dual
phosphoinositide-3-kinase/mTOR inhibitor. Biochem Biophys Res
Commun. 406:194–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu S, Wang XJ, Liu Y and Cui YF:
PI3K/AKT/mTOR signaling is involved in
(−)-epigallocatechin-3-gallate-induced apoptosis of human
pancreatic carcinoma cells. Am J Chin Med. 41:629–642. 2013.
View Article : Google Scholar
|
|
37
|
Li RA, Traver D, Matthes T and Bertrand
JY: Ndrg1b and fam49ab modulate the PTEN pathway to control T-cell
lymphopoiesis in the zebrafish. Blood. 128:3052–3060.
2016.PubMed/NCBI
|
|
38
|
Yang JM, Schiapparelli P, Nguyen HN,
Igarashi A, Zhang Q, Abbadi S, Amzel LM, Sesaki H,
Quiñones-Hinojosa A and Iijima M: Characterization of PTEN
mutations in brain cancer reveals that pten mono-ubiquitination
promotes protein stability and nuclear localization. Oncogene.
36:3673–3685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ciechanover A: Proteolysis: From the
lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol.
6:79–87. 2005. View
Article : Google Scholar : PubMed/NCBI
|