Post-translational protein modification (also known
as PTM) is one of the important regulatory mechanisms of cellular
proteins with a number of biological functions. Any protein in the
proteome can be modified following translation or during
translation. Different types of modifications alter the charge
state, hydrophobicity, conformation and stability of the protein,
and ultimately affect its function. Protein modification is
reversible and has different functions in different organelles
(1). Specific protein modification
controls almost all physiological processes, including immune
function (2), and the position,
duration and intensity of exact physiological process to ensure the
rapid and dynamic response of the cells on the extracellular and
intracellular stimulation (3). To
date, >450 unique protein modifications have been identified,
including phosphorylation, acetylation, ubiquitination and
SUMOylation, which can alter the activity of target proteins,
intracellular distribution, protein interactions and protein
longevity through post-translational modification (4). Phosphorylation is one of the most
common and most extensively studied protein modifications and there
are >500 different kinases in mammals to catalyze protein
phos-phorylation (5).
Phosphorylation occurs mainly in the serine, threonine and tyrosine
residues of target substrate proteins (6). The stability of proteins, protein
interactions, cellular localization of proteins and enzyme activity
are determined according to the different substrates and
phosphorylation sites (7).
Ubiquitination is also a widely studied method of
post-translational protein modification which regulates many
biological processes, including immune responses (8), apoptosis (9) and cancer (10). It is mainly catalyzed by three
enzymes, the ubiquitin activating enzyme E1, the ubiquitin
conjugating enzyme E2 and the ubiquitin ligase E3, in which the E3
ligase determine the substrate specificity. Ubiquitin molecules
bind to target proteins to form poly-ubiquitin chains, which can be
identified by the 26S proteasome, causing protein degradation. The
specific regulatory mechanism mediated by ubiquitination varies
with the different structure of the poly-ubiquitin chain (11). Competing with ubiquitination,
acetylation is a modification of the lysine residues of the target
protein (12). Both serine and
threonine are substrates of acetylation and phosphorylation.
SUMOylation has attracted increasing attention as a
widely used post-translational protein modification. As this
pathway exists in almost all eukaryotes and is essential for the
maintenance of genomic integrity, transcriptional regulation, gene
expression and the regulation of intracellular signal transduction.
The association between SUMOylation and other post-translational
protein modifications, such as phosphorylation (13), ubiquitination (14,15),
methylation (16) and acetylation
(17-20) is currently one of the most
important issues. SUMOylation regulates a number of biological
processes, including DNA damage repair, immune responses,
carcinogenesis, cell cycle progression and apoptosis. Small
ubiquitin-like modifier (SUMO) is also involved in the regulation
of mitochondrial division, ion channels and biological rhythms.
Therefore, multiple layers of regulation or SUMOylation may play
important role in the complex protein regulatory networks. The
disorder of SUMOylation can lead to the development of certain
diseases and tumors. SUMO can thus be used as a potential
therapeutic target for cancer (21-25).
SUMO protein is a type of protein which is similar
to ubiq-uitin in molecular structure. However, the amino sequence
and surface charge distribution of SUMO differ from those of
ubiquitin, and thus they have different functions. The amino acid
sequence homology of SUMO and ubiquitin molecules is only 18%, but
the three-dimensional structure of the two is very similar which
contains a typical ββαββαβ fold and a double glycerin C terminal
(26). SUMO molecules are highly
conserved in evolution, widely found in protozoa, metazoan, plants
and fungi. SUMO protein was first discovered in 1996 and there are
three types of SUMO isoforms in mammals, which are SUMO-1, SUMO-2
and SUMO-3 (27,28). SUMO-2 and SUMO-3 are very similar
as regards their amino acid sequence and are often written as
SUMO-2/3. SUMO-1 mainly modifies the physiological state proteins,
while SUMO-2/3 mainly modifies stress proteins (29). SUMO4 belongs to another SUMO
protein family. SUMO4 mRNA is only found in the kidneys, spleen and
lymph nodes, while SUMO 1-3 is widely expressed in human tissues.
SUMO4-related research is limited and its protein expression in
vivo has not yet been fully determined (30,31).
Eight types of SUMO protein isoforms have been found in
Arabidopsis thaliana (32).
Similar to ubiquitin, SUMO protein can modify one or more lysine
residues of the target protein, and the poly-SUMO protein chain is
formed on the substrate molecule (33).
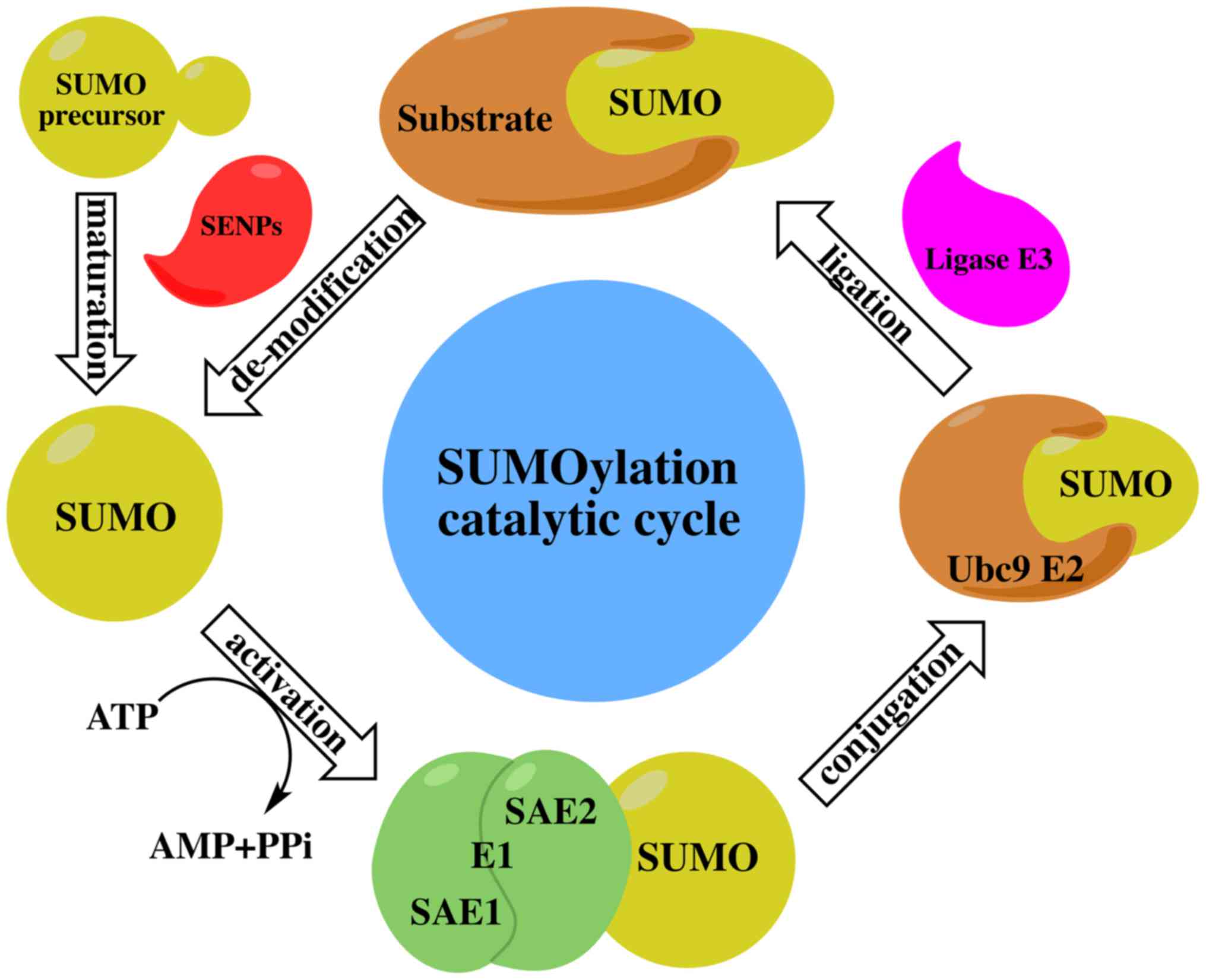
The SUMO cycle and the ubiquitin cycle are similar,
but distinct in function. The ubiquitin-modified target protein
mainly makes the target protein recognized and is degraded by the
proteasome, while the SUMO-modified protein is more stable and
SUMOylation modulates protein-protein interactions in order to
mediate the localization and functional regulation of target
proteins. All SUMO proteins undergo the same enzyme catalytic
mechanism with substrate protein attachment or dissociation. The
SUMO cycle consists of maturation, activation, conjugation,
ligation and de-modification (Fig.
1) as described below:
The SUMO protein has a molecular weight of about 11
kDa and is an inactive precursor molecule at the beginning. It is
activated by ubiquitin-like specific protease 1 (Ulp1) or
sentrin-specific protease 1 (SENP; in humans) to cut the four amino
acids at the C terminal and exposed a terminal diglycine GG motif
(34).
SUMO E1-activating enzyme is a 110 kDa protein that
contains two subunits, which are SUMO-activating enzyme E1 (SAE1 or
Aos1) and SUMO-activating enzymeE2 (SAE2 or Uba2). These two
subunits usually form a heterodimer of SAE1-SAE2 or Aos1-Uba2. The
heterodimer is mediated by ATP-dependent SUMO adenine nucleotide
intermediates to active the SUMO protein by connecting its C
terminal carboxyl group with the cysteine residue of SAE2/Uba2
through the thioester bond (35).
There is only one SUMO-conjugating enzyme E2 (SUMO
E2 or Ubc9) which can be located at the cytoplasmic side of the
nuclear pore complex (NPC) or the nucleoplasm side of the NPC. The
activated SUMO protein is transferred to the conserved number 93
cysteine residue of Ubc9 via transesterification reaction to form
an E2-SUMO thioester compound. Finally, Ubc9 completes the
SUMOylation by connecting the SUMO molecules to the lysine residues
of target proteins via the isopeptide bond (36).
Although experiments have indicated that SUMO E1 and
Ubc9 are sufficient to SUMOylate the substrates, SUMO E3 ligase is
essential in the process of SUMO protein targeting the substrate
(37). SUMO E3 ligase recognizes
substrates and participates in the promotion of SUMOylation through
two different mechanisms. SUMO E3 ligase promotes SUMO protein
dissociation from Ubc9 by stabilizing the substrate and SUMO-E2
complex contact, and then connecting the SUMO protein to the
substrate (38). Through the
accurate positioning of the SUMO-E2 complex, SUMO E3 ligase
decreases the distance between the thioester bond of SUMO-E2
complex and the substrate lysine residue, making it close enough to
enhance the specificity of the substrate (39).
There are approximately three classes of SUMO E3
ligase. Protein inhibitor of activated STAT (PIAS 1-4) (40) and MMS21 (NSE2) (41) are one class of SUMO E3 ligase
existing in mammalian cells. Ran binding protein 2 (RanBP2 or
NUP358) is kind of long cytoplasmic fragments of the nuclear pore
complex. The activity of the RanBP2 SUMO E3 ligase is located in
approximately 100 residues of the internal repeat domain (termed
R1-M-IR2). Which are related to SUMO-modified RanGAP1, as well as
Ubc9 (38). This ternary complex
indicates the multi-subunit function of RanBP2 SUMO E3 ligase to
stimulate SUMO E2 dissociation with SUMO protein (42). Another type of SUMO E3 ligase is
poly-comb protein PC2 or chromobox4 (CBX4), which can promote the
ligation of SUMO protein and C-terminal binding protein 1 (CtBP1).
It also contributes to the localization of activated Ubc9 and
target proteins (43,44). Tripartite motif (TRIM) is the
fourth class of SUMO E3 ligase and includes at least eight
different TRIM family members (45). TRIM enzyme requires both the RING
domain and the B-boxes zinc binding domain to stimulate the SUMO
complex and target protein binding (46).
SUMOylation is a reversible process in which
de-modification involves the SUMO terminal glycine being removed
from the lysine residues of the target protein by specific
proteases. Thus far, the SUMO protease family has been found to
have six SENPs (SENP-1,2,3,5,6,7) in mammals or humans (47). SENP1 and SENP2 can dissociate SUMO1
and SUMO2/3 proteins, while SENP3 and SENP5 are mainly dissociated
by SUMO2/3 protein. SUMO2/3 poly chain is dissociated by SENP6 and
SENP7 (48). SENPs are mainly
located in nuclear or nuclear-related structures. It has recently
been found that SUMO protease includes desumoylating isopeptidase 1
(DESI1), desumoylating isopeptidase 2 (DESI2) and
ubiquitin-specific protease-like 1 (USPL1) (49). DESI1 and DESI2 are also expressed
in the cytoplasm, and USPL1 dissociation enzymes are found in Cajal
bodies in the nucleus. The localization of these enzymes in the
cell differs and is substrate-specific. Nuclear SENP does not
catalyze the deSUMOylation of the cytoplasmic protein. There are
two deSUMOylation isomerases, Ulp1 and Ulp2, in yeast and
prokaryotes. The two enzymes have different substrate specificity
and distribution in cells. Ulp1 is mainly distributed around the
nucleus. Experiments have indicated that Ulp1 is connected with the
nuclear pore, while Ulp2 is unevenly distributed in the cytoplasm.
One of the basic functions of Ulp1 is to process the C end of SUMO
precursor and Ulp2 is mainly mediated deSUMOylation (50-52).
SENP8 is a novel SUMO protease which mainly processes the full
length NEDD8 to the mature form or neddylation (53).
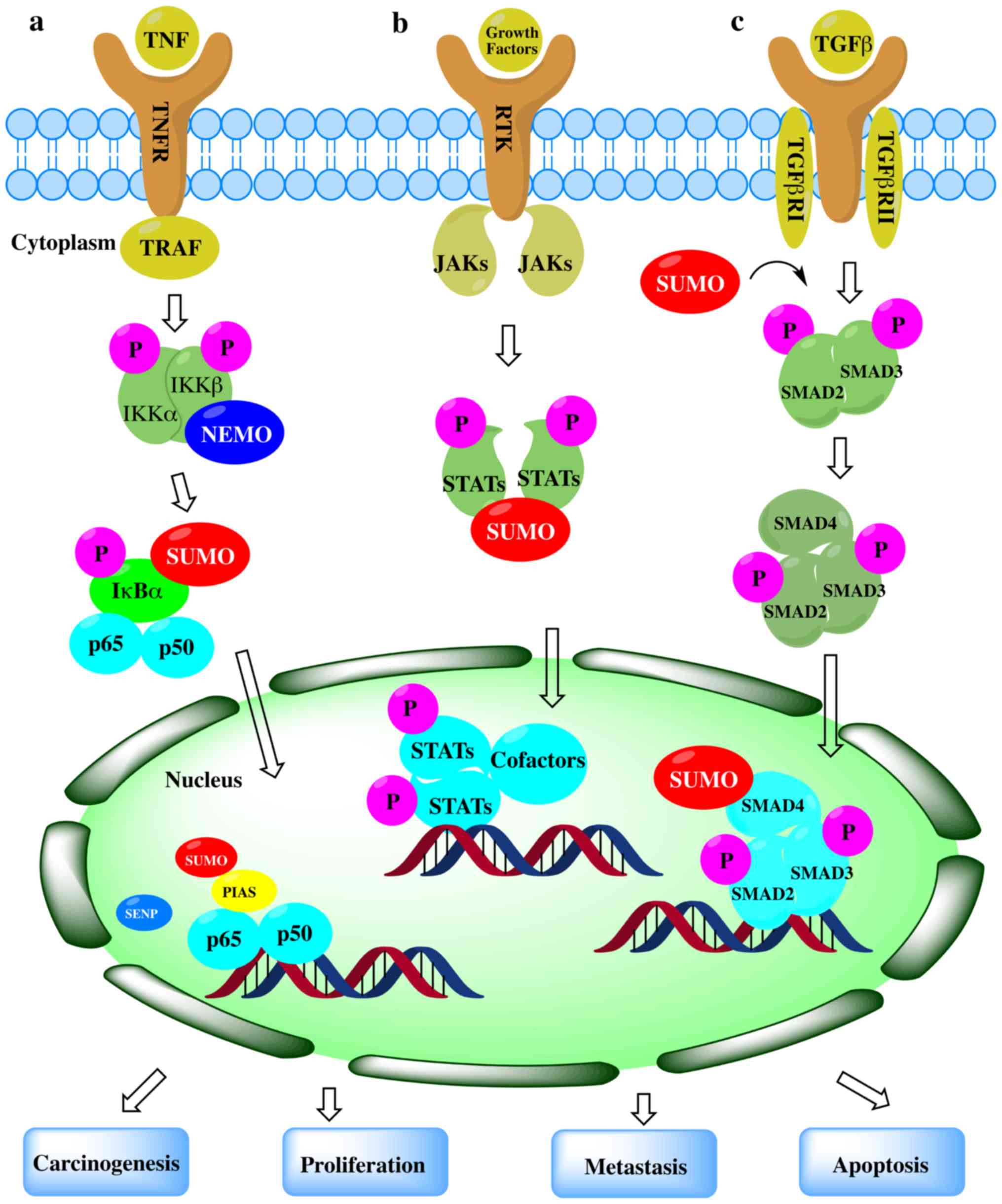
Sumoylation is an important post-translational
modification that fine-tunes virtually all cell function and
pathological processes. The important role of SUMOylation in human
tumorigenesis has gradually emerged. Alterations in the expression
or activity of different components in the SUMO signaling pathway
may alter the nature of the cell completely. The SUMO pathway can
induce cell proliferation, apoptosis resistance and the potential
of metastasis by regulating proteins involved in the carcinogenesis
(54-58) (Fig.
2). Abnormal SUMOylation can lead to the development of a
number of diseases, including cancer. Although the association
between the expression of various components in the SUMO signaling
pathway and cancer progression or metastasis is not yet fully
understood, an increasing number of studies have shown that
SUMOylation plays an important role in cancer (25, and refs. therein).
SUMOylation is widely involved in DNA damage
response (DDR) and regulates DNA damage sensing and repair protein,
which is mainly found in the nucleus (59), particularly in chromatin (60) and nuclear bodies (61). SUMO-modified proteins are
ultimately required to perform specific targeting functions.
SUMOylation can block the binding sites of substrate proteins and
cell interactions, and can influence the function of the proteins
by blocking protein-interaction domains. For example, SUMOylation
blocks the dimerization of FoxM1 and enhances its transcriptional
activity. As the SUMOylation of FoxM1 peaks during the G2 and M
phase, these findings contribute to the understanding of the role
of SUMOylation during cell-cycle progression (62). SUMO attachment severely impairs
E2-25K (UBE2K) ubiquitin thioester and then disrupts the activity
of ubiquitin-conjugating enzyme E2-25K (Hip2). It has been found
that the protein secondary structure elements are also part of SUMO
attachment signals (63).
SUMOylation can also produce new docking sites to facilitate the
interaction with other proteins. RAD51 interacts non-covalently
with SUMO and it interacts more efficiently with SUMO-modified
bloom syndrome protein (BLM) at damaged replication forks in
vitro. BLM SUMOylation can promote RAD51 function (64). The covalent binding of SUMO protein
to the substrate can lead to the conformational change of the
substrate, which affects the protein interaction, enzyme activity
and cell localization.
Some proteins contain SUMO-interaction motifs (SIMs)
and these functional domains usually contain a number of
hydrophobic residues, some acidic residues or regions nearby. SIMs
can facilitate the non-covalent interaction between the target
protein and SUMO protein (65).
They can also promote the interaction between SIM-containing
proteins and covalent SUMOylation proteins (66). For example, SIMs can enhance the
function of SUMO E3 ligase chromebox homologue 4 (CBX4) (67). Protein complexes, nucleosomes,
chromatin and other nuclear structures can become more robust
through multiple SUMO-SIM interactions. This spatial SUMO
regulation through substrate binding and dissociation may be an
important factor in the specificity of dynamic SUMO systems, since
the enzyme structure of the SUMO system is not as complex as the
ubiquitin system (68).
Based on these mechanisms, SUMO can rapidly regulate
a number of cellular processes, such as gene expression,
transcriptional regulation (69),
DDR (70), nucleocytoplasmic
transport (71), cell signaling,
mRNA maturation, meiosis, mitosis, chromatin remodeling, ion
channel activity, cell cycle regulation (72) and cell growth and apoptosis
(73). Since SUMO affects the
process and function of most intracellular pathways, the SUMO
proteins play an important role in certain diseases, particularly
cancer.
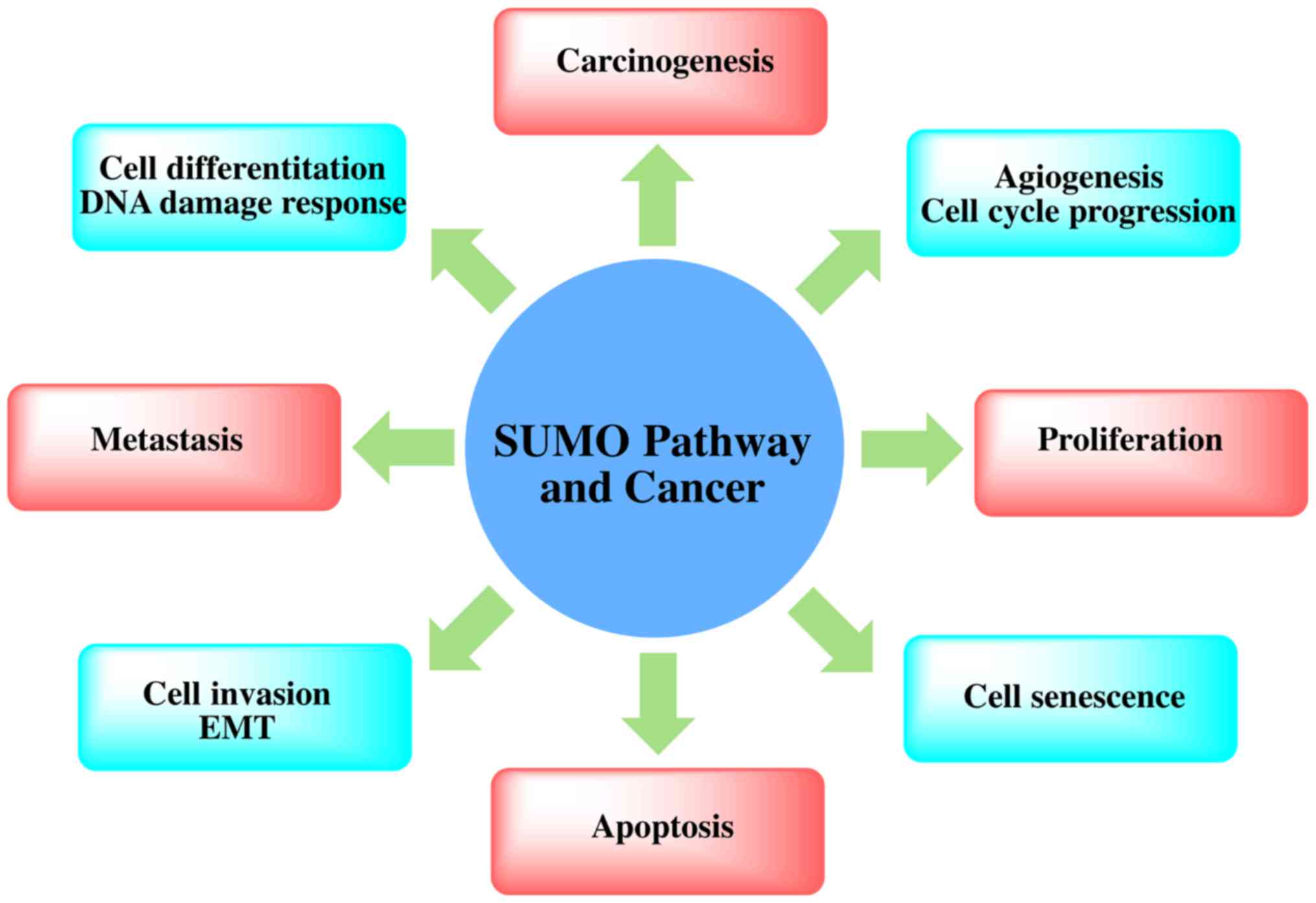
Recent proteomics data have confirmed that there are
at least 1,000 types of human-derived proteins, and as many as
3,000 sites will be modified by SUMO (74). Although the function of these
SUMO-modified proteins is not yet clear, many functions are found
in all normal cells, including some cancer hallmark functions.
SUMOylated proteins are directly or indirectly related to cell
apoptosis, inflammatory reactions, immune regulation, DNA damage
signaling pathways and gene network regulation, angiogenesis and
metastasis-related regulation, the replication of DNA, cell
division and cell cycle regulation (22) (Fig.
3). The mechanism of SUMOylation in telomere maintenance and
chromosome replication has a further connection with carcinogenesis
(75).
The key to cell differentiation is the synthesis of
specific proteins. The essence of synthetic-specific proteins is
the differential expression of tissue-specific genes (or luxury
genes) in time and space due to the combinatorial regulation of
gene expression. Thus, cell differentiation is the result of gene
expression. Cell carcinogenesis is the performance of normal cell
differentiation which is out of control (76). However, the little is known about
the mechanisms through which SUMOylation controls gene
activity.
Metal toxicants, such as chromium and arsenic are
closely related to carcinogenesis through the upregulation of the
overall modification of many cellular proteins by SUMO2/3. Androgen
receptor (AR) has an established a role in prostate carcinogenesis
(77). SUMOylation has diverse
effects on AR transcriptional activity via the direct modification
of the AR or AR co-regulators (78,79).
DNA endonuclease Mus81 is involved in homologous recombination
repair, and is among the identified proteins whose SUMOylation is
increased following treatment with arsenic trioxide
(As2O3). Mus81 SUMOylation is important for
normal mitotic chromosome congression, and cells expressing
SUMO-resistant Mus81 mutants exhibit compromised DNA damage
responses related to tumorigenesis (80). SENP1 exhibits carcinogenic
properties associated with the promotion of androgen
receptor-dependent and -independent cell proliferation, the
stabilization of hypoxia-inducible factor (HIF)1α, an increase in
vascular endothelial growth factor (VEGF) expression and the
support of angiogenesis (81).
Ubc9, PIAS1 and SENP1 are highly expressed in human prostate cancer
specimens and are associated with hypoxia-inducing factor 1alpha
(HIF1α) expression, and SENP1 enhances prostate epithelial cell
proliferation. SENP1 overexpression induces the transformation of
the normal prostate gland to high-grade prostatic intraepithelial
neoplasia both in vitro and in vivo (82). Increased SUMOylation and
deSUMOylation levels at the same time perhaps indicate that cancer
cells need to 'speed up' the SUMO cycle, to increase SUMO-modified
proteins and to modify the frequency and increase the turnover of
the enzyme.
SUMOylation can stabilize PES1 by inhibiting its
ubiquitination, which is stimulated by estrogen (83). PES1 is a component of the PeBoW
complex which is required for the formation of the 60S ribosomal
subunits. The control of ribosome biogenesis is a critical cellular
nodal point and the deregulation of ribosome may cause
carcinogenesis (84). DNA
replication is highly conserved and controlled, the dysregulation
of which can lead to genomic instability and even carcinogenesis.
USP7 as a replisome-enriched SUMO deubiquitinase which can lead to
the deubiquitination of SUMOylated proteins at the replication fork
and plays a pivotal role in the control of DNA replication
(85,86). Epithelial-mesenchymal transition
(EMT) plays an essential role in organogenesis and contributes to a
host of pathologies, including carcinogenesis. SIRT1 plays an
important role in tumorigenesis and opposed ovarian cancer
metastasis by impeding EMT both in vitro and in vivo.
SUMO E3 ligase PIAS4 is induced by hypoxia and prevents Sp1 from
binding to the SIRT1 promoter in cancer cells (87). Amplified in breast cancer 1 (AIB1)
is a transcriptional co-activator of nuclear receptors, which is
implicated in breast carcinogenesis. AIB1 is covalently modified by
SUMO-1 and PIAS1 may play a crucial role in the regulation of AIB1
transcriptional activity through SUMOylation (88). The extracellular signal-regulated
kinase (ERK) and mitogen-activated protein kinase (MAPK) cascade
(Raf-MEK-ERK) mediates mitogenic signaling, and is frequently
hyperactivated by Ras oncogenes in human cancer and is
related to carcinogenesis (89).
Oncogenic Ras efficiently activates the ERK pathway both by
activating Raf and by inhibiting MEK SUMOylation, thereby inducing
carcinogenesis (90). The SMC
protein complexes play important roles in chromosome dynamics and
the MAGEG1 protein is part of this complex. MAGE proteins play
important roles in carcinogenesis and apoptosis (91).
Heat shock proteins (HSPs) constitute a large family
of proteins involved in protein folding and maturation, which play
a significant role in cellular proliferation, differentiation and
carcinogenesis (92). A recent
study found that HSPgp96 promoted hepatocellular carcinogenesis
(93). Heat shock (HS) is type of
stress response of cells and can increase the levels of SUMO
conjugates (94). HS-triggered
SUMOylation targets promoters and enhancers of actively transcribed
genes where it restricts the transcriptional activity of the
HS-induced genes using ChIP-seq in cells. The silencing of
SUMOylation machinery either by the depletion of UBC9 or PIAS1
enhances the expression of HS-induced genes (95). The deSUMOylation of lens
epithelium-derived growth factor (LEDGF) by Sumo-specific
protease-1 regulates its transcriptional activation of small heat
shock protein and the cellular response (96). Heat shock can cause the
accumulation of SUMO-2/3 conjugates which is blocked by proteasome
inhibition (97). HSP27 increases
the number of cell proteins modified by SUMO-2/3 and blocks heat
shock factor 1 (HSF1) transactivation capacity (98). SUMO-2 and SUMO-3 are required for
cells to survive heat shock. In summary, SUMO is polymerized into
polySUMO chains in response to heat shock and is redistributed to a
regulated wide range of protein functions, including folding,
transcription, translation, cell cycle regulation, DNA replication
and apoptosis (99). SUMO-1
localization on chromatin is dynamic throughout the cell cycle by
using chromatin affinity purification coupled with next-generation
sequencing, which is consistent with the reversible nature of
SUMOylation (100). SUMOylation
occurs on many target promoters to regulate transcriptional
activation which is consistent with the rules of group modification
(101).
Ubc9 is overexpressed in ovarian cancer cell lines
and tissues and is associated with the downregulation of Bcl-2 in
tumorigenesis (106). HPV
oncoproteins induce the upregulation of UBC9 during the very early
steps of head and neck tumorigenesis via the autophagic process
(107). Akt SUMOylation has been
found to regulate cell proliferation and tumorigenesis through the
direct phosphorylation of Ubc9 at Thr35 and the phosphorylation of
SUMO1 at Thr76 (108). Akt
SUMOylation is promoted by SUMO E3 ligase PIAS1 and is reversed by
SENP1. The expression of PIAS1 and SUMO1 increases Akt activity,
whereas the expression of SENP1 reduces Akt1 activity. The loss of
SUMOylation markedly reduces Akt1 E17K-mediated cell proliferation
and tumorigenesis (109). Tissue
microarray analysis has revealed that the expression of Ubc9 is
increased during the transformation process of normal colonic
epithelial cells into the early stages of cancer and subsequently
into advanced colon cancer. Ubc9 is also upregulated in advanced
melanomas, prostate intraepithelial neoplasia and primary prostate
cancer. The level of Ubc9 is five-fold higher in tumor tissue than
in matched normal breast tissue. However, the expression of Ubc9 is
downregulated in metastatic breast cancer, prostate cancer and lung
cancer, compared with corresponding normal tissues and primary
tumors (110). MicroRNAs (miRNAs
or miRs) miR-30e and miR-214 can negatively regulate UBC9
expression, and more importantly, the expression of these two
factors in some tumors is downregulated (111).
TRIM family proteins have both SUMO E3 ligase and
ubiquitin E3 ligase activities, and are involved in multiple
cellular processes including carcinogenesis (45,115,116). TRIM contains a common domain
structure composed of a RING finger, one or two B-box motifs and a
coiled-coil motif. TRIM29 overexpression enhances cell growth and
transforming activity and promotes tumor growth by reducing the
acetylation of p53 (117). TRIM40
promotes the neddylation of inhibitor of nuclear factor κB kinase
subunit γ and consequently causes the inhibition of NF-κB activity.
Nuclear factor-κB (NF-κB) is an important transcription factor for
carcinogenesis in chronic inflammatory diseases and plays a key
role in promoting inflammation-associated carcinoma in the
gastrointestinal tract (118).
TRIM45 overexpression also suppresses cell growth by inhibiting the
NF-κB signal (119). TRIM24
functions as an oncogene in colorectal carcinogenesis by using a
lentivirus-mediated RNA interference system (120). The TRIM66 expression level is
higher in osteosarcoma tissues than in normal tissues and it acts
as an oncogene by suppressing the apoptotic pathway and promoting
TGF-β signaling in osteosarcoma carcinogenesis (121). TRIM59 is upregulated in bladder
cancer tissues and is oncogenically active via the TGF-β/Smad2/3
signaling pathway (122).
The SENPs deconjugate modified proteins and are
important for maintaining SUMO homeostasis. Alterations in SENP1
levels can transform normal prostate epithelia to a dysplastic
state. The enhanced SUMO conjugation of cellular substrates in
breast cancer cells promotes tumorigenesis (123). The catalytic activity of SENP is
inhibited in hypoxic cell extracts and oxygen controls SENP
activity to hypoxic reprogramming of metabolism (124). The Pin1 prolyl isomerase
regulates phosphorylation signaling and its upregulation promotes
oncogenesis. SENP1-mediated deSUMOylation increases both Pin1
protein stability and Pin1 levels in human breast cancer specimens
(125). SENP2 overexpression
suppresses the growth and colony-forming ability of hepatocellular
carcinoma cells by modulating the stability of β-catenin (126). The upregulation of SENP3/SMT3IP1
promotes epithelial ovarian cancer progression and may serve as a
potential biomarker for prognosis (127). The overexpression of SENP5 is
associated with the differentiation of oral squamous cell carcinoma
(128). SENP5 also enhances cell
growth in osteosarcoma cells (129) and promotes tumorigenesis in
hepatocellular carcinoma (130).
SENP6 promotes gastric cancer cell growth via the deSUMOylation of
the transcription factor fork head box protein M1 (FoxM1). The
expression of two SENP7 genes in breast cancer is opposite.
The short splice variant SENP7S gene is highly expressed in
normal breast tissue, while the long splice variant SENP7L
gene is highly expressed in breast cancer tissues. SENP7L promotes
gene expression and favors aberrant proliferation and initiates EMT
in breast cancer (131).
Proliferating cell nuclear antigen (PCNA) is an
essential factor for DNA replication and repair. Ubiquitin and SUMO
compete for the modification of PCNA and regulate the accuracy of
replication and repair, contributing to overall genomic stability
(132). It is interesting to
determine the mechanisms through which the SUMO modification of
human PCNA maintains genomic stability. One route is preventing
replication fork collapse to DNA double-strand breaks (DSBs)
(133). SUMO-PCNA signals for the
recruitment of the anti-recombinogenic DNA helicase Srs2 to sites
of replication, which need the Srs2 carboxy-terminal domain which
harbors tandem receptor motifs (134).
BRCA1 participates in the DNA damage response and
mutations in BRCA1 are associated with a high risk of breast and
ovarian cancer. SUMO modification of the BRCA1/BARD1 heterodimer
greatly increases its ligase activity in vitro (135). Hybrid SUMO-ubiquitin chains are
synthesized by SUMO-targeted ubiquitin E3 ligase RNF4 which are
recognized by RAP80 to promote BRCA1 recruitment and DNA DSB repair
(136). PIAS1 and PIAS4 are
required for effective ubiquitin-adduct formation mediated by RNF8,
RNF168 and BRCA1 at sites of DNA damage (137). Deubiquitylation enzyme (DUB)
ataxin-3 counteracts RNF4 activity during DSBs and is essential for
a mediator of DNA damage checkpoint 1 (MDC1)-dependent signaling
and repair of DSBs (138). The
recruitment of RNF4 to DSBs requires its RING and SUMO interaction
motif (SIM) domains and DNA damage factors such as NBS1, MDC1,
RNF8, 53BP1 and BRCA1. RNF4 plays a role in the integration of SUMO
modification and ubiquitin signaling in the cellular response to
DSBs (139). RNF4 targets
proteins to the proteasome and regulates the turnover of the
DSB-responsive factors MDC1 and replication protein A (RPA) at DNA
damage sites (140).
Hypoxia (low oxygen supply) aids tumor metastasis,
in part by promoting EMT in cancer cells. SENP1 has been shown to
enhance the invasion and lung metastasis of triple-negative breast
cancer (TNBC) cells in a xenograft tumor model with nude mice
(163). SENP1 promotes prostate
cancer progression and bone metastasis by regulating two bone
remodeling proteins, matrix metalloproteinase (MMP)-2 and MMP-9,
through the HIF1α signaling pathway (164). SENP2 inhibits bladder cancer cell
invasion and metastasis through the deactivation of the promotor of
MMP13 through deSUMOylation of TBL1/TBLR1 (165).
CBX4 participates in the polycomb repressive complex
(PRC1) which suppresses metastasis via the recruitment of histone
deacetylase 3 (HDAC3) to the key transcription factor Runx2
promoter in colorectal carcinoma (166). CBX4 also enhances hypoxia-induced
VEGF expression and angiogenesis in hepatocellular carcinoma cells
(167) by enhancing the
SUMOylation of HIF-1α. Cbx4 expression promotes the
progression and metastasis of orthotopically transplanted tumors in
nude mice (168). Smad nuclear
interacting protein 1 (SNIP1) is a transcription repressor for the
TGF-β and NF-κB signaling pathways and its SUMOylation is enhanced
by SUMO E3 ligase PIAS proteins and inhibited by SUMO proteases
SENP1/2. The SUMOylation of SNIP1 enhances TGF-β-regulated cell
migration and invasion (169).
Hepatocyte growth factor (HGF)/c-Met signaling is implicated in the
process of EMT in hepatocellular carcinoma. SENP1 silencing via
lentivirus-mediated small hairpin RNA (shRNA) transduction has been
shown to inhibit EMT and to reduce the HGF-induced proliferation
and migration of hepatocellular carcinoma cells (170). N-Myc downstream-regulated gene 2
(NDRG2) protein (171) is
covalently modified by SUMO1 and inhibits the growth, metastasis
and invasion of human lung adenocarcinomas cells; RNF4 increases
the efficiency of this process (172). SUMO and SUMO E3 ligases play a
critical role in the spatial and temporal regulation of homologous
recombination repair during DSB in heterochromatin (173). SAE2 is highly expressed and the
downregulation of SAE2 expression inhibits the migration and
invasion of small cell lung cancer (174). SENP1 enhances the progression and
metastasis of pancreatic ductal adenocarcinoma via the upregulation
of MMP-9, and its expression positively correlates with the lymph
node metastasis of cancer cells (175). Bioinformatics analysis has
revealed that the expression level of SENP5 positively correlates
with the prognosis and survival rate of patients with breast
cancer. Patients with a low expression of SENP5 have a good
prognosis and a higher survival rate. SENP5 silencing inhibits the
anchorage-independence growth, proliferation, migration and
invasion of breast cancer cell lines through the regulation of the
TGFβRI and MMP-9 levels (176).
The mRNA expression of SENP6 in breast cancer tissues is lower than
that in normal tissues (177).
SENP 2 suppresses cell migration and invasion partly
through inhibiting the expression of MMP13 in bladder cancer cells
(178). The Rho GDP dissociation
inhibitor (RhoGDI) can affect actin polymerization and cell
motility by binding to small GTPases and maintaining them in a
biologically inactive state in the cytoplasm (179). RhoGDI interacts with X-linked
inhibitor of apoptosis protein (XIAP) and negatively modulates
RhoGDI SUMOylation and cancer cell migration, invasion and
metastasis (180). The Rho-like
GTPase, Rac1, induces cytoskeletal rearrangements and it interacts
with PIAS3, which is required for increased Rac activation and
optimal cell migration and invasion (181). The expression of galectin-1
belongs to the lectin family, participating in malignant tumor
development through the regulation of HIF for the cellular response
to hypoxia. PHD3-SUMO conjugation represses HIF1 transcriptional
activity (182). Galectin-1
mediates the HIF-1-induced migration and invasion of colorectal
cancer cells during hypoxia (183). Ubc9 promotes breast cancer cell
invasion and metastasis by interacting with metastasis-related
genes, such as CDC42/CXCR4 and regulating the expression of several
miRNAs, such as for example, the downregulation of miR-224
(184). The upregulation of Ubc9
expression promotes the invasion and metastasis of lung cancer
cells (185).
In a previous study, the whole genome shRNA library
screened out a potent synthetic lethal effect between encoding SUMO
E1 subunits SAE1/SAE2 genes and K-ras genes. The
SAE1 and SAE2 genes were able to selectively inhibit
the K-Ras mutant following shRNA interference or silencing,
but not to the non-carcinogenic K-Ras wild-type. The
SAE gene is critical for K-Ras-induced tumor growth.
Therefore, it can be inferred that SAE1/2 can be used to evaluate
the invasive and metastastic ability of the mutated K-ras
gene. It was also suggested that the SUMO activating enzyme E1 may
be a good target for developing new methods for the treatment of
human malignancies in a specific genetic context (186).
However, it is of interest to determine the reasons
why advanced-stage cancer is refractory to conventional
chemotherapy and radiation therapy. As previously demonstrated,
Ubc9 is expressed in high levels in melanoma-positive lymph nodes
and plays an important role in the progression and metastasis of
melanoma. In that study, Ubc9 exerted protective effects, which
prevented advanced-stage melanomas from undergoing
chemotherapy-induced apoptosis (187). The upregulation of Ubc9 mRNA and
the downregulation of miR-214 are greatly associated with the
enhanced invasion of glioma. miR-214 overexpression suppresses the
endogenous Ubc9 protein and inhibits glioma cell proliferation
(188). Ubc9 protein is
overexpressed in some breast cancer cell lines and tissues. Ubc9 is
associated with alterations in the tumor cellular response to
anticancer drugs, as well as tumor growth by regulating Bcl-2
expression through the ER signaling pathway (189).
Metastasis and not the primary tumor is the cause of
the majority of most cancer-related deaths (190). A large proportion of solid tumors
are derived from epithelial cells. Metastasis involves the physical
translocation of a cancer cell to a distant organ and the ability
of the cancer cell to develop into a metastatic lesion via EMT. The
TGF-β family signaling is essential for EMT and angiogenesis
(191). The function of TGF-β is
paradoxical, which inhibits tumor growth at the early stage and
promote EMT and metastasis at the late stage. TGF-β was modified by
SUMO and amplifies TGFβ signaling at multiple levels under various
situations (192).
As regards aerobic respiration in cells, the hypoxic
signal is a key signaling pathway in regulating gene expression and
metabolic process (such as the Warburg effect) and promotes
angiogenesis and metastasis in cancer cells (193). HIF controls the hypoxia-signaling
cascade and has a direct association with SUMOylation (194). Hypoxia can profoundly affect
SUMOylation and it has been shown that the protein expression of
the SUMO1 (195) is increased
under hypoxic conditions. SUMO-1 promotes glycolysis during periods
of hypoxic stress (196).
RWD-domain-containing SUMOylation enhancer (RSUME) enhances SUMO
conjugation by interacting with the SUMO conjugase Ubc9, increases
Ubc9 thioester formation and is overexpressed under conditions of
hypoxic stress. Hypoxia-induced RSUME protein SUMOylation can
enhance the stability and activity of the HIF1α protein (197,198). SUMO E3 ligase PIAS4 (87) and SENP1 (199) are induced by hypoxia, which is
positively associated with cancer aggressiveness. In hepatocellular
carcinoma, the SUMOylation of CBX4-dependent HIF1α protein
increases the transcriptional activity of HIF1, thereby increasing
the expression of vascular endothelial growth factor and
angiogenesis (167). Hypoxia aids
tumor metastasis, in part by promoting EMT in cancer cells. These
results can correspond with the NF-κB and TGF-β pathway observed
above.
The present research suggests that SUMOylation is
neither a tumor promoting factor nor a tumor suppressor factor, and
that it plays a key role in all cells. Although an increasing
number of studies have shown that SUMOylation in tumor cells is
greater than that in normal cells (104,152,157,164,175), the drugs or factors which have an
effect on the SUMOylation pathway in cancer cells also have the
same effect on normal cells.
Cellular senescence is usually irreversible, and
promotes cell cycle arrest and limits cell proliferation.
Senescence is generally due to the shortening of telomeres or
cellular stress responses, such as DNA damage, oxidative stress and
the expression of some oncogenes (200). Emerging evidence indicates that
cellular senescence plays a key role in tumor suppression; however,
the molecular signaling pathway involved has not yet been
determined. During the process of cellular senescence, cellular
protein secretion is also altered, which is termed the
senescence-associated secretory phenotype (SASP). SASP in cells
promotes the secretion of a series of inflammatory cytokines,
chemokines, growth factors and matrix remodeling factors or matrix
metalloproteinases, which alter the local tissue environment and
contributes to chronic inflammation and cancer (201). p53 promotes cellular senescence,
as well as programmed cell death (apoptosis) as a tumor suppressor
(202). The SUMO-1 conjugation
with p53 results in p53 stabilization and activation, which causes
the induction of senescence (203). By inhibiting or consuming UBC9
protein, hypoSUMOylation leads to the cessation of senescence, such
as the growth of human fibroblasts. SUMO protein is selectively
retained at the histone maintenance of a repressive environment at
histone and tRNA loci is a distinguishing feature of the senescent
state. Senescence is related to the widespread increase of SUMO
protein. A large number of SUMO chromatin proteins are retained in
some selected genomic loci, such as histone or tRNA genes in
senescent cells (204). It can be
seen that there is a reciprocal association between SUMO and
senescence. Oncogenic mutations or DNA damage signals induce SUMO
disorder that ultimately leads to cell senescence, which is the
current study on the effects of tumor or tumor microenvironment. In
turn, senescence phenotype has an important influence on the SUMO
balance.
Synthetic lethality interactions are genetic
interactions of two mutations whereby the presence of either
mutation alone has no effect on cell viability, but the combination
of the two mutations results in cell death. The presence of one of
these mutations in cancer cells, but not in normal cells can
therefore create opportunities to selectively kill cancer cells and
spare normal cells.
From the above-mentioned studies, SUMO modification
may improve the stability of complex signaling pathways through a
wide range of regulatory mechanisms. Studies on the role of SUMO
modification in NF-B, TGF-β and JAK/STAT signaling pathways have
shown that SUMO proteins play a role in promoting or antagonizing
the output of the pathway by targeting not only one, but often more
proteins in the signaling pathway components. SUMO modification is
an important post-translational modification. It is not only the
key factor regulating cell activities, but also plays a role in the
pathological processes, which has been widely accepted and
confirmed by extensive research. SUMO modification is closely
associated with carcinogenesis, and the proliferation and
metastasis of tumors; however, the underlying molecular mechanisms
remain poorly understood. SUMOylation is upregulated significantly
in the majority of cancers, and may thus be a potential target for
cancer therapy. The SUMO pathways have profound impacts on
protein-protein interactions and some SUMO inhibitors have been
developed (208,209). However, a large number of
clinical trials are warranted in order to verify these findings and
provide useful information for the diagnosis and prognosis of
cancer.
Not applicable.
The authors are grateful to the National Natural
Science Foundation of China (grant nos. 81670594 and 31270532) and
the Cuiying Scientific and Technological Innovation Program of
Lanzhou University Second Hospital for their financial support.
Not applicable.
ZJH and YHF participated in the writing of the
manuscript. BHG contributed to the editing of the manuscript. HC
and YML served as scientific advisors. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zamaraev AV, Kopeina GS, Prokhorova EA,
Zhivotovsky B and Lavrik IN: Post-translational modification of
caspases: The other side of apoptosis regulation. Trends Cell Biol.
27:322–339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu J, Qian C and Cao X:
Post-translational modification control of innate immunity.
Immunity. 45:15–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bode AM and Dong Z: Post-translational
modification of p53 in tumorigenesis. Nat Rev Cancer. 4:793–805.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Venne AS, Kollipara L and Zahedi RP: The
next level of complexity: Crosstalk of posttranslational
modifications. Proteomics. 14:513–524. 2014. View Article : Google Scholar
|
|
5
|
Woolfrey KM and Dell'Acqua ML:
Coordination of protein phosphorylation and dephosphorylation in
synaptic plasticity. J Biol Chem. 290:28604–28612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olsen JV, Blagoev B, Gnad F, Macek B,
Kumar C, Mortensen P and Mann M: Global, in vivo, and site-specific
phosphorylation dynamics in signaling networks. Cell. 127:635–648.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson LN: The regulation of protein
phosphorylation. Biochem Soc Trans. 37:627–641. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang X and Chen ZJ: The role of
ubiquitylation in immune defence and pathogen evasion. Nat Rev
Immunol. 12:35–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vucic D, Dixit VM and Wertz IE:
Ubiquitylation in apoptosis: A post-translational modification at
the edge of life and death. Nat Rev Mol Cell Biol. 12:439–452.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fulda S, Rajalingam K and Dikic I:
Ubiquitylation in immune disorders and cancer: From molecular
mechanisms to therapeutic implications. EMBO Mol Med. 4:545–556.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Komander D and Rape M: The ubiquitin code.
Annu Rev Biochem. 81:203–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choudhary C, Weinert BT, Nishida Y, Verdin
E and Mann M: The growing landscape of lysine acetylation links
metabolism and cell signalling. Nat Rev Mol Cell Biol. 15:536–550.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo M and Huang BX: Integration of
phosphoproteomic, chemical, and biological strategies for the
functional analysis of targeted protein phosphorylation.
Proteomics. 13:424–437. 2013. View Article : Google Scholar
|
|
14
|
Kim W, Bennett EJ, Huttlin EL, Guo A, Li
J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, et al: Systematic
and quantitative assessment of the ubiquitin-modified proteome. Mol
Cell. 44:325–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamoliatte F, McManus FP, Maarifi G,
Chelbi-Alix MK and Thibault P: Uncovering the SUMOylation and
ubiquitylation crosstalk in human cells using sequential peptide
immunopurification. Nat Commun. 8:141092017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Biggar KK and Li SS: Non-histone protein
methylation as a regulator of cellular signalling and function. Nat
Rev Mol Cell Biol. 16:5–17. 2015. View Article : Google Scholar
|
|
17
|
Choudhary C, Kumar C, Gnad F, Nielsen ML,
Rehman M, Walther TC, Olsen JV and Mann M: Lysine acetylation
targets protein complexes and co-regulates major cellular
functions. Science. 325:834–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drazic A, Myklebust LM, Ree R and Arnesen
T: The world of protein acetylation. Biochim Biophys Acta.
1864:1372–1401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Menzies KJ, Zhang H, Katsyuba E and Auwerx
J: Protein acetylation in metabolism - metabolites and cofactors.
Nat Rev Endocrinol. 12:43–60. 2016. View Article : Google Scholar
|
|
20
|
Verdin E and Ott M: 50 years of protein
acetylation: From gene regulation to epigenetics, metabolism and
beyond. Nat Rev Mol Cell Biol. 16:258–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bettermann K, Benesch M, Weis S and
Haybaeck J: SUMOylation in carcinogenesis. Cancer Lett.
316:113–125. 2012. View Article : Google Scholar
|
|
22
|
Eifler K and Vertegaal AC:
SUMOylation-mediated regulation of cell cycle progression and
cancer. Trends Biochem Sci. 40:779–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flotho A and Melchior F: Sumoylation: A
regulatory protein modification in health and disease. Annu Rev
Biochem. 82:357–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rabellino A, Andreani C and Scaglioni PP:
The role of PIAS SUMO E3-ligases in cancer. Cancer Res.
77:1542–1547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seeler JS and Dejean A: SUMO and the
robustness of cancer. Nat Rev Cancer. 17:184–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y and Dasso M: SUMOylation and
deSUMOylation at a glance. J Cell Sci. 122:4249–4252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mahajan R, Delphin C, Guan T, Gerace L and
Melchior F: A small ubiquitin-related polypeptide involved in
targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell.
88:97–107. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matunis MJ, Coutavas E and Blobel G: A
novel ubiquitin-like modification modulates the partitioning of the
Ran-GTPase-activating protein RanGAP1 between the cytosol and the
nuclear pore complex. J Cell Biol. 135:1457–1470. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saitoh H and Hinchey J: Functional
heterogeneity of small ubiquitin-related protein modifiers SUMO-1
versus SUMO-2/3. J Biol Chem. 275:6252–6258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Owerbach D, McKay EM, Yeh ET, Gabbay KH
and Bohren KM: A proline-90 residue unique to SUMO-4 prevents
maturation and sumoylation. Biochem Biophys Res Commun.
337:517–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang CY, Yang P, Li M and Gong F:
Characterization of a negative feedback network between SUMO4
expression and NFkappaB transcriptional activity. Biochem Biophys
Res Commun. 381:477–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miura K, Jin JB and Hasegawa PM:
Sumoylation, a post-translational regulatory process in plants.
Curr Opin Plant Biol. 10:495–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tatham MH, Jaffray E, Vaughan OA, Desterro
JM, Botting CH, Naismith JH and Hay RT: Polymeric chains of SUMO-2
and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and
Ubc9. J Biol Chem. 276:35368–35374. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nayak A and Müller S: SUMO-specific
proteases/isopeptidases: SENPs and beyond. Genome Biol. 15:4222014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Desterro JM, Rodriguez MS, Kemp GD and Hay
RT: Identification of the enzyme required for activation of the
small ubiquitin-like protein SUMO-1. J Biol Chem. 274:10618–10624.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tatham MH, Kim S, Jaffray E, Song J, Chen
Y and Hay RT: Unique binding interactions among Ubc9, SUMO and
RanBP2 reveal a mechanism for SUMO paralog selection. Nat Struct
Mol Biol. 12:67–74. 2005. View Article : Google Scholar
|
|
37
|
Bernier-Villamor V, Sampson DA, Matunis MJ
and Lima CD: Structural basis for E2-mediated SUMO conjugation
revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and
RanGAP1. Cell. 108:345–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Werner A, Flotho A and Melchior F: The
RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase.
Mol Cell. 46:287–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cappadocia L, Pichler A and Lima CD:
Structural basis for catalytic activation by the human ZNF451 SUMO
E3 ligase. Nat Struct Mol Biol. 22:968–975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rytinki MM, Kaikkonen S, Pehkonen P,
Jääskeläinen T and Palvimo JJ: PIAS proteins: Pleiotropic
interactors associated with SUMO. Cell Mol Life Sci. 66:3029–3041.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stephan AK, Kliszczak M and Morrison CG:
The Nse2/Mms21 SUMO ligase of the Smc5/6 complex in the maintenance
of genome stability. FEBS Lett. 585:2907–2913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reverter D and Lima CD: Insights into E3
ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex.
Nature. 435:687–692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang SH and Sharrocks AD: The SUMO E3
ligase activity of Pc2 is coordinated through a SUMO interaction
motif. Mol Cell Biol. 30:2193–2205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kagey MH, Melhuish TA and Wotton D: The
polycomb protein Pc2 is a SUMO E3. Cell. 113:127–137. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hatakeyama S: TRIM family proteins: Roles
in autophagy, immunity, and carcinogenesis. Trends Biochem Sci.
42:297–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koliopoulos MG, Esposito D, Christodoulou
E, Taylor IA and Rittinger K: Functional role of TRIM E3 ligase
oligomerization and regulation of catalytic activity. EMBO J.
35:1204–1218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hickey CM, Wilson NR and Hochstrasser M:
Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol.
13:755–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mendes AV, Grou CP, Azevedo JE and Pinto
MP: Evaluation of the activity and substrate specificity of the
human SENP family of SUMO proteases. Biochim Biophys Acta.
1863:139–147. 2016. View Article : Google Scholar
|
|
49
|
Shin EJ, Shin HM, Nam E, Kim WS, Kim JH,
Oh BH and Yun Y: DeSUMOylating isopeptidase: A second class of SUMO
protease. EMBO Rep. 13:339–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yeh ET: SUMOylation and De-SUMOylation:
Wrestling with life's processes. J Biol Chem. 284:8223–8227. 2009.
View Article : Google Scholar :
|
|
51
|
Kim JH and Baek SH: Emerging roles of
desumoylating enzymes. Biochim Biophys Acta. 1792:155–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang CJ, Wu D, Khan FA and Huo LJ:
DeSUMOylation: An important therapeutic target and protein
regulatory event. DNA Cell Biol. 34:652–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Enchev RI, Schulman BA and Peter M:
Protein neddylation: Beyond cullin-RING ligases. Nat Rev Mol Cell
Biol. 16:30–44. 2015. View Article : Google Scholar
|
|
54
|
Bergink S and Jentsch S: Principles of
ubiquitin and SUMO modifications in DNA repair. Nature.
458:461–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thomson TM and Guerra-Rebollo M: Ubiquitin
and SUMO signalling in DNA repair. Biochem Soc Trans. 38:116–131.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ulrich HD: Ubiquitin and SUMO in DNA
repair at a glance. J Cell Sci. 125:249–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jackson SP and Durocher D: Regulation of
DNA damage responses by ubiquitin and SUMO. Mol Cell. 49:795–807.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sarangi P and Zhao X: SUMO-mediated
regulation of DNA damage repair and responses. Trends Biochem Sci.
40:233–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Seeler JS and Dejean A: Nuclear and
unclear functions of SUMO. Nat Rev Mol Cell Biol. 4:690–699. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Stielow B, Sapetschnig A, Krüger I, Kunert
N, Brehm A, Boutros M and Suske G: Identification of SUMO-dependent
chromatin-associated transcriptional repression components by a
genome-wide RNAi screen. Mol Cell. 29:742–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhong S, Müller S, Ronchetti S, Freemont
PS, Dejean A and Pandolfi PP: Role of SUMO-1-modified PML in
nuclear body formation. Blood. 95:2748–2752. 2000.PubMed/NCBI
|
|
62
|
Schimmel J, Eifler K, Sigurðsson JO,
Cuijpers SA, Hendriks IA, Verlaan-de Vries M, Kelstrup CD,
Francavilla C, Medema RH, Olsen JV, et al: Uncovering SUMOylation
dynamics during cell-cycle progression reveals FoxM1 as a key
mitotic SUMO target protein. Mol Cell. 53:1053–1066. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pichler A, Knipscheer P, Oberhofer E, van
Dijk WJ, Körner R, Olsen JV, Jentsch S, Melchior F and Sixma TK:
SUMO modification of the ubiquitin-conjugating enzyme E2-25K. Nat
Struct Mol Biol. 12:264–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ouyang KJ, Woo LL, Zhu J, Huo D, Matunis
MJ and Ellis NA: SUMO modification regulates BLM and RAD51
interaction at damaged replication forks. PLoS Biol.
7:e10002522009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Keusekotten K, Bade VN, Meyer-Teschendorf
K, Sriramachandran AM, Fischer-Schrader K, Krause A, Horst C,
Schwarz G, Hofmann K, Dohmen RJ, et al: Multivalent interactions of
the SUMO-interaction motifs in RING finger protein 4 determine the
specificity for chains of the SUMO. Biochem J. 457:207–214. 2014.
View Article : Google Scholar :
|
|
66
|
Song J, Durrin LK, Wilkinson TA, Krontiris
TG and Chen Y: Identification of a SUMO-binding motif that
recognizes SUMO-modified proteins. Proc Natl Acad Sci USA.
101:14373–14378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Merrill JC, Melhuish TA, Kagey MH, Yang
SH, Sharrocks AD and Wotton D: A role for non-covalent SUMO
interaction motifs in Pc2/CBX4 E3 activity. PLoS One. 5:e87942010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rodríguez JA: Interplay between nuclear
transport and ubiquitin/SUMO modifications in the regulation of
cancer-related proteins. Semin Cancer Biol. 27:11–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Müller S, Ledl A and Schmidt D: SUMO: A
regulator of gene expression and genome integrity. Oncogene.
23:1998–2008. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nie M and Boddy MN: Cooperativity of the
SUMO and ubiquitin pathways in genome stability. Biomolecules.
6:142016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Melchior F, Schergaut M and Pichler A:
SUMO: Ligases, isopeptidases and nuclear pores. Trends Biochem Sci.
28:612–618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Eifler K and Vertegaal AC: Mapping the
SUMOylated landscape. FEBS J. 282:3669–3680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Choi SG, Kim H, Jeong EI, Lee HJ, Park S,
Lee SY, Lee HJ, Lee SW, Chung CH and Jung YK: SUMO-Modified FADD
recruits cytosolic Drp1 and caspase-10 to mitochondria for
regulated necrosis. Mol Cell Biol. 37:372017. View Article : Google Scholar
|
|
74
|
Hendriks IA and Vertegaal AC: A
comprehensive compilation of SUMO proteomics. Nat Rev Mol Cell
Biol. 17:581–595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Peuscher MH and Jacobs JJ:
Posttranslational control of telomere maintenance and the telomere
damage response. Cell Cycle. 11:1524–1534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
von Wangenheim KH and Peterson HP: The
role of cell differentiation in controlling cell multiplication and
cancer. J Cancer Res Clin Oncol. 134:725–741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vlachostergios PJ and Papandreou CN: The
role of the small ubiquitin-related modifier (SUMO) pathway in
prostate cancer. Biomolecules. 2:240–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zheng Z, Cai C, Omwancha J, Chen SY,
Baslan T and Shemshedini L: SUMO-3 enhances androgen receptor
transcriptional activity through a sumoylation-independent
mechanism in prostate cancer cells. J Biol Chem. 281:4002–4012.
2006. View Article : Google Scholar
|
|
79
|
Bawa-Khalfe T, Cheng J, Wang Z and Yeh ET:
Induction of the SUMO-specific protease 1 transcription by the
androgen receptor in prostate cancer cells. J Biol Chem.
282:37341–37349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hu L, Yang F, Lu L and Dai W:
Arsenic-induced sumoylation of Mus81 is involved in regulating
genomic stability. Cell Cycle. 16:802–811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bawa-Khalfe T, Yang FM, Ritho J, Lin HK,
Cheng J and Yeh ET: SENP1 regulates PTEN stability to dictate
prostate cancer development. Oncotarget. 8:17651–17664. 2017.
View Article : Google Scholar :
|
|
82
|
Bawa-Khalfe T, Cheng J, Lin SH, Ittmann MM
and Yeh ET: SENP1 induces prostatic intraepithelial neoplasia
through multiple mechanisms. J Biol Chem. 285:25859–25866. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li S, Wang M, Qu X, Xu Z, Yang Y, Su Q and
Wu H: SUMOylation of PES1 upregulates its stability and function
via inhibiting its ubiquitination. Oncotarget. 7:50522–50534.
2016.PubMed/NCBI
|
|
84
|
Finkbeiner E, Haindl M, Raman N and Muller
S: SUMO routes ribosome maturation. Nucleus. 2:527–532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lecona E, Rodriguez-Acebes S, Specks J,
Lopez-Contreras AJ, Ruppen I, Murga M, Muñoz J, Mendez J and
Fernandez-Capetillo O: USP7 is a SUMO deubiquitinase essential for
DNA replication. Nat Struct Mol Biol. 23:270–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Smits VA and Freire R: USP7/HAUSP: A SUMO
deubiquitinase at the heart of DNA replication. BioEssays.
38:863–868. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sun L, Li H, Chen J, Iwasaki Y, Kubota T,
Matsuoka M, Shen A, Chen Q and Xu Y: PIASy mediates hypoxia-induced
SIRT1 transcriptional repression and epithelial-to-mesenchymal
transition in ovarian cancer cells. J Cell Sci. 126:3939–3947.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li S, Yang C, Hong Y, Bi H, Zhao F, Liu Y,
Ao X, Pang P, Xing X, Chang AK, et al: The transcriptional activity
of co-activator AIB1 is regulated by the SUMO E3 ligase PIAS1. Biol
Cell. 104:287–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
90
|
Kubota Y, O'Grady P, Saito H and Takekawa
M: Oncogenic Ras abrogates MEK SUMOylation that suppresses the ERK
pathway and cell transformation. Nat Cell Biol. 13:282–291. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Taylor EM, Copsey AC, Hudson JJ, Vidot S
and Lehmann AR: Identification of the proteins, including MAGEG1,
that make up the human SMC5-6 protein complex. Mol Cell Biol.
28:1197–1206. 2008. View Article : Google Scholar :
|
|
92
|
Wu J, Liu T, Rios Z, Mei Q, Lin X and Cao
S: Heat shock proteins and cancer. Trends Pharmacol Sci.
38:226–256. 2017. View Article : Google Scholar
|
|
93
|
Rachidi S, Sun S, Wu BX, Jones E, Drake
RR, Ogretmen B, Cowart LA, Clarke CJ, Hannun YA, Chiosis G, et al:
Endoplasmic reticulum heat shock protein gp96 maintains liver
homeostasis and promotes hepatocellular carcinogenesis. J Hepatol.
62:879–888. 2015. View Article : Google Scholar :
|
|
94
|
Pinto MP, Carvalho AF, Grou CP,
Rodríguez-Borges JE, Sá-Miranda C and Azevedo JE: Heat shock
induces a massive but differential inactivation of SUMO-specific
proteases. Biochim Biophys Acta. 1823:1958–1966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Niskanen EA, Malinen M, Sutinen P,
Toropainen S, Paakinaho V, Vihervaara A, Joutsen J, Kaikkonen MU,
Sistonen L and Palvimo JJ: Global SUMOylation on active chromatin
is an acute heat stress response restricting transcription. Genome
Biol. 16:1532015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ishihara K, Fatma N, Bhargavan B,
Chhunchha B, Kubo E, Dey S, Takamura Y, Kumar A and Singh DP: Lens
epithelium-derived growth factor deSumoylation by Sumo-specific
protease-1 regulates its transcriptional activation of small heat
shock protein and the cellular response. FEBS J. 279:3048–3070.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Castorálová M, Březinová D, Svéda M, Lipov
J, Ruml T and Knejzlík Z: SUMO-2/3 conjugates accumulating under
heat shock or MG132 treatment result largely from new protein
synthesis. Biochim Biophys Acta. 1823:911–919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Brunet Simioni M, De Thonel A, Hammann A,
Joly AL, Bossis G, Fourmaux E, Bouchot A, Landry J, Piechaczyk M
and Garrido C: Heat shock protein 27 is involved in SUMO-2/3
modification of heat shock factor 1 and thereby modulates the
transcription factor activity. Oncogene. 28:3332–3344. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Golebiowski F, Matic I, Tatham MH, Cole C,
Yin Y, Nakamura A, Cox J, Barton GJ, Mann M and Hay RT: System-wide
changes to SUMO modifications in response to heat shock. Sci
Signal. 2:ra242009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu HW, Zhang J, Heine GF, Arora M, Gulcin
Ozer H, Onti-Srinivasan R, Huang K and Parvin JD: Chromatin
modification by SUMO-1 stimulates the promoters of translation
machinery genes. Nucleic Acids Res. 40:10172–10186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Aguilar-Martinez E, Chen X, Webber A,
Mould AP, Seifert A, Hay RT and Sharrocks AD: Screen for
multi-SUMO-binding proteins reveals a multi-SIM-binding mechanism
for recruitment of the transcriptional regulator ZMYM2 to
chromatin. Proc Natl Acad Sci USA. 112:E4854–E4863. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Amente S, Lavadera ML, Palo GD and Majello
B: SUMO-activating SAE1 transcription is positively regulated by
Myc. Am J Cancer Res. 2:330–334. 2012.PubMed/NCBI
|
|
103
|
Kessler JD, Kahle KT, Sun T, Meerbrey KL,
Schlabach MR, Schmitt EM, Skinner SO, Xu Q, Li MZ, Hartman ZC, et
al: A SUMOylation-dependent transcriptional subprogram is required
for Myc-driven tumorigenesis. Science. 335:348–353. 2012.
View Article : Google Scholar
|
|
104
|
Hoellein A, Fallahi M, Schoeffmann S,
Steidle S, Schaub FX, Rudelius M, Laitinen I, Nilsson L, Goga A,
Peschel C, et al: Myc-induced SUMOylation is a therapeutic
vulnerability for B-cell lymphoma. Blood. 124:2081–2090. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
González-Prieto R, Cuijpers SA, Kumar R,
Hendriks IA and Vertegaal AC: c-Myc is targeted to the proteasome
for degradation in a SUMOylation-dependent manner, regulated by
PIAS1, SENP7 and RNF4. Cell Cycle. 14:1859–1872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mo YY, Yu Y, Theodosiou E, Ee PL and Beck
WT: A role for Ubc9 in tumorigenesis. Oncogene. 24:2677–2683. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mattoscio D, Casadio C, Miccolo C, Maffini
F, Raimondi A, Tacchetti C, Gheit T, Tagliabue M, Galimberti VE, De
Lorenzi F, et al: Autophagy regulates UBC9 levels during
viral-mediated tumorigenesis. PLoS Pathog. 13:e10062622017.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lin CH, Liu SY and Lee EH: SUMO
modification of Akt regulates global SUMOylation and substrate
SUMOylation specificity through Akt phosphorylation of Ubc9 and
SUMO1. Oncogene. 35:595–607. 2016. View Article : Google Scholar
|
|
109
|
Li R, Wei J, Jiang C, Liu D, Deng L, Zhang
K and Wang P: Akt SUMOylation regulates cell proliferation and
tumorigenesis. Cancer Res. 73:5742–5753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Moschos SJ, Jukic DM, Athanassiou C,
Bhargava R, Dacic S, Wang X, Kuan SF, Fayewicz SL, Galambos C,
Acquafondata M, et al: Expression analysis of Ubc9, the single
small ubiquitin-like modifier (SUMO) E2 conjugating enzyme, in
normal and malignant tissues. Hum Pathol. 41:1286–1298. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu F, Zhu S, Ding Y, Beck WT and Mo YY:
MicroRNA-mediated regulation of Ubc9 expression in cancer cells.
Clin Cancer Res. 15:1550–1557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gylfe AE, Kondelin J, Turunen M,
Ristolainen H, Katainen R, Pitkänen E, Kaasinen E, Rantanen V,
Tanskanen T, Varjosalo M, et al: Identification of candidate
oncogenes in human colorectal cancers with microsatellite
instability. Gastroenterology. 145:540–3.e22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Packham S, Warsito D, Lin Y, Sadi S,
Karlsson R, Sehat B and Larsson O: Nuclear translocation of IGF-1R
via p150 (Glued) and an importin-β/RanBP2-dependent pathway in
cancer cells. Oncogene. 34:2227–2238. 2015. View Article : Google Scholar
|
|
114
|
Ritterhoff T, Das H, Hofhaus G, Schröder
RR, Flotho A and Melchior F: The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3
ligase is a disassembly machine for Crm1-dependent nuclear export
complexes. Nat Commun. 7:114822016. View Article : Google Scholar
|
|
115
|
Hatakeyama S: TRIM proteins and cancer.
Nat Rev Cancer. 11:792–804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Watanabe M and Hatakeyama S: TRIM proteins
and diseases. J Biochem. 161:135–144. 2017.PubMed/NCBI
|
|
117
|
Sho T, Tsukiyama T, Sato T, Kondo T, Cheng
J, Saku T, Asaka M and Hatakeyama S: TRIM29 negatively regulates
p53 via inhibition of Tip60. Biochim Biophys Acta. 1813:1245–1253.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Noguchi K, Okumura F, Takahashi N, Kataoka
A, Kamiyama T, Todo S and Hatakeyama S: TRIM40 promotes neddylation
of IKKγ and is downregulated in gastrointestinal cancers.
Carcinogenesis. 32:995–1004. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shibata M, Sato T, Nukiwa R, Ariga T and
Hatakeyama S: TRIM45 negatively regulates NF-κB-mediated
transcription and suppresses cell proliferation. Biochem Biophys
Res Commun. 423:104–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang J, Zhu J, Dong M, Yu H, Dai X and Li
K: Knockdown of tripartite motif containing 24 by lentivirus
suppresses cell growth and induces apoptosis in human colorectal
cancer cells. Oncol Res. 22:39–45. 2014. View Article : Google Scholar
|
|
121
|
Chen Y, Guo Y, Yang H, Shi G, Xu G, Shi J,
Yin N and Chen D: TRIM66 overexpresssion contributes to
osteosarcoma carcinogenesis and indicates poor survival outcome.
Oncotarget. 6:23708–23719. 2015.PubMed/NCBI
|
|
122
|
Chen W, Zhao K, Miao C, Xu A, Zhang J, Zhu
J, Su S and Wang Z: Silencing Trim59 inhibits invasion/migration
and epithelial-to-mesenchymal transition via TGF-β/Smad2/3
signaling pathway in bladder cancer cells. Onco Targets Ther.
10:1503–1512. 2017. View Article : Google Scholar :
|
|
123
|
Bawa-Khalfe T and Yeh ET: SUMO losing
balance: SUMO proteases disrupt SUMO homeostasis to facilitate
cancer development and progression. Genes Cancer. 1:748–752. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kunz K, Wagner K, Mendler L, Hölper S,
Dehne N and Müller S: SUMO signaling by hypoxic inactivation of
SUMO-specific isopeptidases. Cell Reports. 16:3075–3086. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chen CH, Chang CC, Lee TH, Luo M, Huang P,
Liao PH, Wei S, Li FA, Chen RH, Zhou XZ, et al: SENP1 deSUMOylates
and regulates Pin1 protein activity and cellular function. Cancer
Res. 73:3951–3962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Shen HJ, Zhu HY, Yang C and Ji F: SENP2
regulates hepatocellular carcinoma cell growth by modulating the
stability of β-catenin. Asian Pac J Cancer Prev. 13:3583–3587.
2012. View Article : Google Scholar
|
|
127
|
Cheng J, Su M, Jin Y, Xi Q, Deng Y, Chen
J, Wang W, Chen Y, Chen L, Shi N, et al: Upregulation of
SENP3/SMT3IP1 promotes epithelial ovarian cancer progression and
forecasts poor prognosis. Tumour Biol. 39:10104283176945432017.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ding X, Sun J, Wang L, Li G, Shen Y, Zhou
X and Chen W: Overexpression of SENP5 in oral squamous cell
carcinoma and its association with differentiation. Oncol Rep.
20:1041–1045. 2008.PubMed/NCBI
|
|
129
|
Wang K and Zhang XC: Inhibition of SENP5
suppresses cell growth and promotes apoptosis in osteosarcoma
cells. Exp Ther Med. 7:1691–1695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Jin ZL, Pei H, Xu YH, Yu J and Deng T: The
SUMO-specific protease SENP5 controls DNA damage response and
promotes tumorigenesis in hepatocellular carcinoma. Eur Rev Med
Pharmacol Sci. 20:3566–3573. 2016.PubMed/NCBI
|
|
131
|
Bawa-Khalfe T, Lu LS, Zuo Y, Huang C, Dere
R, Lin FM and Yeh ET: Differential expression of SUMO-specific
protease 7 variants regulates epithelial-mesenchymal transition.
Proc Natl Acad Sci USA. 109:17466–17471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Stelter P and Ulrich HD: Control of
spontaneous and damage-induced mutagenesis by SUMO and ubiquitin
conjugation. Nature. 425:188–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Gali H, Juhasz S, Morocz M, Hajdu I,
Fatyol K, Szukacsov V, Burkovics P and Haracska L: Role of SUMO
modification of human PCNA at stalled replication fork. Nucleic
Acids Res. 40:6049–6059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Armstrong AA, Mohideen F and Lima CD:
Recognition of SUMO-modified PCNA requires tandem receptor motifs
in Srs2. Nature. 483:59–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Morris JR, Boutell C, Keppler M, Densham
R, Weekes D, Alamshah A, Butler L, Galanty Y, Pangon L, Kiuchi T,
et al: The SUMO modification pathway is involved in the BRCA1
response to genotoxic stress. Nature. 462:886–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Guzzo CM, Berndsen CE, Zhu J, Gupta V,
Datta A, Greenberg RA, Wolberger C and Matunis MJ: RNF4-dependent
hybrid SUMO-ubiquitin chains are signals for RAP80 and thereby
mediate the recruitment of BRCA1 to sites of DNA damage. Sci
Signal. 5:ra882012. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Galanty Y, Belotserkovskaya R, Coates J,
Polo S, Miller KM and Jackson SP: Mammalian SUMO E3-ligases PIAS1
and PIAS4 promote responses to DNA double-strand breaks. Nature.
462:935–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Pfeiffer A, Luijsterburg MS, Acs K,
Wiegant WW, Helfricht A, Herzog LK, Minoia M, Böttcher C, Salomons
FA, van Attikum H, et al: Ataxin-3 consolidates the MDC1-dependent
DNA double-strand break response by counteracting the SUMO-targeted
ubiquitin ligase RNF4. EMBO J. 36:1066–1083. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yin Y, Seifert A, Chua JS, Maure JF,
Golebiowski F and Hay RT: SUMO-targeted ubiquitin E3 ligase RNF4 is
required for the response of human cells to DNA damage. Genes Dev.
26:1196–1208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Galanty Y, Belotserkovskaya R, Coates J
and Jackson SP: RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes
DNA double-strand break repair. Genes Dev. 26:1179–1195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
He X, Riceberg J, Pulukuri SM, Grossman S,
Shinde V, Shah P, Brownell JE, Dick L, Newcomb J and Bence N:
Characterization of the loss of SUMO pathway function on cancer
cells and tumor proliferation. PLoS One. 10:e01238822015.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhang J, Huang FF, Wu DS, Li WJ, Zhan HE,
Peng MY, Fang P, Cao PF, Zhang MM, Zeng H, et al: SUMOylation of
insulin-like growth factor 1 receptor, promotes proliferation in
acute myeloid leukemia. Cancer Lett. 357:297–306. 2015. View Article : Google Scholar
|
|
143
|
You L, Liu C, Tang H, Liao Y and Fu S:
Advances in targeting insulin-like growth factor signaling pathway
in cancer treatment. Curr Pharm Des. 20:2899–2911. 2014. View Article : Google Scholar
|
|
144
|
Oh Y and Chung KC: Small ubiquitin-like
modifier (SUMO) modification of zinc finger protein 131 potentiates
its negative effect on estrogen signaling. J Biol Chem.
287:17517–17529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Schulz S, Chachami G, Kozaczkiewicz L,
Winter U, Stankovic-Valentin N, Haas P, Hofmann K, Urlaub H, Ovaa
H, Wittbrodt J, et al: Ubiquitin-specific protease-like 1 (USPL1)
is a SUMO isopeptidase with essential, non-catalytic functions.
EMBO Rep. 13:930–938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Xu J, Sun HY, Xiao FJ, Wang H, Yang Y,
Wang L, Gao CJ, Guo ZK, Wu CT and Wang LS: SENP1 inhibition induces
apoptosis and growth arrest of multiple myeloma cells through
modulation of NF-κB signaling. Biochem Biophys Res Commun.
460:409–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Huang HJ, Zhou LL, Fu WJ, Zhang CY, Jiang
H, Du J and Hou J: β-catenin SUMOylation is involved in the
dysregulated proliferation of myeloma cells. Am J Cancer Res.
5:309–320. 2014.
|
|
148
|
Toropainen S, Malinen M, Kaikkonen S,
Rytinki M, Jääskeläinen T, Sahu B, Jänne OA and Palvimo JJ: SUMO
ligase PIAS1 functions as a target gene selective androgen receptor
coregulator on prostate cancer cell chromatin. Nucleic Acids Res.
43:848–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang W, Chen Y, Wang S, Hu N, Cao Z, Wang
W, Tong T and Zhang X: PIASxα ligase enhances SUMO1 modification of
PTEN protein as a SUMO E3 ligase. J Biol Chem. 289:3217–3230. 2014.
View Article : Google Scholar
|
|
150
|
Wen D, Xu Z, Xia L, Liu X, Tu Y, Lei H,
Wang W, Wang T, Song L, Ma C, et al: Important role of SUMOylation
of Spliceosome factors in prostate cancer cells. J Proteome Res.
13:3571–3582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Huang W, He T, Chai C, Yang Y, Zheng Y,
Zhou P, Qiao X, Zhang B, Liu Z, Wang J, et al: Triptolide inhibits
the proliferation of prostate cancer cells and down-regulates
SUMO-specific protease 1 expression. PLoS One. 7:e376932012.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Shao DF, Wang XH, Li ZY, Xing XF, Cheng
XJ, Guo T, Du H, Hu Y, Dong B, Ding N, et al: High-level SAE2
promotes malignant phenotype and predicts outcome in gastric
cancer. Am J Cancer Res. 5:140–154. 2014.
|
|
153
|
Paakinaho V, Kaikkonen S, Makkonen H,
Benes V and Palvimo JJ: SUMOylation regulates the chromatin
occupancy and anti-proliferative gene programs of glucocorticoid
receptor. Nucleic Acids Res. 42:1575–1592. 2014. View Article : Google Scholar :
|
|
154
|
Hua G, Ganti KP and Chambon P:
Glucocorticoid-induced tethered transrepression requires
SUMOylation of GR and formation of a SUMO-SMRT/NCoR1-HDAC3
repressing complex. Proc Natl Acad Sci USA. 113:E635–E643. 2016.
View Article : Google Scholar :
|
|
155
|
Bies J, Sramko M and Wolff L:
Stress-induced phosphorylation of Thr486 in c-Myb by p38
mitogenactivated protein kinases attenuates conjugation of
SUMO-2/3. J Biol Chem. 288:36983–36993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Du JX, McConnell BB and Yang VW: A small
ubiquitin-related modifier-interacting motif functions as the
transcriptional activation domain of Krüppel-like factor 4. J Biol
Chem. 285:28298–28308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Han Y, Huang C, Sun X, Xiang B, Wang M,
Yeh ET, Chen Y, Li H, Shi G, Cang H, et al: SENP3-mediated
de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated
with accelerated cell proliferation under mild oxidative stress. J
Biol Chem. 285:12906–12915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Carter S, Bischof O, Dejean A and Vousden
KH: C-terminal modifications regulate MDM2 dissociation and nuclear
export of p53. Nat Cell Biol. 9:428–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Dadke S, Cotteret S, Yip SC, Jaffer ZM,
Haj F, Ivanov A, Rauscher F III, Shuai K, Ng T, Neel BG, et al:
Regulation of protein tyrosine phosphatase 1B by sumoylation. Nat
Cell Biol. 9:80–85. 2007. View Article : Google Scholar
|
|
160
|
Jin L, Shen K, Chen T, Zhang H and Yu W:
SUMO-1 gene silencing inhibits proliferation and promotes apoptosis
of human gastric cancer SGC-7901 Cells. Cell Physiol Biochem.
41:987–998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wang B, Tang J, Liao D, Wang G, Zhang M,
Sang Y, Cao J, Wu Y, Zhang R, Li S, et al: Chromobox homolog 4 is
correlated with prognosis and tumor cell growth in hepatocellular
carcinoma. Ann Surg Oncol. 20(Suppl 3): S684–S692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Bellail AC, Olson JJ and Hao C: SUMO1
modification stabilizes CDK6 protein and drives the cell cycle and
glioblastoma progression. Nat Commun. 5:42342014. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wang Z, Jin J, Zhang J, Wang L and Cao J:
Depletion of SENP1 suppresses the proliferation and invasion of
triple-negative breast cancer cells. Oncol Rep. 36:2071–2078. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wang Q, Xia N, Li T, Xu Y, Zou Y, Zuo Y,
Fan Q, Bawa-Khalfe T, Yeh ET and Cheng J: SUMO-specific protease-1
promotes prostate cancer progression and metastasis. Oncogene.
32:2493–2498. 2013. View Article : Google Scholar
|
|
165
|
Tan M, Gong H, Wang J, Tao L, Xu D, Bao E,
Liu Z and Qiu J: SENP2 regulates MMP13 expression in a bladder
cancer cell line through SUMOylation of TBL1/TBLR1. Sci Rep.
5:139962015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wang X, Li L, Wu Y, Zhang R, Zhang M, Liao
D, Wang G, Qin G, Xu RH and Kang T: CBX4 suppresses metastasis via
recruitment of HDAC3 to the Runx2 promoter in colorectal carcinoma.
Cancer Res. 76:7277–7289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Li J, Xu Y, Long XD, Wang W, Jiao HK, Mei
Z, Yin QQ, Ma LN, Zhou AW, Wang LS, et al: Cbx4 governs HIF-1α to
potentiate angiogenesis of hepatocellular carcinoma by its SUMO E3
ligase activity. Cancer Cell. 25:118–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Mei Z, Jiao H, Wang W, Li J, Chen G and Xu
Y: Polycomb chromobox 4 enhances migration and pulmonary metastasis
of hepatocellular carcinoma cell line MHCC97L. Sci China Life Sci.
57:610–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liu S, Long J, Yuan B, Zheng M, Xiao M, Xu
J, Lin X and Feng XH: SUMO modification reverses inhibitory effects
of Smad nuclear interacting protein-1 in TGF-β responses. J Biol
Chem. 291:24418–24430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhang W, Sun H, Shi X, Wang H, Cui C, Xiao
F, Wu C, Guo X and Wang L: SENP1 regulates hepatocyte growth
factor-induced migration and epithelial-mesenchymal transition of
hepatocellular carcinoma. Tumour Biol. 37:7741–7748. 2016.
View Article : Google Scholar
|
|
171
|
Hu W, Fan C, Jiang P, Ma Z, Yan X, Di S,
Jiang S, Li T, Cheng Y and Yang Y: Emerging role of N-myc
downstream-regulated gene 2 (NDRG2) in cancer. Oncotarget.
7:209–223. 2016.
|
|
172
|
Tantai J, Pan X and Hu D: RNF4-mediated
SUMOylation is essential for NDRG2 suppression of lung
adenocarcinoma. Oncotarget. 7:26837–26843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Ryu T, Spatola B, Delabaere L, Bowlin K,
Hopp H, Kunitake R, Karpen GH and Chiolo I: Heterochromatic breaks
move to the nuclear periphery to continue recombinational repair.
Nat Cell Biol. 17:1401–1411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Liu X, Xu Y, Pang Z, Guo F, Qin Q, Yin T,
Sang Y, Feng C, Li X, Jiang L, et al: Knockdown of SUMO-activating
enzyme subunit 2 (SAE2) suppresses cancer malignancy and enhances
chemotherapy sensitivity in small cell lung cancer. J Hematol
Oncol. 8:672015. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Ma C, Wu B, Huang X, Yuan Z, Nong K, Dong
B, Bai Y, Zhu H, Wang W and Ai K: SUMO-specific protease 1
regulates pancreatic cancer cell proliferation and invasion by
targeting MMP-9. Tumour Biol. 35:12729–12735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Cashman R, Cohen H, Ben-Hamo R, Zilberberg
A and Efroni S: SENP5 mediates breast cancer invasion via a TGFβRI
SUMOylation cascade. Oncotarget. 5:1071–1082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Mooney SM, Grande JP, Salisbury JL and
Janknecht R: Sumoylation of p68 and p72 RNA helicases affects
protein stability and transactivation potential. Biochemistry.
49:1–10. 2010. View Article : Google Scholar
|
|
178
|
Tan MY, Mu XY, Liu B, Wang Y, Bao ED, Qiu
JX and Fan Y: SUMO-specific protease 2 suppresses cell migration
and invasion through inhibiting the expression of MMP13 in bladder
cancer cells. Cell Physiol Biochem. 32:542–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Garcia-Mata R, Boulter E and Burridge K:
The 'invisible hand': Regulation of RHO GTPases by RHOGDIs. Nat Rev
Mol Cell Biol. 12:493–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Yu J, Zhang D, Liu J, Li J, Yu Y, Wu XR
and Huang C: RhoGDI SUMOylation at Lys-138 increases its binding
activity to Rho GTPase and its inhibiting cancer cell motility. J
Biol Chem. 287:13752–13760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Castillo-Lluva S, Tatham MH, Jones RC,
Jaffray EG, Edmondson RD, Hay RT and Malliri A: SUMOylation of the
GTPase Rac1 is required for optimal cell migration. Nat Cell Biol.
12:1078–1085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Núñez-O'Mara A, Gerpe-Pita A, Pozo S,
Carlevaris O, Urzelai B, Lopitz-Otsoa F, Rodríguez MS and Berra E:
PHD3-SUMO conjugation represses HIF1 transcriptional activity
independently of PHD3 catalytic activity. J Cell Sci. 128:40–49.
2015. View Article : Google Scholar
|
|
183
|
Zhao XY, Chen TT, Xia L, Guo M, Xu Y, Yue
F, Jiang Y, Chen GQ and Zhao KW: Hypoxia inducible factor-1
mediates expression of galectin-1: The potential role in
migration/invasion of colorectal cancer cells. Carcinogenesis.
31:1367–1375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zhu S, Sachdeva M, Wu F, Lu Z and Mo YY:
Ubc9 promotes breast cell invasion and metastasis in a
sumoylation-independent manner. Oncogene. 29:1763–1772. 2010.
View Article : Google Scholar :
|
|
185
|
Li H, Niu H, Peng Y, Wang J and He P: Ubc9
promotes invasion and metastasis of lung cancer cells. Oncol Rep.
29:1588–1594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Luo J, Emanuele MJ, Li D, Creighton CJ,
Schlabach MR, Westbrook TF, Wong KK and Elledge SJ: A genome-wide
RNAi screen identifies multiple synthetic lethal interactions with
the Ras oncogene. Cell. 137:835–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Moschos SJ, Smith AP, Mandic M,
Athanassiou C, Watson-Hurst K, Jukic DM, Edington HD, Kirkwood JM
and Becker D: SAGE and antibody array analysis of
melanoma-infiltrated lymph nodes: Identification of Ubc9 as an
important molecule in advanced-stage melanomas. Oncogene.
26:4216–4225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Zhao Z, Tan X, Zhao A, Zhu L, Yin B, Yuan
J, Qiang B and Peng X: microRNA-214-mediated UBC9 expression in
glioma. BMB Rep. 45:641–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Lu Z, Wu H and Mo YY: Regulation of bcl-2
expression by Ubc9. Exp Cell Res. 312:1865–1875. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Morrison CD, Parvani JG and Schiemann WP:
The relevance of the TGF-β Paradox to EMT-MET programs. Cancer
Lett. 341:30–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Gilkes DM, Semenza GL and Wirtz D: Hypoxia
and the extracellular matrix: Drivers of tumour metastasis. Nat Rev
Cancer. 14:430–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Núñez-O'Mara A and Berra E: Deciphering
the emerging role of SUMO conjugation in the hypoxia-signaling
cascade. Biol Chem. 394:459–469. 2013.PubMed/NCBI
|
|
195
|
Shao R, Zhang FP, Tian F, Anders Friberg
P, Wang X, Sjöland H and Billig H: Increase of SUMO-1 expression in
response to hypoxia: Direct interaction with HIF-1alpha in adult
mouse brain and heart in vivo. FEBS Lett. 569:293–300. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Agbor TA, Cheong A, Comerford KM, Scholz
CC, Bruning U, Clarke A, Cummins EP, Cagney G and Taylor CT: Small
ubiquitin-related modifier (SUMO)-1 promotes glycolysis in hypoxia.
J Biol Chem. 286:4718–4726. 2011. View Article : Google Scholar :
|
|
197
|
Antico Arciuch VG, Tedesco L, Fuertes M
and Arzt E: Role of RSUME in inflammation and cancer. FEBS Lett.
589:3330–3335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Carbia-Nagashima A, Gerez J, Perez-Castro
C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F and Arzt E:
RSUME, a small RWD-containing protein, enhances SUMO conjugation
and stabilizes HIF-1alpha during hypoxia. Cell. 131:309–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Xu Y, Zuo Y, Zhang H, Kang X, Yue F, Yi Z,
Liu M, Yeh ET, Chen G and Cheng J: Induction of SENP1 in
endothelial cells contributes to hypoxia-driven VEGF expression and
angio-genesis. J Biol Chem. 285:36682–36688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Childs BG, Baker DJ, Kirkland JL, Campisi
J and van Deursen JM: Senescence and apoptosis: Dueling or
complementary cell fates. EMBO Rep. 15:1139–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Davalos AR, Coppe JP, Campisi J and
Desprez PY: Senescent cells as a source of inflammatory factors for
tumor progression. Cancer Metastasis Rev. 29:273–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Lujambio A, Akkari L, Simon J, Grace D,
Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA,
Krizhanovsky V, et al: Non-cell-autonomous tumor suppression by
p53. Cell. 153:449–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Ivanschitz L, Takahashi Y, Jollivet F,
Ayrault O, Le Bras M and de Thé H: PML IV/ARF interaction enhances
p53 SUMO-1 conjugation, activation, and senescence. Proc Natl Acad
Sci USA. 112:14278–14283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Neyret-Kahn H, Benhamed M, Ye T, Le Gras
S, Cossec JC, Lapaquette P, Bischof O, Ouspenskaia M, Dasso M,
Seeler J, et al: Sumoylation at chromatin governs coordinated
repression of a transcriptional program essential for cell growth
and proliferation. Genome Res. 23:1563–1579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Jiang Z, Fan Q, Zhang Z, Zou Y, Cai R,
Wang Q, Zuo Y and Cheng J: SENP1 deficiency promotes ER
stress-induced apoptosis by increasing XBP1 SUMOylation. Cell
Cycle. 11:1118–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Xia W, Tian H, Cai X, Kong H, Fu W, Xing
W, Wang Y, Zou M, Hu Y and Xu D: Inhibition of SUMO-specific
protease 1 induces apoptosis of astroglioma cells by regulating
NF-κB/Akt pathways. Gene. 595:175–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Sudharsan R and Azuma Y: The SUMO ligase
PIAS1 regulates UV-induced apoptosis by recruiting Daxx to
SUMOylated foci. J Cell Sci. 125:5819–5829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Zhou Y, Ji C, Cao M, Guo M, Huang W, Ni W,
Meng L, Yang H and Wei JF: Inhibitors targeting the SUMOylation
pathway: A patent review 2012–2015 (Review). Int J Mol Med.
41:3–12. 2018.
|
|
209
|
Scott DE, Bayly AR, Abell C and Skidmore
J: Small molecules, big targets: Drug discovery faces the
protein-protein interaction challenge. Nat Rev Drug Discov.
15:533–550. 2016. View Article : Google Scholar : PubMed/NCBI
|