Introduction
Prostate cancer (PCa) is a leading cause of
mortality in Western countries. In Japan, PCa incidence and
mortality rates are on the increase, despite efforts to screen and
diagnose patients early and despite extensive efforts to treat this
disease (1,2). There are a number of modalities for
localized PCa. However, androgen deprivation therapy remains the
standard method used to treat patients with advanced PCa. During
hormone therapy, PCa responds well to androgen ablation initially.
However, this response is gradually lost and the disease becomes
castration-resistant prostate cancer (CRPC), defined as disease
progression, even in the presence of castration levels of
circulating androgens (3,4). The androgen receptor (AR) remains
functional and is important in the mechanisms of CRPC (5), even at this stage.
Evidence for persistent hormone dependence in CRPC
facilitated the development of novel anti-androgens capable of
blocking testosterone synthesis by the testes as well as adrenal
glands and prostate tumor tissue. Abiraterone acetate is an oral,
selective and irreversible inhibitor of CYP17, a critical enzyme in
androgen biosynthesis, which blocks non-gonadal androgen
production. Abiraterone at a dose of 1000 mg/day in combination
with prednisone at a dose of 10 mg/day was approved by the Food and
Drug Administration (FDA) in April, 2011, and by the European
Medicines Agency (EMA) in July, 2011 for the treatment of
metastatic CRPC. The approvals were based on the results of the
COU-AA-301 trial conducted in patients who received previous
docetaxel chemotherapy, which demonstrated a survival benefit for
the experimental arm versus the placebo (6). New anti-androgens with improved
binding properties have also been developed. One of these agents is
enzalutamide, an oral AR antagonist small molecule that binds to
ARs with a higher affinity compared to bicalutamide and blocks AR
nuclear translocation, co-activator recruitment and DNA binding
without agonist activity when AR is overexpressed (7). Results of the AFFIRM trial showed
that enzalutamide was approved by the FDA in August, 2012 (8). These novel compounds were reported to
have survival benefits. However, the CRPC is likely to eventually
progress to a phase of disease that is resistant to these types of
therapy.
One of the mechanisms of androgen-independent (AI)
progression of PCa is neuroendocrine differentiation (NED).
Neuroendocrine (NE) cells originally exist in the normal prostate
acini and duct and they regulate prostatic growth, differentiation
and secretion. The lack of AR expression is indicative of the AI
activity of NE cells. Clusters of malignant NE cells are found
among adenocarcinoma cells in most PC, sharing a common clonal
origin. The prostate NE cells affect the target cells in an AI
manner. Therefore, the NE pathway is thought to be one of the most
significant mechanisms for AIPC or CRPC (9). NE cells are detected by
immunohistochemical staining (IHS) using antibodies against
neuropeptides, of which CGA and NSE are the most thoroughly
investigated and are believed to be the best markers for prostatic
NED (9). Using CGA and NSE as NE
markers, Tanaka et al (10)
reported that lesions predominantly composed of a neuroendocrine
cell tumor were found in 4 of 20 autopsy cases (Japanese patients
with PCa). However, AR is expressed in almost all types of cancer
of the prostate, before and after androgen ablation therapy
(11). Specific downregulation of
the AR with anti-AR antibodies or siRNAs results in AI prostate
cancer (or CRPC), cell growth inhibition and a reduction in PSA
expression (12,13). In addition, recent clinical data,
such as abiraterone acetate and enzalutamide, support the AR
function in CRPC.
The aims of this study were to compare the AR
expression and NED between hormone-sensitive PCa (HSPC) and CRPC
patients using IHS and to analyze the diagnostic and prognostic
significance of this expression in CRPC.
Materials and methods
Patients and PCa tissues
PCa tissues were retrospectively obtained using
prostate needle biopsies from 20 HSPC patients at initial
diagnosis. Consecutive CRPC specimens were obtained using prostate
needle biopsies in 24 patients and transurethral resections (TUR)
in 4 patients (CRPC biopsy). Cores (≥10) were obtained using
Transrectal Ultrasound-Guided Prostate (TRUS) biopsies. CRPC was
defined as three increases in the PSA level at least 1 month apart,
or evidence of a new clinical disease while the patient was
receiving androgen deprivation therapy and the testosterone levels
were at castrate levels (14).
Patient characteristics are shown in Table I. The median follow-up following
CRPC biopsies was 15.6 months (range, 1–57). From the
formalin-fixed paraffin-embedded tissue samples, multiple
4-μm sections were prepared using a microtome and
transferred to glass slides (Fisherbrand Superfrost Plus; Fisher
Scientific, Pittsburg, PA, USA). Chromogranin A (CGA),
neuron-specific enolase (NSE) and AR were immunohistochemically
stained in the representative positive cores or TUR specimens for
PCa.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| HSPC
| CRPC
|
|---|
| Variables | Mean | SD | Mean | SD |
|---|
| Age (years) | 74.1 | 6.2 | 73.3 | 7.1 |
| iPSA (ng/ml) | 1110 | 2141.3 | 412.4 | 750.3 |
| rPSA (ng/ml) | 77.3 | 175.0 | | |
| Gleason score | 7.9 | 1.5 | | |
| Time to CRPC
(months) | n.a. | | 33.5 | 51 |
| No. | | | | |
| M+ | 10 | | 25 | |
| M− | 10 | | 3 | |
Antibodies
Antibodies for the IHS were: Anti-CGA, a rabbit
polyclonal antibody (A0430; dilution, 1:800) (Dako, Carpinteria,
CA, USA); anti-NSE antibody, a mouse monoclonal antibody (M0873;
dilution, 1:200) (Dako) and anti-AR antibody, a mouse monoclonal
antibody (BSB6076; dilution, 1:50) (BioSB).
IHS
The glass slides were incubated with the primary
antibodies at an optimized titer and were diluted using Universal
Blocking Reagent (BioGenex, Fremont, CA, USA) for 30 min, then
washed three times with KN buffer (KN-09001; Pathology Institute,
Toyama, Japan). The sections were then incubated with a
horse-radish peroxidase dextran polymer-labeled goat anti-mouse
antibody or goat anti-rabbit antibody (ChemMate EnVision kit;
dilution 1:100) (Dako) for 30 min at room temperature (RT). The
sections were then washed three times in KN buffer and incubated at
RT with 3,3′-diaminobenzidine (DAB; Dako) for 10 min. The sections
were rinsed several times with distilled water and were
counterstained with Mayer’s hematoxylin, dehydrated, cleared and
mounted with resinous mounting medium (15).
The staining intensity was graded as negative, 0;
positive, 1+ and strongly positive, 2+ (Fig. 1). The proportion of 1+ and 2+ areas
in the PCa cells was determined. These analyses were carried out by
an experienced pathologist (T.T.). PCa was regarded as NED if ≥50%
of the tumor cells were positive for CGA and/or NSE. In addition,
PCa was regarded as strong NED if ≥50% of the tumor cells were 2+
for CGA and/or NSE.
Statistical analysis
Statistical analysis was performed using the
Chi-square test to compare the two groups. The cause-specific
survival was estimated for each study group using the Kaplan-Meier
method and the differences were assessed using the Wilcoxon test.
For the multivariate analysis, the Cox proportional hazard model
was used to identify independent variables to predict
cause-specific survival following CRPC biopsy. P<0.05 was
considered to indicate a statistically significanct difference. We
used the JMP software version 8.0.1 (SAS Institute, Tokyo, Japan)
for the statistical analyses. The study was approved by the
institutional review board of the University of Toyama.
Results
Immunohistological staining
intensity
Distribution of the staining intensity is shown in
Table II. CRPC exhibited more
cases with strong NE intensity. The AR expression was positive in
all the HSPC. However, 9/28 (32.1%) CRPC patients were negative for
AR, which was more frequent compared to HSPC patients (P=0.0049,
Table III). NED was positive in
9/20 HSPC (45.0%) and in 20/28 CRPC (71.4%). CRPC showed a tendency
to have more NED compared to HSPC (P=0.0649; Table III).
 | Table IIDistribution of IHS intensity. |
Table II
Distribution of IHS intensity.
| HSPC
| CRPC
|
|---|
| IHS intensity | CGA (No.) | NSE (No.) | AR (No.) | CGA (No.) | NSE (No.) | AR (No.) |
|---|
| 0 | 5 | 11 | 0 | 5 | 8 | 9 |
| 1+ | 11 | 4 | 4 | 8 | 4 | 3 |
| 2+ | 4 | 5 | 16 | 15 | 16 | 16 |
 | Table IIIAR expression and NED in HSPC and
CRPC. |
Table III
AR expression and NED in HSPC and
CRPC.
| HSPC (No.) | CRPC (No.) | P-value (Chi-square
test) |
|---|
| AR expression | | | |
| Negative | 0 | 9 | 0.0049 |
| Positive | 20 | 19 | |
| NED | | | |
| Negative | 11 | 8 | 0.0649 |
| Positive | 9 | 20 | |
In CRPC, 11/19 patients (57.9%) were NED-positive
when the AR was positive. However, NED was positive in the CRPC
with a negative AR expression (n=9). Therefore, NED was
significantly associated with a negative AR expression in CRPC
(P=0.0292, Table IV).
 | Table IVAssociation between NED and androgen
receptor expression in CRPC. |
Table IV
Association between NED and androgen
receptor expression in CRPC.
| NED
| |
|---|
| AR expression | Negative (No.) | Positive (No.) | P-value (Chi-square
test) |
|---|
| Negative | 0 | 9 | 0.0292 |
| Positive | 8 | 11 | |
Survival after CRPC biopsy
Table V shows the
treatment modalities in CRPC patients before and after CRPC biopsy.
Prior to CRPC biopsy, the main treatments were hormonal
manipulations, whereas cytotoxic agents were mainly used following
CRPC biopsy (Table V).
 | Table VTreatments before and after CRPC
biopsy. |
Table V
Treatments before and after CRPC
biopsy.
| Treatments | Before CRPC biopsy
(No. of patients) | After CRPC biopsy
(No. of patients) |
|---|
| Radiation | 3 | 7 |
| Radical
prostatectomy | 2 | 0 |
| Alternative
anti-androgen | 13 | 6 |
| Estramustine
monophosphate | 5 | 20 |
| Cisplatin | 0 | 2 |
| Low-dose
prednisone | 1 | 2 |
| Low-dose
dexamethasone | 2 | 5 |
|
Ethinylestradiol | 2 | 0 |
| Paclitaxel | 0 | 11 |
| Docetaxel | 0 | 16 |
| Etoposide | 1 | 2 |
| Angiotensin II
receptor blockers | 0 | 2 |
The cause-specific survival was available in 27
patients with CRPC. Fig. 2 shows
survival curves based on the AR expression in CRPC. Nine CRPC
patients were negative for the AR at CRPC biopsy. AR expression was
not associated with prostate cancer-specific survival in this
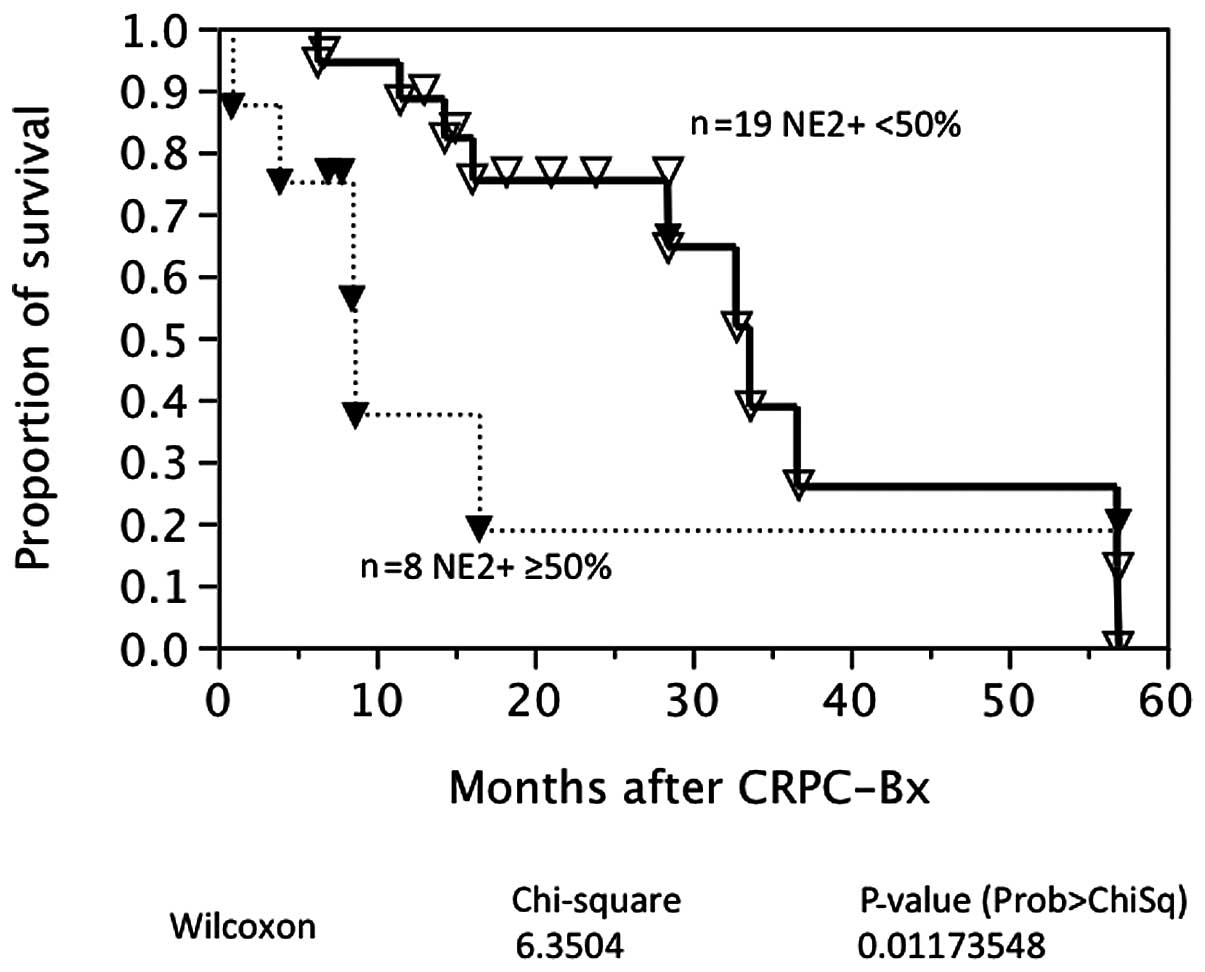
univariate analysis. By contrast, strong NED was associated with a
poorer prognosis following CRPC biopsy (P=0.0117; Fig. 3). A multivariate analysis
demonstrated that age, AR expression and a strong NED were
independent characteristics for prognosis following CRPC biopsy
(Table VI).
 | Table VIA multivariate analysis of the
prognostic features after CRPC biopsy. |
Table VI
A multivariate analysis of the
prognostic features after CRPC biopsy.
|
Characteristics | CI Lower to upper
limits | Chi-square | P-value (Prob >
ChiSq) | Hazard ratio |
|---|
| Age at CRPC
biopsya (years) | 0.00233 to
0.25589 | 3.99141 | 0.04573b | 1.13021 |
| iPSAa (ng/ml) | −0.00051 to
0.00010 | 1.02092 | 0.31230 | 0.99987 |
| rPSAa (ng/ml) | −0.02385 to
0.01026 | 0.36536 | 0.54554 | 0.99509 |
| M at CRPC biopsy
+/− | −2.67880 to
0.06644 | 3.35616 | 0.06695 | 8.30273 |
| AR +/− | −2.68520 to
−0.09616 | 4.59882 | 0.03199b | 12.21495 |
| Strong NED +/− | 0.42617 to
3.21225 | 7.58827 | 0.00587b | 26.75226 |
Discussion
In the present study, the AR was positive for the
HSPC. However, 9/28 patients (32.1%) with CRPC were negative for
the AR. Therefore, a significant number of CRPC cases did not have
the AR in the primary prostate. The information for AR expression
in CRPC is limited. Generally, it has been reported that the AR is
expressed in nearly all types of cancers of the prostate, before
and after androgen ablation therapy (11). The high levels of AR expression and
the development of hypersensitive receptors have been recognized as
a feature associated with the development of CRPC (13,16).
Hobisch et al (17) showed
that metastases from PCa expressed the AR following endocrine
therapy. From a Rapid Autopsy Program, Shah et al (18) indicated that the majority of
patients continued to express substantial quantities of AR despite
having undergone a long-term androgen ablation. The patients
demonstrated marked differences in AR expression between different
tissue sites (2- to 50-fold) and several patients demonstrated a
high amount of AR staining, although they were no longer responding
to androgen-deprivation therapy. In the Japanese studies, Takeda
et al (19) demonstrated
that patients with metastatic PCa with ≥48% AR-positive cells had a
significantly better outcome, in terms of progression-free and
cause-specific survival compared to patients with <48% AR
content. Masai et al (20)
have shown that 33% of cancer cells were positive for the AR in
regrowing prostates from 8 relapsed cancers after endocrine
therapy. The AR-positive cells were more frequently found in
untreated PCa. However, the frequency of AR loss was not shown in
the Japanese series. Matei et al (21) previously reported that the AR was
positive in neoplastic cells in 20/47 patients (53%) with CRPC.
Therefore, 47% of CRPC cases lost AR expression, which is higher
compared to our series. These results indicate that loss of the AR
may be identified in a subset of CRPC and should alert physicians
to the use of androgen- or AR-targeted therapies without conducting
an evaluation of AR expression. It is important to analyze
expression profiles prior to targeted therapies. For example, the
expression of the estrogen and progesterone receptors and HER2 are
frequently evaluated in breast cancer tissues to estimate the
response to treatment. Trastuzumab is used for HER2+ breast cancer
therapy (15).
NED was found in 9/20 HSPC (45%) and 20/28 CRPC
cases (71%) in the present study. Its frequency was slightly higher
in CRPC. Strong NED was found in 9/28 CRPC (32.1%). NED was
determined using IHS or serum NE marker concentrations (9). Using IHS, the NE cells were
identified in ∼10–100% of the untreated PCa tissues. This large
discrepancy in prevalence between studies is explained in part by
the lack of quantitative and consistent tissue-imaging techniques.
McWilliam et al (22) found
NED in 52% of PCa tissues using IHS for CGA and NSE. They also
demonstrated a significant correlation between NED and worsening
tumor differentiation, the presence of bone metastasis and poor
patient survival. Kokubo et al (23) showed that 22% of stage D2 PCa
overexpressed CGA by IHS and that CGA positivity was correlated
with a shorter time to recurrence after hormone therapy. Kamiya
et al (24) demonstrated
that the cause-specific survival was significantly poorer after
hormone therapy in stage D2 PCa with strongly positive staining for
independent CGA and combined CGA with NSE. In the setting of CRPC,
Mucci et al (25) reported
that NE expression was heterogeneous and observed in 4/12 autopsy
cases (33%) when the tumor sites per case were considered among
rapid autopsies from men with hormone refractory PCa. Tanaka et
al (10) reported that lesions
predominantly composed of a neuroendocrine cell tumor were found in
4/20 autopsy cases (20%) of Japanese PCa patients. Therefore, our
finding is consistent with findings of previous studies. However,
information is limited in terms of the survival significance of NED
in CRPC. Tanaka et al (10)
also demonstrated that the survival was brief after relapse,
although the duration of control by employing endocrine therapy
varied in the neuroendocrine cell tumors found at autopsy. An
evaluation of NED was performed only within the primary prostate in
this study. The state of NED in metastases was unknown unlike the
autopsy series. However, NED at CRPC biopsy was a strong and
independent prognostic factor in the Cox proportional hazard model.
NED was associated with a negative AR expression in this study,
which is consistent with previous findings (9) and the significance of NED in the
mechanisms of CRPC was confirmed. In addition, these results
support the importance of performing a CRPC biopsy. In the present
study, we found two neuroendocrine cancers by CRPC biopsy and
patients underwent etoposide and cisplatin (EP) therapy (26,27)
instead of taxane-based chemotherapy.
There are, however, limitations to this study. The
study design was retrospective. The results may be different if
performed in a prospective manner. The study sample size was small;
however, this number of CRPC specimens is believed to be the best
in Japan. The results may have been different if the study was
conducted on a larger and different population. Quantification of
the staining intensity was visually performed, which is a
traditional method for IHS. This may be better analyzed by multiple
pathologists or by using computer-aided methods to avoid
inter-observer or inter-institutional variations (28). If NED was otherwise determined, the
results could also have been affected. The CRPC biopsy findings
were obtained only from the prostate. Therefore, the expression
profiles in metastatic cites were not evaluated, unlike the autopsy
series.
In conclusion, the AR was lost in a subset of CRPC,
in which AR-targeted therapy might not be effective. NED was
observed more frequently in CRPC vs. HSPC. NED was associated with
a loss of the AR and worse prognosis in CRPC. Thus, CRPC biopsy may
be useful to better characterize the diseases.
References
|
1
|
Suzuki H, Komiya A, Kamiya N, Imamoto T,
Kawamura K, Miura J, Suzuki N, Nakatsu H, Hata A and Ichikawa T:
Development of a nomogram to predict probability of positive
initial prostate biopsy among Japanese patients. Urology.
67:131–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yano M, Imamoto T, Suzuki H, Fukasawa S,
Kojima S, Komiya A, Naya Y and Ichikawa T: The clinical potential
of pretreatment serum testosterone level to improve the efficiency
of prostate cancer screening. Eur Urol. 51:375–380. 2007.
View Article : Google Scholar
|
|
3
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small
EJ, Beer TM, Wilding G, Martin A and Hussain M; Prostate Cancer
Clinical Trials Working Group: Design and end points of clinical
trials for patients with progressive prostate cancer and castrate
levels of testosterone: recommendations of the Prostate Cancer
Clinical Trials Working Group. J Clin Oncol. 26:1148–1159. 2008.
View Article : Google Scholar
|
|
4
|
Scher HI, Buchanan G, Gerald W, Butler LM
and Tilley WD: Targeting the androgen receptor: improving outcomes
for castration-resistant prostate cancer. Endocr Relat Cancer.
11:459–476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Attar RM, Takimoto CH and Gottardis MM:
Castration-resistant prostate cancer: locking-up the molecular
escape routes. Clin Cancer Res. 15:3251–3255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng
T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL,
Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D,
Loriot Y, Chieffo N, Kheoh T, Haqq CM and Scher HI; COU-AA-301
Investigators: Abiraterone and increased survival in metastatic
prostate cancer. N Engl J Med. 364:1995–2005. 2011.
|
|
7
|
Tran C, Ouk S, Clegg NJ, Chen Y, Watson
PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A,
Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT,
Scher HI, Jung ME and Sawyers CL: Development of a
second-generation antiandrogen for treatment of advanced prostate
cancer. Science. 324:787–790. 2009. View Article : Google Scholar
|
|
8
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller MD, de Wit R, Mulders P, Chi KN, Shore ND,
Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M,
Hainsworth JD, Hirmand M, Selby B, Seely L and de Bono JS; AFFIRM
Investigators: Increased survival with enzalutamide in prostate
cancer after chemotherapy. N Engl J Med. 367:1187–1197. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komiya A, Suzuki H, Imamoto T, Kamiya N,
Nihei N, Naya Y, Ichikawa T and Fuse H: Neuroendocrine
differentiation in the progression of prostate cancer. Int J Urol.
16:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka M, Suzuki Y, Takaoka K, Suzuki N,
Murakami S, Matsuzaki O and Shimazaki J: Progression of prostate
cancer to neuroendocrine cell tumor. Int J Urol. 8:431–436. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grossmann ME, Huang H and Tindall DJ:
Androgen receptor signaling in androgen-refractory prostate cancer.
J Natl Cancer Inst. 93:1687–1697. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zegarra-Moro OL, Schmidt LJ, Huang H and
Tindall DJ: Disruption of androgen receptor function inhibits
proliferation of androgen-refractory prostate cancer cells. Cancer
Res. 62:1008–1013. 2002.PubMed/NCBI
|
|
13
|
Chen CD, Welsbie DS, Tran C, Baek SH, Chen
R, Vessella R, Rosenfeld MG and Sawyers CL: Molecular determinants
of resistance to antiandrogen therapy. Nat Med. 10:33–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crook JM, O’Callaghan CJ, Duncan G,
Dearnaley DP, Higano CS, Horwitz EM, Frymire E, Malone S, Chin J,
Nabid A, Warde P, Corbett T, Angyalfi S, Goldenberg SL,
Gospodarowicz MK, Saad F, Logue JP, Hall E, Schellhammer PF, Ding K
and Klotz L: Intermittent androgen suppression for rising PSA level
after radiotherapy. N Engl J Med. 367:895–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagata T, Shimada Y, Sekine S, Hori R,
Matsui K, Okumura T, Sawada S, Fukuoka J and Tsukada K: Prognostic
significance of NANOG and KLF4 for breast cancer. Breast Cancer.
Apr 17–2012.(Epub ahead of print).
|
|
16
|
Bonkhoff H and Berges R: From pathogenesis
to prevention of castration resistant prostate cancer. Prostate.
70:100–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hobisch A, Culig Z, Radmayr C, Bartsch G,
Klocker H and Hittmair A: Distant metastases from prostatic
carcinoma express androgen receptor protein. Cancer Res.
55:3068–3072. 1995.PubMed/NCBI
|
|
18
|
Shah RB, Mehra R, Chinnaiyan AM, Shen R,
Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA,
Kim R, Rubin MA and Pienta KJ: Androgen-independent prostate cancer
is a heterogeneous group of diseases: lessons from a rapid autopsy
program. Cancer Res. 64:9209–9216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takeda H, Akakura K, Masai M, Akimoto S,
Yatani R and Shimazaki J: Androgen receptor content of prostate
carcinoma cells estimated by immunohistochemistry is related to
prognosis of patients with stage D2 prostate carcinoma. Cancer.
77:934–940. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masai M, Sumiya H, Akimoto S, Yatani R,
Chang CS, Liao SS and Shimazaki J: Immunohistochemical study of
androgen receptor in benign hyperplastic and cancerous human
prostates. Prostate. 17:293–300. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matei DV, Renne G, Pimentel M, Sandri MT,
Zorzino L, Botteri E, De Cicco C, Musi G, Brescia A, Mazzoleni F,
Tringali V, Detti S and de Cobelli O: Neuroendocrine
differentiation in castration-resistant prostate cancer: a
systematic diagnostic attempt. Clin Genitourin Cancer. 10:164–173.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McWilliam LJ, Manson C and George NJ:
Neuroendocrine differentiation and prognosis in prostatic
adenocarcinoma. Br J Urol. 80:287–290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kokubo H, Yamada Y, Nishio Y, Fukatsu H,
Honda N, Nakagawa A, Saga S, Tsuzuki T and Hara K:
Immunohistochemical study of chromogranin A in Stage D2 prostate
cancer. Urology. 66:135–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamiya N, Suzuki H, Kawamura K, Imamoto T,
Naya Y, Tochigi N, Kakuta Y, Yamaguchi K, Ishikura H and Ichikawa
T: Neuroendocrine differentiation in stage D2 prostate cancers. Int
J Urol. 15:423–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mucci NR, Akdas G, Manely S and Rubin MA:
Neuroendocrine expression in metastatic prostate cancer: evaluation
of high throughput tissue microarrays to detect heterogeneous
protein expression. Hum Pathol. 31:406–414. 2000. View Article : Google Scholar
|
|
26
|
Amato RJ, Logothetis CJ, Hallinan R, Ro
JY, Sella A and Dexeus FH: Chemotherapy for small cell carcinoma of
prostatic origin. J Urol. 147:935–937. 1992.PubMed/NCBI
|
|
27
|
Komiya A, Yasuda K, Nozaki T, Fujiuchi Y,
Hayashi S and Fuse H: Small cell carcinoma of the prostate after
high-dose-rate brachytherapy for low-risk prostatic adenocarcinoma.
Oncol Lett. 5:53–56. 2013.PubMed/NCBI
|
|
28
|
Rizzardi AE, Johnson AT, Vogel RI,
Pambuccian SE, Henriksen J, Skubitz AP, Metzger GJ and Schmechel
SC: Quantitative comparison of immunohistochemical staining
measured by digital image analysis versus pathologist visual
scoring. Diagn Pathol. 7:422012. View Article : Google Scholar
|