Introduction
Pancreatic carcinoma is one of the most aggressive
tumors and continues to constitute a major health problem. The
overall 5-year survival rate of pancreatic cancer patients remains
poor (<5%) despite treatment improvement (1,2). The
resistance of pancreatic carcinoma to aggressive treatment regimens
represents a major challenge, whereas pancreatic carcinoma cell
resistance to apoptosis contributes to treatment failure (3,4).
Apoptosis is initiated by caspase activation in
mitochondria (5). Resistance to
apoptosis has been associated with the overexpression of extrinsic
and intrinsic apoptosis signaling cascades (6). The X-linked inhibitor of apoptosis
protein (XIAP) belongs to the inhibitor of apoptosis proteins
(IAPs) family, a group of structurally related proteins inducing
caspase inhibition that block apoptosis at the core of the
apoptotic machinery by inhibiting caspase-3, -7 and -9 activation,
thereby modulating resistance to apoptosis in pancreatic carcinoma
(7).
Although not expressed in normal pancreatic ductal
cells, XIAP overexpression is known to occur in pancreatic
carcinoma cells, as noted in previous studies (7). The aim of this study was to
investigate the association of resistance to apoptosis with disease
survival by assessing the predictive power of XIAP expression on
pancreatic carcinoma outcome.
Materials and methods
Tissue collection
In total, 54 patients (34 men and 20 women; age,
34–79 years) who underwent macroscopically curative resection for
pancreatic carcinoma between 2003 and 2009 in The Fourth Hospital
of Hebei Medical University (Shijiazhuang, China) were included in
this study. Clinical data were collected for the patients, and
histopathological and tumor-node-metastasis (TNM) classifications
were defined in accordance with the International Union Against
Cancer (UICC) Classification of 2002 (8). Vascular invasion or lymph node
metastases and differentiated grade were also determined using
hematoxylin and eosin (H&E) staining. The specimens were fixed
immediately in 10% formalin for immunostaining. The procedures were
supervised and approved by the Human Tissue Research Committee of
the hospital. Informed consent was obtained from the patients.
Measurement of XIAP level in pancreatic
cancer tissue
The specimens were fixed in 10% formalin and
embedded in paraffin. Five serial 4-μm sections were cut
from the tissue blocks. The deparaffinized sections were stained
with the anti-XIAP antibody (BD Biosciences, Franklin Lakes, NJ,
USA) at a dilution of 1:100 overnight at 4°C followed by incubation
with a biotinylated secondary anti-mouse IgG antibody for 1 h at
room temperature. The sections were subsequently incubated with
horseradish peroxidase (HRP)-conjugated streptavidin and were
developed using 3,3′-diaminobenzidine (DAB). XIAP staining was
performed using the methods previously described. Grading was
performed by two pathologists with no background on the clinical
data of patients (9). XIAP
staining was scored as: 0, 0–25%; 1, ≥25–50%; 2, ≥50–75% and 3,
≥75%.
Statistical analysis
Statistical analysis was performed using the
χ2 test to determine the XIAP expression frequency
between normal and pancreatic cancer tissues. Spearman’s
correlation analysis was used to assess the correlation between
XIAP expression and patient clinical data. Post-operative survival
was estimated using the Kaplan-Meier method and differences between
survival curves were compared using the log-rank test. Multivariate
survival analysis was performed using the Cox proportional hazards
model. Statistical analyses were performed using the SPSS 13.0
software (SPSS, Inc, Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
XIAP staining assessment in normal and
pancreatic cancer tissues
XIAP was mostly located in the cytoplasm, showing
diffuse distribution. The cytoplasm of positive cells was stained
yellow or brown (Fig. 1). Compared
to the lower XIAP staining frequency in normal pancreatic tissue
observed in 7 of 14 cases, XIAP was expressed in 48 of 54
pancreatic cancer tissues (P=0.001). These findings demonstrate
that XIAP expression varied in normal and pancreatic cancer
tissues.
Correlation between XIAP expression and
post-operative survival
A total of 54 patients including 18 of stage I, 19
of stage II, 4 of stage III and 13 of stage IV were included in
this study and a review was conducted every 3 months between 2003
and 2009. Two patients were lost during the follow-up period and
the remaining 52 patients were included in the subsequent analysis.
Forty-five patients had succumbed to the disease by the end of the
follow-up period. The median follow-up time for the 52 patients was
14.39±13.37 months. Adjuvant chemo- or radiation therapy was not
administered following carcinoma resection. Data collected during
the follow-up were analyzed for clinical characteristics as
described in Materials and methods. No statistically significant
difference was observed for gender or age, while borderline
statistically significant difference was observed for tumor size
and histological grade (P=0.069 and 0.079, respectively). A
significant difference was observed in the TNM classification and
tumor invasion status, P<0.000, respectively (Table I).
 | Table IUnivariate analysis of clinical
characteristics associated with post-operative survival in
pancreatic cancer patients. |
Table I
Univariate analysis of clinical
characteristics associated with post-operative survival in
pancreatic cancer patients.
| Characteristics | No. of cases | Survival rate
(%) | P-value |
|---|
| Gender | | | 0.810 |
| Male | 34 | 20.6 | |
| Female | 20 | 5.0 | |
| Age (years) | | | 0.883 |
| <60 | 21 | 14.3 | |
| ≥60 | 33 | 15.2 | |
| Diameter of tumor
(cm) | | | 0.069 |
| <5 | 26 | 19.2 | |
| ≥5 | 28 | 10.7 | |
| TNM
classification | | | 0.000 |
| I–II | 37 | 18.9 | |
| III–IV | 17 | 5.9 | |
| Histological
grade | | | 0.079 |
| Well | 37 | 16.2 | |
| Moderately and
poorly | 17 | 11.8 | |
| Invasion or
metastasis | | | 0.000 |
| Yes | 30 | 23.3 | |
| No | 24 | 4.2 | |
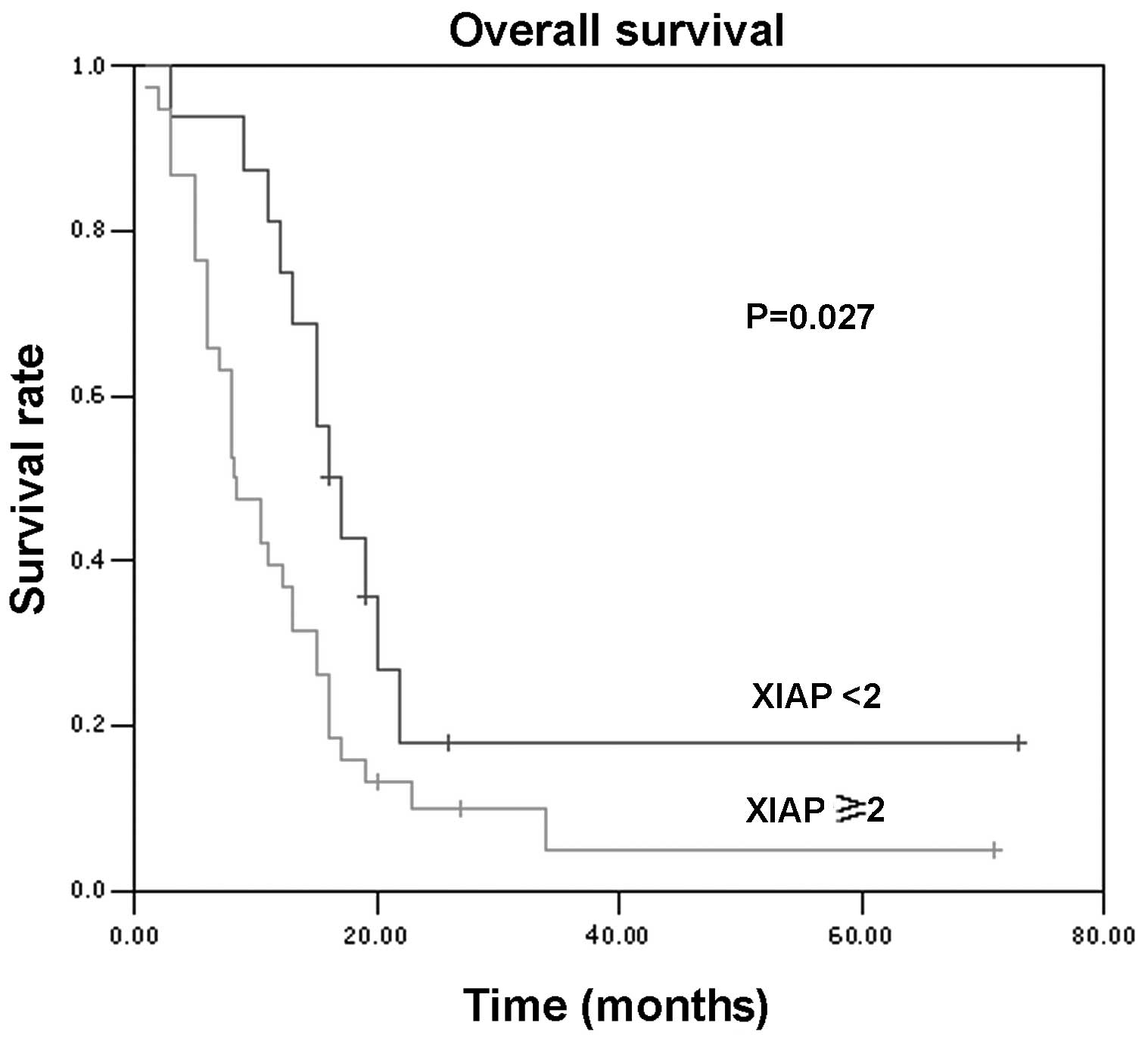
XIAP expression levels were graded with a score of
0–3. Patients were divided into two subgroups for survival
analysis, with a scored of 0 and 1 for the low-expression group and
a score of 2 and 3 for the high-expression group. The cumulative
survival rate was plotted as a Kaplan-Meier curve (Fig. 2), and the log-rank test
demonstrated a statistically significant difference (P=0.027)
between the low- and high-expression groups, demonstrating the
usefulness of XIAP levels in pancreatic cancer prognosis.
Effect of XIAP expression on the
predictive power of traditional clinical characteristics
A multivariate analysis, including the predictors
mentioned above, using the Cox proportional hazard method was used
to evaluate the effect of XIAP expression on the predictive power
of traditional clinical parameters. The analysis demonstrated that
the XIAP level and TNM classification were independent risk factors
for the post-operative survival rate of pancreatic cancer patients
(Table II). Of the predictors,
XIAP expression status was strongly associated with the cancer
survival rate, at a hazard ratio of 1.771 (95% CI, 1.099–2.852).
Results of the univariate and multivariate analyses suggested that
the XIAP level in pancreatic cancer tissues constitutes one of the
most significant predictors of the post-operative survival rate of
pancreatic cancer patients.
 | Table IIMultiple analysis of prognostic
factors associated with post-operative survival in pancreatic
cancer patients using the COX proportional hazard model. |
Table II
Multiple analysis of prognostic
factors associated with post-operative survival in pancreatic
cancer patients using the COX proportional hazard model.
| Characteristics | Relative risk | 95% CI | P-value |
|---|
| Age | 1.004 | 0.978–1.030 | 0.770 |
| Gender | 1.131 | 0.515–2.481 | 0.759 |
| Diameter of
tumors | 0.918 | 0.829–1.018 | 0.105 |
| Histological
grade | 0.675 | 0.353–1.290 | 0.234 |
| TNM stage | 1.652 | 1.014–2.694 | 0.044 |
| Invasion or
metastasis | 2.229 | 0.805–6.167 | 0.123 |
| XIAP expression | 1.771 | 1.099–2.852 | 0.019 |
Correlation of clinical characteristics
and XIAP levels
The correlation of clinical characteristics and XIAP
levels was investigated in pancreatic cancer patients using the
Spearman’s correlation analysis (Table
III). Gender, age, tumor size and TNM classification were not
correlated with XIAP levels, while tumor invasion status and
histological grade were significantly correlated with XIAP
expression. The data suggest that XIAP is able to modify tumor
development in combination with other predictors.
 | Table IIIComparison of XIAP expression with
clinical characteristics in cancer tissue. |
Table III
Comparison of XIAP expression with
clinical characteristics in cancer tissue.
| | XIAP expression
| |
|---|
| Characteristics | No. | 0 | 1 | 2 | 3 | P-value |
|---|
| Gender | | | | | | |
| Male | 34 | 4 | 3 | 15 | 12 | 0.406 |
| Female | 20 | 2 | 4 | 9 | 5 | |
| Age (years) | | | | | | |
| <60 | 21 | 3 | 5 | 8 | 5 | 0.116 |
| ≥60 | 33 | 3 | 2 | 16 | 12 | |
| Tumor diameter
(cm) | | | | | | |
| <5 | 26 | 5 | 4 | 9 | 8 | 0.268 |
| ≥5 | 28 | 1 | 3 | 15 | 9 | |
| TNM
classification | | | | | | |
| I+II | 37 | 5 | 6 | 16 | 10 | 0.152 |
| III+IV | 17 | 1 | 1 | 8 | 7 | |
| Invasion or
metastasis | | | | | | |
| No | 30 | 6 | 4 | 13 | 7 | 0.040 |
| Yes | 24 | 0 | 3 | 11 | 10 | |
| Histological
grade | | | | | | |
| Well | 12 | 2 | 2 | 6 | 2 | 0.014 |
| Moderately | 25 | 3 | 5 | 11 | 6 | |
| Poorly | 17 | 1 | 0 | 7 | 9 | |
Discussion
In the present study, we used immunohistochemical
methods with a monoclonal antibody against XIAP with paraffin-
embedded pancreatic tissue sections for the assessment of this
apoptosis inhibitor in pancreatic cancer patients. Consistent with
the findings of previous studies, different expression frequencies
were found in normal and pancreatic cancer tissues (7). XIAP expression was also found to be
associated with the outcome of pancreatic cancer patients. Similar
results have also been demonstrated in other types of cancer where
XIAP overexpression was correlated with worse prognosis (10–12).
XIAP levels constituted a risk factor for pancreatic carcinogenesis
and a good marker for predicting the outcome of post-operative
pancreatic cancer patients.
IAP is a family of endogenous caspase inhibitors
that share a common baculoviral IAP repeat (BIR) domain. XIAP
carrying three functional domains including BIR, linker and
Ring-finger domains, potentially constitutes the best-characterized
IAP proteins with respect to its structure and biochemical
mechanisms. XIAP exhibits its effect on resistance to apoptosis by
inhibiting caspases-3, -7 and -9, but not caspases-1, -6, -8 and
-10 (7,13,14).
XIAP is a potential target for pancreatic carcinoma treatment due
to the fact that XIAP blocks apoptosis at the core of the apoptotic
machinery. XIAP inhibitors increase apoptosis sensitivity in
pancreatic carcinoma cells (7),
while XIAP knockdown inhibits pancreatic cancer cell proliferation
in vitro and in vivo (15). XIAP overexpression shortens the
survival time of pancreatic cancer patients probably by modifying
the resistance to apoptosis and the proliferation capacity of
pancreatic carcinoma cells.
The clinical significance of XIAP expression was
investigated in this study. The expression of XIAP in pancreatic
cancer was not significantly correlated with gender, age, tumor
size or TNM classification. However, increased XIAP expression was
detected in less differentiated pancreatic carcinoma tissues,
confirming the data provided by Jian et al (16). Furthermore, the prevalence of XIAP
expression in cases with a tumor invasion status suggested a
correlation of XIAP expression with a more aggressive phenotype of
pancreatic cancer.
The balance of anti- to pro-apoptotic regulators has
been shown to control the relative sensitivity or resistance of
various cells to apoptotic stimuli. Smac/DIABLO inhibits XIAP and
enhances apoptosis sensitivity by binding with the BIR structure of
human IAP family genes. An inverse correlation between XIAP
expression and Smac/DIABLO mitochondrial release has been observed
following apoptosis induction in colon cancer cells, lymphoma cells
and keratinocytes (17–19). The delicate balance between XIAP
and Smac/DIABLO expression was gradually disturbed in certain types
of cancer, resulting in a relative increase of the anti-apoptotic
XIAP levels exceeding the pro-apoptotic Smac/DIABLO levels, a fact
which may be responsible for the carcinogenesis and marked
resistance to apoptosis of these types of cancer (10,20).
In this study, XIAP expression was also found to have a significant
inverse correlation with Smac expression in pancreatic cancer
tissue (P=0.002), however, Smac expression was not associated with
pancreatic carcinoma outcome (data not shown).
In conclusion, to the best of our knowledge, the
correlation of XIAP expression with prognosis was analyzed for the
first time in patients with pancreatic cancer. Data in this study
obtained by univariate and multivariate analysis showed that
patients in the high XIAP expression subgroups exhibited a
significantly shorter overall survival. Additional studies are
required to determine the key factors involved in the process of
XIAP-related resistance to apoptosis.
Acknowledgements
This study was supported by the
Natural Science Foundation of the Hebei Province (no. C
2006000856).
References
|
1
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
3
|
Schneider G, Siveke JT, Eckel F and Schmid
RM: Pancreatic cancer: basic and clinical aspects.
Gastroenterology. 128:1606–1625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gukovskaya AS and Pandol SJ: Cell death
pathways in pancreatitis and pancreatic cancer. Pancreatology.
4:567–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotheraphy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neesse A, Gress TM and Michl P:
Therapeutic targeting of apoptotic pathways: novel aspects in
pancreatic cancer. Curr Pharm Biotechnol. May 2–2011.(Epub ahead of
print).
|
|
7
|
Vogler M, Walczak H, Stadel D, Haas TL,
Genze F, Jovanovic M, Bhanot U, Hasel C, Möller P, Gschwend JE,
Simmet T, Debatin KM and Fulda S: Small molecule XIAP inhibitors
enhance TRAIL-induced apoptosis and antitumor activity in
preclinical models of pancreatic carcinoma. Cancer Res.
69:2425–2434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobin LH and Wittekind CH: International
Union Against Cancer (UICC), TNM Classification of Malignant
Tumors. 6th edition. John Wiley & Sons; New York: 2002
|
|
9
|
Wang J, Liu Y, Ji R, Gu Q, Zhao X, Liu Y
and Sun B: Prognostic value of the X-linked inhibitor of apoptosis
protein for invasive ductal breast cancer with triple-negative
phenotype. Hum Pathol. 41:1186–1195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan Y, Mahotka C, Heikaus S, Shibata T,
Wethkamp N, Liebmann J, Suschek CV, Guo Y, Gabbert HE, Gerharz CD
and Ramp U: Disturbed balance of expression between XIAP and
Smac/DIABLO during tumour progression in renal cell carcinomas. Br
J Cancer. 91:1349–1357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Augello C, Caruso L, Maggioni M, Donadon
M, Montorsi M, Santambrogio R, Torzilli G, Vaira V, Pellegrini C,
Roncalli M, Coggi G and Bosari S: Inhibitors of apoptosis proteins
(IAPs) expression and their prognostic significance in
hepatocellular carcinoma. BMC Cancer. 9:1252009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa Y, Abe S, Kurata M, Hasegawa M,
Yamamoto K, Inoue M, Takemura T, Suzuki K and Kitagawa M: IAP
family protein expression correlates with poor outcome of multiple
myeloma patients in association with chemotherapy-induced
overexpression of multidrug resistance genes. Am J Hematol.
81:824–831. 2006. View Article : Google Scholar
|
|
13
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riedl SJ, Renatus M, Schwarzenbacher R,
Zhou Q, Sun C, Fesik SW, Liddington RC and Salvesen GS: Structural
basis for the inhibition of caspase-3 by XIAP. Cell. 104:791–800.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang C, Tan T, Yi XP, Shen H and Li YX:
Lentivirus-mediated shRNA targeting XIAP and survivin inhibit
SW1990 pancreatic cancer cell proliferation in vitro and
in vivo. Mol Med Rep. 4:667–674. 2011.PubMed/NCBI
|
|
16
|
Jian ZY, Li YX, Li XG and Chen JY:
Expression and significance of XIAP in pancreatic carcinoma
tissues. Chin J Gen Surg. 14:385–387. 2005.(In Chinese).
|
|
17
|
Tillman DM, Izeradjene K, Szucs KS,
Douglas L and Houghton JA: Rottlerin sensitizes colon carcinoma
cells to tumor necrosis factor-related apoptosis-inducing
ligand-induced apoptosis via uncoupling of the mitochondria
independent of protein kinase C. Cancer Res. 63:5118–5125.
2003.
|
|
18
|
Chow KU, Nowak D, Boehrer S, Ruthardt M,
Knau A, Hoelzer D, Mitrou PS and Weidmann E: Synergistic effects of
chemotherapeutic drugs in lymphoma cells are associated with
down-regulation of inhibitor of apoptosis proteins (IAPs),
prostate-apoptosis-response-gene 4 (Par-4), death-associated
protein (Daxx) and with enforced caspase activation. Biochem
Pharmacol. 66:711–724. 2003. View Article : Google Scholar
|
|
19
|
Takasawa R and Tanuma S: Sustained release
of Smac/DIABLO from mitochondria commits to undergo UVB-induced
apoptosis. Apoptosis. 8:291–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Zhu J, Tang Y, Li F, Zhou H, Peng
B, Zhou C and Fu R: X-linked inhibitor of apoptosis positive
nuclear labeling: a new independent prognostic biomarker of breast
invasive ductal carcinoma. Diagn Pathol. 6:492011. View Article : Google Scholar : PubMed/NCBI
|