Introduction
Pancreatic carcinoma frequently infiltrates the
anterior and posterior tissues lying beyond the thin adjacent
pancreatic parenchyma. The pancreas is fixed posteriorly and the
fusion fascia lies between the pancreatic parenchyma and
retroperitoneal organs such as the kidney, adrenal gland and
inferior vena cava (IVC). The fusion fascia consists of peritoneal
and retroperitoneal elements (1).
Pancreatic carcinoma rarely infiltrates the IVC and adrenal gland,
which lie beyond this fascia. Therefore, the selection of the
retropancreatic dissection plane and concomitant resection of
retroperitoneal structures is controversial in pancreatic cancer
patients. In the present study, we assessed the role of the fusion
fascia in the prevention of the infiltration of tissues located
posterior to the pancreas by pancreatic carcinoma.
Materials and methods
Patient characteristics
This study included 140 patients who underwent
pancreatic carcinoma resection at our institution between 1997 and
2011. The age of the patients was 33–80 years (median, 63 years)
and the male/female ratio was 84/56. Ninety-one patients underwent
pancreatoduodenectomy (PD), 3 underwent total pancreatectomy and 46
underwent distal pancreatectomy (DP). According to the TNM
classification of the UICC (2),
the tumor was T1, T2, T3 and T4 in 5, 2, 115 and 18 patients,
respectively. The disease was stage IA, IB, IIA, IIB, III and IV in
4, 2, 31, 73, 14 and 15 patients, respectively. The histological
type was well-, moderately and poorly differentiated in 31, 87 and
21 patients, respectively (Table
I). This study was approved by the ethics committee of Kanazawa
University. Informed consent was obtained from all patients.
 | Table IPatient baseline characteristics. |
Table I
Patient baseline characteristics.
| Age in years
(range) | 63.2 (33–80) |
| Gender | |
| Male | 84 |
| Female | 56 |
| Surgical
procedure | |
| PD | 91 |
| TP | 3 |
| DP | 46 |
| TNM
classification | |
| T1 | 5 |
| T2 | 2 |
| T3 | 115 |
| T4 | 18 |
| Stage | |
| IA | 4 |
| IB | 2 |
| IIA | 32 |
| IIB | 73 |
| III | 14 |
| IV | 15 |
| Histological
differentiation | |
| High | 31 |
| Moderate | 87 |
| Poor | 21 |
Grading
Resected specimens were cut into 5-mm stepwise
tissue blocks following fixation with 10% neutral buffered
formaldehyde solution. Then, consecutive 5-μm serial
sections were cut from the blocks. PD specimens were cut
perpendicular to the body axis, whereas DP specimens were cut
perpendicular to their own longitudinal axis. Retropancreatic
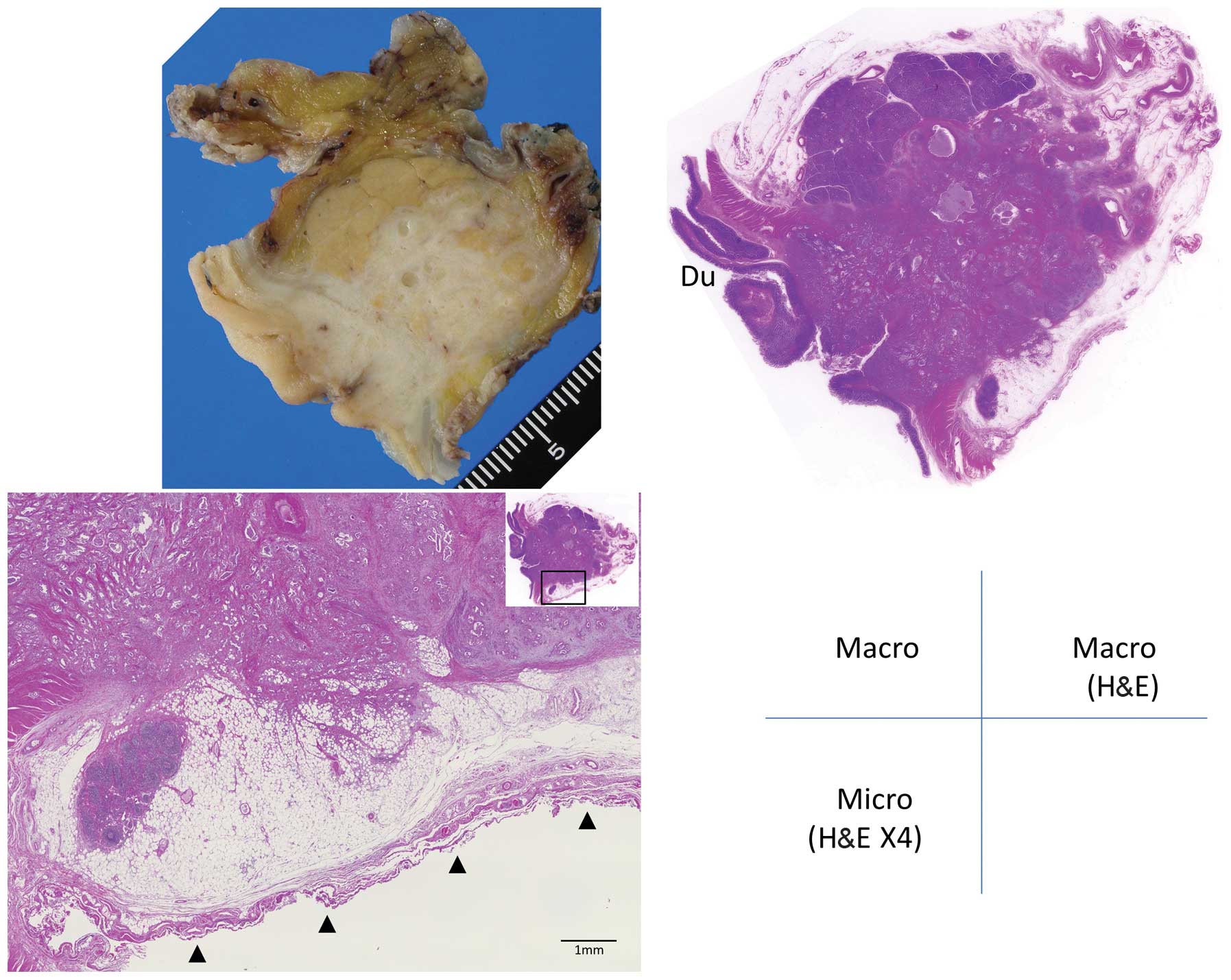
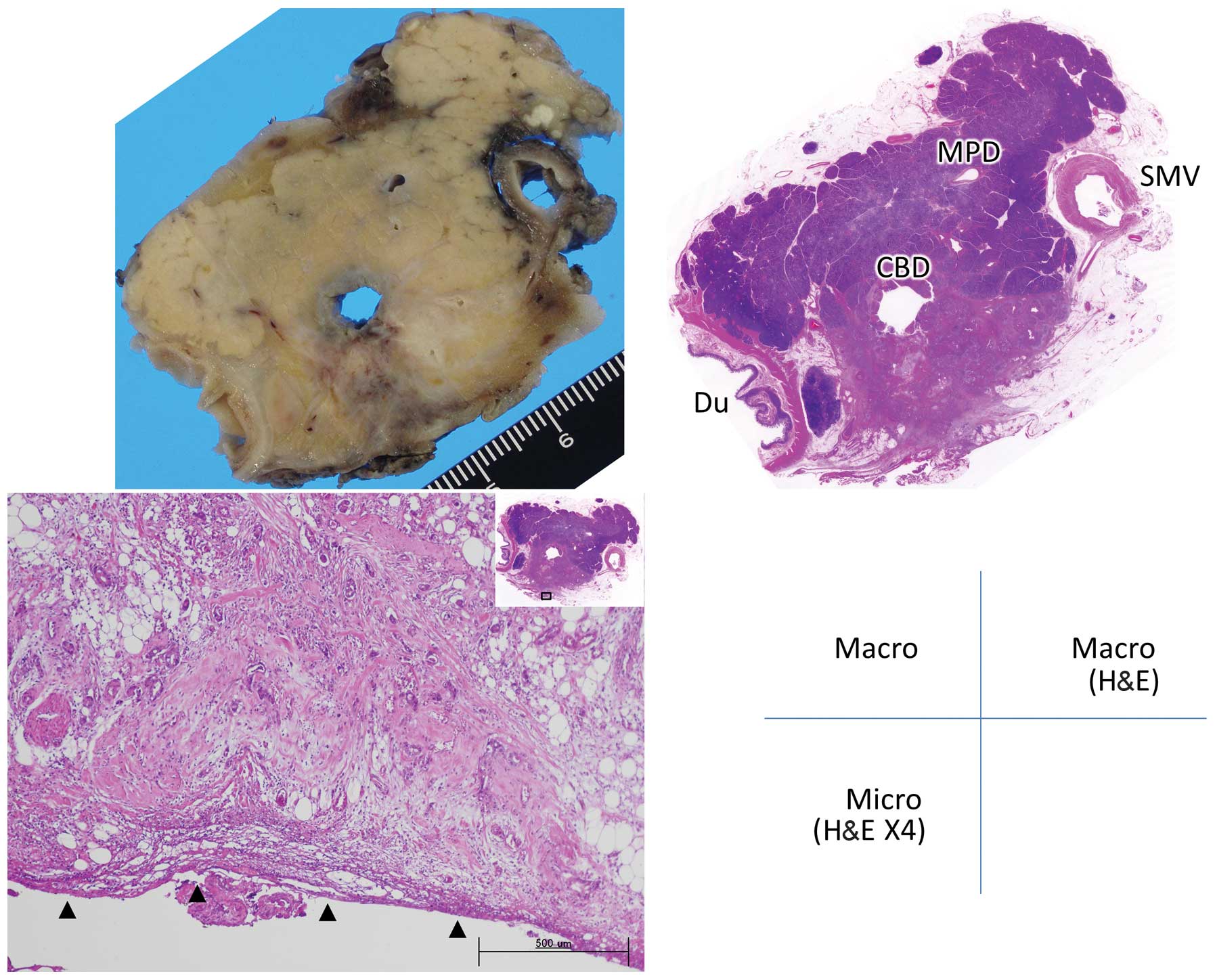
infiltration was graded as follows: Grade 0, carcinoma confined
within the pancreatic parenchyma; grade 1, infiltration beyond the
parenchyma but within the fusion fascia (including tumors abutting
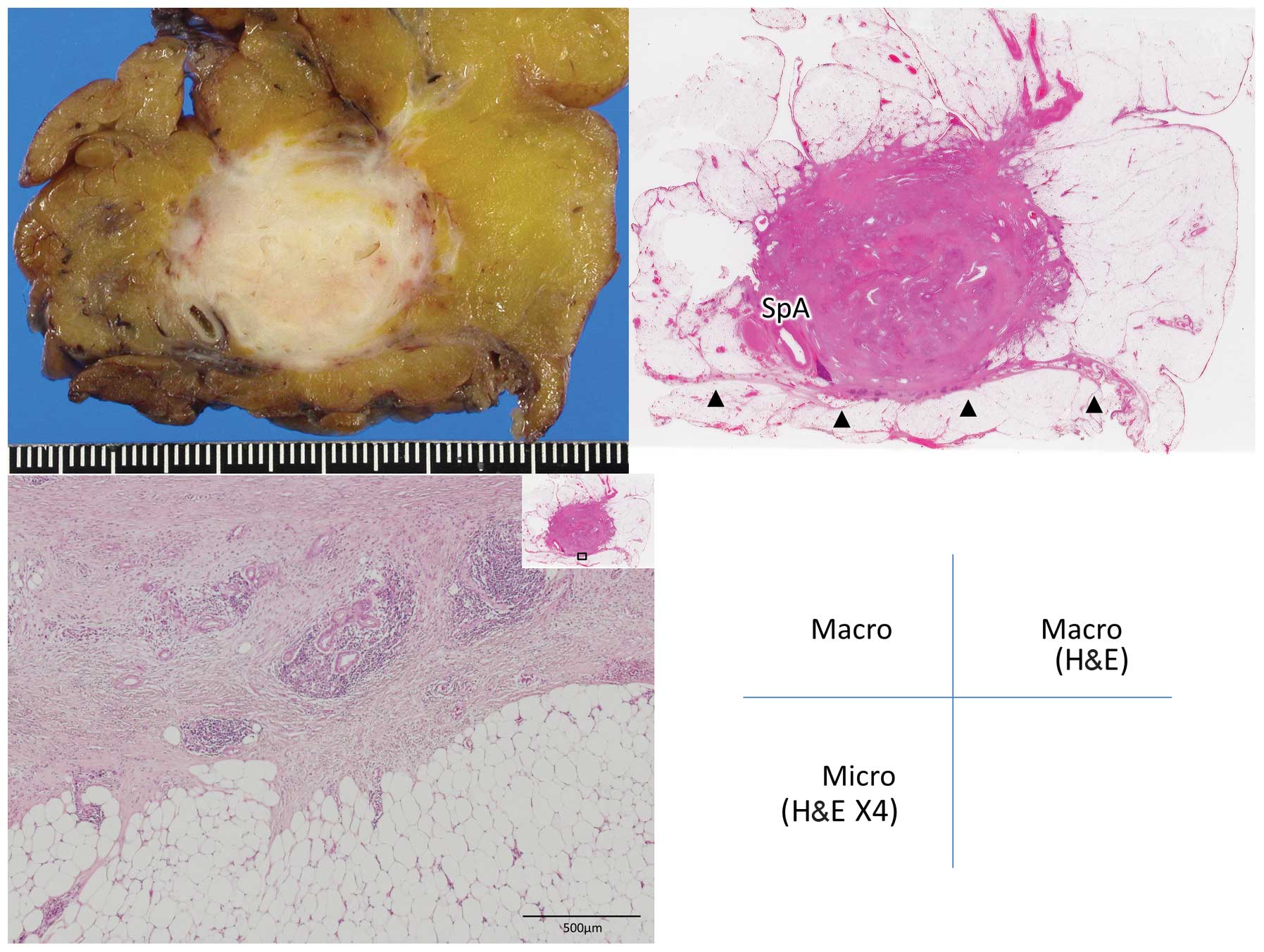
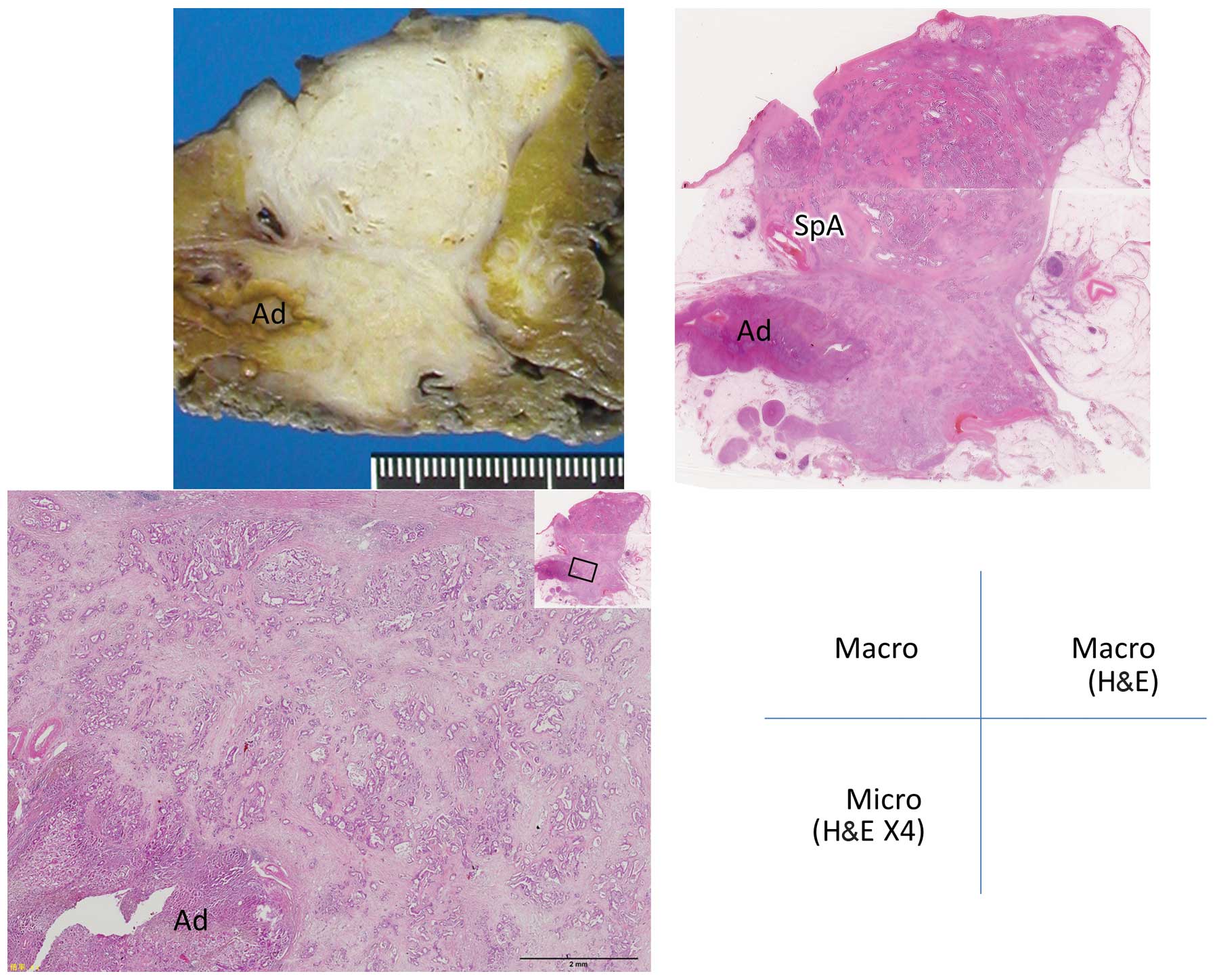
the fascia) (Figs. 1 and 2); and grade 2, infiltration of
retroperitoneal tissues beyond the fusion fascia (Figs. 3 and 4).
Results
The extent of retropancreatic infiltration was grade
0 in 24% and grade 1 in 73% of the patients. Grade 2 was observed
in merely 3% of the patients. There was no significant difference
in grade 2 infiltration between patients undergoing PD and those
undergoing DP (Table II).
 | Table IIGrade of infiltration by pancreatic
carcinoma. |
Table II
Grade of infiltration by pancreatic
carcinoma.
| Surgical
procedure | No. of patients | Grade 0, n (%) | Grade 1, n (%) | Grade 2, n (%) |
|---|
| PD | 91 | 21 (23) | 68 (75) | 2 (2) |
| TP | 3 | 0 | 3 (100) | 0 |
| DP | 46 | 13 (28) | 31 (68) | 2 (4) |
| Total | 140 | 34 (24) | 102 (73) | 4 (3) |
Grade 2 infiltration was observed in a total of 4
patients, 2 of which had undergone PD and the other 2 DP. In those
patients, the tumor had infiltrated the IVC, adrenal gland and
retropancreatic adipose tissue located posterior to the fusion
fascia (Table III). Combined
resection of the involved tissues was performed in all 4
patients.
 | Table IIIDetails of grade 2 cases. |
Table III
Details of grade 2 cases.
| Case no. | Surgical
procedure | T | N | M | Stage | Histological
type | Tumor size (max
mm) | Extrapancreatic
involvement |
|---|
| 1 | PD | 3 | 1 | 0 | IIB | Por | 37 | IVC |
| 2 | PD | 3 | 1 | 0 | IIB | Mod | 38 | IVC |
| 3 | DP | 3 | 3 | 1 | IV | Mod | 40 | Adrenal gland |
| 4 | DP | 3 | 1 | 0 | IIB | Por | | Adipose tissue |
Discussion
Pancreatic carcinoma has the worst prognosis among
all gastrointestinal cancers, with a frequency of local recurrence
and liver metastases (3–5). Retropancreatic infiltration beyond
the parenchyma is common; however, involvement of retroperitoneal
organs is rare. Thus, the optimum retropancreatic dissection plane
and the need for concomitant resection of retroperitoneal organs
such as the adrenal gland, kidney or IVC, remains a controversial
subject (6).
During embryological development, the
retroperitoneal organs are covered by the anterior renal fascia and
the pancreas is covered by the proper peritoneum. The pancreas
becomes fixed posteriorly along with rotation of the mesentery
during the 6th–12th weeks of gestation (7), after which time the proper pancreatic
peritoneum and the anterior renal fascia fuse. This fused membrane
plays the role of the retroperitoneal fascia as well as of the
proper pancreatic peritoneum.
In the present study, we classified retropancreatic
infiltration into 3 grades and identified tumor infiltration beyond
the posterior border of the pancreatic parenchyma in >70% of our
cases. By contrast, infiltration beyond the fusion fascia (grade 2)
was a rare finding (3%). Tumor infiltration was confined to the
anterior side of the fusion fascia in the majority of cases, even
if the carcinoma was identified as adjacent to the IVC or adrenal
gland on preoperative imaging. Thus, the fusion fascia may act as a
barrier against infiltration by pancreatic carcinoma.
Kocher’s maneuver is usually performed during PD.
Following this traditional mobilization procedure, the fusion
fascia may be identified as a thin and translucent fibrous membrane
covering the surface of the IVC. However, the pancreatic head is
resected without the fusion fascia by the traditional Kocher’s
maneuver. It is not customary to intentionally select this
dissection plane, since the fusion fascia is too thin to identify,
similar to the peritoneum of the abdominal wall. However,
dissection including the fusion fascia is recommended, since this
fascia acts as the proper peritoneum of the pancreas and delineates
the retroperitoneum.
A dissection plane including the fusion fascia is
readily identified anterior to the IVC or the left renal vein.
Following exposure of the adventitia of these veins, this plane may
be precisely identified.
A microscopically negative resection margin (R0) is
the most important prognostic factor for pancreatic carcinoma;
however, the posterior margin has little impact on the prognosis
compared to the mesopancreatic margin following PD for pancreatic
ductal adenocarcinoma (8). Even in
the case of a very short distance between the retropancreatic
margin and the leading edge of the tumor, an R0 may be obtained by
resection including the fusion fascia. Therefore, resection
including the fusion fascia is recommended at the posterior margin
of the pancreas, since tumor infiltration is confined to the
anterior surface of the fusion fascia in the majority of cases.
In conclusion, the fusion fascia lies between the
pancreas and the retroperitoneal organs and provides protection
against retropancreatic infiltration of pancreatic carcinoma.
Pancreatic carcinoma should be resected en bloc with the
retropancreatic fusion fascia when surgical resection is performed
with curative intent.
References
|
1.
|
Perlemuter L and Waligora J: Cahiers
d’Anatomie. 2. 3rd edition. Masson et Cie; Paris: 1975
|
|
2.
|
Unio Internationalis Contra Cancrum
(UICC): TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; New York: 2010
|
|
3.
|
Kayahara M, Nagakawa T, Ueno K, et al: An
evaluation of radical resection for pancreatic cancer based on the
mode of recurrence as determined by autopsy and diagnostic imaging.
Cancer. 72:2118–2123. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kayahara M, Nagakawa T, Ueno K, et al:
Surgical strategy for carcinoma of the pancreas head area based on
clinicopathologic analysis of nodal involvement and plexus
invasion. Surgery. 117:616–623. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nagakawa T, Ohta T, Kayahara M, et al:
Clinicopathological evaluation of long-term survivors treated for
cancer of the head of pancreas. Hepatogastroenterology.
45:1865–1869. 1998.PubMed/NCBI
|
|
6.
|
Strasberg SM, Linehan DC and Hawkins WG:
Radical antegrade modular pancreatosplenectomy procedure for
adenocarcinoma of the body and tail of the pancreas: ability to
obtain negative tangential margins. J Am Coll Surg. 204:244–249.
2007. View Article : Google Scholar
|
|
7.
|
Moore KL and Persaud TVN: The Developing
Human: Clinically Oriented Embryology. 8th edition. Saunders;
Philadelphia: 2008
|
|
8.
|
Jamieson NB, Foulis AK, Oien KA, et al:
Positive mobilization margins alone do not influence survival
following pancreaticoduodenectomy for pancreatic ductal
adenocarcinoma. Ann Surg. 251:1003–1010. 2010. View Article : Google Scholar
|