Introduction
Keratocystic odontogenic tumor (KCOT), previously
referred to as odontogenic keratocyst, is a relatively rare benign
intraosseous neoplasm of odontogenic origin that is
histopathologically characterized by the presence of a lining of
parakeratinized stratified squamous epithelium. Squamous cell
lesions, such as squamous cell carcinoma (SCC) and intraepithelial
squamous neoplasia, usually lack melanocytes within the tumor.
However, a few reports on invasive SCC or intraepithelial squamous
neoplasia with melanocytic hyperplasia, namely pigmented SCC, have
been reported in certain organs, such as skin, oral mucosa, nasal
cavity and uterine cervix (1–4). The
squamous epithelium of KCOT usually does not contain melanocytes,
however, cases of pigmented KCOT have been documented, albeit
extremely rarely (5–8). In the present study, we described an
additional case of pigmented KCOT and review the
clinicopathological features of this extremely rare lesion. This
study was approved by the ethics committee of Shiga University of
Medical Science. Informed consent was obtained from the
patient.
Case report
A 23-year-old Japanese female presented with right
odontalgia. X-ray demonstrated a relatively well-circumscribed
round unilocular radiolucency, measuring ∼20 mm, that impacted the
third molar in her right mandible. Surgical resection of the
mandibular cystic lesion was performed following a clinical
diagnosis of KCOT.
The formalin-fixed, paraffin-embedded tissue block
of the resected mandibular specimen was cut into 3-μm
sections, deparaffinized and rehydrated. The sections were stained
with hematoxylin and eosin (H&E) and submitted for
immunostaining. Immunohistochemical analyses were performed using
an autostainer (Benchmark XT system; Ventana Medical System,
Tucson, AZ, USA) according to the manufacturer’s instructions. The
following primary antibodies were used: a mouse monoclonal antibody
against HMB-45 (HMB-45; Novocastra Laboratories, Ltd., Newcastle
upon Tyne, UK), a mouse monoclonal antibody against Melan-A (A103;
Novocastra LAboratories) and a rabbit polyclonal antibody against
S-100 protein (Nichirei Biosciences, Inc., Tokyo, Japan).
Results
Histopathological study of the resected mandibular
cyst showed that the cyst was covered by a parakeratinized
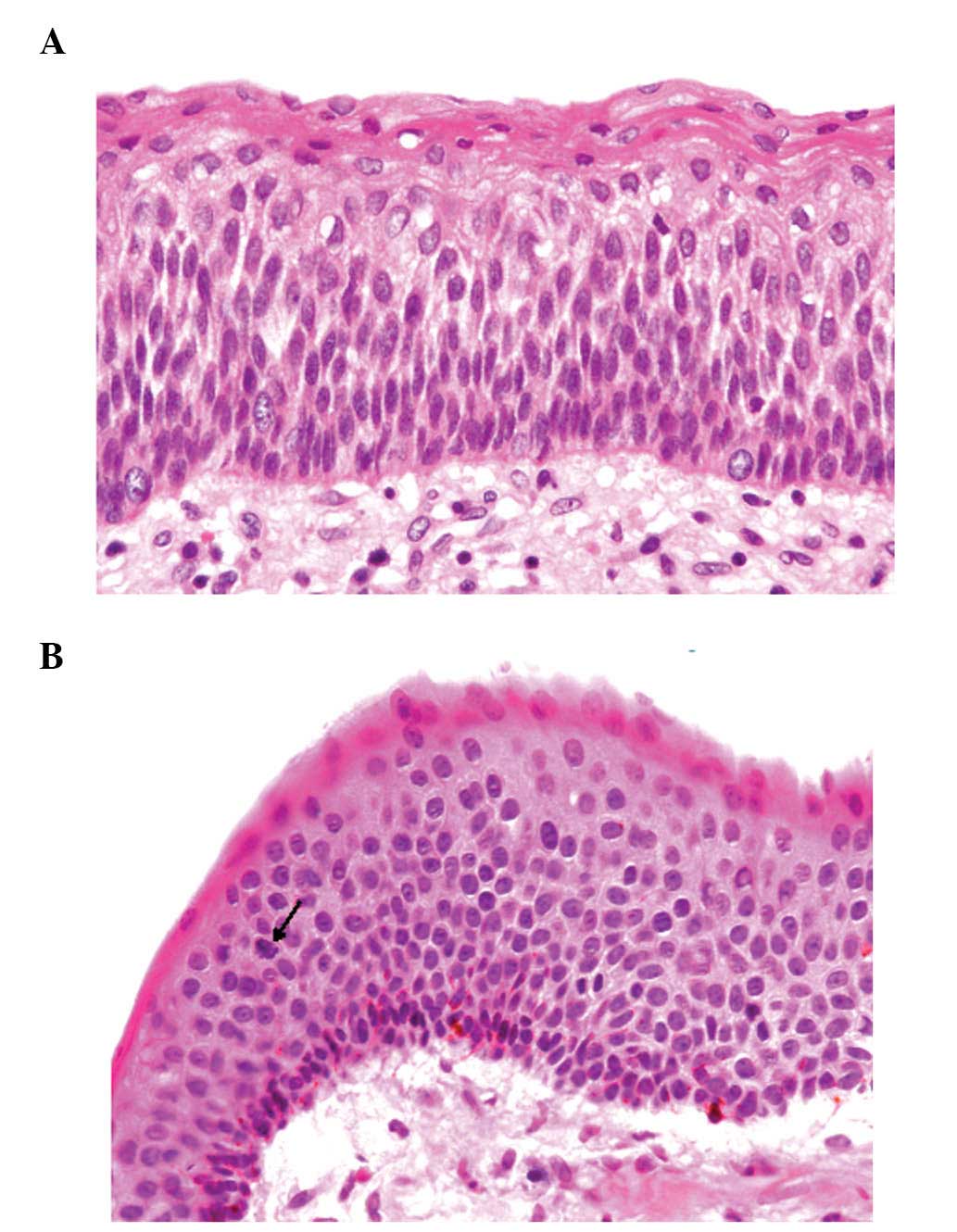
stratified squamous epithelium without rete ridges (Fig. 1A). The squamous epithelial cells
had slightly enlarged hyperchromatic nuclei (Fig. 1A). On the luminal surface, a wavy
layer of parakeratin was observed. Mitotic figures were found in
the suprabasal layer of the squamous epithelium (Fig. 1B). Mild lymphocytic infiltration
was present in the cyst wall. The above-mentioned histopathological
features are typical of KCOT. Besides these findings, dendritic
melanocytes without atypia were observed in approximately half of
the squamous epithelium of the cyst wall (Fig. 1B). Basal cells that contained
melanin pigment within the cytoplasm were rarely identified.
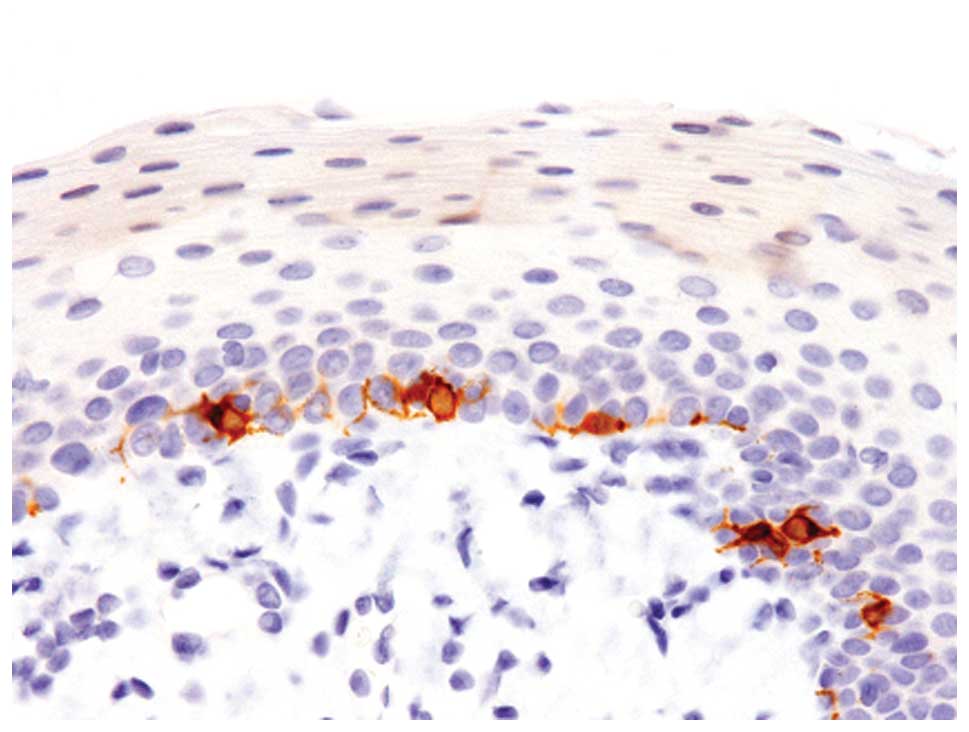
Immunohistochemical analyses revealed that these
melanocytes were positive for S-100 protein, Melan-A and HMB-45
(Fig. 2).
According to these histopathological and
immunohistochemical findings, an ultimate diagnosis of pigmented
KCOT was made.
Discussion
A noteworthy finding of this study is the presence
of melanocytes in the squamous epithelium of KCOT, referred to as
pigmented KCOT. Table I summarizes
the clinicopathological features of the previously reported cases
of pigmented KCOT as well as the present case (5–8).
This lesion mostly occurs in young persons (average age 18, years;
range, 11–26) and shows female predominance (male:female ratio of
2:5). The majority of cases were solitary (6/9 cases) and involved
the mandibula (only one multiple case was shown to involve the
maxilla as well as the mandibula). The reported incidences of
pigmented KCOT are 0.9% (1/104 cases) and 0.36% (1/278 cases)
(5,6). These patients were of West Indian and
African origins, respectively (Table
I). However, Takeda et al(7) reported that the prevalence of
pigmented KCOT was 10.6% (5/47 cases) in the Japanese population
and this difference in frequency of pigmented KCOT may be related
to ethnicity (7).
 | Table IClinicopathological features of
pigmented keratocystic odontogenic tumor. |
Table I
Clinicopathological features of
pigmented keratocystic odontogenic tumor.
| Case | Age/gender | Location | Number | Ethnicity | Refs. |
|---|
| 1 | N/A | N/A | Multiple | West Indian | 5 |
| 2 | N/A | N/A | Solitary | African | 6 |
| 3 | 16/male | Mandibula | Solitary | Japanese | 7 |
| 4 | 15/female | Mandibula | Solitary | Japanese | 7 |
| 5 | 26/female | Mandibula | Solitary | Japanese | 7 |
| 6 | 15/male | Mandibula | Multiple | Japanese | 7 |
| 7 | 11/female | Mandibula and
maxilla | Multiple | Japanese | 7 |
| 8 | 20/female | Mandibula | Solitary | Japanese | 8 |
| Present case | 23/female | Mandibula | Solitary | Japanese | |
Takeda et al(7) reported a series of pigmented KCOT and
they described two distinct histopathological patterns of this
lesion. In the first pattern, numerous melanocytes are scattered in
the basal layer and inconspicuous melanin pigment is present in the
basal squamous cells, as observed in the present case. In the other
pattern, dendritic melanocytes are inconspicuous within the lining
epithelium, but basal squamous cells contain abundant melanin
pigment within the cytoplasm (7).
In the series, four of five cases showed the first pattern
(7).
Pigmented jaw lesions have been documented in other
odontogenic tumors in addition to KCOT, such as calcifying
epithelial odontogenic tumor, adenomatoid odontogenic tumor and
dentigerous cyst (9). The
mechanisms by which melanocytes and/or melanin pigment appear in
odontogenic tumors remain unclear. However, potential explanations
have been suggested. One possible origin of melanocytes involves
the migration of melanocytes through the mesenchyme, while another
proposal is that melanocytes form part of the oral epithelium
(9).
In conclusion, we have described the ninth reported
case of pigmented KCOT. The mechanism by which melanocytes and/or
melanin pigment appear in KCOT and the ethnic difference in the
prevalence of pigmented KCOT remain unclear. Additional
clinicopathological studies are needed to clarify these issues.
References
|
1.
|
Morgan MB, Lima-Maribona J, Miller RA,
Kilpatrick T and Tannenbaum M: Pigmented squamous cell carcinoma of
the skin: morphologic and immunohistochemical study of five cases.
J Cutan Pathol. 27:381–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mathews A, Abraham EK, Amman S and Nair
MK: Pigmented squamous cell carcinoma of nasal cavity.
Histopathology. 33:184–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mikami T, Furuya I, Kumagai A, et al:
Pigmented squamous cell carcinoma of oral mucosa: clinicopathologic
study of 3 cases. J Oral Maxillofac Surg. 70:1232–1239. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ishida M, Kagotani A, Yoshida K and Okabe
H: Pigmented cervical intraepithelial neoplasia. Int J Gynecol
Pathol. (In press).
|
|
5.
|
Browne RM: The odontogenic keratocyst.
Histologic features and their correlation with clinical behavior.
Br Dent J. 131:249–259. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Brannon RB: The odontogenic keratocyst. A
clinicopathologic study of 312 cases. Part II Oral Surg Oral Med
Oral Pathol. 43:233–255. 1977.PubMed/NCBI
|
|
7.
|
Takeda Y, Kuroda M, Kuroda M, Suzuki A and
Fujioka Y: Melanocytes in odontogenic keratocyst. Acta Pathol Jpn.
35:899–903. 1985.PubMed/NCBI
|
|
8.
|
Macleod RI, Fanibunda KB and Soames JV: A
pigmanted odontogenic keratocyst. Br J Oral Maxillofac Surg.
23:216–219. 1985. View Article : Google Scholar
|
|
9.
|
Takeda Y and Yamamoto H: Case report of a
pigmented dentigerous cyst and a review of the literature on
pigmented odontogenic cysts. J Oral Sci. 42:43–46. 2000. View Article : Google Scholar : PubMed/NCBI
|