Introduction
Immune checkpoint inhibitor in non-small cell lung
cancer (NSCLC) has been a breakthrough in the treatment of
metastatic NSCLC. Among antibodies against two classes of immune
checkpoints, anti-PD-1 or anti-PD-L1, nivolumab, and pembrolizumab
have been established as novel and promising treatment of NSCLC
(1–3). The US FDA approved pembrolizumab as a
first-line therapy in October 2016. Since tumor size can increase
initially due to infiltration of immune cells such as cytotoxic T
lymphocytes surrounding tumors before significant shrinkage of the
tumor with immunotherapy, several studies reported
pseudoprogression in patients with different types of solid tumors
treated with immune checkpoint inhibitors (4–6).
Pseudoprogression is unique response pattern which
is observed in patients who are treated with immunotherapeutic
agents (7). The initial progression
due to infiltration of immune cells such as cytotoxic T lymphocytes
surrounding tumors eventually regress. The prevalence of
pseudoprogression is quite high in malignant melanoma, but
pseudoprogression in NSCLC is not frequently observed (8,9). In
addition, intestinal perforation related with pseudoprogression has
not been reported yet. In this case, we firstly described the case
of pseudoprogression in NSCLC treated with pembrolizumab which was
presented as intestinal perforation.
Case report
A 54-year-old man presented with acute periumbilical
pain in February 2018. He had been diagnosed with metastatic NSCLC
with adrenal metastasis in August 2017 at Chungbuk National
University Hospital. The patient had received second-line therapy
with pembrolizumab (200 mg every 3 weeks) since October 2017 after
failing first-line therapy with pemetrexed and cisplatin
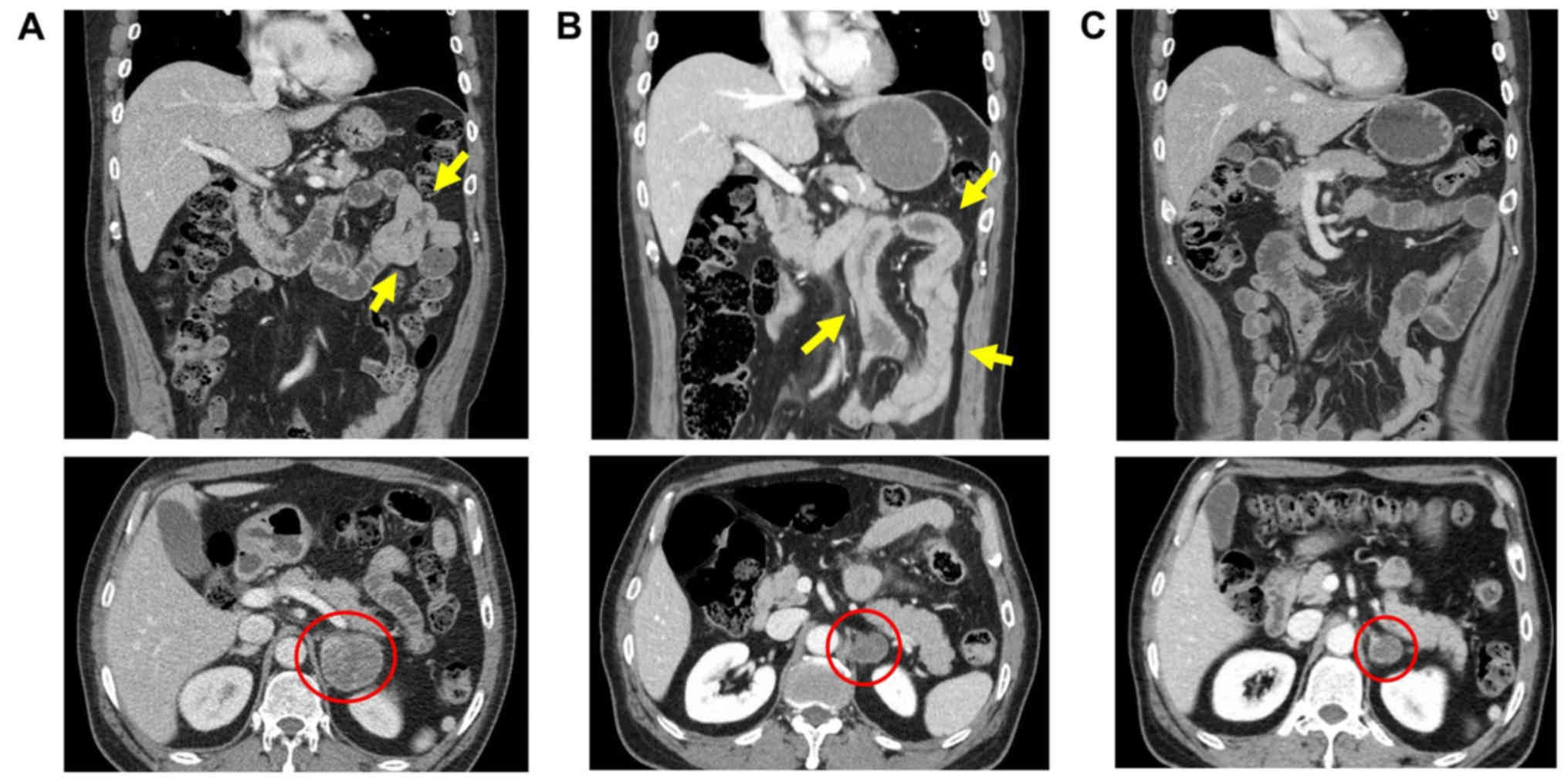
chemotherapy. The abdominal computed tomography (CT) scan showed
diffuse enhancing wall thickening at the duodenum through the
proximal jejunum and signs of free air in the peritoneal cavity
suggesting intestinal perforation (Fig.
1B). The patient underwent emergency laparotomy with segmental
resection and anastomosis of the perforated small bowel.
Microscopic examination of the resected specimen revealed diffuse
transmural involvement of the tumor and massive infiltration of
inflammatory cells (Fig. 2A).
Immunohistochemistry (IHC) analysis indicated that the infiltrating
cells primarily consisted of lymphocytes, especially CD8-positive
cells (Fig. 2B and C).
Histopathological findings including IHC staining for TTF-1, CK7,
and CK20 for tumor cells were the same as those of the primary lung
mass (data not shown). Formalin-fixed, paraffin embedded (FFPE)
tissue were cut using a microtome (RM 2245; Leica Biosystems,
Nussloch, Germany) and stained with hematoxylin and eosin
(H&E). IHC staining methods were used according to the
manufacturer's protocols; LCA (X16/99, 1:400; Novocastra), CD8
(1A5, 1:50; Cell Marque), TTF-1 (SPT24, 1:250; Cell Marque), CK7
(OV-TL 12/3-0, 1:100; Cell Marque), and CK20 (KS20.8, 1:100;
Novocastra) using Benchmark XT autostainer (Ventana). The slides
were counterstained with Harris haematoxylin. Retrospective review
of the baseline CT scan revealed a suspicious focal wall thickening
in the proximal jejunum that was missed at baseline (Fig. 1A). The primary lung tumor and a
metastatic adrenal mass achieved partial response (PR) after two
cycles of treatment and remained in PR state at the time of
presentation (Fig. 1B).
Tumor-related or gastrointestinal symptoms suggestive of intestinal
pathology did not develop until the occurrence of the perforation.
Treatment was skipped during the postoperative period, and a
follow-up CT scan at 3 weeks after surgery showed spontaneous
resolution of the small bowel wall thickening (Fig. 1C). The patient recovered without
events, and pembrolizumab treatment was resumed 4 weeks after
surgery.
Discussion
Pseudoprogression is observed in 6.7 to 10% of
patients with melanoma treated with immune checkpoint inhibitors;
however, the frequency of pseudoprogression in other solid tumors
including NSCLC is below 5% (8).
Although the mechanism of pseudoprogression is not completely
understood, it is thought to be caused by infiltration of
inflammatory cells leading to transient enlargement of existing
tumor masses or appearance of new lesions that eventually regress
(10). The pathologic findings in
the present case revealed extensive infiltration of immune cells,
including cytotoxic CD8-positive T cells, into tumor tissue, which
is in line with the immune infiltration theory. However, because
tumor volume is largely determined by tumor cells, as shown in this
case, further studies are needed to elucidate the mechanism by
which the cancer cells transiently proliferate during the course of
immunotherapy. Novel response criteria developed for the response
evaluation of immunotherapy require confirmation of progressive
disease to prevent inappropriate discontinuation of effective
therapy (11,12). Discriminating pseudoprogression from
true progression is often challenging in clinical practice. In
general, patients with pseudoprogression tend to be clinically
stable without the aggravation of tumor-related symptoms (11). In the present case, the patient had
no gastrointestinal symptoms such as vomiting or diarrhea until the
occurrence of the intestinal perforation and rapidly recovered
after the operation. There was the possibility that anti-tumor
effect of pembrolizumab resulted in intestinal perforation rather
than pseudoprogression, the diffuse wall thickening of the small
bowel after pembrolizumab (Fig. 1B)
in current case remains to be explained. Any tumor marker was not
analyzed in the patients, tumor markers could be measured for
future cases.
Although pseudoprogression is not frequent in NSCLC,
clinicians should be aware of a possible unusual presentation
during immunotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
HKK, HSH, CGW and OJL acquired, analyzed and
interpreted the data, and drafted the manuscript. SWB, YJ, YY, JK,
JYA and TGL interpreted the data and drafted the manuscript. KHL
contributed to the conception and design of the study, analyzed and
interpreted the data, and drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent to
publish his data.
Competing interests
KL has been personally compensated for participating
in advisory boards for Boehringer Ingelheim, AstraZeneca and Eli
Lilly and Company. The other authors declare that they have no
competing interests.
References
|
1
|
Smit EF and Baas P: Lung cancer in 2015:
Bypassing checkpoints, overcoming resistance, and honing in on new
targets. Nat Rev Clin Oncol. 13:75–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-programmed-death-receptor-1 treatment with pembrolizumab
in ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motzer RJ, Rini BI, McDermott DF, Redman
BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S,
Logan TF, et al: Nivolumab for metastatic renal cell carcinoma:
Results of a randomized phase II trial. J Clin Oncol. 33:1430–1437.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Giacomo AM, Danielli R, Guidoboni M,
Calabrò L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli
M, Altomonte M and Maio M: Therapeutic efficacy of ipilimumab, an
anti-CTLA-4 monoclonal antibody, in patients with metastatic
melanoma unresponsive to prior systemic treatments: Clinical and
immunological evidence from three patient cases. Cancer Immunol
Immunother. 58:1297–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiou VL and Burotto M: Pseudoprogression
and immune-related response in solid tumors. J Clin Oncol.
33:3541–3543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HK, Heo MH, Lee HS, Sun JM, Lee SH,
Ahn JS, Park K and Ahn MJ: Comparison of RECIST to immune-related
response criteria in patients with non-small cell lung cancer
treated with immune-checkpoint inhibitors. Cancer Chemother
Pharmacol. 80:591–598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribas A, Chmielowski B and Glaspy JA: Do
we need a different set of response assessment criteria for tumor
immunotherapy? Clin Cancer Res. 15:7116–7118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|