Introduction
Centrally located hepatocellular carcinoma (HCC) is
defined as HCC closely adjacent to the central hepatic segments,
including Couinaud’s segments IVa, IVb, V and VIII, without usually
including malignancies within the caudate lobe (1). Centrally located HCC is adjacent to
the portal vein, bile duct, hepatic vein and inferior vena cava.
Due to the close proximity to these major vessels, the treatment of
such cancers remains challenging. Radiofrequency ablation (RFA) and
percutaneous ethanol injection are less effective for larger (>5
cm) compared to smaller tumors (2–4).
Centrally located HCC had long been considered unsuitable for
surgical resection and the traditional treatment for such cancers
included an extended right or left hepatectomy (5–7).
However, extended hepatectomy is associated with higher morbidity
and mortality, mainly due to the increased risk of postoperative
liver failure (8,9).
Over the last few years, with the advancement in the
surgical techniques for liver cancer, mesohepatectomy has become a
feasible option for patients with centrally located HCC,
particularly for those with post-hepatitis cirrhosis. Previous
clinical studies demonstrated that mesohepatectomy may be superior
to extended hepatectomy, as it reduces the volume of the resected
liver (10,11). However, mesohepatectomy is a
technically demanding procedure and may cause injuries to blood
vessels and bile ducts, resulting in increased blood loss;
therefore, it is less frequently used (11,12)
and the application of mesohepatectomy in the treatment of
centrally located tumors has not been adequately assessed.
The aim of this study was to review the surgical
techniques, clinicopathological characteristics and outcomes of 24
patients who underwent mesohepatectomy, in order to determine
whether this treatment is safe and effective for patients with
centrally located HCC. We also summarized the surgical procedures
and our experience with effective mesohepatectomy for the treatment
of centrally located HCC.
Patients and methods
Patient information
A total of 24 patients, including 19 men and 5 women
(mean age, 53 years; range, 27–74 years), with pathologically
diagnosed primary HCC were included in this study. Ten of the
tumors were completely encapsulated, whereas the remaining 14
tumors were not or only partially encapsulated. Of the 24 patients,
19 were hepatitis B surface antigen (HBsAg)-positive, 2 were
hepatitis C virus (HCV)-positive and 2 patients were both HBsAg-
and HCV-positive. All 24 patients were cirrhotic, with the
cirrhosis in 18 patients being rated as mild-to-moderate, in 4
patients as severe, while the remaining 2 patients had no obvious
liver cirrhosis. Six of these patients also had mild-to-moderate
esophageal varices. The α-fetoprotein levels, which may be elevated
in a subset of HCC patients and are useful for monitoring response
to treatment and as an early screening test (13), were >400 μg/ml in 19 patients.
The median tumor diameter was 6.3 cm (range, 2–14 cm). According to
the Child-Pugh classification, 21 patients were classed as A and
the remaining 3 patients were classed as B. Child-Pugh class B
patients were not subjected to mesohepatectomy until their scores
had decreased to A with treatment (14,15).
All the patients were administered preoperative liver-protecting
and anticoagulation treatments for 4–5 days prior to
mesohepatectomy.
Surgical procedure applied for the
isolation of the hepatic artery, portal vein, bile duct and hepatic
vein from the tumor
Under general anesthesia, the mesohepatectomy was
initiated with a right subcostal or a bilateral subcostal incision
with midline extension, or a chevron incision. After the operative
field was exposed sufficiently by retractor traction, the texture
of the liver, the degree of severity of the cirrhosis and the size
and location of the tumors were probed using the fingers. The
round, falciform, right triangle, coronary, caudate and hepatocolic
ligaments were incised to fully mobilize the right lobe of the
liver. After the right side of the hepatic inferior vena cava was
separated and exposed, the short hepatic veins were ligated.
Subsequently, the hepatic artery, portal vein and bile duct were
separated from the tumor. A long suture was placed behind the right
hepatic vein to block the hepatoduodenal ligament. The perihepatic
ligaments and the bare area of the right lobe of the liver were
divided from top to bottom, up to the inferior vena cava. The
hepatic vena cava gap was separated from top to bottom, up to 3~4
cm (Fig. 1), using a right angle
clamp. The anterior, left, right and posterior walls of the right
hepatic vein were fully exposed to separate and suspend the right
hepatic vein with a vascular suture. The left hepatic lobe was
mobilized up to the root of the left hepatic vein and the liver
cavity was expanded to 3–4 cm. The common trunk of the left and
right hepatic veins was divided and vertically clamped with a
Simpson clamp (2).
Mesohepatectomy and resectional surface
treatment
Intraoperative ultrasonography (US) was used to
precisely locate the hepatic veins (15). The hepatic venous circulation was
reconstituted according to the preoperative computed tomography
(CT) examination. Using an electric or ultrasonic knife, an
incision of 2–3 cm in depth was performed in the liver parenchyma
along the middle hepatic vein; the vein was separated, ligated and
severed between its junction with the inferior vena cava and the
common trunk of the left and right hepatic veins. During this
process, the right hepatic vein should be well protected. Following
complete removal of the middle lobe, bleeding and oozing from the
cross sections was fully controlled by clipping or ligating the
bleeding sites and biliary leaks, but the resectional surfaces were
not sutured compulsively. Of the 24 patients, 8 underwent
hepatectomy under intraoperative US guidance.
The surgical approach was selected based on the
location and size of the tumor. The blood flow through the hepatic
artery and portal vein was intermittently blocked for 20 min,
restored for 5 min and then the cycle was repeated. The blood
inflow from the hepatic artery and portal vein in one patient was
interrupted 3 times. The average occlusion time was 23 min (15–60
min). The right hepatic vein and the common trunk of the left and
right hepatic veins were clamped with a Satinsky clamp to block the
inflow of blood from the hepatic veins, which significantly
improved the safety and simplicity of the operative procedure.
Occlusion of the hepatic artery, portal vein and bile duct was
performed on 17 patients. While 14 of the 17 cases were subjected
to occlusion once, 2 patients were subjected to occlusion twice and
1 patient was treated with occlusion 3 times, whereas 4 patients
did not undergo occlusion. Total hepatic vascular exclusion (HVE)
was performed on 3 patients, once in each patient.
Results
Procedures
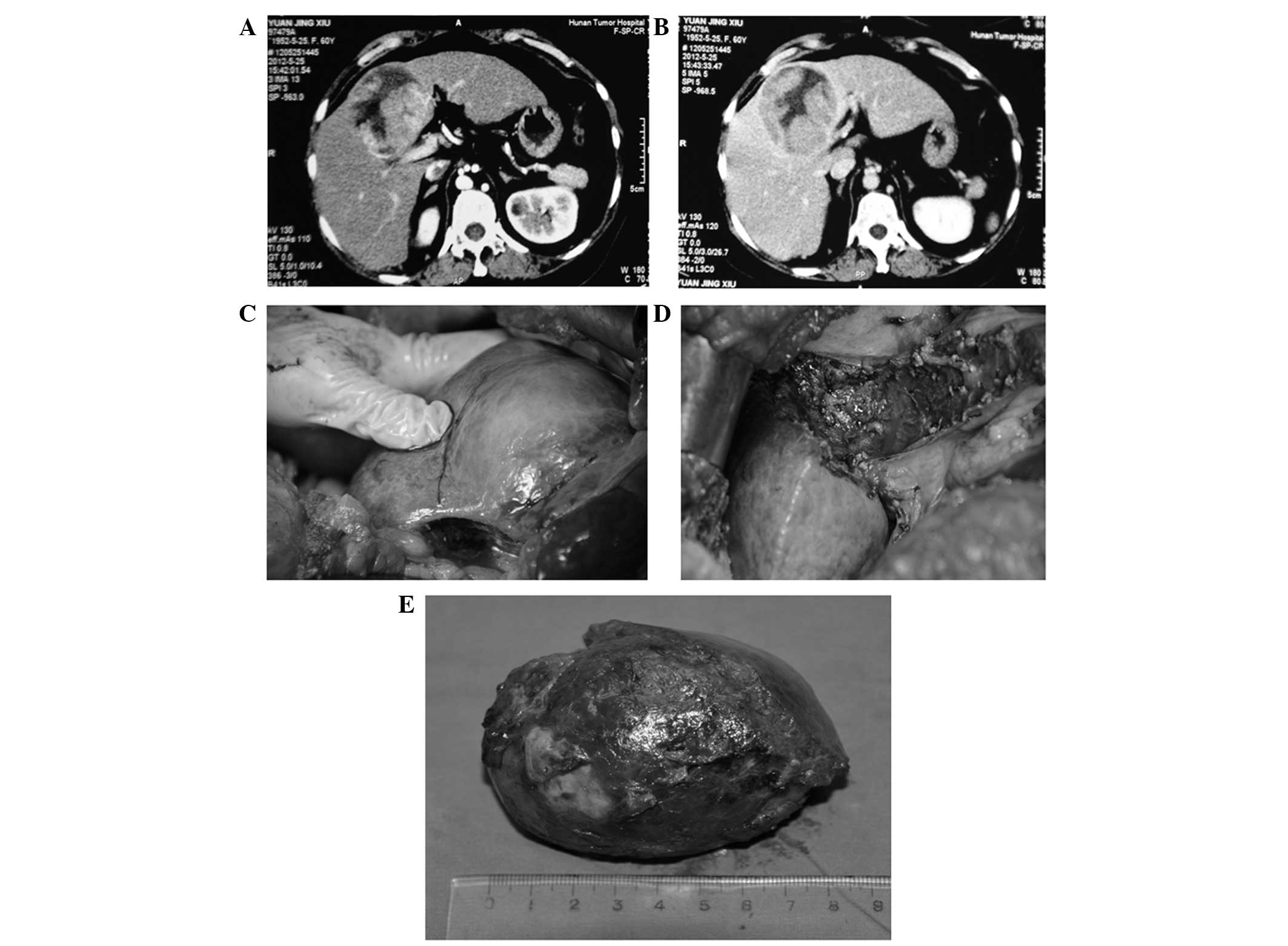
Of the 24 patients, 9 underwent hepatectomy of
Couinaud’s segments IV, V and VIII with concurrent cholecystectomy
(Figs. 1 and 2); 8 underwent hepatectomy of segments
IVb, V and VIII (Fig. 3), of whom
7 also received a cholecystectomy (Fig. 4); 4 underwent hepatectomy of
segments IVa, V and VIII; and 3 underwent hepatectomy of segments
I, IV, V and VIII with concurrent cholecystectomy (Fig. 5).
All the tumors were completely excised and there was
no intraoperative mortality. The average intraoperative blood loss
was 480 ml (range, 200–2,200 ml). The serum alanine
aminotransferase levels returned to normal or preoperative levels
in 11±3 days post-operation. The total bilirubin decreased to
normal or preoperative levels in 8±2 days post-operation. The
incidence of complications associated with the operation was 33%
(8/24), including 4 cases of reactive pleural effusion, 3 of
biliary fistula and 1 of upper gastrointestinal bleeding. Of the 4
patients with reactive pleural effusion, 2 were treated by pleural
puncture and the other 2 were received symptomatic treatment.
Patients with other complications also received symptomatic
treatment. A total of 23 of the 24 patients (96%) were followed up
postoperatively. The 1- and 3-year overall survival rates of these
patients were 76 and 46%, respectively.
Discussion
The middle segments of the liver include the medial
section of the left lobe and the anterior section of the right
lobe. The visceral surface of the middle lobe of the liver is
adjacent to the entrance of the hepatic artery, portal vein and
bile duct and its diaphragmatic surface is adjacent to the entrance
of the hepatic veins, where they merge into the inferior vena cava.
The dorsal side of the middle lobe of the liver is where 15 short
hepatic veins empty into the inferior vena cava. Due to the unique
anatomical location of centrally located HCC, minimally invasive
methods such as RFA, cryotherapy and microwave ablation may readily
cause heat injuries to the surrounding blood vessels (2–4).
Centrally located HCCs tend to invade blood vessels and lead to
intrahepatic or extrahepatic metastasis. Therefore, for patients
with centrally located HCC, provided their general medical
conditions allow surgical intervention and they do not have any
concurrent heart, lung, kidney and brain disease or significant
severe liver dysfunction, surgical resection is the first choice of
treatment, despite a high degree of difficulty and the risks
associated with this technically demanding procedure (6). Centrally located HCCs are deeply
located, closely adjacent to several major blood vessels and bile
ducts; therefore, the selection of the most appropriate surgical
approach is crucial for treatment success.
A prerequisite for any successful mesohepatectomy is
a well-exposed operation field. To obtain such a field, a bilateral
subcostal chevron incision should be adopted, with traction using
an all-round retractor. Furthermore, surgeons have to be familiar
with the liver anatomy and vascular distribution. During the
operation, hepatic vein flow should be accurately assessed by
combination of enhanced hepatic CT and magnetic resonance imaging.
The hepatic artery, portal vein and bile duct should be protected
to avoid any damage and the hepatic arteriovenous vessels of the
right and middle lobes of the liver should be fully preserved
(16). Injuries in hepatic venous
vessels should be repaired with vascular sutures. The wedge area of
the middle lobe of the liver is formed by the middle point of the
gallbladder bed and the edge of the superior and inferior vena
cava. The majority of HCC cases are also complicated by
post-hepatitis cirrhosis, fibrosis, compensatory hyperplasia and
displacement; therefore, it may be difficult to accurately locate
the area to be resected. Intraoperative US is routinely used to
probe the middle hepatic vein to determine the boundaries of the
middle lobe and the affected area to be removed (15). In our study, the cancer was
successfully localized by intraoperative US in 8 patients. Finally,
a suitable blockage of blood inflow is key to a successful
mesohepatectomy and postoperative patient recovery. In case of
massive intraoperative bleeding, it is extremely difficult to
protect these major blood vessels, bile ducts and residual
liver.
The middle lobe of the liver is closely adjacent to
several major blood vessels. Once uncontrollable massive hemorrhage
occurs, injuries to these blood vessels by hasty clamping or blind
suturing is unavoidable and these injuries may lead to further
severe complications. To reduce intraoperative hemorrhage, we
adopted the Pringle maneuver to interrupt the blood flow through
the hepatic artery, portal vein and total HVE (8). We also adopted the HVE method
reported by Curley et al (2) to selectively separate the hepatic
veins and block the inflow of blood. The Pringle maneuver was used
during hepatectomy on 18 patients, while HVE was used on 4
patients. The rate of successful inflow occlusion was 83.33%
(20/24). Intermittent inflow occlusion may effectively reduce liver
damage caused by ischemia-reperfusion (16). Blockage of blood inflow through the
hepatic veins at the right hepatic vein and the common trunk of
left and right hepatic veins have significantly improved the safety
and simplicity of the operative procedure.
Generally, it is difficult to suture the resectional
surfaces of centrally located HCC, since its base consists of the
vena cava, portal vein, hepatic artery and bile duct and its
bilateral sections are adjacent to the right and left hepatic veins
(8). To avoid injuries to the
porta hepatis, the suture needle should not be pricked into the
liver parenchyma too deeply. We recommend that the resectional
surfaces should be left open after the bleeding is controlled. The
hepatic resectional surfaces should not be sutured compulsively to
avoid compression of the left and right hepatic veins; otherwise,
the hepatic venous flow may be interrupted (17). We used biological fibrin glue or
styptic powder on the resectional surfaces and the surface was
further covered with absorbable hemostatic gauze. For patients with
suspected bile duct injuries, the bile ducts were cut open to place
a T-tube drain. External T-tube drainage was established in 7
patients (18). One month after
the placement, the T-tubes were smoothly pulled out under
cholangiographic guidance. Of the 24 patients, 1 was subjected to
hepatectomy of segments II, III and VI and Roux-en-Y
choledojejunostomy of the right bile duct due to invasion of the
left bile duct by recurrent cancer. For resection of centrally
located HCC, an incision was performed at the entrance of the
hepatic vein and extended further towards the two lateral sides.
After the hepatic artery, portal vein and bile duct were clearly
exposed, the incision was extended to the entrance of the hepatic
artery, portal vein, bile duct, where it was terminated. The
openings between the two lateral incisions were kept wide at the
top and narrow at the bottom to avoid injuries to the blood vessels
and bile ducts. Lang et al (5) also suggested that mesohepatectomy is
a safe and effective operative procedure for the treatment of
centrally located HCC complicated by hepatitis or cirrhosis.
In summary, despite the unique anatomical location
of centrally located HCC and technical complexity of the operating
procedure, mesohepatectomy is an effective surgical treatment for
patients with centrally located HCCs. The keys for a successful
mesohepatectomy include proper preoperative assessment and
preparation, precise resection along the middle hepatic vein and
suitable inflow occlusion. Mesohepatectomy may be particularly
beneficial or even curative for HCC patients with post-hepatitis
cirrhosis, as this procedure preserves more functional liver
parenchyma compared to conventional or extended lobectomy and
reduces the incidence of liver failure. Therefore, we recommend
mesohepatectomy as treatment for patients with centrally located
HCC with concomitant cirrhosis.
References
|
1
|
Hu RH, Lee PH, Chang YC, Ho MC and Yu SC:
Treatment of centrally located hepatocellular carcinoma with
central hepatectomy. Surgery. 133:251–256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Curley SA, Izzo F, Ellis LM, Nicolas
Vauthey J and Vallone P: Radiofrequency ablation of hepatocellular
cancer in 110 patients with cirrhosis. Ann Surg. 232:381–391. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGahan JP, Brock JM, Tesluk H, Gu WZ,
Schneider P and Browning PD: Hepatic ablation with use of
radio-frequency electrocautery in the animal model. J Vasc Interv
Radiol. 3:291–297. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tateishi R, Shiina S, Teratani T, et al:
Percutaneous radiofrequency ablation for hepatocellular carcinoma.
An analysis of 1000 cases. Cancer. 103:1201–1209. 2005.
|
|
5
|
Lang H, Broelsch CE, Bertona C and
Bourquain H: Extended left hepatectomy with an inferior right liver
vein: improved operation planning by 3-D reconstruction and
computer-assisted imaging. J Am Coll Surg. 205:626–627. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poon RT, Fan ST, Lo CM, et al: Extended
hepatic resection for hepatocellular carcinoma in patients with
cirrhosis: is it justified? Ann Surg. 236:602–611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chedid AD, Chedid MF, Kruel CR, Girardi FM
and Krue CD: Extended right hepatectomy with total caudate lobe
resection and biliary tree resection for a large colorectal liver
metastasis involving both the right and left hepatic lobes and the
umbilical fissure: a case report. Am Surg. 71:447–449. 2005.
|
|
8
|
Scudamore CH, Buczkowski AK, Shayan H, Ho
SG, Legiehn GM, Chung SW and Owen DA: Mesohepatectomy. Am J Surg.
179:356–360. 2000. View Article : Google Scholar
|
|
9
|
Vauthey JN, Pawlik TM, Abdalla EK, et al:
Is extended hepatectomy for hepatobiliary malignancy justified? Ann
Surg. 239:722–730; discussion 730–732. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehrabi A, Mood ZA, Roshanaei N, et al:
Mesohepatectomy as an option for the treatment of central liver
tumors. J Am Coll Surg. 207:499–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sotiropoulos GC, Lang H, Molmenti EP,
Kaiser GM, Paul A and Broelsch CE: Partial or complete
mesohepatectomy combined with resection of the hilar bifurcation in
cases of Klatskin tumors: a reasonable strategy? Am J Surg.
198:297–298. 2009. View Article : Google Scholar
|
|
12
|
Wu CC, Ho WL, Chen JT, et al:
Mesohepatectomy for centrally located hepatocellular carcinoma: an
appraisal of a rare procedure. J Am Coll Surg. 188:508–515. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riaz A, Ryu RK, Kulik LM, et al:
Alpha-fetoprotein response after locoregional therapy for
hepatocellular carcinoma: oncologic marker of radiologic response,
progression and survival. J Clin Oncol. 27:5734–5742. 2009.
View Article : Google Scholar
|
|
14
|
Ishii H, Ogino S, Ikemoto K, et al:
Mesohepatectomy with total caudate lobectomy of the liver for
hepatocellular carcinoma. World J Surg Oncol. 11:822013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kishi Y, Hasegawa K, Sugawara Y and Kokudo
N: Hepatocellular carcinoma: current management and future
development-improved outcomes with surgical resection. Int J
Hepatol. 2011:7281032011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Fu SY, Lau WY, et al: Selective
main portal vein clamping to minimize the risk of recurrence after
curative liver resection for hepatocellular carcinoma.
Hepatogastroenterology. 59:1560–1565. 2012.PubMed/NCBI
|
|
17
|
Chang YC, Nagasue N, Chen CS and Lin XZ:
Simplified hepatic resections with the use of a Chang’s needle. Ann
Surg. 243:169–172. 2006.PubMed/NCBI
|
|
18
|
Soulez G, Lerouge S, Allard L, et al:
Vulnerable carotid atherosclerotic plaque creation in a swine
model: evaluation of stenosis creation using absorbable and
permanent suture in a diabetic dyslipidemic model. J Vasc Interv
Radiol. 23:1700–1708. 2012. View Article : Google Scholar
|