Introduction
Prostate carcinoma (CaP) is the fourth most
frequently diagnosed cancer among Malaysian men, preceded by lung,
colorectal and nasopharyngeal cancers (1). However, consistent with the global
tendency of an increasing median age of patient populations due to
increased overall longevity, the incidence of CaP in Malaysia is
also on the increase (2). Among
the major ethnic groups in Malaysia, the incidence of CaP was found
to increase after the age of 45 years and is highest among
Malaysian Chinese (MC) compared to Malaysian Indian (MI) and Malay
men (1).
There is a clear disparity in CaP incidence and
mortality worldwide and the major determining factors are being
actively investigated. Although socioeconomic status and access to
healthcare are often associated with disparities in the diagnosis,
treatment and survival of CaP patients of different ethnic
backgrounds, contributing genetic differences have also been
identified (3, 4). Some of the tools used to characterize
gene alterations and identify potential driver genes include
genome-wide association studies, karyotyping of chromosomal copy
number, as well as exome and whole-genome sequencing. In addition
to the search for underlying genetic events that initiate cancer or
distinguish aggressive from indolent tumors, the search for genetic
alterations that may explain the ethnic disparities in CaP is
currently actively pursued (3,
5, 6).
The variant allele on 8q24, which increases the risk
for CaP, particularly in men of African ancestry, is one of the
most convincing risk alleles for CaP (7, 8).
Another allele associated with an increased risk of CaP men of
African ancestry is the rs743572 single-nucleotide polymorphism of
CYP17 (9). More recently,
evaluations of the transmembrane protease, serine 2
(TMPRSS2)-erythroblast transformation-specific (ETS)-related
gene (ERG) fusion in different populations have highlighted
the differences in frequency between ethnic groups. Recurrent gene
fusions between regulatory sequences of an androgen receptor
(AR)-regulated gene, such as TMPRSS2, solute carrier family
45 member 3 or N-myc downstream-regulated gene 1, and an ETS
gene family member, such as ERG, ETS translocation
variant (ETV)1, 4 and 5 as the 3′;
fusion partner, result in androgen-dependent expression of ETS
transcription factors. Among these genetic alterations, the
TMPRSS2-ERG fusion, detected in 50–70% of CaP patients from
Western countries, is the most prevalent (10, 11). The frequency of TMPRSS2-ERG
gene fusions detected in CaPs of African Americans (AA) (31–43%) is
often lower compared to that of Caucasian Americans (CA) (50–66%)
(5, 12). Interestingly, ERG overexpression is
more frequently detected in the index tumors of CA (63.3%),
compared to those of AA patients (28.6%) (5). However, evaluations of
TMPRSS2-ERG gene fusions, either by immunohistochemistry
(IHC) detection of ERG expression alone or in combination with
fluorescence in situ hybridization (FISH), in different
populations worldwide demonstrated lower frequencies compared to
that detected CA and Europeans (12–20).
The aberrant overexpression of an ERG oncoprotein as
a result of TMPRSS2-ERG fusion exerts a profound effect on
cellular pathways associated with cancer initiation and progression
(10, 21–24).
Evidence of the association between ERG-positive prostatic
intraepithelial neoplasia lesions and ERG-positive prostate tumors
highlights the significance of ERG activation in the early stages
of tumor development (25). ERG
overexpression inhibits prostate epithelial differentiation, while
promoting epithelial-to-mesenchymal transition (26, 27). In addition, ERG regulates target
genes with functions in DNA damage repair, epigenetic silencing and
inflammation, which affect pathways associated with tumor cell
growth, proliferation and invasion (24). For example, the cooperation of ERG
with phosphatase and tensin homolog (PTEN) deletion and activation
of AKT has been shown to promote neoplastic transformation
(28, 29). A better understanding of how ERG
interacts with cancer genes that contribute to cancer progression
has led to the development of various treatment strategies that
target ERG and its downstream effectors (30). The ability to clearly detect ERG
expression in prostate tumors in contrast to normal glands by IHC
using specific monoclonal antibodies (MAbs) has improved the
diagnosis of the majority of CaPs (25, 31). The high concordance between the
evaluations of TMPRSS2-ERG fusion by FISH and ERG protein
expression by IHC supports the reliability and accuracy of ERG IHC
as a surrogate for FISH detection (25, 31–33).
Furthermore, the evaluation of prostate tumors for ERG expression,
together with PTEN deletion and integrity of AR signaling pathways,
may help the prognostic stratification of patients and the
selection of treatment options (34, 35).
To date, no study has evaluated the frequency of ERG
alterations in CaP patients in Malaysia, which has a population
comprising diverse ethnic groups. The major ethnic groups in
Malaysia are Malays (55%), Chinese (24%) and Indians (7.2%)
(36). In order to better
understand the role of ERG in the etiology of CaP initiation and
progression, we used the detection of ERG by IHC as a surrogate for
ERG fusion events to evaluate the prevalence of ERG
expression in a multiethnic cohort of Malaysian CaP patients.
Materials and methods
Specimens
Transrectal ultrasound (TRUS)-guided biopsies were
performed on 120 patients who were diagnosed with CaP based on
clinical findings at the Sime Darby Medical Centre, Subang Jaya,
Malaysia. The specimens were collected between 2011 and 2013,
following approval by an independent Ethics Committee of Sime Darby
Healthcare (ethics reference no. 201309.5). The TRUS-guided biopsy
entails targeting the suspected prostatic lesion, as well as random
sampling of the prostatic gland. Typically, 12–18 biopsy cores were
collected from each prostate. Occasionally, 24–36 biopsy cores were
collected from patients with significantly larger prostates, or
from whom a second biopsy was required. The biopsy specimens were
fixed with formalin and embedded in paraffin blocks.
IHC detection for ERG expression
Anti-ERG-MAb 9FY was obtained (cat. no. CM421C;
Biocare Medical, Concord, CA, USA). Sections (4-µm) were cut from
formalin-fixed paraffin-embedded (FFPE) blocks, mounted on slides
and deparaffinized. IHC was performed using a Ventana Benchmark
Ultra autostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA)
using Ventana reagents. Briefly, the sectioned specimens were
processed for antigen retrieval using CC1 antigen retrieval
solution prediluted in Tris/borate/EDTA buffer (pH 8.0–8.5) and
incubated at 95°C for 48 min. The sections were then put through
peroxidase inhibition prior to incubation with ERG-MAb at a
dilution of 1:100 for 20 min at room temperature. ERG expression
was detected by using OptiView HQ universal Linker and OptiView HRP
Multimer (Ventana Medical Systems, Inc.), incubated consecutively
at room temperature for 8 min. The color was developed using Bluing
reagent for 4 min and the sections were counterstained with
hematoxylin. The ERG protein expression status and Gleason scores
of the prostate sections were evaluated by a trained pathologist.
Depending on the amount and intensity of the ERG IHC staining, the
specimens were scored as follows: 0, negative; 1+, mild; 2+,
moderate; and 3+, strong staining. Positive staining of endothelial
cells in the specimens served as a built-in control for the
staining.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software for Windows (IBM, Inc., New York, NY, USA). The Pearson's
Chi-square and Kruskal-Wallis tests were used to determine the
statistical associations of ERG expression with ethnicity, age and
Gleason sum score. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient demographics and Gleason score
of prostate specimens
The ERG oncoprotein expression status was evaluated
in tumor specimens from 120 patients by demographic distribution
and tumor Gleason scores (Table
I). The mean age of the patients was 69 years (range, 52–91
years). MC patients represented the largest ethnic group in this
study (68.3%), followed by MI (25%) and Malay patients (6.7%). Both
the left and right lobes of the prostate were biopsied in all the
patients. Among the 120 patients, 71 were found to have tumors in
both lobes, whereas 20 patients had tumors in only the left lobe
and 29 had tumors in only the right lobe of the prostate.
Specifically, of the 191 tumor sections that were evaluated, 91
were from the left lobe and 100 were from the right lobe of the
prostate (Table I and Fig. 1). The ERG expression status for
each patient was scored as positive if ERG oncoprotein expression
was detected in tumor sections from either lobe, taking into
account inter-tumoral heterogeneity within the same prostate
(25).
 | Table IDemographics of prostate cancer
patients (n=120) and Gleason scores of tumor specimens (n=191). |
Table I
Demographics of prostate cancer
patients (n=120) and Gleason scores of tumor specimens (n=191).
| Variables | No. | % |
|---|
| Age (years) | | |
|
<69 | 60 |
50.0 |
|
≥69 | 60 |
50.0 |
| Ethnicity | | |
|
Chinese | 82 |
68.3 |
|
Malay | 8 |
6.7 |
|
Indian | 30 |
25.0 |
| Sections from right
lobe (n=100) | | |
| Gleason
scores | | |
| ≤6 | 32 |
32.0 |
| 7
(3+4) | 18 |
18.0 |
| 7
(4+3), 8–10 | 50 |
50.0 |
| Sections from left
lobe (n=91) | | |
| Gleason
scores | | |
| ≤6 | 27 |
29.7 |
| 7
(3+4) | 20 |
22.0 |
| 7
(4+3), 8–10 | 44 |
48.3 |
| Total prostate
sections (n=191) | | |
| Total
Gleason scores | | |
| ≤6 | 59 |
30.9 |
| 7
(3+4) | 38 |
19.9 |
| 7
(4+3), 8–10 | 94 |
49.2 |
Prevalence of ERG expression in
different ethnic groups
The evaluation of ERG oncoprotein expression by IHC
in the multiethnic cohort of Malaysian CaP patients revealed an
overall frequency of 39.2%, with positive ERG expression in 47 of
the 120 patients. ERG-positive tumors were detected in 31.4%
(60/191) of the individual tumor sections examined. The status and
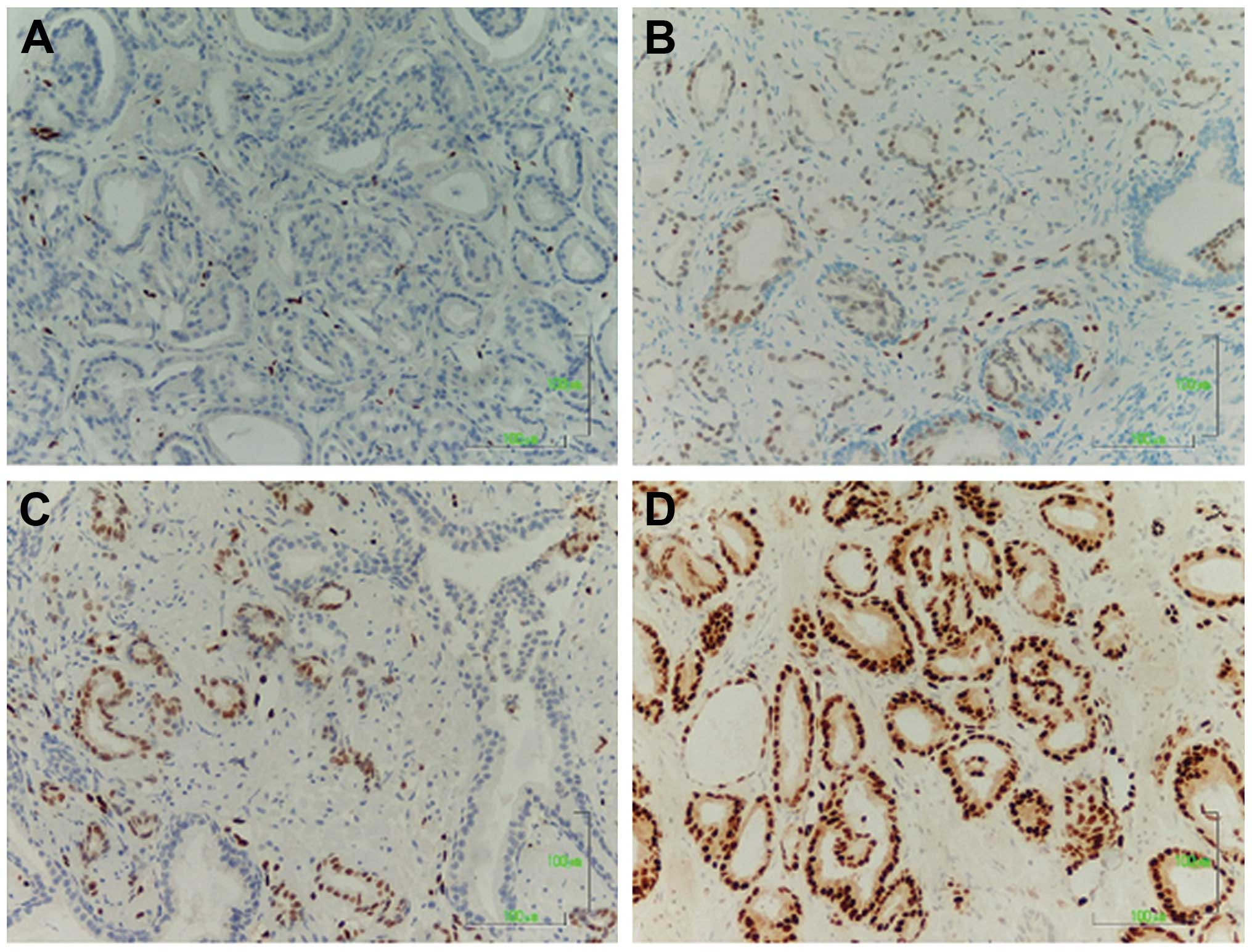
intensity of ERG staining are detailed in the heatmap in Fig. 1 and sections representative for
each level of expression are shown in Fig. 2.
Among the MC patients, who formed the majority
ethnic group of this study, 27 of 82 cases (32.9%) were
ERG-positive (Table II). Prostate
tumor sections were evaluated from either the right or left lobe of
the prostate in 29 cases and from both lobes in 53 cases (Fig. 1). Positive ERG expression was
detected in 35 of the 135 sections (25.9%) examined (Table II). Of the 27 cases positive for
ERG expression, ERG was detected in either the right or the left
lobe of the prostate in 19 and in both lobes in 8 cases.
 | Table IIAssociation of erythroblast
transformation-specific-related gene (ERG) oncoprotein expression
status with ethnicity, age and Gleason score, as evaluated by
patient and by individual tumor sections. |
Table II
Association of erythroblast
transformation-specific-related gene (ERG) oncoprotein expression
status with ethnicity, age and Gleason score, as evaluated by
patient and by individual tumor sections.
| Evaluation by
patient (n=120) | Evaluation by
individual tumor sections (n=191) |
|---|
|
|
|
|---|
| ERG expression | | ERG expression | |
|---|
|
| |
| |
|---|
| Variables | Negative (%) | Positive (%) | P-value | Negative (%) | Positive (%) | P-value |
|---|
| Ethnicity | | |
0.004a,b | | |
<0.001a |
|
Chinese | 55 (67.1) | 27 (32.9) | | 100 (74.1) | 35 (25.9) | |
|
Malay | 7 (87.5) | 1 (12.5) | | 13 (92.9) | 1 (7.1) | |
|
Indian | 11 (36.7) | 19 (63.3) | | 18 (42.9) | 24 (57.1) | |
|
Total | 73 (60.8) | 47(39.2) | | 131 (68.6) | 60 (31.4) | |
| Age (years) | | |
0.040a,c | | |
0.015a,c |
|
<69 | 31 (51.7) | 29 (48.3) | | 56 (60.2) | 37 (39.8) | |
|
≥69 | 42 (70.0) | 18 (30.0) | | 75 (76.5) | 23 (23.5) | |
| Gleason score | | |
0.813c | | |
0.476c |
| ≤6 | 22 (62.9) | 13 (37.1) | | 41 (69.5) | 18 (30.5) | |
| 7
(3+4) | 15 (55.6) | 12 (44.4) | | 23 (60.5) | 15 (39.5) | |
| 7
(4+3), 8–10 | 36 (62.1) | 22 (37.9) | | 67 (71.3) | 27 (28.7) | |
Surprisingly, 19 of 30 (63.3%) MI patients were
positive for ERG expression (Table
II). Biopsy specimens were examined from either the right or
left lobe of the prostate in 18 and from both lobes in 12 cases.
Positive ERG expression was detected in 24 of 42 (57.1%) individual
tumor sections (Table II). Of the
19 MI patients with a positive ERG expression status, ERG was
detected in either the right or the left lobe of the prostate in 14
patients and in both lobes in 5 cases.
Among the 8 Malay patients evaluated, only 1 (12.5%)
was positive for ERG (Table II).
Prostate tumor sections from both lobes of the prostate were
evaluated for 6 of the 8 Malay patients. Only 1 of the 14 (7.1%)
sections examined was positive for ERG expression (Table II).
Analysis of the association of the ERG
expression status of patients with age and Gleason score
The association of ERG expression status of patients
with age and Gleason score of tumors was evaluated by statistical
analysis. The results revealed a positive correlation between
positive ERG expression of tumors and younger patients, when
evaluated either by patient (P=0.04) or by individual tumor
sections (P=0.015; Table II). We
also observed a correlation between higher intensity of ERG
staining with younger patients as a whole (P=0.032; Table III). The evaluation of the
association between ERG expression status and Gleason score, either
by patient or by individual tumor sections, did not reveal a
significant correlation (Table
II).
 | Table IIIAssociation of erythroblast
transformation-specific-related gene (ERG) oncoprotein staining
intensity in the examined sections with ethnicity, age and Gleason
score. |
Table III
Association of erythroblast
transformation-specific-related gene (ERG) oncoprotein staining
intensity in the examined sections with ethnicity, age and Gleason
score.
| ERG staining
intensity (%) | |
|---|
|
| |
|---|
| Variables | Negative (0) | Weak (1+) | Strong (2+ and
3+) | P-value |
|---|
| Ethnicity | | | |
<0.001a,b |
|
Chinese | 100 (74.1) | 7 (5.2) | 28 (20.7) | |
|
Malay | 13 (92.9) | 0 (0.0) | 1 (7.1) | |
|
Indian | 18 (42.9) | 7 (16.6) | 17 (40.5) | |
| Age (years) | | | |
0.032a,c |
|
<69 | 56 (60.2) | 7 (7.5) | 30 (32.3) | |
|
≥69 | 75 (76.5) | 7 (7.2) | 16 (16.3) | |
| Gleason score | | | |
0.397b |
| ≤6 | 41 (69.5) | 7 (11.9) | 11 (18.6) | |
| 7
(3+4) | 23 (60.5) | 2 (5.3) | 13 (34.2) | |
| 7
(4+3), 8–10 | 67 (71.3) | 5 (5.3) | 22 (23.4) | |
Discussion
In this study, we evaluated the expression of ERG
oncoprotein in a multiethnic cohort of patients as a surrogate for
the detection of TMPRSS2-ERG fusion events. We examined 191
sections of FFPE prostate tumor specimens isolated by TRUS-guided
biopsy from 120 patients. The ethnic distribution of this study
cohort, which consisted of 82 MC (68.3%), 30 MI (25.0%) and 8 Malay
men (6.7%), is representative of patient enrollment at the hospital
where this study was conducted and does not mirror the ethnic
distribution of the overall Malaysian population. However, it does
represent the overall incidence of CaP diagnosed in the country,
with the highest incidence among MC, followed by MI, and the lowest
among Malays (1).
The overall frequency of ERG oncoprotein expression
in the cohort of Malaysian CaP patients, as determined by IHC, was
39.2%, which was considerably lower compared to the frequency of
50–70% detected in Western countries. The prevalence of ERG among
MC, the largest ethnic group analyzed, was 32.9%. Although this
frequency of ERG-positive expression in the MC population is
marginally higher compared to the frequencies of 15.9–29.7%
reported for populations from Korea, Japan and China, it remains
within a similar range (12,
14, 15, 18,
20) (Table IV).
 | Table IVSummary of the frequency of
erythroblast transformation-specific-related gene (ERG) oncoprotein
expression status in different populations worldwide. |
Table IV
Summary of the frequency of
erythroblast transformation-specific-related gene (ERG) oncoprotein
expression status in different populations worldwide.
| Population | Sample | Assay method for
ERG detection | Frequency, %
(no./total) | (Refs.) |
|---|
| USA |
|
NSeg | RP | FISH | 41.6 (217/521) | (45) |
| CA | Biopsy | FISH | 46.0 (46/100) | (46) |
|
NSeg | RP, WM | IHC, FISH | 65.1 (86/132) | (25) |
| CA | RP | FISH | 50.0 (21/42) | (12) |
| AA | RP | FISH | 31.3 (20/64) | (12) |
| CA | RP, WM | IHC, FISH | 65.9 (60/91) | (5) |
| AA | RP, WM | IHC, FISH | 42.9 (39/91) | (5) |
| UK | TURP | FISH | 30.1 (134/445) | (37) |
| Sweden | TURP | FISH, RT-PCR | 17.5 (62/354) | (47) |
| TURP | FISH, RT-PCR | 16.9 (46/272) | (48) |
| Germany | PCa, LNMets,
Mets | IHC, FISH | 45.3 (120/265) | (32) |
| RP | FISH | 58.7 (44/75) | (49) |
| Japan | RP | FISH | 15.9 (7/44) | (12) |
| RP | IHC | 16.3 (15/92) | (17) |
| RP and biopsy | IHC | 20.1 (42/209) | (16) |
| RP | RT-PCR | 27.8 (54/194) | (15) |
| Korea | RP | FISH | 20.9 (53/254) | (13) |
| RP | IHC | 24.4 (73/303) | (18) |
| China | NS | FISH | 7.5 (7/93) | (14) |
| | IHC | 10.2 (9/88) | (50) |
| TURP | FISH | 23.2 (44/190) | (20) |
| India | RP | IHC, FISH | 26.7 (8/30) | (19) |
| Malaysia | | | | |
|
NSeg | TRUS-biopsy | IHC | 39.2 (47/120) | Present study |
| MC | | | 32.9 (27/82) | |
| MI | | | 63.3 (19/30) | |
|
Malay | | | 12.5 (1/8) | |
Interestingly, we detected a disproportionately
higher frequency (63.3%) of ERG-positive tumors among MI patients
in this study. This is in comparison to a previous study on Indian
CaP patients without prior hormonal treatment from New Delhi,
India, in which ERG-positive tumors were detected in 8 of the 30
cases (27%) examined (19).
However, the higher prevalence of ERG-positive cases in this study
may be attributed to the limitations inherent in a small sample
size. Whether the higher prevalence of ERG-positive tumors among MI
patients indicates a regional variation where TMPRSS2-ERG
fusion contributes more significantly to the progression of the
disease compared to other populations of the same ethnicity,
requires confirmation by studies on larger populations. Among the
three ethnic groups, Malay CaP patients exhibited the lowest
frequency (12.5%) of ERG-positive tumors. However, the results
obtained from the small sample of Malay patients analyzed in this
study require further confirmation in studies involving larger
cohorts.
Efforts to identify the correlation of
TMPRSS2-ERG fusion or ERG overexpression with
clinicopathological characteristics have yielded variable results,
which is likely due to the heterogeneity of patient cohorts
evaluated in different studies. In certain studies, a higher
Gleason score and a lower tumor differentiation exhibited a
significant correlation with ERG gene alterations or with
ERG-positive immunostaining (25,
37–39). Other studies have reported the
association of a lower Gleason score with a higher number of
TMPRSS2-ERG fusion events (13, 40). However, other studies have reported
a significant association of TMPRSS2-ERG fusion with tumors
of higher stage and lymph node metastasis (41) or higher pathological stage
(42), but no association between
TMPRSS2-ERG fusion and Gleason score. In a comparison
between patients of different ethnic backgrounds, Rosen et
al (5) reported a correlation
between ERG-negative status and high-grade CaP tumors among AA but
not among CA patients. There was no significant correlation between
Gleason score and ERG expression or intensity when evaluated
against either tumor sections or patients in our study. However, a
significant association between younger patients (aged <69
years) and a positive ERG expression status, as well as ERG
intensity, was observed in our Malaysian cohort as a whole. This
correlation was also observed in studies among Japanese and
European CaP patients (17,
43), which suggests that ERG
rearrangement may be particularly important in patients with
early-onset CaP.
The effect of multiple factors, including diet,
genetics and environmental factors, may contribute to the
significant disparity in the frequency of CaP globally. The
TMPRSS2-ERG gene fusion alteration, which is frequent among
Western Caucasian populations, has been found to be less frequent
among South Asian and East Asian populations. Whether other genomic
alteration events typified by the fusion of other ETS gene
family members, such as ETV1 and ETV4, to
androgen-regulated promoters (10,
23), amplification of the 8q24
loci (44), PTEN deletion
(20), or yet to be identified
genetic events, are more prevalent in Asian populations remains to
be investigated. A more comprehensive study, including a larger
number of Malay and MI patients should be undertaken, not only to
confirm the frequency of TMPRSS2-ERG fusion events, but to
gain better understanding of the underlying genetics of CaP in the
Malaysian population.
Acknowledgements
This study was funded by the sponsors of the Cancer
Research Initiatives Foundation (CARIF). We are grateful to Mr.
Vijaya Kumar T. Krishnan and Ms. Mary Catherine D. Cruz from the
Histopathology Laboratory, Sime Darby Medical Centre, for their
technical assistance. The Henry M. Jackson Foundation for the
Advancement of Military Medicine has filed a patent application on
the mouse monoclonal anti-ERG antibody, 9FY, on which S.T., A.D.
and S.S. are co-inventors and have been licensed to the Biocare
Medical. This study was conducted independently of any involvement
from Biocare Medical.
References
|
1
|
Zainal Ariffin O and Nor Saleha IT:
Nationa. Cancer Registry Report 2007. Malaysia Cancer Statistics -
Data and Figure. National Cancer Registry, Ministry of Health,
Putrajaya Malaysia: 2011
|
|
2
|
Othman NH, Nor ZM and Biswal BM: Is
Kelantan joining the global cancer epidemic? - experience from
hospital Universiti Sains Malaysia; 1987–2007. Asian Pac J Cancer
Prev. 9:473–478. 2008.PubMed/NCBI
|
|
3
|
Farrell J, Petrovics G, McLeod DG and
Srivastava S: Genetic and molecular differences in prostate
carcinogenesis between African American and Caucasian American men.
Int J Mol Sci. 14:15510–15531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mononen N and Schleutker J: Polymorphisms
in genes involved in androgen pathways as risk factors for prostate
cancer. J Urol. 181:1541–1549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosen P, Pfister D, Young D, et al:
Differences in frequency of ERG oncoprotein expression between
index tumors of Caucasian and African American patients with
prostate cancer. Urology. 80:749–753. 2012. View Article : Google Scholar
|
|
6
|
Martin DN, Starks AM and Ambs S:
Biological determinants of health disparities in prostate cancer.
Curr Opin Oncol. 25:235–241. 2013.PubMed/NCBI
|
|
7
|
Amundadottir LT, Sulem P, Gudmundsson J,
et al: A common variant associated with prostate cancer in European
and African populations. Nat Genet. 38:652–658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freedman ML, Haiman CA, Patterson N, et
al: Admixture mapping identifies 8q24 as a prostate cancer risk
locus in African-American men. Proc Natl Acad Sci USA.
103:14068–14073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taioli E, Sears V, Watson A, et al:
Polymorphisms in CYP17 and CYP3A4 and prostate cancer in men of
African descent. Prostate. 73:668–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rubin MA, Maher CA and Chinnaiyan AM:
Common gene rearrangements in prostate cancer. J Clin Oncol.
29:3659–3668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosen P, Sesterhenn IA, Brassell SA,
McLeod DG, Srivastava S and Dobi A: Clinical potential of the ERG
oncoprotein in prostate cancer. Nat Rev Urol. 9:131–137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Magi-Galluzzi C, Tsusuki T, Elson P, et
al: TMPRSS2-ERG gene fusion prevalence and class are significantly
different in prostate cancer of Caucasian, African-American and
Japanese patients. Prostate. 71:489–497. 2011. View Article : Google Scholar
|
|
13
|
Lee K, Chae JY, Kwak C, Ku JH and Moon KC:
TMPRSS2-ERG gene fusion and clinicopathologic characteristics of
Korean prostate cancer patients. Urology. 76(1268): e7–e13.
2010.PubMed/NCBI
|
|
14
|
Mao X, Yu Y, Boyd LK, et al: Distinct
genomic alterations in prostate cancers in Chinese and Western
populations suggest alternative pathways of prostate
carcinogenesis. Cancer Res. 70:5207–5212. 2010. View Article : Google Scholar
|
|
15
|
Miyagi Y, Sasaki T, Fujinami K, et al: ETS
family-associated gene fusions in Japanese prostate cancer:
analysis of 194 radical prostatectomy samples. Mod Pathol.
23:1492–1498. 2010. View Article : Google Scholar
|
|
16
|
Furusato B, van Leenders GJ, Trapman J, et
al: Immunohistochemical ETS-related gene detection in a Japanese
prostate cancer cohort: diagnostic use in Japanese prostate cancer
patients. Pathol Int. 61:409–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimura T, Furusato B, Miki J, et al:
Expression of ERG oncoprotein is associated with a less aggressive
tumor phenotype in Japanese prostate cancer patients. Pathol Int.
62:742–748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suh JH, Park JW, Lee C and Moon KC: ERG
immunohistochemistry and clinicopathologic characteristics in
Korean prostate adenocarcinoma patients. Korean J Pathol.
46:423–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rawal S, Young D, Williams M, et al: Low
frequency of the ERG oncogene alterations in prostate cancer
patients from India. J Cancer. 4:468–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi M, Yang X, Zhang F, et al: ERG
rearrangement is associated with prostate cancer-related death in
Chinese prostate cancer patients. PLoS One. 9:e849592014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petrovics G, Liu A, Shaheduzzaman S, et
al: Frequent overexpression of ETS-related gene-1 (ERG1) in
prostate cancer transcriptome. Oncogene. 24:3847–3852. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomlins SA, Rhodes DR, Perner S, et al:
Recurrent fusion of TMPRSS2 and ETS transcription factor genes in
prostate cancer. Science. 310:644–648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dobi A, Sreenath T and Srivastava S:
Androgen-dependent oncogenic activation of ETS transcription
factors by recurrent gene fusions in prostate cancer: biological
and clinical implicationsAndrogen-Responsive Genes in Prostate
Cancer. WangZ: Springer; New York, NY: pp. 307–328. 2013,
View Article : Google Scholar
|
|
24
|
Sreenath TL, Dobi A, Petrovics G and
Srivastava S: Oncogenic activation of ERG: a predominant mechanism
in prostate cancer. J Carcinog. 10(37)2011.PubMed/NCBI
|
|
25
|
Furusato B, Tan SH, Young D, et al: ERG
oncoprotein expression in prostate cancer: clonal progression of
ERG-positive tumor cells and potential for ERG-based
stratification. Prostate Cancer Prostatic Dis. 13:228–237. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun C, Dobi A, Mohamed A, et al:
TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer
activates C-MYC and abrogates prostate epithelial differentiation.
Oncogene. 27:5348–5353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gupta S, Iljin K, Sara H, et al: FZD4 as a
mediator of ERG oncogene-induced WNT signaling and
epithelial-to-mesenchymal transition in human prostate cancer
cells. Cancer Res. 70:6735–6745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carver BS, Tran J, Gopalan A, et al:
Aberrant ERG expression cooperates with loss of PTEN to promote
cancer progression in the prostate. Nat Genet. 41:619–624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
King JC, Xu J, Wongvipat J, et al:
Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in
prostate oncogenesis. Nat Genet. 41:524–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rahim S and Uren A: Emergence of ETS
transcription factors as diagnostic tools and therapeutic targets
in prostate cancer. Am J Transl Res. 5:254–268. 2013.PubMed/NCBI
|
|
31
|
Park K, Tomlins SA, Mudaliar KM, et al:
Antibody-based detection of ERG rearrangement-positive prostate
cancer. Neoplasia. 12:590–598. 2010.PubMed/NCBI
|
|
32
|
Braun M, Goltz D, Shaikhibrahim Z, et al:
ERG protein expression and genomic rearrangement status in primary
and metastatic prostate cancer - a comparative study of two
monoclonal antibodies. Prostate Cancer Prostatic Dis. 15:165–169.
2012. View Article : Google Scholar
|
|
33
|
Svensson MA, Perner S, Ohlson AL, et al: A
comparative study of ERG status assessment on DNA, mRNA, and
protein levels using unique samples from a Swedish biopsy cohort.
Appl Immunohistochem Mol Morphol. 22:136–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gsponer JR, Braun M, Scheble VJ, et al:
ERG rearrangement and protein expression in the progression to
castration-resistant prostate cancer. Prostate Cancer Prostatic
Dis. 17:126–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fontugne J, Lee D, Cantaloni C, et al:
Recurrent prostate cancer genomic alterations predict response to
brachytherapy treatment. Cancer Epidemiol Biomarkers Prev.
23:594–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hasan ARb: Statistica. Handbook Malaysia
2013. Department of Statistics; Malaysia: 2013
|
|
37
|
Attard G, Clark J, Ambroisine L, et al:
Duplication of the fusion of TMPRSS2 to ERG sequences identifies
fatal human prostate cancer. Oncogene. 27:253–263. 2008. View Article : Google Scholar
|
|
38
|
Rajput AB, Miller MA, De Luca A, et al:
Frequency of the TMPRSS2:ERG gene fusion is increased in moderate
to poorly differentiated prostate cancers. J Clin Pathol.
60:1238–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Demichelis F, Fall K, Perner S, et al:
TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a
watchful waiting cohort. Oncogene. 26:4596–4599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Darnel AD, Lafargue CJ, Vollmer RT, Corcos
J and Bismar TA: TMPRSS2-ERG fusion is frequently observed in
Gleason pattern 3 prostate cancer in a Canadian cohort. Cancer Biol
Ther. 8:125–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perner S, Demichelis F, Beroukhim R, et
al: TMPRSS2:ERG fusion-associated deletions provide insight into
the heterogeneity of prostate cancer. Cancer Res. 66:8337–8341.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mehra R, Tomlins SA, Shen R, et al:
Comprehensive assessment of TMPRSS2 and ETS family gene aberrations
in clinically localized prostate cancer. Mod Pathol. 20:538–544.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schaefer G, Mosquera JM, Ramoner R, et al:
Distinct ERG rearrangement prevalence in prostate cancer: higher
frequency in young age and in low PSA prostate cancer. Prostate
Cancer Prostatic Dis. 16:132–138. 2013.PubMed/NCBI
|
|
44
|
Fromont G, Godet J, Peyret A, et al: 8q24
amplification is associated with Myc expression and prostate cancer
progression and is an independent predictor of recurrence after
radical prostatectomy. Hum Pathol. 44:1617–1623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gopalan A, Leversha MA, Satagopan JM, et
al: TMPRSS2-ERG gene fusion is not associated with outcome in
patients treated by prostatectomy. Cancer Res. 69:1400–1406. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mosquera JM, Mehra R, Regan MM, et al:
Prevalence of TMPRSS2-ERG fusion prostate cancer among men
undergoing prostate biopsy in the United States. Clin Cancer Res.
15:4706–4711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Setlur SR, Mertz KD, Hoshida Y, et al:
Estrogen-dependent signaling in a molecularly distinct subclass of
aggressive prostate cancer. J Natl Cancer Inst. 100:815–825. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sboner A, Demichelis F, Calza S, et al:
Molecular sampling of prostate cancer: a dilemma for predicting
disease progression. BMC Med Genomics. 3(8)2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hofer MD, Kuefer R, Maier C, et al:
Genome-wide linkage analysis of TMPRSS2-ERG fusion in familial
prostate cancer. Cancer Res. 69:640–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xue L, Mao X, Ren G, et al: Chinese and
Western prostate cancers show alternate pathogenetic pathways in
association with ERG status. Am J Cancer Res. 2:736–744.
2012.PubMed/NCBI
|