Introduction
Soft tissue sarcomas (STS) are a heterogeneous group
of rare tumors that arise from mesenchymal cells at various body
sites (1, 2). The malignant precursor cell(s) may
differentiate along one or several lineages, such as muscle,
adipose, fibrous, cartilage, nerve or vascular tissue. STS has
>50 distinct histological subtypes, with leiomyosarcoma,
liposarcoma, synovial sarcoma, undifferentiated pleomorphic sarcoma
and malignant peripheral nerve sheath tumors being among the most
common (3). STS occurs accounts
for ∼1% of malignancies in adults and 2% of the overall cancer
mortality (4, 5). Approximately half of the patients
diagnosed with STS present with advanced/metastatic cancer and
eventually succumb to their disease (1, 6).
The median overall survival for advanced-stage disease was reported
to range between 11 and 18 months (7, 8).
For advanced-stage STS, judicious use of cytotoxic
therapy provides meaningful palliation and may prolong survival.
The selection of systemic therapy may be individualized based upon
several factors, including tumor histology, health status and
preferences of the patients (2,
8, 9). The European Society for Medical
Oncology and the National Comprehensive Cancer Network treatment
guidelines recommend anthracycline-based chemotherapy, primarily
with doxorubicin, either as monotherapy or in combination with
ifosfamide, as first-line treatment for the most advanced STS
subtypes (9, 10). Pazopanib is a novel treatment
modality and has been approved by the Food and Drug Administration
and the European Medicines Agency as second-line treatment after a
phase III trial of this drug reported a statistically significant
increase in progression-free survival. Of note, only 6% of the
patients exhibit a tumor response and in the majority of the cases,
stable disease may be achieved. There remains the question of
whether this drug may be used in highly symptomatic cases, as in
some cases response rate may be a more important goal than survival
benefit. In this study, we present 2 case reports of patients with
STS who achieved a nearly complete radiological response to
pazopanib.
Case 1
A 25-year-old female patient presented with a soft
tissue mass at the level of right knee that had developed 3 years
prior. The patient's past history was negative for systemic
diseases and the laboratory evaluation was normal. We used positron
emission tomography (PET)- computed tomography (CT) for initial
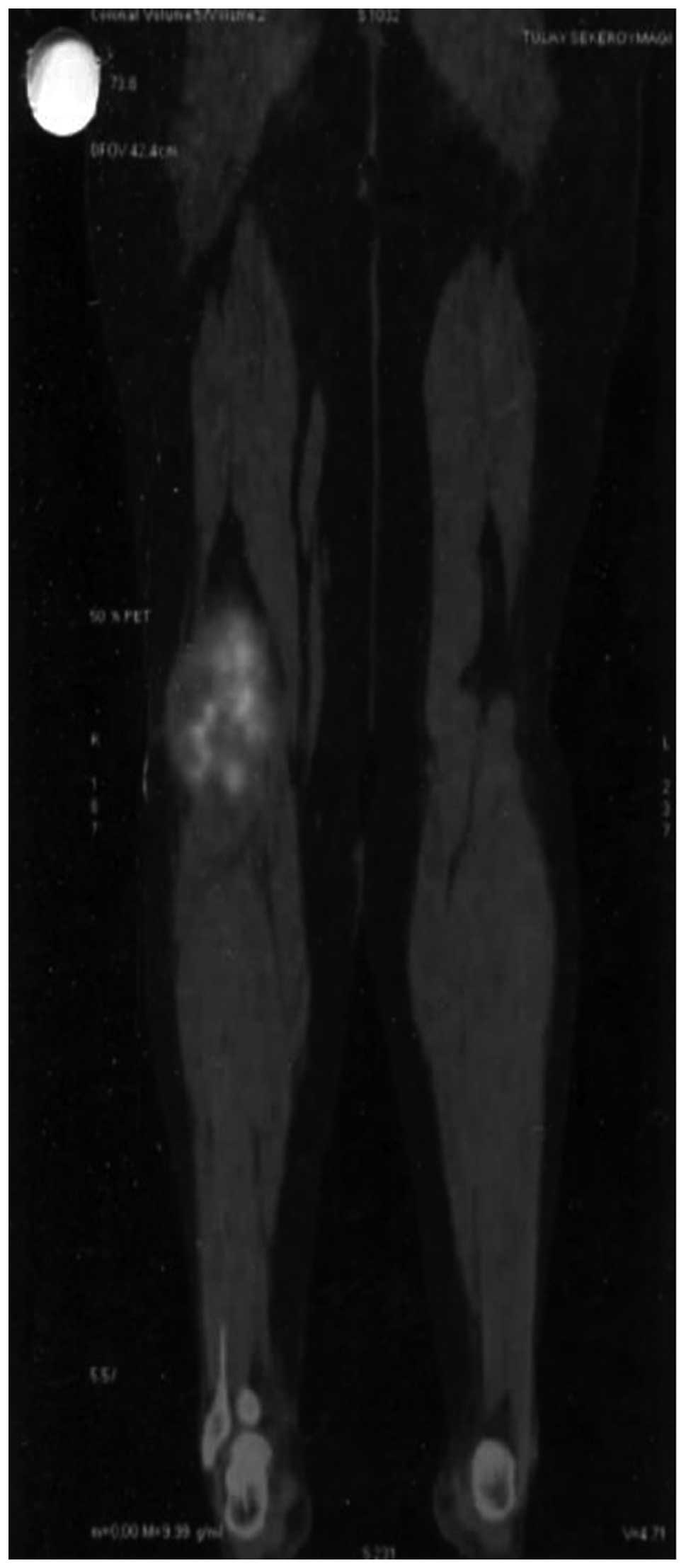
staging and to exclude systemic metastases. The PET-CT showed a
hypermetabolic lesion close to the right knee joint, filling the
popliteal area, sized 110×82×160 mm (maximum standard uptake value,
10.7) (Fig. 1). The
histopathological evaluation was compatible with a malignant
mesenchymal tumor, namely a synovial sarcoma. The patient was
treated with systemic neoadjuvant chemotherapy, followed by
surgical resection in August, 2010. The patient also received
adjuvant radiotherapy.
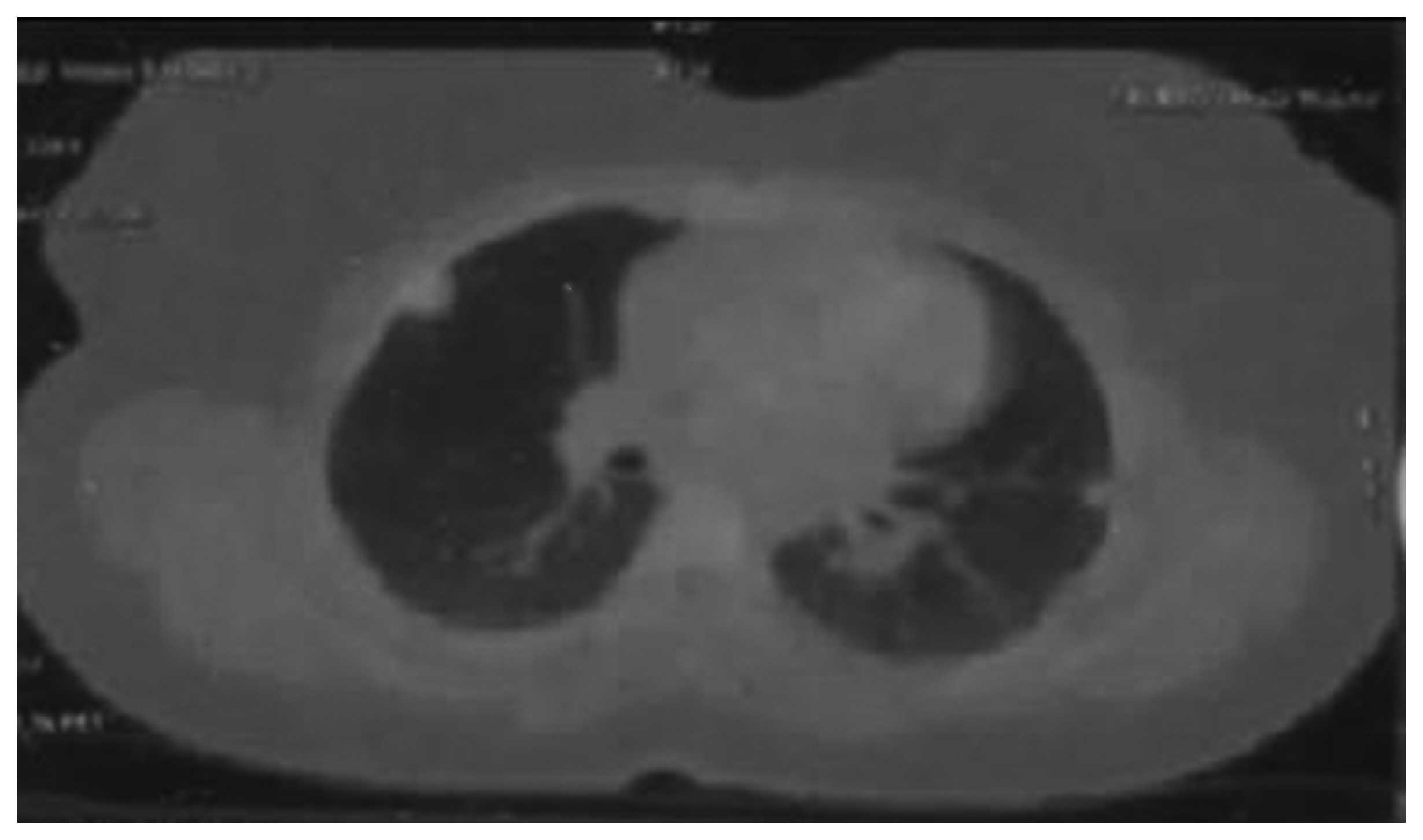
In September, 2012, the patient presented with
dyspnea and her detailed evaluation with PET-CT revealed lung
metastases, accompanied by right iliac chain and right inguinal
lymphadenopathy (Fig. 2). The
patient received treatment with a gemcitabine-docetaxel combination
regimen, but the disease progressed. Subsequently, pazopanib (800
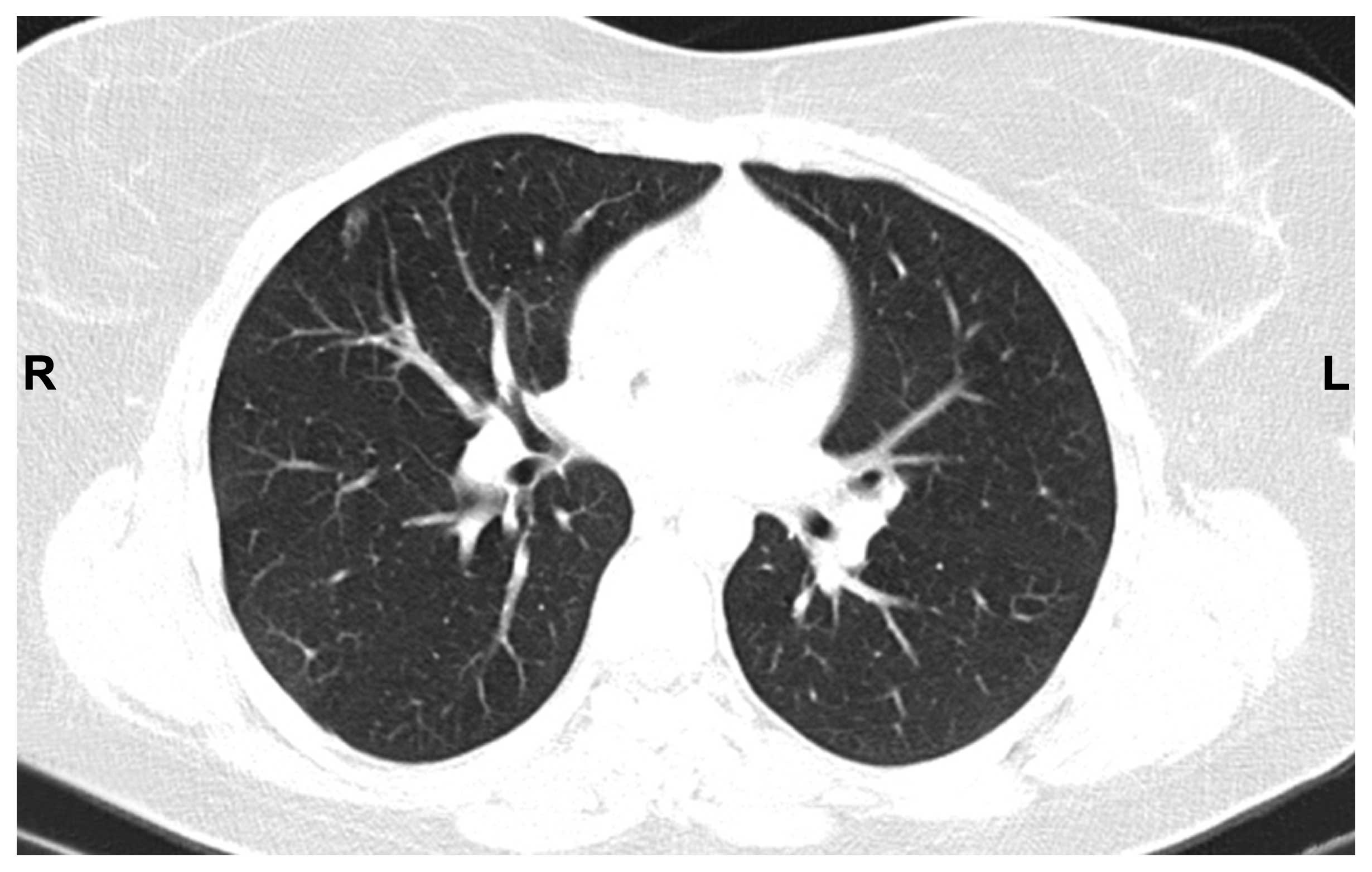
mg/day) was initiated in January, 2013. The radiological evaluation
in July, 2013 was compatible with nearly complete response
(Figs. 3).
Case 2
A 50-year-old female patient presented with a right
breast mass in July, 2010. The preoperative biopsy was compatible
with the diagnosis of primary breast leiomyosarcoma and a right
mastectomy was performed. The patient was treated with 6 courses of
doxorubicin-based adjuvant chemotherapy. During surveillance, the
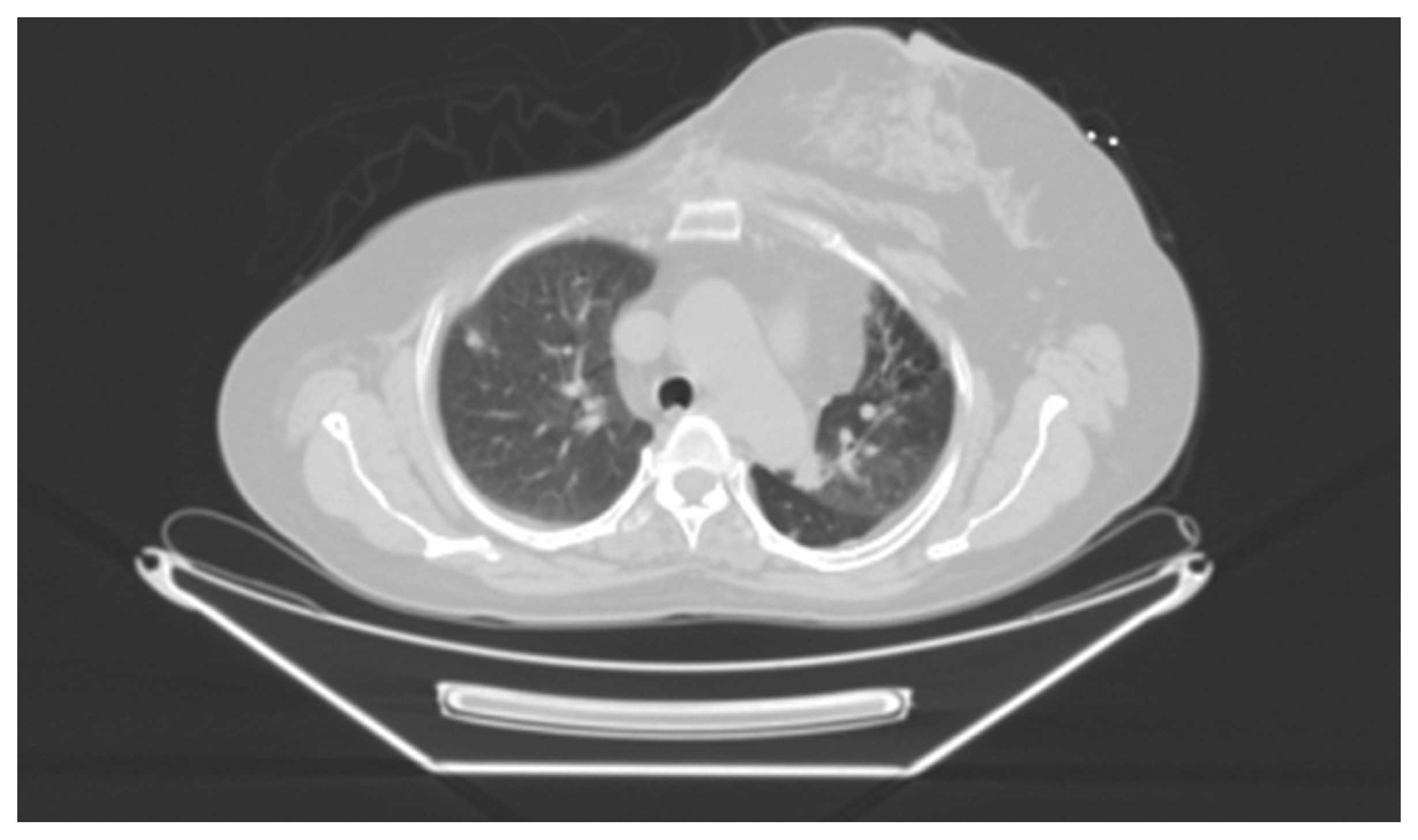
patient presented with progressive dyspnea in September, 2012 and
the PET-CT scan revealed lytic bone lesions and hypermetabolic
metastatic lung nodules (Fig. 4).
At the second-line setting, the patient was treated with
docetaxel-gemcitabine and the progression-free survival with this
regimen was 6 months; the maximal response, as evaluated by PET-CT,
was stable disease. The patient received palliative radiotherapy to
the bone metastases in March, 2013. The radiological evaluation
following radiotherapy showed progression of the lung metastases.
Subsequently, pazopanib (800 mg/day) was initiated in May, 2013.
The radiological evaluation in July, 2013 was compatible with
nearly complete response (Fig.
5).
Discussion
Metastatic STS is an incurable disease and very
little improvement regarding the treatment of advanced STS has been
achieved over the last 2 decades, excluding imatinib treatment for
gastrointestinal stromal tumors (GIST). Pazopanib, which is a
synthetic indazolpyrimidine, is a multitargeted tyrosine kinase
inhibitor, exhibiting activity against the vascular endothelial
growth factor receptors 1, 2 and 3, and the platelet-derived growth
factor receptor (11).
Single-agent pazopanib was found to be effective against various
STS subtypes in a phase II clinical trial (12). Subsequently, a registration phase
III study (PALETTE trial) was designed to compare pazopanib (800 mg
daily) vs. placebo in 369 patients with a variety of histological
subtypes, excluding liposarcomas or GIST, in the second-line
setting (13). There was a
statistically significant increase in PFS in the pazopanib group
(4.6 vs. 1.6 months) and the survival benefit was consistent across
all histological subtypes (14).
However, although pazopanib significantly increased PFS in that
study, the objective response rate was quite low and the best
overall response was partial response in 6% of the pazopanib group.
When evaluating pazopanib in the light of those trials, we may not
recommend pazopanib for patients in need of fast symptomatic
relief. However, when we used pazopanib in our first 2 cases as
third-line treatment, a nearly complete response was achieved. On
analyzing the demographic, clinical and histopathological
characteristics of these 2 cases, including drug-related side
effects, we were unable to identify a common point that may help
explain why these 2 patients exhibited such a strong response to
pazopanib.
In conclusion, we reported 2 cases that exhibited an
unexpected, nearly complete response to anti-angiogenic treatment,
with the aim to raise the question of whether we can define a
specific group of patients who respond well to treatment with
pazopanib.
References
|
1
|
Clark MA, Fisher C, Judson I and Thomas
JM: Soft-tissue sarcomas in adults. N Engl J Med. 353:701–711.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Casali PG and Blay
JYESMO/CONTICANET/EUROBONET Consensus Panel of Experts: Soft tissue
sarcomas: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 21 (Suppl 5):v198–v203. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cormier JN and Pollock RE: Soft tissue
sarcomas. CA Cancer J Clin. 54:94–109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain A, Sajeevan KV, Babu KG and
Lakshmaiah KC: Chemotherapy in adult soft tissue sarcoma. Indian J
Cancer. 46:274–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weiss SW and Goldblum JR: General
considerationsEnzinger and Weiss's Soft Tissue Tumors. Weiss SW and
Goldblum JR: 4th. CV Mosby; St. Louis, MO: pp. 1–19. 2001
|
|
6
|
Schöffski P: Pazopanib in the treatment of
soft tissue sarcoma. Expert Rev Anticancer Ther. 12:711–723. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Italiano A, Mathoulin-Pelissier S, Cesne
AL, Terrier P, Bonvalot S, Collin F, Michels JJ, Blay JY, Coindre
JM and Bui B: Trends in survival for patients with metastatic
soft-tissue sarcoma. Cancer. 117:1049–1054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grimer R, Judson I, Peake D and Seddon B:
Guidelines for the management of soft tissue sarcomas. Sarcoma.
2010:5061822010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spira AI and Ettinger DS: The use of
chemotherapy in soft-tissue sarcomas. Oncologist. 7:348–359. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
NCCN, . NCCN Clinical Practice Guidelines
in Oncology: Soft Tissue Sarcoma, Volume V.1. 2011. National
Comprehensive Cancer Network; 2011
|
|
11
|
Schutz FA, Choueiri TK and Sternberg CN:
Pazopanib: clinical development of a potent anti-angiogenic drug.
Crit Rev Oncol Hematol. 77:163–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sleijfer S, Ray-Coquard I, Papai Z, et al:
Pazopanib, a multikinase angiogenesis inhibitor, in patients with
relapsed or refractory advanced soft tissue sarcoma: a phase II
study from the European organisation for research and treatment of
cancer-soft tissue and bone sarcoma group (EORTC study 62043). J
Clin Oncol. 27:31262009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van der Graaf WT, Blay JY, Chawla SP, et
al: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a
randomised, double-blind, placebo-controlled phase 3 trial. Lancet.
379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Der Graaf W, Blay JY, Chawla SP, et
al: PALETTE: Final overall survival (OS) data and predictive
factors for OS of EORTC 62072/GSK VEG110727, a randomized,
double-blind phase III trial of pazopanib versus placebo in
advanced soft tissue sarcoma (STS) patients. J Clin Oncol. 30:abs.
100092012.
|