Introduction
Similar to the hemangiopericytoma of soft tissues in
pathological features, first reported by Stout and Murray (1), intracranial meningeal hemangiopericytoma
(M-HPC) is a rare mesenchymal tumor, possibly of pericytic origin
in the meninges, which was initially described by Begg and Garret
(2) in 1954. The study by Cushing and
Eisenhardt (3) was the first to
report a dural-based hemangiopericytoma, which was described as a
variant of meningioma. M-HPC, constituting ~0.4% of all the primary
central nervous system tumors, is a distinctive, well-defined
clinicopathological entity characterized by a propensity for local
recurrence and extraneural metastasis (4). In the 2007 World Health Organization
Classification of Tumors of the Central Nervous System, M-HPC was
identified as a distinct entity in the group of mesenchymal
non-meningothelial tumors (4).
M-HPC shares similar clinical manifestations and
radiological findings with meningioma and the newly recognized
solitary fibrous tumor of the meninges, which makes it difficult to
differentiate this entity from those prognostically favorable
mimics prior to surgery. Preoperative detection and identification
of M-HPC is important for improved clinical risk stratification,
more optimal selection of therapy, and improved treatment response
prediction and prognosis evaluation. Regardless of an enhanced
understanding of the aggressive biological behavior of this type of
tumor, the treatment of M-HPC remains a great challenge.
In the present study, a histopathologically and
immunohistochemically confirmed case is described of M-HPC with
recurrences at the primary and distant intracranial sites and
extraneural metastases to multiple organs. The radiological
features and treatment outcome is summarized.
Case report
Patient
A 36-year-old female presented with a 3-year history
of weakness and numbness of the left lower limb and a recent
headache. The patient had no history of trauma or fever. The
neurological examination revealed that power was decreased in the
left lower limb (grade III) when compared to the right lower limb
(grade IV). Atrophy of the muscles of the limbs was found. The
sensory system examination revealed a decrease in the sensations of
the left lower limb. The laboratory findings were normal.
Computed tomography (CT) of the head revealed a
right frontal isodense mass adjacent to the falx cerebri without
calcification, however, minimal peritumoral edema was observed.
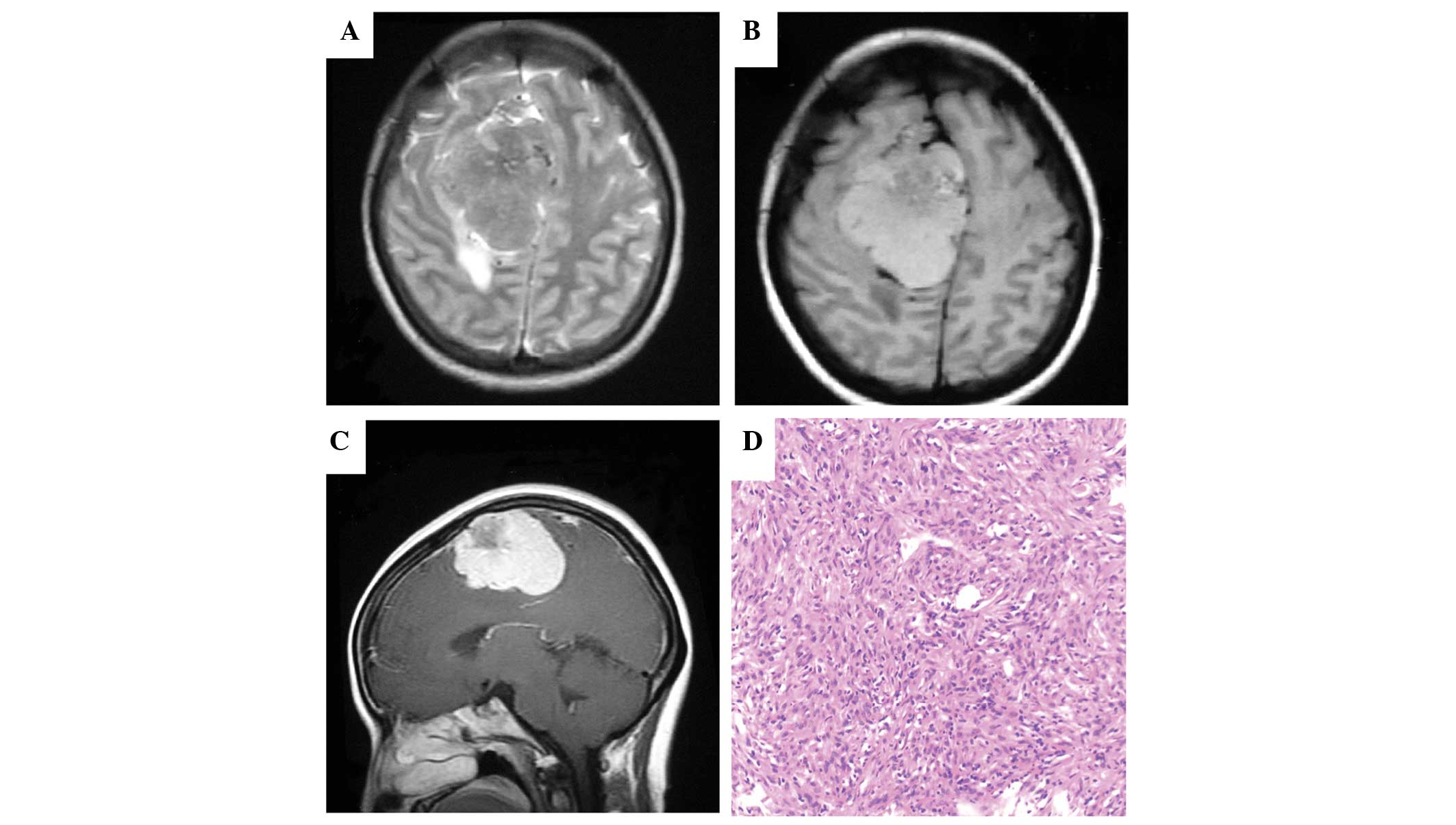
Magnetic resonance imaging (MRI) demonstrated a large extra-axial
dural-based tumor (5.8×5.2×4.2 cm) in the right frontal region. On
T2-weighted imaging, the mass exhibited a predominant isointensity
with minimal peritumoral edema and mass effect extending across the
midline (Fig. 1A). On T1-weighted
imaging, the lobulated lesion demonstrated a mixed intensity in
comparison to the surrounding brain (Fig.
1B). Intense inhomogeneous contrast enhancement with cystic
components and lobulated contour of the mass were noted on
contrast-enhanced MRI (Fig. 1C). A
possible diagnosis of M-HPC was made. Preoperative embolization at
the time of cerebral angiography was used to reduce blood loss and
brain injury during the surgery.
Immunohistochemical staining
Tissue blocks of the primary and recurrent tumors
and the biopsy bone specimen (right ilium) were available for
histopathological and immunohistochemical studies. Hematoxylin and
eosin (H&E) stains were routinely performed.
Immunohistochemical stains were performed with the Dako EnVision
System (Peroxidase, DAB; Dako North America, Inc., Carpinteria, CA,
USA), and the following antibodies were used for
immunohistochemistry: Vimentin (1:100, V9; DakoCytomation, Glostrup
Denmark), glial fibrillary acidic protein (1:400, 6F2; Antibody
Diagnostics, Stanford, CT, USA), cluster of differentiation (CD34)
(1:100, QBEnd10; Immunotech, Marseille, France), epithelial
membrane antigen (1:100, E29; DakoCytomation), S-100 (1:50, 4C4.9;
rabbit polyclonal; DakoCytomation), factor XIIIa (1:500;
Calbiochem, San Diego, CA, USA), CD99 (1:100, clone O13; Signet
Laboratories, Dedham, MA, USA), B-cell lymphoma 2 (bcl-2) (1:200;
DakoCytomation) and Ki-67 (1:50; MIB-1, DakoCytomation). The
immunohistochemical results were graded subjectively according to
extent as negative (–), focal (+) or diffuse (++).
Gross total resection was performed and
histopathological examinations of the primary M-HPC revealed a
typically cellular tumor composed of round to slightly spindled
cells in a jumbled arrangement (Fig.
1D). Additionally, the characteristic ‘staghorn’ vascular
pattern was revealed. Calcification was not demonstrated as
epithelioid features were distinctly absent. No high-grade HPC
feature was defined, and all the features were compatible with
M-HPC. The immunohistochemical staining revealed positive staining
for vimentin (diffuse), factor XIIIa (diffuse), CD99 (focal), bcl-2
(focal) and CD34 (focal), and negative for epithelial membrane
antigen, glial fibrillary acidic protein and S-100 protein. The
proliferation index evaluated with antibody against Ki-67 antigen
reached 8–15% (Table I). Single-dose,
image-guided radiosurgery to the tumor bed was undertaken. The
symptoms were resolved following surgery.
 | Table I.Immunohistochemical features of
M-HPC. |
Table I.
Immunohistochemical features of
M-HPC.
| Specimen | Vim | CD34 | CD99 | bcl-2 | XIIIa | S-100 | EMA | GFAP | Ki-67(%) |
|---|
| Primary | ++ | + | + | + | ++ | – | – | – | 8–15 |
| Recurrence | + | + | ++ | + | N/A | – | – | – | 15 |
| Metastase | + | + | ++ | – | N/A | – | – | – | 10 |
Follow-up
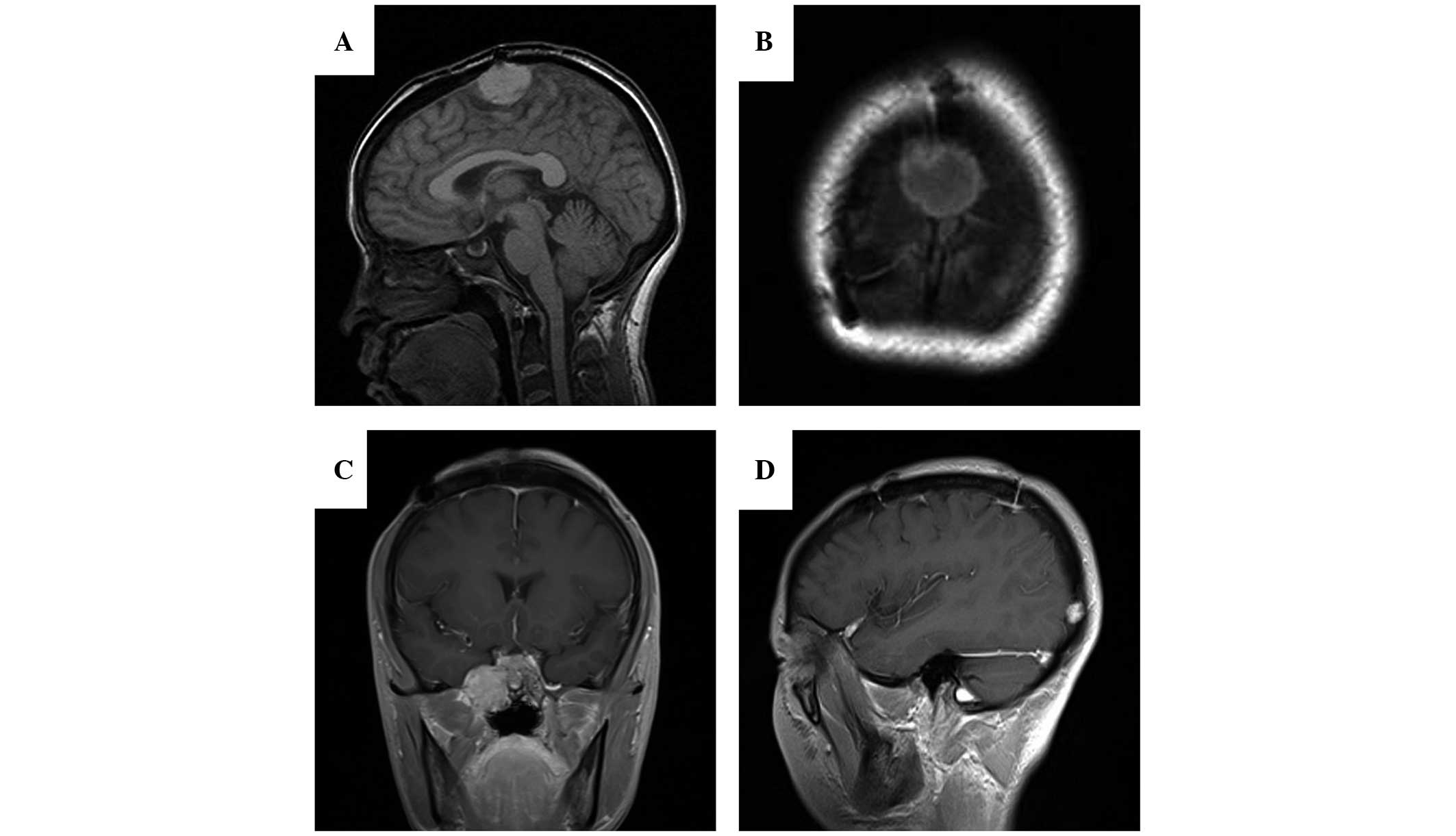
Four years later, a follow-up MRI revealed a local
relapse within the original site (Fig. 2A
and B) and two recurrent tumors at the right parasellar region
(Fig. 2C) and right occipital region
(Fig. 2D), respectively. A second
total-resection surgery was performed. The resected-recurrent tumor
shared similar histopathological features with the proliferation
index of 15% in comparison to the primary tumor.
Series MRI and CT scan performed later disclosed
multiple extracranial metastases to the ilium, costal bone,
bilateral kidneys and spine with compression fractures of C4, T4
and T6 (Fig. 3A–G). The corresponding
coronal volume-rendered single photon emission-CT images showed
multiple areas of increased tracer uptake. CT-guided aspiration of
the mass in the left ilium was performed and histological
examination revealed a spindle cell tumor compatible with an M-HPC,
with proliferation index of 10%. The immunohistochemical findings
of the primary, recurrent tumors and metastases are summarized in
Table I.
The patient received two cycles of chemotherapy with
oral 150 mg/m2 temozolomide on days 1–7 and 15–21 and 5
mg/kg bevacizumab intravenously on days 8 and 22, repeated at
28-day intervals. Two months later, in the absence of unacceptable
toxicity, the patient continued to receive treatment with 4 mg
zoledronic acid once every 3 weeks for 10 cycles to prevent
skeletal relevant events and to palliate bone pain. At the end of
the treatment, stable disease was obtained and it lasted over one
year from then on. Currently, the patient continues to be
clinically and radiographically stable on MRI and CT.
Discussion
Intracranial M-HPC is a rare, but distinct highly
cellular and vascularized mesenchymal tumor with a high recurrent
rate of >91% after 15 years (5)
and high metastasis rate of 64% (6).
Bone, liver, lung, central nervous system and abdominal cavity are
the most commonly reported sites of metastasis in HPC. The present
study described a case of primary M-HPC with recurrence at the
initial and distant intracranial sites and extraneural
multiple-organ metastases in a 36-year-old female. The metastasis
of M-HPC was extremely extensive, and to the best of our knowledge,
this is the first case of M-HPC with delayed metastasis to the
bilateral kidneys.
Preoperative detection and identification of M-HPC
is important with regards to therapeutic and prognostic value due
to its more aggressive biology, which is distinct from that of
meningioma. Clinically and radiographically, it is often
challenging for differential diagnosis of M-HPC from meningioma.
However, multimodality imaging, such as CT and MRI, can demonstrate
the important characteristics of these tumors and may provide
certain diagnostic clues. Clinically, M-HPC typically occurs at a
younger age than meningiomas, and slightly more often in males
compared to females (4). The clinical
course of M-HPC is often shorter than that of meningiomas due to
its faster growth rate. Radiographically, M-HPC is usually a
sharply demarcated extra-axial mass with dural attachment,
multilobulated margin and marked contrast enhancement on CT and MR
imaging (7,8). On T1-weighted imaging, this lesion is
isointense to slightly hyperintense, which may have resulted from
the nature of hypercellularity and hypervascularity. In the study
by Chen et al (8), all the
eight cases of M-HPC demonstrated multiple signal-intensity voids
of vessels on MRI. As opposed to meningioma, M-HPC may present
adjacent bony erosion, but lacks calcification and hyperostosis of
the involved bone, which is indicative of meningioma. The present
case represented several imaging features that are suggestive of
the diagnosis. Additionally, this case clarified the requirement
for detailed staging and long-term follow-up. However, these
radiological profiles are not sufficiently distinctive to permit
the exclusion of meningioma. The correct diagnosis primarily relies
on histological and immunophenotypical confirmation.
The total removal of the tumor followed by
postoperative-adjuvant radiotherapy is the mainstay of treatment.
Postoperative-adjuvant radiotherapy has been reported to be
effective in local-recurrence control (6,9–13), although controversy exists in its
associations with the reduction of metastasis development (9) and survival benefit (13–16).
The propensity of M-HPC in producing metastases in
extraneural organs is the principal cause of failure in the
treatment. The development of M-HPC metastasis resulted in a
significant reduction in the survival time with an average survival
time of 24 months after discovery (6). The role of chemotherapy in the treatment
of the metastatic M-HPC remains controversial with varied responses
in an extremely limited number of studies (9,14,17,18).
Certain novel drugs, including anti-vascular endothelial growth
factor receptor drugs (19–21) and a tyrosine kinase inhibitor
(22), have been initiated to treat
this disease. Temozolomide has demonstrated activity against
numerous types of cancers (23–25). In
the present case, multiple metastases were discovered in multiple
organs, including rib, ilium, spine and the bilateral kidneys with
vertebral compression fractures. Palliative radiation therapy, with
a dose of 40 Gy/10 fractions, resulted in alleviation of the
involved bone pain. Four cycles of chemotherapy with temozolomide
and bevacizumab were initiated, followed by 10 cycles of zoledronic
acid. The patient continued to be clinically and radiographically
stable on follow-up MRI and CT. The case provides evidence that a
multimodality approach of systemic therapy with temozolomide and
bevacizumab, in combination with palliative radiation therapy, may
be a promising therapeutic strategy when metastatic M-HPC is
encountered. However, limited to the rarity of the condition and
available data reported previously, the optimal systemic treatment
strategy has not been defined.
In conclusion, M-HPC shares similar clinical
manifestations and radiological findings with meningioma, but it is
a rare, distinct clinicopathological entity with high metastatic
potential and tendency for aggressive-local recurrence.
Preoperative CT and MRI could provide certain diagnostic clues and
useful information for more optimal treatment planning. However,
the treatment of M-HPC, particularly in metastatic settings,
remains a challenge. Novel drugs, including temozolomide and
bevacizumab, as a component of multimodality therapy, may deserve
further investigation. Increasing the knowledge regarding the
nature of this entity, underlying molecular pathogenesis and
affected signaling pathways makes molecularly-targeted therapy of
this lesion possible.
Acknowledgements
The authors would like to thank Professor Fucheng Ma
at the Department of Pathology, Xijing Hospital (Xian, China) for
his help in the present study.
References
|
1
|
Stout AP and Murray MR:
Hemangiopericytoma: A vascular tumor featuring Zimmermanns
pericytes. Ann Surg. 116:26–33. 1942. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Begg CF and Garret R: Hemangiopericytoma
occurring in the meninges: case report. Cancer. 7:602–606. 1954.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cushing H and Eisenhardt L: Meningiomas:
Their Classification, Regional Behavior, Life History, and Surgical
End ResultsCharles C Thomas; Springfield, IL: 1938, PubMed/NCBI
|
|
4
|
Giannini C, Rushing EJ and Hainfellner:
HemangiopericytomaIn: WHO Classification of Tumors of the Central
Nervous System. Louis DN, Ohgaki H, Wiestler OD and Cavenee WK:
IARC Press; Lyon: pp. 178–180. 2007
|
|
5
|
Vuorinen V, Sallinen P, Haapasalo H, et
al: Outcome of 31 intracranial hemangiopericytomas: poor predictive
value of cell proliferation indices. Acta Neurochir (Wien).
138:1399–1408. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guthrie BL, Ebersold MJ, Scheithauer BW
and Shaw EG: Meningeal hemangiopericytoma: histopathological
features, treatment, and long-term follow-up of 44 cases.
Neurosurgery. 25:514–522. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiechi MV, Smirniotopoulos JG and Mena H:
Intracranial hemangiopericytomas: MR and CT features. AJNR Am J
Neuroradiol. 17:1365–1371. 1996.PubMed/NCBI
|
|
8
|
Chen Q, Chen XZ, Wang JM, Li SW, Jiang T
and Dai JP: Intracranial meningeal hemangiopericytomas in children
and adolescents: CT and MR imaging findings. AJNR Am J Neuroradiol.
33:195–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dufour H, Métellus P, Fuentes S, et al:
Meningeal hemangiopericytoma: a retrospective study of 21 patients
with special review of postoperative external radiotherapy.
Neurosurgery. 48:756–763. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang SD and Sakamoto GT: The role of
radiosurgery for hemangiopericytomas. Neurosurg Focus. 14:e142003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar N, Kumar R, Kapoor R, et al:
Intracranial meningeal hemangiopericytoma: 10 years experience of a
tertiary care Institute. Acta Neurochir (Wien). 154:1647–1651.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rutkowski MJ, Jian BJ, Bloch O, et al:
Intracranial hemangiopericytoma: clinical experience and treatment
considerations in a modern series of 40 adult patients. Cancer.
118:1628–1636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stessin AM, Sison C, Nieto J, Raifu M and
Li B: The role of postoperative radiation therapy in the treatment
of meningeal hemangiopericytoma: experience from the SEER database.
Int J Radiat Oncol Biol Phys. 85:784–790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH, Jung HW, Kim YS, et al: Meningeal
hemangiopericytomas: long-term outcome and biological behavior.
Surg Neurol. 59:47–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rutkowski MJ, Sughrue ME, Kane AJ, et al:
Predictors of mortality following treatment of intracranial
hemangiopericytoma. J Neurosurg. 113:333–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schiariti M, Goetz P, El-Maghraby H,
Tailor J and Kitchen N: Hemangiopericytoma: long-term outcome
revisited. Clinical article. J Neurosurg. 114:747–755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bastin KT and Mehta MP: Meningeal
hemangiopericytoma: defining the role for radiation therapy. J
Neurooncol. 14:277–287. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fountas KN, Kapsalaki E, Kassam M, et al:
Management of intracranial meningeal hemangiopericytomas: outcome
and experience. Neurosurg Rev. 29:145–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delgado M, Pérez-Ruiz E, Alcalde J, et al:
Anti-angiogenic treatment (sunitinib) for disseminated malignant
haemangiopericytoma: a case study and review of the literature.
Case Rep Oncol. 4:55–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirn DH and Kramer A: Long-term freedom
from disease progression with interferon alfa therapy in two
patients with malignant hemangiopericytoma. J Natl Cancer Inst.
88:764–765. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Domont J, Massard C, Lassau N, et al:
Hemangiopericytoma and antiangiogenic therapy: clinical benefit of
antiangiogenic therapy (sorafenib and sunitinib) in relapsed
malignant haemangioperyctoma/solitary fibrous tumour. Invest New
Drugs. 28:199–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peters KB, McLendon R, Morse MA and
Vredenburgh JJ: Treatment of recurrent intracranial
hemangiopericytoma with SRC-related tyrosine kinase targeted
therapy: a case report. Case Rep Oncol. 3:93–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yovine A, Riofrio M, Blay JY, et al: Phase
II study of ecteinascidin-743 in advanced pretreated soft tissue
sarcoma patients. J Clin Oncol. 22:890–899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park MS, Patel SR, Ludwig JA, et al:
Activity of temozolomide and bevacizumab in the treatment of
locally advanced, recurrent, and metastatic hemangiopericytoma and
malignant solitary fibrous tumor. Cancer. 117:4939–4947. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tatar Z, Thivat E, Planchat E, et al:
Temozolomide and unusual indications: review of literature. Cancer
Treat Rev. 39:125–135. 2013. View Article : Google Scholar : PubMed/NCBI
|