Introduction
Radiation pneumonitis (RP) is the inflammation of
the normal lung tissue within the radiation field following
radiation therapy. RP is one of the most common complications and
one of the most important dose-limiting toxicities in the treatment
of thoracic tumors by radiotherapy (1). Although significant progress has been
made in the treatment technologies, a considerable number of
patients experience RP following thoracic irradiation. Studies have
demonstrated that ~10–20% of lung cancer patients develop severe RP
(grade ≥3) following radiotherapy, of whom 50% succumb to this
complication (2). The main risk
factors of RP include patient-related factors, such as gender,
smoking and pulmonary function, and treatment-related factors, such
as radiation dose, irradiated lung volume, surgery and chemotherapy
(3–7).
However, it remains difficult to predict the occurrence of RP for
any individual patient. Based on the development of the human
genome project and pharmacogenomics, it is reported that
single-nucleotide polymorphisms (SNPs) in inflammation-related, DNA
repair-related, stress response-related and angiogenesis-related
genes, may be used as biomarkers to predict the development of
RP.
Inflammation-related genes
Inflammation is a defensive reaction that results
from tissue damage or cellular injury, and is also a key process
underlying radiation-induced toxicity (8,9). Several
studies have been conducted to evaluate inflammation-related
biomarkers, focusing mostly on genes as described below.
Transforming growth factor-β1
(TGF-β1)
TGF-β1 is a type of cytokine that has been widely
investigated and plays an important role in the processes of cell
proliferation and differentiation, tissue fibrosis and inflammation
(10–12). Early in 1998, Anscher et al
(13) found that patients who
developed RP following radiotherapy had higher plasma TGF-β1 levels
compared with those prior to radiotherapy, unlike patients who did
not develop RP. As a result, the plasma TGF-β1 levels may be used
as a prediction index of RP.
In recent years, with the advances in molecular
biology and genetics, the association between RP and individual
differences caused by gene polymorphisms has become a research
focus. Yuan et al (14)
analyzed the correlation between genetic variants of TGF-β1 in 164
cases of lung cancer patients (77.4% Caucasian) who developed RP
following radiotherapy, and found that the TC/CC genotypes in
TGF-β1 rs1982073 (T>C) were associated with a decreased risk of
grade ≥3 RP (HR=0.390, 95% CI: 0.197–0.774, P=0.007) and grade ≥2
RP (HR=0.489, 95% CI: 0.227–0.861, P=0.013) compared with the TT
genotype. However, in 2010, Wang and Bi (15) found no association between TGF-β1
rs1982073 and grade ≥2 RP in Chinese patients (P=0.84) and the
plasma TGF-β1 levels were not dependent on this gene polymorphism.
Subsequently, a study by Niu et al (16) validated and supported Wang's view and
discovered that the AG/GG genotypes of TGF-β1 rs11466345 (A>G)
were associated with an increased risk of grade ≥3 RP in Chinese
lung cancer patients (HR=2.264, 95% CI: 1.126–4.552, P=0.022).
These studies suggested the presence of significant interethnic
differences in the SNPs of TGF-β1.
Abnormal cell lineage protein 28
(Lin28) B
Lin28 (including Lin28A and Lin28B) is a type of
protein that binds RNA and is involved in the processes of cell
growth, tumorigenesis and tissue inflammation (17–19). Lin28
binds with miRNA precursors and regulates the biosynthesis of
miRNA, particularly the let-7 family miRNAs; therefore, Lin28 is
the post-transcriptional inhibitor of let-7 (20). It was previously demonstrated that,
inhibiting the expression of Lin28 may increase the synthesis of
let-7 and reduce the expression of K-Ras, leading to high
radiosensitivity of lung cancer cells (21). Therefore, Lin28-let-7 may be a
regulatory site of overcoming the low tumor radiosentivity caused
by activated Ras signaling. In addition, Iliopoulos et al
(19) found that nuclear factor
(NF)-κB may directly stimulate the transcription of Lin28 and
decrease let-7 miRNA levels, leading to high interleukin (IL)-6
levels. Although the mechanisms underlying the association between
Lin28 and inflammatory response are not clear, we may infer that
Lin28 plays an important role in inflammatory response based on the
regulation of IL-6 expression.
A previous study evaluated the association between
Lin28 polymorphisms and the risk of grade ≥3 RP in 362 cases of
non-small-cell lung cancer (NSCLC) patients receiving definitive
radiotherapy (22). Lin28B rs314280
(G>A) AG/AA (HR=2.23, 95% CI: 1.01–4.94, P=0.048) and rs314276
(C>A) AC/AA (HR=2.00, 95% CI: 1.11–3.62, P=0.022) are risk
genotypes of grade ≥3 RP. Among the treatment-related factors, only
mean lung dose (MLD) was found to be associated with the occurrence
of grade ≥3 RP. The highest-risk patients were those with the two
risk genotypes and MLD ≥19.0 Gy.
Pro- and anti-inflammatory genes
There are numerous pro- and anti-inflammatory
cytokines in the human body. Whether the inflammation occurs and to
what severity, depends on the balance between the two types of
cytokines. Thus, damage caused by radiotherapy, such as RP, likely
results from the interaction between pro- and anti-inflammatory
cytokines. Hildebrandt et al (23) investigated 59 SNPs in 37
inflammation-related genes and found that 12 SNPs were associated
with RP, including 7 SNPs in pro-inflammatory genes and 5 SNPs in
anti-inflammatory genes (Table I).
These SNPs were all associated with an increased risk of RP, with
the exception of nitric oxide synthase 3 (NOS3) rs1799983. The
study also demonstrated a dose-related effect in
inflammation-related SNPs. The higher the number of risk genotypes
a patient carries, the higher the risk for RP. Another study also
identified the association between inflammation-related SNPs and
toxicity following radiotherapy in NSCLC patients (24) by evaluating 11,930 SNPs in 904
inflammation-related genes. Following double screening of the
discovery and validation phases and polygenic risk score analysis,
they observed that DEAD box polypeptide 58 rs11795343 affected the
risk of RP. However, the specific mechanisms underlying the
association between inflammation-related SNPs and RP remain
unclear.
 | Table I.Effects of gene polymorphisms on the
risk of radiation pneumonitis. |
Table I.
Effects of gene polymorphisms on the
risk of radiation pneumonitis.
| Gene | SNP ID | Base change | No. | Ethnic group | Treatment | Tumor type
(stage) | End point | Impact on RP | Refs. |
|---|
| TGF-β1 | rs1982073 | T>C | 164 | 77.4% Caucasian,
16.5% black, 6.1% other | RT, RCT | NSCLC (I–IV) | Grade ≥2 and grade
≥3 | CT/CC decreased
risk | (14) |
|
|
|
| 179 | Chinese | RT, RCT | NSCLC (I–IV) | Grade ≥2 | No significant
correlation | (15) |
|
|
|
| 167 | Chinese | RT, RCT | NSCLC (I–IV) | Grade ≥3 | No significant
correlation | (16) |
|
| rs11466345 | A>G | 167 | Chinese | RT, RCT | NSCLC (I–IV) | Grade ≥3 | AG/GG increased
risk | (16) |
| Lin28B | rs314280 | G>A | 362 | 82.0% Caucasian,
18.0% black | RT, RCT | NSCLC (I–IV) | Grade ≥3 | AG/AA increased
risk | (22) |
|
| rs314276 | C>A | 362 | 82.0% Caucasian,
18.0% black | RT, RCT | NSCLC (I–IV) | Grade ≥3 | AC/AA increased
risk | (22) |
| NOS3 | rs1799983 | G>T | 173 | Non-Hispanic
Caucasian | RT, RCT | NSCLC (IIIA and
IIIB) | Grade ≥2 | Decreased risk | (23) |
| IL1A | rs1800587 | C>T |
|
|
|
|
| Increased risk |
|
|
| rs17561 | G>T |
|
|
|
|
|
|
|
| IL8 | rs4073 | T>A |
|
|
|
|
|
|
|
| TNFRSF1B | rs1061622 | T>G |
|
|
|
|
|
|
|
| MIF | rs755622 | C>G |
|
|
|
|
|
|
|
| TNF | rs1799724 | C>T |
|
|
|
|
|
|
|
| IL4 | rs2243250 | C>T |
|
|
|
|
|
|
|
|
| rs2070874 | C>T |
|
|
|
|
|
|
|
| IL13 | rs20541 | C>T |
|
|
|
|
|
|
|
|
| rs180925 | C>T |
|
|
|
|
|
|
|
| NFKBIA | rs8904 | C>T |
|
|
|
|
|
|
|
| ATM | rs189037 | G>A | 253 | Chinese | RT, RCT | LC (I–IV) | Grade ≥2 | GA/AA increased
risk | (33) |
|
|
|
| 362 | 82% non-Hispanic
Caucasian, 17.7% black, 0.3% other | RT, RCT | NSCLC (I–IV) | Grade ≥3 | GA/GG decreased
risk | (35) |
|
| rs228590 | C>T | 362 | 82% non-Hispanic
Caucasian, 17.7% black, 0.3% other | RT, RCT | NSCLC (I–IV) | Grade ≥3 | CT/TT decreased
risk | (35) |
|
| rs373759 | G>A | 253 | Chinese | RT, RCT | LC (I–IV) | Grade ≥2 | GA/AA increased
risk | (33) |
| p53 | rs1042522 | G>C | 253 | Chinese | RT, RCT | LC (I–IV) | Grade ≥2 | Arg/Arg increased
risk | (34) |
| RAD51 | rs1801320 | G>C | 196 | 71.9% Caucasian,
21.4% black, 6.7% other | RT, RCT | NSCLC (I–IV) | Grade ≥1 | CG/CC decreased
risk | (37) |
| LIG4 | rs1805388 | C>T | 195 | 71.3% Caucasian,
21.0% black, 7.7% other | RT, RCT | NSCLC (I–IV) | Grade ≥3 | CT/TT increased
risk | (38) |
| XRCC1 | rs25487 | A>G | 165 | 72.7% Caucasian,
19.4% black, 7.9% other | RT, RCT | NSCLC (I–III) | Grade ≥2 | AA decreased
risk | (40) |
| APEX1 | rs1130409 | T>G | 165 | 72.7% Caucasian,
19.4% black, 7.9% other | RT, RCT | NSCLC (I–III) | Grade ≥2 | GG increased
risk | (40) |
|
|
|
| 126 | Chinese | RT, RCT | NSCLC (I–IV) | Grade ≥3 | GG/GT increased
risk | (41) |
| NEIL1 | rs4462560 | C>G | 187 | Chinese | RT, RCT | ESCC (I–IV) | Grade ≥2 | GC/CC decreased
risk | (43) |
| MTHFR | rs1801131 | A>C | 136 | Caucasian | RT, RCT,
surgery | LC (IIB-IV) | Grade ≥2 and grade
≥3 | AC/CC decreased
risk | (48) |
| HSPB1 | rs2868371 | C>G | 271 | 80% Caucasian, 20%
other | RT, RCT | NSCLC (I–IV) | Grade ≥2 and grade
≥3 | CC increased
risk | (53) |
| VEGF | rs2010963 | G>C | 195 | 71.3% Caucasian,
21.0% black, 7.7% other | RT, RCT | NSCLC (I–IV) | Grade ≥3 | CC increased
risk | (60) |
|
| rs3025039 | C>T |
|
| RT, RCT | NSCLC (I–IV) | Grade ≥3 | TT increased
risk |
|
DNA repair-related genes
Ionizing radiation may cause DNA damage, including
DNA strand breaks, base change, ribose destruction and dimer
formation. Radiotherapy-induced DNA damage mainly consists of
strand breaks and base alterations; therefore, the repair pathways
involved in DNA damage are DNA double-strand break repair (DSBR)
and base excision repair (BER) (25).
The genetic variants of DNA repair-related genes may affect the
capacity of the DNA repair pathways. Insufficient DNA repair
capacity leads to increased DNA damage and high tissue
radiosensitivity, resulting in severe radiation-related
complications (26,27).
DNA DSBR genes
The ataxia telangiectasia mutated (ATM) gene is
located on chromosome 11q22–23 and its mutations lead to
ataxia-telangiectasia. The ATM protein is the main receptor for
radiation-induced DNA injury that may detect and repair DNA DSBs
and plays an important role in the DSBR pathway (28,29).
Furthermore, the ATM protein is a type of serine/threonine kinase,
which may phosphorylate several intermediates involved in cellular
stress responses, modulation of cell cycle regulation point and
apoptosis (30).
Studies in vivo and in vitro
demonstrated that ATM heterozygosity or decreased expression may
cause high radiosensitivity among individuals or cells (31,32); thus,
ATM may be a key checkpoint of radiosensitivity. In 2009, Zhang
et al (33) found that ATM
rs189037 (G>A) and rs373759 (G>A) exhibited a significant
correlation with grade ≥2 RP in Chinese patients (n=253) and the
two SNPs are in linkage disequilibrium. ATM rs189037 is located in
the core region of ATM promoter and its GA/AA genotypes may cause a
decline in ATM mRNA expression, resulting in hypersensitivity to
radiation and increased risk of RP. In addition, this research team
assessed the association between p53 gene polymorphisms and the
risk of RP; by using ATM rs189037 and P53 as genetic markers, they
were able to predict 63.2% of the patients with RP following
radiotherapy (34). Xiong et
al (35) demonstrated that ATM
rs189037 AG/GG, rs228590 CT/TT and rs189037G/rs228590T/rs1801516G
(G-T-G) haplotype exerted a negative effect on grade ≥3 RP in both
univariate and multivariate analyses in Caucasians. However, there
was no statistically significant association between the ATM
rs189037 and the risk of grade ≥2 RP. This result conflicted with
the findings of Zhang et al (33), who found ATM rs189037 to have a
significant correlation with grade ≥2 RP, mainly because the
variant allele frequencies and the incidence of severe RP were
different between Chinese and non-Hispanic whites.
DNA DSB repair has two main pathways: Homologous
recombination (HR) and non-homologous end-joining (NHEJ) (36). Thus, the genetic variants involved in
the two pathways were considered to be associated with the risk of
RP. Yin et al (37) found that
the HR gene RAD51 rs1801320 (G>C) C allele was associated with a
lower risk of grade ≥1 RP (HR=0.52, 95% CI: 0.31–0.86, P=0.010)
compared with the GG genotype, and the NHEJ gene DNA ligase 4
(LIG4) rs1805388 (C>T) T allele increased the risk of severe RP
(HR=1.96, 95% CI: 1.00–3.85, P=0.048) in patients with NSCLC
(38).
BER genes
DNA BER is the major repair pathway of DNA
single-strand breaks, including the apurinic/apyrimidinic (AP) site
break and DNA base injury caused by radiation (25). The main enzymes involved in this
pathway are DNA glycosylase, AP endonuclease, DNA polymerase and
DNA ligase. AP endonuclease 1 (APEX1) may detect and incise the AP
sites in the early stages of DNA damage and plays a role in the
inflammatory response by regulating NF-κB (39). X-ray repair cross-complementing 1
(XRCC1) usually forms a complex with poly(ADP-ribose) polymerase,
DNA ligase 3 and DNA polymerase β, connects and fills the DNA
incision in the final stage. Yin et al (40) reported that the XRCC1 rs25487 (A>G)
AA genotype was associated with a low risk of grade ≥2 RP (HR=0.48,
95% CI: 0.24–0.97, P=0.041), whereas the APEX1 rs1130409 (T>G)
GG genotype was associated with an increased risk of grade ≥2 RP
(HR=3.61, 95% CI: 1.64–7.93, P=0.001) in whites. In 2014, Li et
al (41) found that the APEX1
rs1130409 G allele was associated with grade ≥3 RP, verifying the
correlation between APEX1 SNPs and the risk of severe RP. The grade
endpoints of the abovementioned studies may have been discrepant
due to the gene heterogeneity and differences in inflammation
sensitivity among different races, but the specific mechanisms
remain unclear.
Nei endonuclease VIII-like 1 (NEIL1) is one of the
genes encoding the human DNA glycosylase involved in the first
reaction of BER. NEIL1 may combine with deoxyuridylate to repair
DNA damage induced by thymidylate synthesis inhibition (42). It was previously suggested that NEIL1
rs4462560 (C>G) GC/CC genotypes may reduce the risk of grade ≥2
RP in patients with esophageal squamous cell carcinoma (43).
Stress response-related genes
At the molecular level, reactive oxygen species
induced by ionizing radiation exert a direct damaging effect on DNA
and tissue, leading to DNA DSBs and production of cytokines or
growth factors, which may cause pulmonary hypoxia, inflammation,
chronic oxidative stress and, eventually, damage repair delay
(44–46). In addition to inflammation and DNA
repair-related genes, stress response-related genes may also be
involved in the regulation of the effect of radiation on lung
tissue.
5,10-Methylenetetrahydrofolate reductase (MTHFR)
plays a key role in folate metabolism, thymidine synthesis,
homocysteine processing and other important metabolic pathways
(47). Folate metabolism is closely
related to DNA synthesis and repair and MTHFR is the key enzyme of
redox reaction in cellular metabolic activity, which may
irreversibly convert MTHFR to 5-methyltetrahydrofolate. Mak et
al (48) investigated the
correlation between the SNPs of MTHFR and superoxide dismutase
(SOD) 2 genes and the risk of RP, and demonstrated that the MTHFR
rs1801131 (A>C) AC/CC genotypes decreased the risk of grade ≥2
RP (HR=0.37, 95% CI: 0.18–0.76, P=0.006) and grade ≥3 RP (HR=0.21,
95% CI: 0.06–0.70, P=0.01). No SOD2 gene polymorphisms were found
to be associated with RP risk in this study. Since the number of
candidate SNPs is limited, this study was unable to fully analyze
the correlation between these two genes and RP risk.
Heat shock proteins (HSPs) may be stimulated by
several stressors, such as drugs or ionizing radiation, and protect
the body from the injury caused by these stressors. The HSP27
protein is widely expressed in the human body at a low level. HSP27
strengthens the cells' ability of resistance when they are exposed
to oxidative stress, cytotoxic agents, thermal shock and apoptosis
(49–51). Furthermore, HSP27 chaperone may also
enhance the antioxidant capacity of the cell by increasing
glutathione and decreasing the toxicity of oxidizing reactions
(52). The plasma HSP27 levels are
regulated by the HSPB1 gene; thus, HSPB1 was considered to modulate
cell radiosensitivity. Pang et al (53) demonstrated that the HSPB1 rs2868371
(C>G) CC genotype was associated with a higher risk of grade ≥3
RP compared with the CG/GG genotype (P=0.02).
Angiogenesis-related genes
Angiogenesis is not only an important physiological
process in normal tissues, but also a necessary step in
carcinogenesis, cancer development and metastasis (54–56).
Pro-angiogenic substances excreted by tumor cells and tumor stromal
cells may promote tumor revascularization. Reactive oxygen species
generated by radiation therapy result in vascular endothelial cell
damage in normal lung tissues and lead to early inflammation and
high vascular permeability (45,57,58).
Subsequently, white blood cells migrate to the sites of
inflammation, a series of inflammatory reactions occur and lead to
increased radiation toxicity in the surrounding normal tissues.
Vascular endothelial growth factor (VEGF) exerts a dual effect on
the occurrence of RP: High VEGF levels stimulate the growth of
endothelial cells and maintain the integrity of vascular
endothelium, which may enhance the resistance to RP; on the other
hand, VEGF may increase the synthesis of inflammatory cytokines
through NF-κB in the damaged endothelial cells, leading to RP
(59). VEGF SNPs were analyzed by Yin
et al (60), who observed that
the rs2010963 (G>C) CC and rs3025039 (C>T) TT genotypes were
associated with a high risk of RP in 195 NSCLC patients.
Summary and radiogenomics
The recent studies on the effects of gene
polymorphisms on RP risk are summarized in Table I and Fig.
1 outlines the mechanisms, pathways and genes involved. These
studies, however, had certain limitations: First, they were all
retrospective studies based on recorded information; thus, it was
difficult to accurately classify the severity of the RP. Second,
the diagnosis of RP was subjective, whereas RP should be
differentially diagnosed from chronic obstructive pulmonary
disease, infectious pneumonia and heart disease, taking into
consideration the patients' overall condition. Therefore, the
diagnosis of RP requires experienced physicians. The standard used
for RP grading in the abovementioned articles was the National
Cancer Institute's Common Terminology Criteria for Adverse Events
(CTCAE), version 3.0 or 4.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
or http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
However, other researchers raised the viewpoint that the use of an
objective evaluation method may more accurately determine the
degree of radiation-induced toxicity, as it may avoid subjective
confounders (61,62). Third, the majority of the studies
adopted the candidate gene approach, with which was easy to
overlook the crucial but rare SNPs. In addition, the sample size
was relatively small, which may may have led to a high
false-positive rate. Finally, some independent factors, which were
shown in previous articles, should be kept consistent in the study
to avoid adversely affecting the results, including smoking, MLD,
and volume of normal lung receiving ≥20Gy radiation.
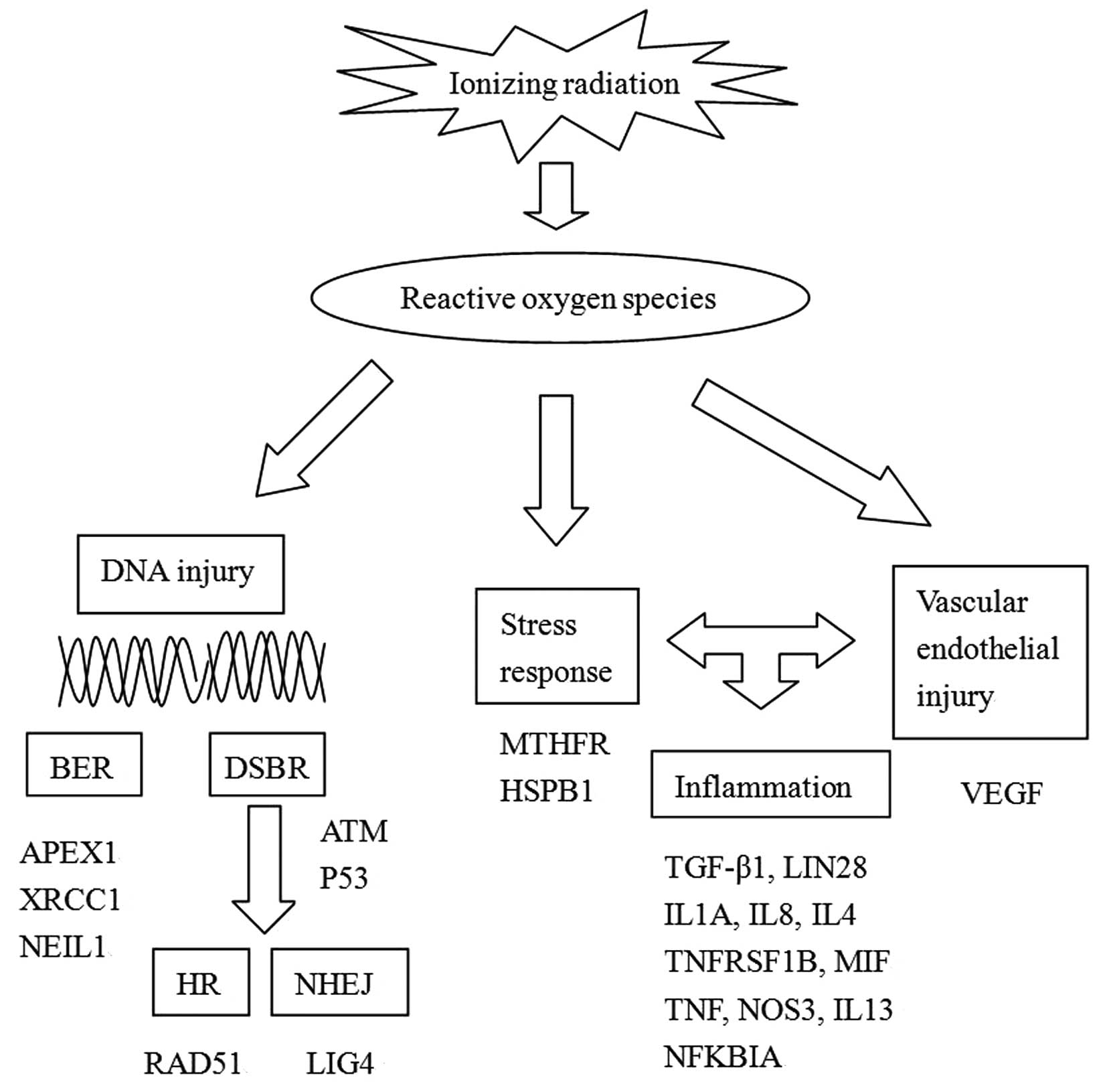 | Figure 1.Mechanisms, pathways and genes
involved in RP and gene polymorphisms. BER, base excision repair;
DSBR, DNA double-strand break repair; APEX1, apurinic/apyrimidinic
endonuclease 1; XRCC1, X-ray repair cross-complementing 1; NEIL1,
nei endonuclease VIII-like 1; ATM, ataxia telangiectasia mutated;
HR, homologous recombination; NHEJ, non-homologous end-joining;
LIG4, DNA ligase 4; MTHFR, 5,10-methylenetetrahydrofolate
reductase; HSPB1, heat shock proteins B1; VEGF, vascular
endothelial growth factor; TGF-β1, transforming growth factor-β1;
Lin28, abnormal cell lineage protein 28; TNF, tumor necrosis
factor; TNFRSF1B, TNF receptor superfamily 1B; MIF, macrophage
migration inhibitory factor; NOS3, nitric oxide synthase 3; NFKBIA,
nuclear factor of κ-light polypeptide gene enhancer in B-cells
inhibitor-α. |
Radiogenomics analyzes the differences in
radiosensitivity caused by gene sequence variations (63). Radiogenomics has two objectives: The
first is to determine a way of predicting the risk of radiation
injuries in patients following radiotherapy, and the second is to
investigate the molecular mechanisms underlying normal tissue
toxicity induced by radiation (64).
Thus, radiogenomics may help achieve treatment individualization
for patients treated with radiotherapy. In 2006, the RAPPER study
(Radiogenomics Assessment of Polymorphisms for Predicting the
Effects of Radiotherapy), which was the first nationwide radiation
genomics program worldwide, was launched in UK (65). In 2009, the International
Radiogenomics Consortium was founded, dedicated to the study of
genetic predictors of adverse reactions following radiotherapy in
various types of tumors (66).
Genome-wide association study (GWAS) is an approach
to radiogenomics research. GWASs do not miss important SNPs, as
they analyze SNPs with a minor allele frequency of ≥1% over the
entire gene (64). Due to the large
number of SNPs, it is necessary to enlarge the sample size or
validate in replication studies to obtain a higher statistical
power (67). Several SNPs identified
in GWASs are located in non-coding regions with unknown functions
(68,69), which may broaden our understanding of
the mechanisms underlying normal tissue toxicity caused by
radiation. Despite GWASs being effective in SNP genotyping, they
are less efficient in distinguishing changes in DNA structure, such
as inversions, deletions and insertions, which may exert an effect
on the response to radiation (70,71).
Currently available published GWAS studies mainly investigate the
complications following radiotherapy in breast and prostate cancer
patients, with only few studies on lung cancer.
Conclusion
Through accurately evaluating the patients'
sensitivity to radiation and effectively predicting the occurrence
of RP, we may determine an optimal individualized treatment plan
for each patient. For patients at high risk of developing RP,
non-radiotherapy or low-dose radiation therapy may be applied to
achieve a relatively high radiation dose based on the low risk of
RP. For low-risk patients, the radiation dose may be increased to
achieve the best therapeutic effect. At present, preliminary
studies have demonstrated that genetic polymorphisms are closely
associated with RP and radiation sensitivity, but the specific
molecular mechanisms remain unclear. With the development of
radiogenomics and the promotion of GWAS, the molecular mechanisms
of gene polymorphisms and radiosensitivity are major issues that
must be addressed in the future, as they may provide a reliable
molecular basis for the personalized therapy of malignant
tumors.
Acknowledgements
This study was supported by grants from the National
Scientific Foundation of China (nos. 81460409 and 81301924), the
Scientific Foundation of Hunan Province (no. 14JJ7016) and the
Science and Technology Plan of Changsha (no. k1403065-31).
Glossary
Abbreviations
Abbreviations:
|
RP
|
radiation pneumonitis
|
|
SNPs
|
single-nucleotide polymorphisms
|
|
BER
|
base excision repair
|
|
DSBR
|
DNA double-strand break repair
|
|
HR
|
homologous recombination
|
|
NHEJ
|
non-homologous end-joining
|
|
NSCLC
|
non-small-cell lung cancer
|
References
|
1
|
Arpin D, Mahé MA, Servois V and Claude L:
Predictive factors for acute radiation pneumonitis. Rev Pneumol
Clin. 65:177–186. 2009.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamashita H, Nakagawa K, Nakamura N, et
al: Exceptionally high incidence of symptomatic grade 2–5 radiation
pneumonitis after stereotactic radiation therapy for lung tumors.
Radiat Oncol. 2:212007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang XJ, Sun JG, Sun J, et al: Prediction
of radiation pneumonitis in lung cancer patients: A systematic
review. J Cancer Res Clin Oncol. 138:2103–2116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giuliani ME, Lindsay PE, Kwan JY, et al:
Correlation of dosimetric and clinical factors with the development
of esophagitis and radiation pneumonitis in patients with
limited-stage small-cell lung carcinoma. Clin Lung Cancer.
16:216–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dang J, Li G, Zang S, Zhang S and Yao L:
Comparison of risk and predictors for early radiation pneumonitis
in patients with locally advanced non-small cell lung cancer
treated with radiotherapy with or without surgery. Lung Cancer.
86:329–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsujino K, Hashimoto T, Shimada T, et al:
Combined analysis of V20, VS5, pulmonary fibrosis score on baseline
computed tomography and patient age improves prediction of severe
radiation pneumonitis after concurrent chemoradiotherapy for
locally advanced non-small-cell lung cancer. J Thorac Oncol.
9:983–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dang J, Li G, Zang S, Zhang S and Yao L:
Risk and predictors for early radiation pneumonitis in patients
with stage III non-small cell lung cancer treated with concurrent
or sequential chemoradiotherapy. Radiat Oncol. 9:1722014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siva S, MacManus M, Kron T, et al: A
pattern of early radiation-induced inflammatory cytokine expression
is associated with lung toxicity in patients with non-small cell
lung cancer. PLoS One. 9:e1095602014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnston CJ, Williams JP, Elder A, Hernady
E and Finkelstein JN: Inflammatory cell recruitment following
thoracic irradiation. Exp Lung Res. 30:369–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pappas PJ, You R, Rameshwar P, et al:
Dermal tissue fibrosis in patients with chronic venous
insufficiency is associated with increased transforming growth
factor-beta1 gene expression and protein production. J Vasc Surg.
30:1129–1145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi W, Twigg S, Chen X, et al: Integrated
actions of transforming growth factor-beta1 and connective tissue
growth factor in renal fibrosis. Am J Physiol Renal Physiol.
288:F800–F809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oberringer M, Meins C, Bubel M and
Pohlemann T: In vitro wounding: Effects of hypoxia and transforming
growth factor beta1 on proliferation, migration and myofibroblastic
differentiation in an endothelial cell-fibroblast co-culture model.
J Mol Histol. 39:37–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anscher MS, Kong FM, Αndrews K, et al:
Plasma transforming growth factor beta1 as a predictor of radiation
pneumonitis. Int J Radiat Oncol Biol Phys. 41:1029–1035. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan X, Liao Z, Liu Z, et al: Single
nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is
associated with the risk of radiation pneumonitis in patients with
non-small-cell lung cancer treated with definitive radiotherapy. J
Clin Oncol. 27:3370–3378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L and Bi N: TGF-beta1 gene
polymorphisms for anticipating radiation-induced pneumonitis in
non-small-cell lung cancer: Different ethnic association. J Clin
Oncol. 28:e621–e622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niu X, Li H, Chen Z, et al: A study of
ethnic differences in TGFβ1 gene polymorphisms and effects on the
risk of radiation pneumonitis in non-small-cell lung cancer. J
Thorac Oncol. 7:1668–1675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin R, Zhou J, Chen C, et al: LIN28 is
involved in glioma carcinogenesis and predicts outcomes of
glioblastoma multiforme patients. PLoS One. 9:e864462014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Zhu L, Wang R, et al: Genetic
variants in let-7/Lin28 modulate the risk of oral cavity cancer in
a Chinese Han population. Sci Rep. 4:74342014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iliopoulos D, Hirsch HA and Struhl K: An
epigenetic switch involving NF-kappaB, Lin28, Let-7 microRNA and
IL6 links inflammation to cell transformation. Cell. 139:693–706.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayr F and Heinemann U: Mechanisms of
Lin28-mediated miRNA and mRNA regulation - a structural and
functional perspective. Int J Mol Sci. 14:16532–16553. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oh JS, Kim JJ, Byun JY and Kim IA:
Lin28-let7 modulates radiosensitivity of human cancer cells with
activation of K-Ras. Int J Radiat Oncol Biol Phys. 76:5–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wen J, Liu H, Wang Q, et al: Genetic
variants of the LIN28B gene predict severe radiation pneumonitis in
patients with non-small cell lung cancer treated with definitive
radiation therapy. Eur J Cancer. 50:1706–1716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hildebrandt MA, Komaki R, Liao Z, et al:
Genetic variants in inflammation-related genes are associated with
radiation-induced toxicity following treatment for non-small cell
lung cancer. PLoS One. 5:e124022010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pu X, Wang L, Chang JY, et al:
Inflammation-related genetic variants predict toxicity following
definitive radiotherapy for lung cancer. Clin Pharmacol Ther.
96:609–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hubenak JR, Zhang Q, Branch CD and
Kronowitz SJ: Mechanisms of injury to normal tissue after
radiotherapy: A review. Plast Reconstr Surg. 133:49e–56e. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hornhardt S, Rößler U, Sauter W, et al:
Genetic factors in individual radiation sensitivity. DNA repair
(Amst). 16:54–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patrono C, Sterpone S, Testa A and Cozzi
R: Polymorphisms in base excision repair genes: Breast cancer risk
and individual radiosensitivity. World J Clin Oncol. 5:874–882.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shiloh Y: ATM and related protein kinases:
Safeguarding genome integrity. Nat Rev Cancer. 3:155–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su D, Ma S, Liu P, et al: Genetic
polymorphisms and treatment response in advanced non-small cell
lung cancer. Lung Cancer. 56:281–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bakkenist CJ and Kastan MB: DNA damage
activates ATM through intermolecular autophosphorylation and dimer
dissociation. Nature. 421:499–506. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Worgul BV, Smilenov L, Brenner DJ, Junk A,
Zhou W and Hall EJ: Atm heterozygous mice are more sensitive to
radiation-induced cataracts than are their wild-type counterparts.
Proc Natl Acad Sci USA. 99:9836–9839. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang SC, Wu CC, Wei YY, Hong JH and Chiang
CS: Inactivation of ataxia telangiectasia mutated gene can increase
intracellular reactive oxygen species levels and alter
radiation-induced cell death pathways in human glioma cells. Int J
Radiat Biol. 87:432–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Yang M, Bi N, et al: ATM
polymorphisms are associated with risk of radiation-induced
pneumonitis. Int J Radiat Oncol Biol Phys. 77:1360–1368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang M, Zhang L, Bi N, et al: Association
of P53 and ATM polymorphisms with risk of radiation-induced
pneumonitis in lung cancer patients treated with radiotherapy. Int
J Radiat Oncol Biol Phys. 79:1402–1407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiong H, Liao Z, Liu Z, et al: ATM
polymorphisms predict severe radiation pneumonitis in patients with
non-small cell lung cancer treated with definitive radiation
therapy. Int J Radiat Oncol Biol Phys. 85:1066–1073. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Srivastava M and Raghavan SC: DNA
double-strand break repair inhibitors as cancer therapeutics. Chem
Biol. 22:17–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yin M, Liao Z, Huang YJ, et al:
Polymorphisms of homologous recombination genes and clinical
outcomes of non-small cell lung cancer patients treated with
definitive radiotherapy. PLoS One. 6:e200552011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin M, Liao Z, Liu Z, et al: Genetic
variants of the nonhomologous end joining gene LIG4 and severe
radiation pneumonitis in nonsmall cell lung cancer patients treated
with definitive radiotherapy. Cancer. 118:528–535. 2012.
|
|
39
|
Tell G, Fantini D and Quadrifoglio F:
Understanding different functions of mammalian AP endonuclease
(APE1) as a promising tool for cancer treatment. Cell Mol Life Sci.
67:3589–3608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yin M, Liao Z, Liu Z, et al: Functional
polymorphisms of base excision repair genes XRCC1 and APEX1 predict
risk of radiation pneumonitis in patients with non-small cell lung
cancer treated with definitive radiation therapy. Int J Radiat
Oncol Biol Phys. 81:e67–e73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Liu G, Xia L, et al: A polymorphism
in the DNA repair domain of APEX1 is associated with the
radiation-induced pneumonitis risk among lung cancer patients after
radiotherapy. Br J Radiol. 87:201400932014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Taricani L, Shanahan F, Pierce RH, Guzi TJ
and Parry D: Phenotypic enhancement of thymidylate synthetase
pathway inhibitors following ablation of Neil1 DNA
glycosylase/lyase. Cell Cycle. 9:4876–4883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Y, Zhu M, Zhang Z, et al: A NEIL1
single nucleotide polymorphism (rs4462560) predicts the risk of
radiation-induced toxicities in esophageal cancer patients treated
with definitive radiotherapy. Cancer. 119:4205–4211. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cadet J and Wagner JR: DNA base damage by
reactive oxygen species, oxidizing agents and UV radiation. Cold
Spring Harb Perspect Biol. 5:a0125592013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stankova J, Lawrance AK and Rozen R:
Methylenetetrahydrofolate reductase (MTHFR): A novel target for
cancer therapy. Curr Pharm Des. 14:1143–1150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mak RH, Alexander BM, Asomaning K, et al:
A single-nucleotide polymorphism in the methylene tetrahydrofolate
reductase (MTHFR) gene is associated with risk of radiation
pneumonitis in lung cancer patients treated with thoracic radiation
therapy. Cancer. 118:3654–3665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mymrikov EV, Seit-Nebi AS and Gusev NB:
Large potentials of small heat shock proteins. Physiol Rev.
91:1123–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Laskowska E: Small heat shock
proteins-role in apoptosis, cancerogenesis and diseases associated
with protein aggregation. Postepy Biochem. 53:19–26. 2007.(In
Polish). PubMed/NCBI
|
|
51
|
Schmid TE and Multhoff G:
Radiation-induced stress proteins - the role of heat shock proteins
(HSP) in anti-tumor responses. Curr Med Chem. 19:1765–1770. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Arrigo AP, Virot S, Chaufour S, et al:
Hsp27 consolidates intracellular redox homeostasis by upholding
glutathione in its reduced form and by decreasing iron
intracellular levels. Antioxid Redox Signal. 7:414–422. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pang Q, Wei Q, Xu T, et al: Functional
promoter variant rs2868371 of HSPB1 is associated with risk of
radiation pneumonitis after chemoradiation for non-small cell lung
cancer. Int J Radiat Oncol Biol Phys. 85:1332–1339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Medinger M and Passweg J: Angiogenesis in
myeloproliferative neoplasms, new markers and future directions.
Memo. 7:206–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mittal K, Ebos J and Rini B: Angiogenesis
and the tumor microenvironment: Vascular endothelial growth factor
and beyond. Semin Oncol. 41:235–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29(Suppl 16): 15–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lum H and Roebuck KA: Oxidant stress and
endothelial cell dysfunction. Am J Physiol Cell Physiol.
280:C719–C741. 2001.PubMed/NCBI
|
|
58
|
Fisher M: Injuries to the vascular
endothelium: Vascular wall and endothelial dysfunction. Rev Neurol
Dis. 5(Suppl 1): S4–S11. 2008.PubMed/NCBI
|
|
59
|
Anbalagan D, Yap G, Yuan Y, et al:
Annexin-A1 regulates microRNA-26b* and microRNA-562 to directly
target NF-κB and angiogenesis in breast cancer cells. PLoS One.
9:e1145072014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yin M, Liao Z, Yuan X, et al:
Polymorphisms of the vascular endothelial growth factor gene and
severe radiation pneumonitis in non-small cell lung cancer patients
treated with definitive radiotherapy. Cancer Sci. 103:945–950.
2012.
|
|
61
|
Kelsey CR, Jackson L, Langdon S, et al: A
polymorphism within the promoter of the TGFβ1 gene is associated
with radiation sensitivity using an objective radiologic endpoint.
Int J Radiat Oncol Biol Phys. 82:e247–e255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
De Ruysscher D, Sharifi H, Defraene G, et
al: Quantification of radiation-induced lung damage with CT scans:
The possible benefit for radiogenomics. Acta Oncol. 52:1405–1410.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kerns SL, West CM, Andreassen CN, et al:
Radiogenomics: The search for genetic predictors of radiotherapy
response. Future Oncol. 10:2391–2406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kerns SL, Ostrer H and Rosenstein BS:
Radiogenomics: Using genetics to identify cancer patients at risk
for development of adverse effects following radiotherapy. Cancer
Discov. 4:155–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Burnet NG, Barnett GC, Elliott RM,
Dearnaley DP, Pharoah PD, Dunning AM, West CM and Investigators R:
RAPPER Investigators: RAPPER: The radiogenomics of radiation
toxicity. Clin Oncol (R Coll Radiol). 25:431–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
West C, Rosenstein BS, Alsner J, et al:
EQUAL-ESTRO: Establishment of a Radiogenomics Consortium. Int J
Radiat Oncol Biol Phys. 76:1295–1296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Borchiellini D, Etienne-Grimaldi MC,
Thariat J and Milano G: The impact of pharmacogenetics on radiation
therapy outcome in cancer patients. A focus on DNA damage response
genes. Cancer Treat Rev. 38:737–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Barnett GC, Thompson D, Fachal L, et al: A
genome wide association study (GWAS) providing evidence of an
association between common genetic variants and late radiotherapy
toxicity. Radiother Oncol. 111:178–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kerns SL, Stock R, Stone N, et al: A
two-stage genome-wide association study to identify single
nucleotide polymorphisms associated with development of erectile
dysfunction following radiation therapy for prostate cancer. Int J
Radiat Oncol Biol Phys. 85:e21–e28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Manolio TA: Bringing genome-wide
association findings into clinical use. Nat Rev Genet. 14:549–558.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tuzun E, Sharp AJ, Bailey JA, et al:
Fine-scale structural variation of the human genome. Nat Genet.
37:727–732. 2005. View
Article : Google Scholar : PubMed/NCBI
|















