Introduction
Adenomas of the colon and rectum are very common
benign neoplasms, but adenomas of the common bile duct (CBD) are
very rare diseases (1,2). Since bile duct adenomas often cause
obstructive jaundice, patients are suspected to have CBD stones or
malignant neoplasms. Adenomas of the bile duct are essentially
benign tumors, although they are occasionally considered to be
premalignant tumors. Intraductal papillary neoplasms of the bile
duct (IPNB) have been proposed to be the biliary counterpart of
intraductal papillary mucinous neoplasms of the pancreas, and the
processes of the adenoma-to-carcinoma sequence in bile duct
neoplasms have been identified (3–5).
Treatments for bile duct adenoma are necessarily based on
diagnostic results, and local resections of the CBD may be
performed if the distal and proximal cut ends are free from the
tumor, and the tumor is diagnosed to be benign. When a bile duct
resection is insufficient for complete resection, or if a malignant
transformation of the tumor is suspected, consequently,
pancreatoduodenectomy should be considered (5).
Fluorine-18 fluorodeoxyglucose positron emission
tomography (18F-FDG PET) is used for cancer diagnosis
and staging, and is often used for CBD tumors. 18F-FDG
PET is known to have 92.3% sensitivity, and 92.9% specificity, in
the diagnosis of bile duct cancer (6), although whether 18F-FDG PET
is able to differentially discriminate between diagnoses of adenoma
and carcinoma of the bile duct remains to be fully elucidated. In
the present study, a case of bile duct adenoma with low-grade
atypia was reported, demonstrating the uptake of
18F-FDG, which was successfully treated by surgical
resection.
Case report
A 64-year-old man was admitted to hospital with
epigastric discomfort and nausea. He had diabetes mellitus and
hypertension, which were controlled by the use of oral medicines. A
physical examination revealed normal findings in the patient's
abdomen. Laboratory analyses, however, revealed increased levels of
total bilirubin (40.4 µmol/l), direct bilirubin (21.0 µmol/l),
alkaline phosphatase (507 units/l), γ-glutamyl transpeptidase (364
units/l), aspartate aminotransferase (1,578 units/l) and alanine
aminotransferase (1,132 units/l). Tumor markers, including
carcinoembryonic antigen and carbohydrate antigen 19-9, were shown
to be within the normal range. Computerized tomography (CT)
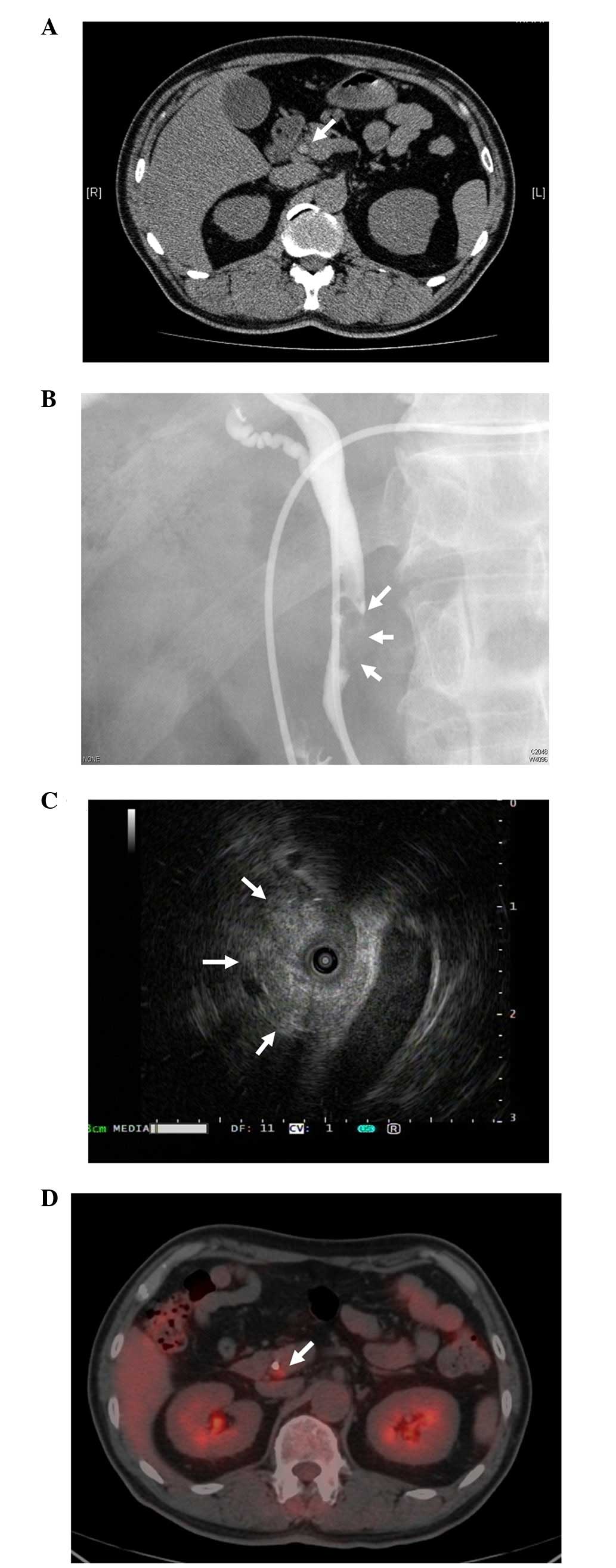
revealed a slight dilation of the CBD, with the identification of a
mass in the distal CBD (Fig. 1A).
Following a diagnosis of obstructive jaundice, endoscopic
nasobiliary drainage was performed after the admission of the
patient. Radiological examinations revealed the presence of a 25 mm
fixed filling defect in the distal CBD, and intraductal
ultrasonography revealed an isoechoic, and partially high echoic,
mass (Figs. 1B and C). A biopsy of
this lesion revealed the presence of tubular adenoma with low-grade
atypia. 18F-FDG PET demonstrated an accumulation of
focal increased tracer in this lesion, with a maximum standard
uptake value (SUVmax) of 3.3, and the position where
uptake of the 18F-FDG occurred was separate from the
drainage tube (Fig. 1D).
On the basis of these findings, a pylorus-preserving
pancreatoduodenectomy and regional lymph-node dissection were
performed. The tumor was impacted in the bile duct lumen, occupying
2.5 cm in length (Fig. 2A).
Histological examinations revealed that the tumor was composed of
relatively uniform tubules, with a bland cellular appearance.
Neither necrotic foci nor mitotic figures were observed.
Furthermore, invasion was not observed in the duct wall, and no
intraductal mass was identified (Fig. 2B
and C). Lymph node metastasis was not detected. A grade B
pancreatic fistula was identified following the surgery, although
the patient was discharged 40 days post-surgery. The patient
remains alive, with no evidence of any recurrence of the tumor, 15
months following the surgery.
Discussion
Benign tumors arising from the extrahepatic biliary
tree are very rare, and are reported to occupy 6% of all tumors of
the bile ducts (2). In benign tumors,
adenomas and papillomas are commonly encountered. Adenomas arise
from the epithelial lining of the biliary duct, and grow in a
tubular, papillary or a tubulopapillary manner. Adenomas of the
bile duct are considered to be premalignant tumors. The
adenoma-to-carcinoma sequence has been well established to occur in
the colon and the rectum, and this also applies in the ampullary
region (7–10). In carcinoma of the ampulla of Vater,
adenomatous areas were revealed to co-exist with high frequency in
>40% of the surgically resected specimens (11,12).
Previously, IPNB have been proposed to be the biliary counterpart
of intraductal papillary mucinous neoplasms of the pancreas
(3,4).
IPNB are a major intraductal neoplasm, which is capable of
progressing to an invasive carcinoma, and the types of
cytoarchitectural atypia in IPNB were characterized as adenoma,
borderline, carcinoma in situ and invasive carcinoma
(3). The development of IPNB was
reported to follow an adenoma-to-carcinoma sequence, which
correlated with the stepwise activation of common oncogenic
pathways, including mutated Kirsten rat sarcoma viral oncogene
homolog, the overexpression of tumor protein 53 and loss of p16
(13). Kim et al (1) summarized the 26 cases of adenomas
arising from CBD, and reported that the histological findings
ranged from adenoma without atypia to carcinoma in situ with
an adenoma component. Therefore, complete resection of the lesion
is required in order to avoid the postoperative development of bile
duct carcinoma.
Appropriate modalities to resect CBD adenoma have
not been clearly defined. Endoscopic resection for bile duct
adenoma has been infrequently reported, and the technique is
considered to be applicable only in a limited number of situations,
for example, for patients for whom surgical resection would pose a
high risk (14,15). Local resection of the CBD may be
performed if the distal and proximal cut ends are free from the
tumor and the tumor is diagnosed to be benign. If the extent of the
bile duct adenoma occupies a range which reaches to the distal CBD
and local resection is impossible, pancreatoduodenectomy should be
considered for complete resection. In adenoma of the bile duct,
predicting the presence of malignant foci preoperatively may be
difficult. Kim et al (1)
reported that radical resection may be required in cases where the
size of the adenoma was >~20 mm, or where malignant
transformation was suspected. In the present case study, the tumor
was located in the distal bile duct in the pancreas, and
consequently, pancreaticoduodectomy was selected as the procedure,
not bile duct resection. Based on the results obtained from the
18F-FDG PET, the regional lymph node was also dissected.
Had the results of the 18F-FDG PET proven to be
negative, lymph node dissection would not have been necessary.
18F-FDG PET has been applied in clinical
practice to detect a wide variety of tumor types, including
lymphoma, lung, esophageal, colon and bile duct cancer (16–19). For
patients with extrahepatic cholangiocarcinoma, 18F-FDG
PET may be used in the diagnosis and staging of the patients
(6,20). Furthermore, Choi EK et al
(21) reported that the
SUVmax value identified from PET-CT scans is a useful
parameter to enable the differentiation of an extrahepatic biliary
malignancy from benign disease. In the meta-analysis, Annunziata
et al (22) reported that the
sensitivity and specificity of 18F-FDG PET were 76 and
74%, respectively, for extrahepatic cholangiocarcinoma. Several
previous studies reported that false-negative results obtained in
cases of 18F-FDG PET were due to the morphology of
extrahepatic cholangiocarcinoma (20,23).
Infiltrative types of cholangiocarcinoma led to discrepancies in
the diagnostic performance due to an insufficient uptake of FDG in
the tumor. However, an explanation of how an uptake of FDG was
observed with benign biliary tumors was not forthcoming, and
neither was it discussed. 18F-FDG PET detects
premalignant colonic adenomas, and a focal FDG accumulation is
detected in >50% of reported cases (24). The degree of FDG uptake was reported
to be correlated with the size of the adenoma, or to the degree of
dysplasia (25). For results obtained
from 18F-FDG-PET of the bile duct adenoma, Dong et
al (26) reported two cases of
IPNB, with uptake of FDG, in 2012. Histological findings revealed
that these cases were adenomas, with a high-grade dysplasia in one
case, and a low-grade dysplasia in the other. The authors reported
that the reason for an uptake of FDG in the adenoma was, primarily,
high-mitotic activity across the entire range, from low-grade to
high-grade dysplasia, and, secondly, the larger tumor size,
composed of a greater number of tumor cells (26). In the present case study, the adenoma
was 25 mm in diameter with low-grade atypia, and it was
hypothesized that the tumor size of the adenoma and the
histological grade of atypia correlated with the extent of FDG
accumulation.
False-positive results obtained with FDG uptake
which are due to inflammatory causes are well recognized.
Wakabayashi et al (27)
reported that, in diagnosing malignant diseases in patients with
biliary stricture, FDG-PET was superior as a method compared with
CT examination in terms of both the sensitivity and the
specificity, and superior to cytological examination of the bile in
terms of its sensitivity. Anderson et al (28) described a false-positive result in a
patient scanned following a cholecystectomy, with FDG uptake
identified during the analysis of residual post-operative
inflammatory changes. In the present case study, the bile duct
drainage tube for obstructive jaundice was already inserted at the
time of PET scan. In a previous report, Choi et al (21) evaluated the clinical value of
18F-FDG PET for differentiating extrahepatic
cholangiocarcinoma from benign disease. In that study, the final
diagnosis was of cholangiocarcinoma in 34 patients, and of benign
disease in five other patients. Of all 39 patients studied, 20 of
them had either an endoscopic or external biliary drainage tube, or
a biliary stent, at the time of the PET scan. Only one patient was
false-positive, with a hyperplastic polyp in the ampulla of Vater,
and the update of FDG was correlated with the drainage tube. In the
present case study, the site of FDG uptake was observed to be
separate from that of the drainage tube. In addition, Kitamura
et al (18) examined the
prognostic value of 18F-FDG PET in extrahepatic bile
duct cancers. The authors reported that no significant correlation
was identified between FDG uptake and the presence of a biliary
drainage tube, or the levels of C-reactive protein. Therefore, it
was not possible to conclude that the presence of the biliary
drainage tube did not affect the measurement of FDG uptake.
In conclusion, a case of bile duct adenoma with
low-grade atypia showing FDG uptake has been reported in the
present study. 18F-FDG PET may be used to detect
premalignant tumors of the bile duct, although whether
18F-FDG PET is able to differentially discriminate
between diagnoses of adenoma and carcinoma of the bile duct remains
to be fully elucidated, and the assessment of further case studies
is required.
References
|
1
|
Kim BS, Joo SH and Joo KR: Carcinoma in
situ arising in a tubulovillous adenoma of the distal common
bile duct: A case report. World J Gastroenterol. 14:4705–4708.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fletcher ND, Wise PE and Sharp KW: Common
bile duct papillary adenoma causing obstructive jaundice: Case
report and review of the literature. Am Surg. 70:448–452.
2004.PubMed/NCBI
|
|
3
|
Abraham SC, Lee JH, Hruban RH, Argani P,
Furth EE and Wu TT: Molecular and immunohistochemical analysis of
intraductal papillary neoplasms of the biliary tract. Hum Pathol.
34:902–910. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zen Y, Fujii T, Itatsu K, Nakamura K,
Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A,
Masuda S, et al: Biliary papillary tumors share pathological
features with intraductal papillary mucinous neoplasm of the
pancreas. Hepatology. 44:1333–1343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim KM, Lee JK, Shin JU, Lee KH, Lee KT,
Sung JY, Jang KT, Heo JS, Choi SH, Choi DW and Lim JH:
Clinicopathologic features of intraductal papillary neoplasm of the
bile duct according to histologic subtype. Am J Gastroenterol.
107:118–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kluge R, Schmidt F, Caca K, Barthel H,
Hesse S, Georgi P, Seese A, Huster D and Berr F: Positron emission
tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and
staging of bile duct cancer. Hepatology. 33:1029–1035. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hasebe T, Sakamoto M, Mukai K, Kawano N,
Konishi M, Ryu M, Fukamachi S and Hirohashi S: Cholangiocarcinoma
arising in bile duct adenoma with focal area of bile duct
hamartoma. Virchows Arch. 426:209–213. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Serafini FM and Carey LC: Adenoma of the
ampulla of Vater: A genetic condition? HPB Surg. 11:191–193. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato T, Konishi K, Kimura H, Maeda K,
Yabushita K, Tsuji M and Miwa A: Adenoma and tiny carcinoma in
adenoma of the papilla of Vater - p53 and PCNA.
Hepatogastroenterology. 46:1959–1962. 1999.PubMed/NCBI
|
|
10
|
Genc H, Haciyanli M, Tavusbay C, Colakoglu
O, Aksöz K, Unsal B and Ekinci N: Carcinoma arising from villous
adenoma of the ampullary bile duct: Report of a case. Surg Today.
37:165–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takashima M, Ueki T, Nagai E, Yao T,
Yamaguchi K, Tanaka M and Tsuneyoshi M: Carcinoma of the ampulla of
Vater associated with or without adenoma: A clinicopathologic
analysis of 198 cases with reference to p53 and Ki-67
immunohistochemical expressions. Mod Pathol. 13:1300–1307. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaiser A, Jurowich C, Schönekäs H,
Gebhardt C and Wünsch PH: The adenoma-carcinoma sequence applies to
epithelial tumours of the papilla of Vater. Z Gastroenterol.
40:913–920. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schlitter AM, Born D, Bettstetter M,
Specht K, Kim-Fuchs C, Riener MO, Jeliazkova P, Sipos B, Siveke JT,
Terris B, et al: Intraductal papillary neoplasms of the bile duct:
Stepwise progression to carcinoma involves common molecular
pathways. Mod Pathol. 27:73–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sturgis TM, Fromkes JJ and Marsh W Jr:
Adenoma of the common bile duct: Endoscopic diagnosis and
resection. Gastrointest Endosc. 38:504–506. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Munshi AG and Hassan MA: Common bile duct
adenoma: Case report and brief review of literature. Surg Laparosc
Endosc Percutan Tech. 20:e193–e194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubota K: From tumor biology to clinical
Pet: A review of positron emission tomography (PET) in oncology.
Ann Nucl Med. 15:471–486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bomanji JB, Costa DC and Ell PJ: Clinical
role of positron emission tomography in oncology. Lancet Oncol.
2:157–164. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitamura K, Hatano E, Higashi T, Seo S,
Nakamoto Y, Narita M, Taura K, Yasuchika K, Nitta T, Yamanaka K, et
al: Prognostic value of (18)F-fluorodeoxyglucose positron emission
tomography in patients with extrahepatic bile duct cancer. J
Hepatobiliary Pancreat Sci. 18:39–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamada H, Hosokawa M, Itoh K, Takenouchi
T, Kinoshita Y, Kikkawa T, Sakashita K, Uemura S, Nishida Y, Kusumi
T and Sasaki S: Diagnostic value of 18F-FDG PET/CT for
lymph node metastasis of esophageal squamous cell carcinoma. Surg
Today. 44:1258–1265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishiyama Y, Yamamoto Y, Kimura N, Miki A,
Sasakawa Y, Wakabayashi H and Ohkawa M: Comparison of early and
delayed FDG PET for evaluation of biliary stricture. Nucl Med
Commun. 28:914–919. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi EK, Yoo IeR, Kim SH, O JH, Choi WH,
Na SJ and Park SY: The clinical value of dual-time point 18F-FDG
PET/CT for differentiating extrahepatic cholangiocarcinoma from
benign disease. Clin Nucl Med. 38:e106–e111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Annunziata S, Caldarella C, Pizzuto DA,
Galiandro F, Sadeghi R, Giovanella L and Treglia G: Diagnostic
accuracy of fluorine-18-fluorodeoxyglucose positron emission
tomography in the evaluation of the primary tumor in patients with
cholangiocarcinoma: A meta-analysis. BioMed Res Int.
2014:2476932014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Albazaz R, Patel CN, Chowdhury FU and
Scarsbrook AF: Clinical impact of FDG PET-CT on management
decisions for patients with primary biliary tumours. Insights
Imaging. 4:691–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Kouwen MC, Nagengast FM, Jansen JB,
Oyen WJ and Drenth JP: 2-(18F)-fluoro-2-deoxy-D-glucose positron
emission tomography detects clinical relevant adenomas of the
colon: A prospective study. J Clin Oncol. 23:3713–3717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yasuda S, Fujii H, Nakahara T, Nishiumi N,
Takahashi W, Ide M and Shohtsu A: 18F-FDG PET detection of colonic
adenomas. J Nucl Med. 42:989–992. 2001.PubMed/NCBI
|
|
26
|
Dong A, Dong H, Zhang L and Zuo C: F-18
FDG uptake in borderline intraductal papillary neoplasms of the
bile duct. Ann Nucl Med. 26:594–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wakabayashi H, Akamoto S, Yachida S, Okano
K, Izuishi K, Nishiyama Y and Maeta H: Significance of
fluorodeoxyglucose PET imaging in the diagnosis of malignancies in
patients with biliary stricture. Eur J Surg Oncol. 31:1175–1179.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anderson CD, Rice MH, Pinson CW, Chapman
WC, Chari RS and Delbeke D: Fluorodeoxyglucose PET imaging in the
evaluation of gallbladder carcinoma and cholangiocarcinoma. J
Gastrointest Surg. 8:90–97. 2004. View Article : Google Scholar : PubMed/NCBI
|