Introduction
Nephrogenic adenoma (NA) is an uncommon benign
lesion of the urothelial mucosa of the urinary tract (1,2). In 1949,
Davis reported the first identified case of NA as a ‘hamartoma of
the urinary bladder’ (3). In 1950,
Fiedman and Kuhlenbeck named this lesion ‘nephrogenic adenoma’
based on its histological similarity to renal tubules (4). It has been reported that NA mainly
arises in the urinary bladder (68.6%) and urethra (13.3%), but also
in the ureter (8.2%), renal pelvis (8.2%) and, rarely, in the
prostate (2%) (5). NAs exhibit a male
predominance, with a male:female ratio of ~3.6:1. When NA occurs in
the female urethra, approximately one-fourth of the cases are
associated with a urethral diverticulum (6,7).
From a study including 134 cases of NA, Lopez
reported that the mean age of NA patients was 66 years, with a
range of 14–96 years (5).
Furthermore, Husainet al reported 18 cases of NA in
pediatric patients, ranging in age from 2 to 19 years (8), whereas Kaoet al reported 21 cases
of pediatric NAs, with an age range of 2–16 years (9). The usual clinical presentation is
hematuria, dysuria and frequency of micturition (10). Several predisposing factors have been
reported to be associated with NA, in adults as well as in
children, including genitourinary trauma, chronic inflammation,
prior surgery, renal calculi, repeated instrumentation, irritated
anatomical anomalies and pelvic irradiation, bacillus
Calmette-Guérin (BCG) immunotherapy and diverticuli (1,8,9,11,12).
We herein present 3 cases of NA arising in the
urinary bladder of elderly male patients.
Case reports
Case 1
A 64-year-old man consulted Toyooka Hospital (Hyogo,
Japan) due to gross hematuria. The patient had undergone
transurethral lithotripsy (TUL) due to a left ureteral stone 6
years prior to the consultation, followed by transurethral
resection of the prostate (TUR-P) for benign prostatic hyperplasia
(BPH) 3 years prior. The patient underwent cystoscopy, which
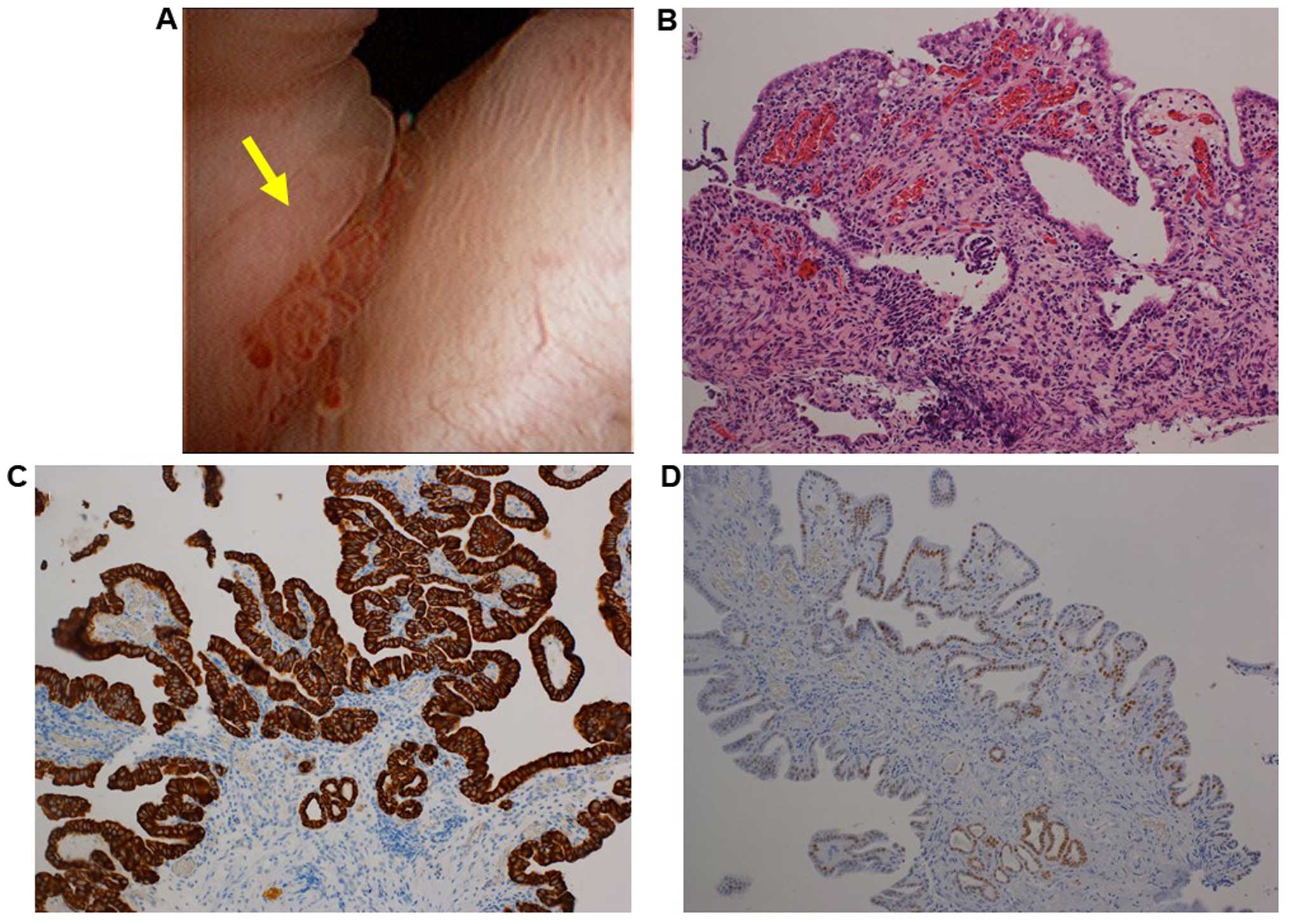
revealed a small papillary lesion in the bladder neck (Fig. 1A). the lesion was resected and
histological examination of the resected lesion revealed papillary
and tubular formations, lined by low cuboidal to flattened
epithelial cells (Fig. 1B). The
epithelial cells were immunohistologically positive for cytokeratin
(CK) 7, paired box (PAX) 8 and P504S, and negative for P63 and
prostate-specific antigen (PSA) (Fig. 1C
and D and data not shown). Therefore, the lesion was diagnosed
as NA. In the 3 years (to date) since the resection of the NA, the
patient has shown no signs of NA recurrence.
Case 2
The urinary bladder of an 82-year-old man had been
examined using a cystoscope at Toyooka Hospital during a follow-up
visit for urothelial carcinoma of the left lateral wall of the
urinary bladder, which had been treated with TUR 1 year prior. The
patient had also been treated with peritoneal dialysis due to
end-stage renal failure from approximately the same time as TUR
onwards. A small sessile papillary lesion was identified in the
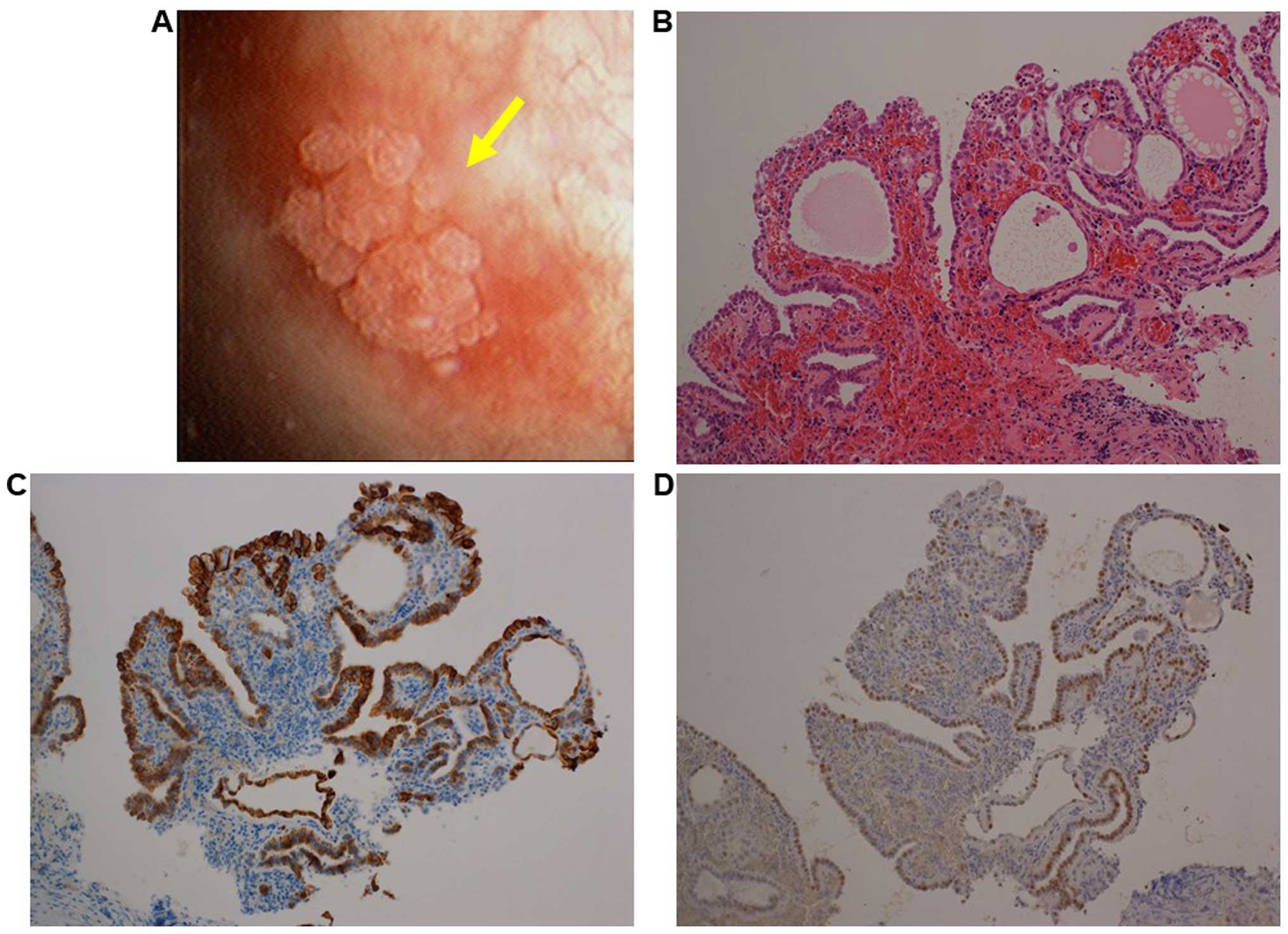
left lateral wall of the urinary bladder (Fig. 2A). The lesion was transurethrally
resected and histological examination revealed a lesion similar to
that described in case 1, mainly composed of papillary and
partially tubular structures lined by low cuboidal to flattened
epithelial cells (Fig. 2B). The
epithelial cells were immunohistologically positive for CK7, PAX8
and P504S, and negative for P63 and PSA (Fig. 2C and D and data not shown). Therefore,
the lesion was diagnosed as NA.
Three years after the TUR for NA the patient
succumbed to candida pneumonia that was unrelated to the NA or
bladder cancer.
Case 3
An 87-year-old man underwent TUR of an urothelial
carcinoma at the trigone of the urinary bladder, followed by
intravesical chemotherapy with mitomycin C at Toyooka Hospital.
Twelve months later, there was a recurrence of the urothelial
carcinoma in the urinary bladder. TUR and intravesical therapy with
BCG were performed. Six months after the recurrence, a papillary
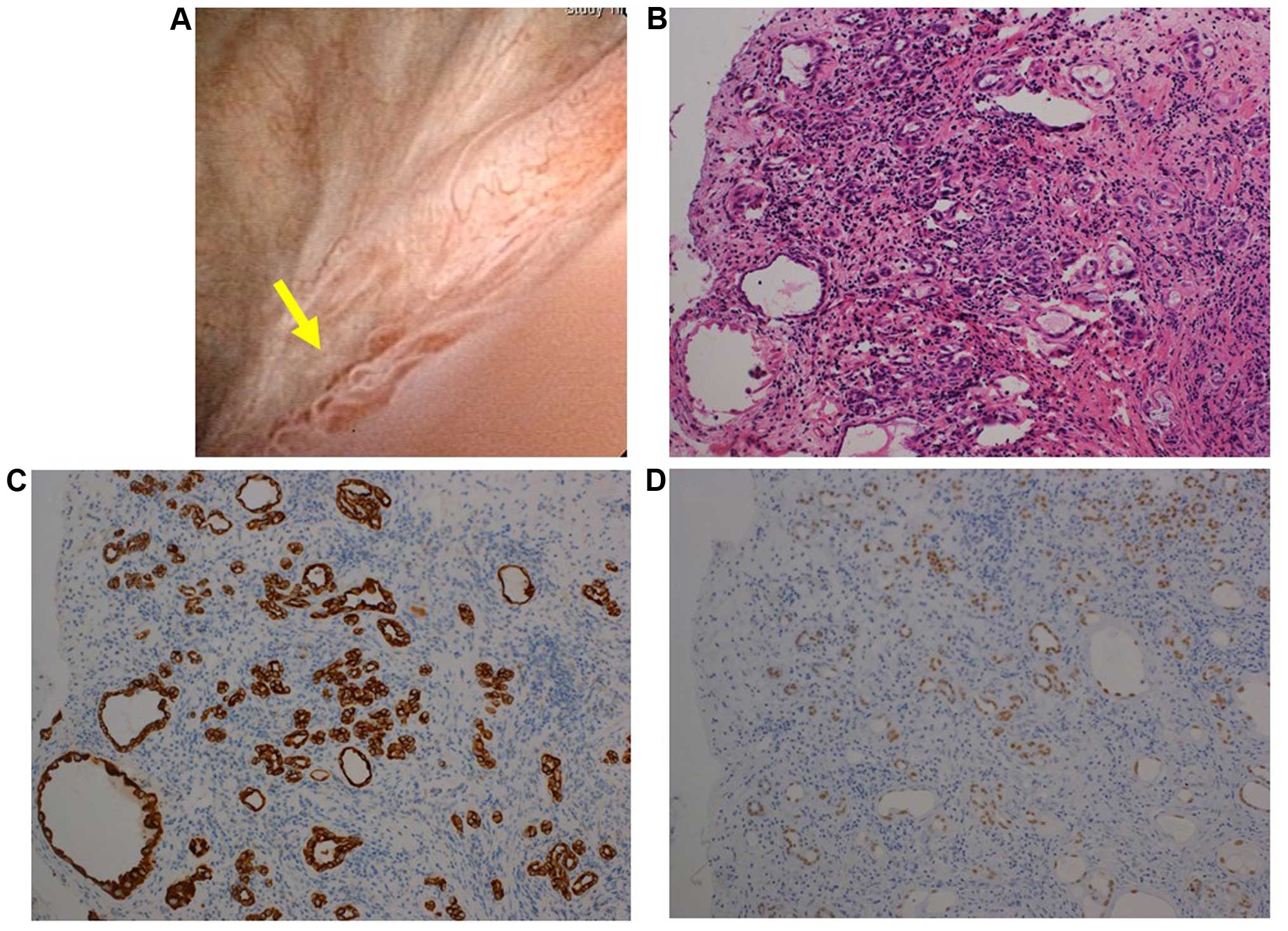
lesion was observed around the right ureteral orifice (Fig. 3A). The lesion was transurethrally
resected. Histologically, part of the lesion exhibited a papillary
pattern, similar to cases 1 and 2, whereas another part exhibited
tubular structures in the interstitial tissue of the urinary
bladder, histologically mimicking invasion by prostate cancer
(Fig. 3B). However,
immunohistochemical staining revealed that the epithelial cells
comprising the tubular structure were positive for CK7 and PAX8,
but not for P63 or PSA (Fig. 3C and D
and data not shown), suggesting the lesion was also a NA.
In the 6 months (to date) since the resection of the
NA, the patient has shown no signs of NA recurrence.
Discussion
Lopezet al analyzed 134 cases of NA with a
mean patient age of 66 years, suggesting that elderly individuals
appear to be more prone to development of NA (5). However, the studies of Husain et
al and Kao et al involved pediatric cases of NA
(8,9).
Several predisposing factors have been reported to be associated
with NA, in adults as well as in children (1,8,9,11,12). Our patients had also undergone various
treatments prior to the development of the NAs; 1 patient was
subjected to TUL due to a ureteral stone and TUR-P due to BPH; 1
patient had received TUR due to urothelial carcinoma in the urinary
bladder; and the third patient had received TUR due to urothelial
carcinoma in the urinary bladder, followed by treatment with BCG.
Regarding the mechanisms underlying the origin of NA, it has been
reported that the NAs arising in female recipients of renal
transplantations from male donors display a male chromosomal status
onin situ hybridization, while NAs arising in the male
recipients of renal transplantations from female donors display a
female chromosomal status (13).
However, the stromal components of NA exhibit the sex chromosome
status of the recipients. These results suggest that epithelial
cells in NA are derived from the renal tubules, such that the
detached epithelial cells in the renal tubules are able to become
engrafted in the injured urinary tract, from which the urothelial
epithelium has disappeared.
It has been reported that the epithelial cells of NA
are similar to those of renal tubules and that the epithelial cells
of NA express PAX2, PAX8 and CK7, but not p63 or PSA (2). The expression pattern of antigens is
similar to that of renal tubules. These results also support the
theory of NAs originating from the renal tubules. All our patients
also exhibited the same patterns of expression of these
antigens.
It has also been reported that NA occasionally
mimics prostatic adenocarcinoma, due to the pseudoinvasive pattern
of small tubules lacking a basal cell layer (14,15). Kunju
presented an image of an NA in the superficial muscle layer of the
urinary bladder (15). In our case 3,
the NA was found in the interstitial tissue in the urinary bladder.
Based on the theory that NA originates in the renal tubules, it is
suggested that epithelial cells from the renal tubules become
embedded in the urothelial tract wall followed by restoration of
the structure of the wall. During the restoration process, the
epithelial cells of the renal tubules may become implanted into the
urothelial tract wall, even as deep as the muscle layer.
In the present study, we presented 3 cases of NA in
elderly male patients with predisposing factors. Since NAs are
benign lesions, it is important to make a definitive differential
diagnosis of NAs based on their morphology, immunohistochemistry
and the patient's history, particularly in cases with any
predisposing factors.
The patients or their families provided informed
consent for the publication of the case details, and the ethics
committee of Toyooka Hospital approved the publication of this
study.
Acknowledgements
We would like to thank Ms. K. Ando (Department of
Stem Cell Disorders, Kansai Medical University) and Mr. Hilary
Eastwick-Field for the preparation of this manuscript. We would
also like to thank Ms. H. Ogaki, Mr. K. Nagaoka, Mr. T. Kuge, Mr.
H. Takenaka and Ms. S. Kawasaki at Toyooka Hospital for their
expert technical assistance.
References
|
1
|
Amin W and Parwani AV: Nephrogenic
adenoma. Pathol Res Pract. 206:659–662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alexiev BA and LeVea CM: Nephrogenic
adenoma of the urinary tract: A review. Int J Surg Pathol.
20:123–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis TA: Hamartoma of the urinary
bladder. Northwest Med. 48:182–185. 1949.PubMed/NCBI
|
|
4
|
Friedman NB and Kuhlenbeck H: Adenomatoid
tumors of the bladder reproducing renal structures (nephrogenic
adenomas). J Urol. 64:657–670. 1950.PubMed/NCBI
|
|
5
|
López JI, Schiavo-Lena M, Corominas-Cishek
A, Yagüe A, Bauleth K, Guarch R, Hes O and Tardanico R: Nephrogenic
adenoma of the urinary tract: Clinical, histological, and
immunohistochemical characteristics. Virchows Arch. 463:819–825.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ford TF, Watson GM and Cameron KM:
Adenomatous metaplasia (nephrogenic adenoma) of urothelium. An
analysis of 70 cases. Br J Urol. 57:427–433. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oliva E and Young RH: Nephrogenic adenoma
of the urinary tract: A review of the microscopic appearance of 80
cases with emphasis on unusual features. Mod Pathol. 8:722–730.
1995.PubMed/NCBI
|
|
8
|
Husain AN, Armin AR and Schuster GA:
Nephrogenic metaplasia of urinary tract in children: Report of
three cases and review of the literature. Pediatr Pathol.
8:293–300. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kao CS, Kum JB, Fan R, Grignon DJ, Eble JN
and Idrees MT: Nephrogenic adenomas in pediatric patients: A
morphologic and immunohistochemical study of 21 cases. Pediatr Dev
Pathol. 16:80–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao GQ, Burstein DE, Miller LK and Unger
PD: Nephrogenic adenoma: Immunohistochemical evaluation for its
etiology and differentiation from prostatic adenocarcinoma. Arch
Pathol Lab Med. 130:805–810. 2006.PubMed/NCBI
|
|
11
|
Stilmant MM and Siroky MB: Nephrogenic
adenoma associated with intravesical bacillus Calmette-Guerin
treatment: A report of 2 cases. J Urol. 135:359–361.
1986.PubMed/NCBI
|
|
12
|
Gujral H, Chen H and Ferzandi TR:
Nephrogenic adenoma in a urethral diverticulum. Female Pelvic Med
Reconstr Surg. 20:e12–e14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazal PR, Schaufler R, Altenhuber-Müller
R, Haitel A, Watschinger B, Kratzik C, Krupitza G, Regele H, Meisl
FT, Zechner O, et al: Derivation of nephrogenic adenomas from renal
tubular cells in kidney-transplant recipients. N Engl J Med.
347:653–659. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Skinnider BF, Oliva E, Young RH and Amin
MB: Expression of alpha-methylacyl-CoA racemase (P504S) in
nephrogenic adenoma: A significant immunohistochemical pitfall
compounding the differential diagnosis with prostatic
adenocarcinoma. Am J Surg Pathol. 28:701–705. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunju LP: Nephrogenic adenoma: Report of a
case and review of morphologic mimics. Arch Pathol Lab Med.
134:1455–1459. 2010.PubMed/NCBI
|