Introduction
Dysplasia that is seen on a biopsy of the cervix is
called cervical intraepithelial neoplasia (CIN). It is grouped into
three categories: CIN I (mild dysplasia), CIN II (moderate to
marked dysplasia) and CIN III (severe dysplasia to carcinoma in
situ). Treating high-grade (HG)-CIN reduces the incidence and
mortality caused by invasive cervical cancer in women with these
lesions (1–3). Women treated for CIN are of reproductive
age (mean age of approximately 30 years), although the disorder may
also arise in much younger women (4,5).
Multiple techniques are available for the
conservative treatment of HG-CIN in women of fertile age (6–9). These
include ablative methods (e.g., cryotherapy, laser vaporization and
cold coagulation), with satisfactory colposcopic examination
results in women for whom invasion has been ruled out and
excisional methods [e.g., the loop electrosurgical excision
procedure (LEEP), laser conization and cold-knife conization] for
women whose colposcopic examinations are unsatisfactory.
Hysterectomy is ruled out as a primary therapy option for HG-CIN
woman of fertile age. Cold knife conization, laser ablation, laser
conization and large loop excision of the transformation zone
(LLETZ; an alternative term for LEEP) are all conservative
treatment methods used to remove or destroy transformation zones
containing abnormal cells and to preserve cervical function at the
same time (10,11). Cold knife conization was closely
associated with preterm delivery, low birth weight and cesarean
sections. LLETZ was also closely associated with preterm delivery,
low birth weight and premature rupture of the membranes. All
excisional procedures to treat CIN are associated with a small, but
real increase in the risk of pregnancy-related morbidity (12–15).
Although no markedly increased risks for obstetric outcomes
following laser ablation were detected (14), the recurrence rate of CIN3 in the
first year following treatment was reported as 22.6% in a different
study (16). Although the reported
primary cure rate of conservative treatment for HG-CIN exceeds 95%,
a significant number of patients show persistent or recurring
disease during the follow-ups (6–9,17,18).
Appropriate management of women with HG-CIN is a critical component
of cervical cancer prevention. Improper management may increase the
risk of both cervical cancer and complications from over-treatment,
such as preterm delivery (14,19).
Photodynamic therapy (PDT) uses photosensitizing
agents, oxygen and light to create a photochemical reaction that
selectively destroys cancer cells. Photosensitizing agents are
drugs, administered in the body, which become concentrated in
cancer cells and are activated only when light of a certain
wavelength is directed to the area affected by the cancer. The
photodynamic reactions between the photosensitizing agent and light
and oxygen kill the cancer cells (20). These patients may find all parts of
their body sensitive to light, and are advised to take precautions
to protect themselves from light for the necessary length of time,
ranging from days to weeks, depending on the photosensitizing drug
used. Patients are also advised to avoid intense direct sunshine
for approximately 6 months following PDT. Due to patient burdens,
this is not the standard treatment for HG-CIN in women of fertile
age.
On the other hand, thermal ablation is a new
heat-based cancer therapy that kills tumor cells by means of heat
induction in a magnetic metal subjected to alternating magnetic
fields. This technique has recently been put into practice, and has
proven to be effective in rat subcutaneous tumor and liver tumor
models (21). Applying this principle
to treat CIN, including cervical dysplasia and cervical carcinoma
in situ, eliminates the need for trachelectomy while
exerting therapeutic effects on deep foci. Thus, this treatment
method offers the advantages of both conization and laser
vaporization. However, its safety and efficacy have yet to be
investigated, even with respect to the development of associated
medical devices.
Developed by AdMeTech Co., Ltd. (Matsuyama, Japan)
to treat CIN, the AMTC400 is a hyperthermia-inducing device that
harnesses the principle of heat induction by alternating magnetic
fields to convert magnetic energy into heat, applying alternating
magnetic fields to a magnetic needle inserted into the uterine
cervix via the applicator tip. The device achieves precise control
of the temperature of the treated area, maintaining temperatures at
~60°C without passing a high-frequency electric current through the
body, ensuring that deep-lying areas are also heated. Classified as
high-temperature hyperthermia, the treatment method used with this
device is one of several possible hyperthermia therapies.
The aim of the present study was to investigate the
therapeutic safety and efficacy of the therapy performed by the
transaction magnetic field induction heating device, AMTC400, in
fertile patients with HG-CIN (excluding carcinoma in
situ).
Patients and methods
The present clinical study was undertaken at the
authors' institution between April 2012 and March 2013 in
compliance with the following protocol. Women of ages ranging from
20 to 39 years were investigated. The inclusion criteria were as
follows: A diagnosis of CIN3 (excluding carcinoma in situ)
based on cytology, histology and colposcopy; location of the lesion
in a visible area; a positive result from a high-risk HPV test; and
consent to the treatment method in question.
The lesions were treated in the uterine cervix of
the patients using AMTC400, the transaction magnetic field
induction heating device developed by AdMeTech Co., Ltd., that uses
heat induced by alternating magnetic fields. Cytological and
high-risk HPV tests were performed approximately 5 weeks
afterwards, with subsequent conization using an ultrasonic scalpel
for a histopathological examination at 6 weeks following treatment
of the lesions.
An uncontrolled open-label study design was
selected, since Japan currently lacks an established standard
therapy for the condition treated in the present investigation that
retains the uterine cervix. Additionally, since this was an
exploratory clinical trial to confirm the safety and efficacy of
the treatment, the target number of patients was set at six, so
that the probability of detecting at least one adverse event whose
incidence rate was 25% would be 80%. The clinical trial described
above was undertaken with the approval of the ethics committee at
Ehime University, Graduate School of Medicine (Ehime, Japan).
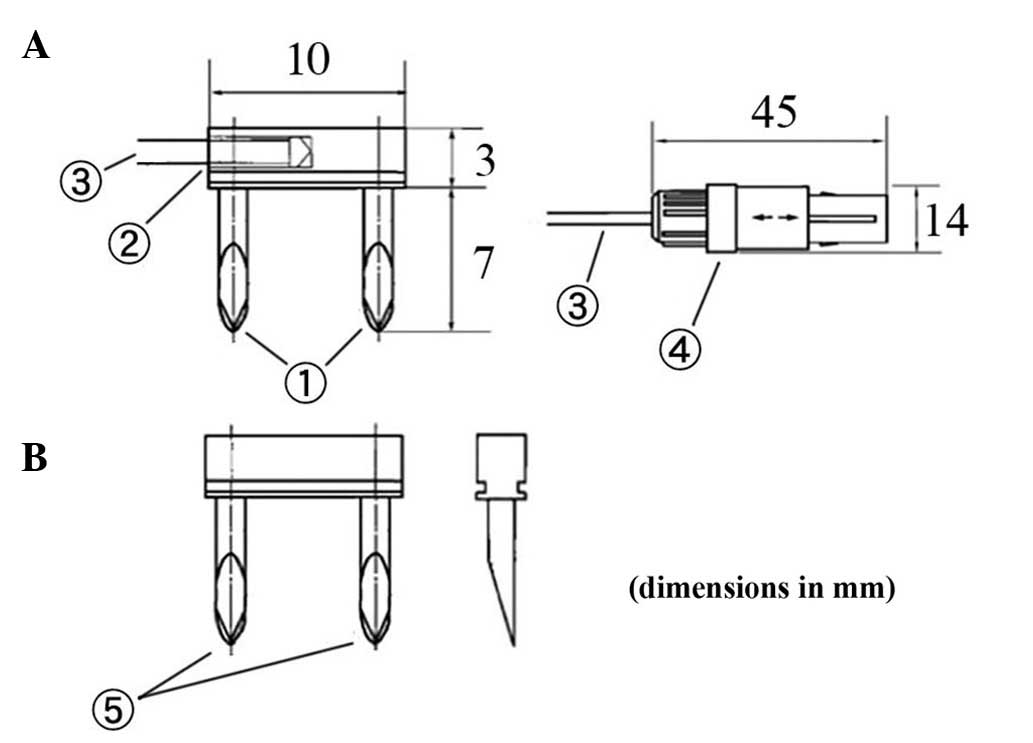
Composition of the device,
AMTC400
The medical device in question is composed of a main
unit that generates high-frequency magnetic fields and heating
needles that are inserted into the affected areas (Figs. 1 and 2).
The heating needles convert alternating magnetic fields into heat.
Two types of needles were used for this trial: One combined with a
temperature-measuring thermocouple for measuring temperature and
heating, and another that simply emits heat (Fig. 2).
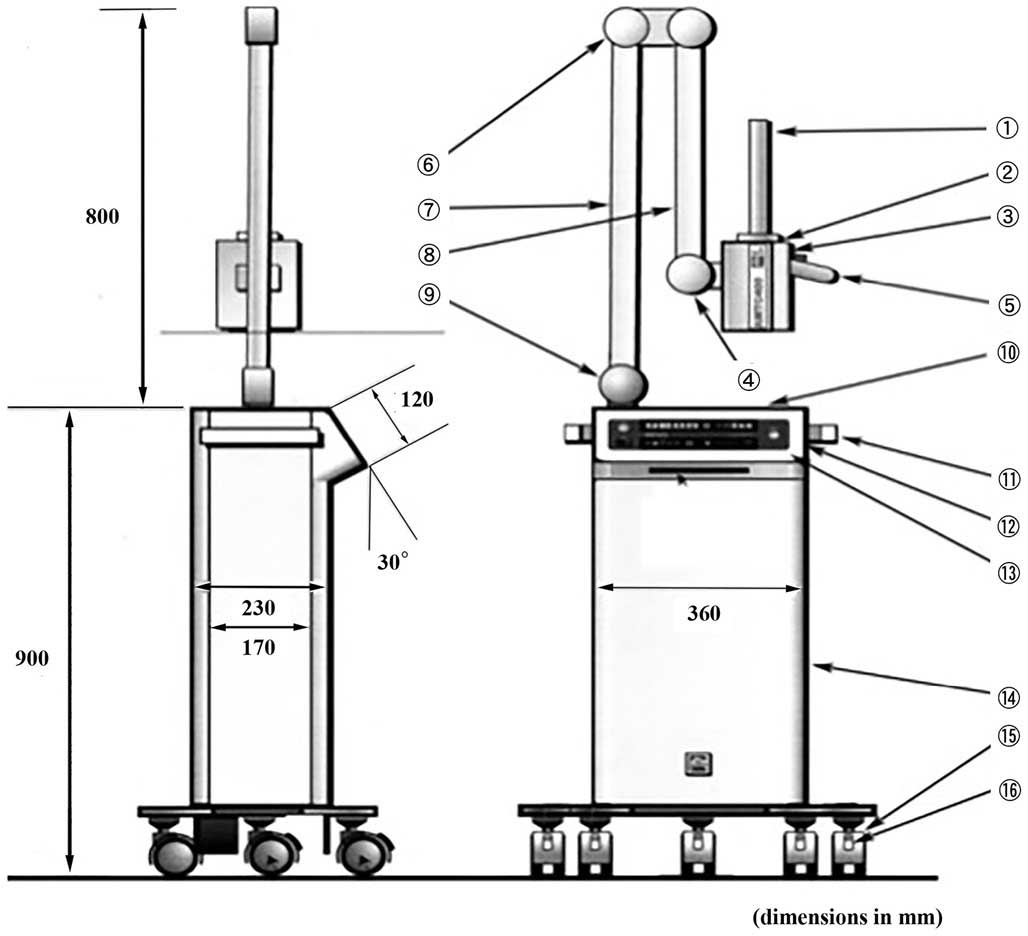 | Figure 1.Structure of the main unit of the
device and part names. 1, applicator: This is the part inserted
into a body cavity in which alternating magnetic fields are
generated; 2, Resonant box: The applicator is attached to the end
of the resonance circuit; 3; Resonant box cover: The box features a
sliding mechanism that moves backwards and forwards over the
resonant box, serving as a cover; 4, Knob used to lock the resonant
box cover: This knob secures the sliding mechanism of the resonant
box; 5, Grip: This is the grip grasped when moving the resonant box
forward or backward; 6, First arm-securing knob: This knob secures
the arm in place to keep it from moving up or down; 7, Arm: This
mechanism supports the resonant box; used to move the box up and
down and right and left; 8, Thermosensor connector: Heating needles
with thermosensors are connected here; 9, Second arm-securing knob:
This knob secures the arm in place to keep it from moving right or
left; 10, Tray: The tray can be used for temporary placement of
thermosensors; 11, side handle: This handle is used when moving the
entire device; 12, Card slot: This slot accepts MMC or SD cards;
13, Control panel: The control panel is used to control the device,
including starting or stopping treatment; 14, Power control unit:
This is the device component used to control electric power and the
microcomputer; 15, Casters: These casters make it easy to move the
device with braking levers; 16, Caster braking levers: Use these
levers to secure the casters and to prevent the device from
moving. |
Treatment procedure
The treatment procedure was to puncture the lesion
with heating needles under colposcopy, and subsequently to insert
the tip of the applicator on the main unit of the device into the
vagina to apply alternating magnetic fields to the heating needles.
The resulting heat emitted by the heating needles denatures the
surrounding lesion. A detailed description of the procedure was as
follows: i) The switch on the control panel on the main unit of the
device was activated to confirm that the device was functioning.
ii) The investigator or the subinvestigator explained to the
patient that hyperthermia using heat induced by alternating
magnetic fields would begin, and instructed the patient to sit in
an examining chair in a lithotomy position. iii) The vaginal canal
was disinfected with a plastic Cusco speculum, the affected area
was subsequently located, and images were captured under
colposcopy. iv) The located affected area was punctured with the
appropriate number of heating needles or
temperature-measuring/heating needles using Kocher clamps with a
space of 5 mm between each needle, since the effect of heat
induction was to reach up to 5 mm wide in the preliminary study
(data not shown). If the lesion was punctured with
temperature-measuring/heating needles, the thermocouple connector
of the thermocouple signal cable was connected to the arm on the
main unit of the device afterwards. v) Two
temperature-measuring/heating needles were used to control the
device. For the first three patients, only
temperature-measuring/heating needles were used to ensure that all
inserted heating needles emitted heat equally. If three or more
temperature-measuring/heating needles were used, the temperature of
each needle was monitored and recorded separately using a
battery-operated 5 V direct current temperature-measuring device
supplied by the sponsor (AdMeTech Co., Ltd.). vi) The applicator
cover and the probe cover were attached to the applicator on the
main unit of the device, and this was inserted into the vagina
until it touched the heating needles. vii) The ‘Start Treatment’
button on the control panel on the device was pushed to start the
device. Once treatment began, the heating needles began to heat.
Their temperature was automatically maintained at 60±5°C, and the
temperature of the affected area was ~55±5°C. viii) Since the
patient's movements may increase the distance between the
applicator and the heating needles, the heat emitted by the heating
needles was reduced during treatment, and the investigator or
subinvestigator was required to keep his or her hand on the
applicator at all times, remaining aware of any sudden movements
and keeping the heating needles and the applicator in contact. ix)
If all devices operated normally, the output of the high-frequency
magnetic fields automatically ceased within 10 min, and the heating
needles stopped emitting heat. x) The applicator was removed from
the vagina, all heating and temperature-measuring/heating needles
were removed, and it was made certain that bleeding had been
arrested. The affected area was disinfected and images were
captured. xi) The Cusco speculum was removed to conclude the
procedure. xii) A final check was performed to ensure the absence
of bleeding 30–60 min following the end of the treatment.
Endpoints for efficacy
Specimens excised during conization 6 weeks
following the treatment were histologically examined and assessed
on the following 6-point scale: Cure, no dysplasia detected;
moderate improvement, CIN1 detected; mild improvement, CIN2
detected; No change, CIN3 (with no carcinoma in situ)
detected; progression, carcinoma in situ detected;
aggravation, minimally invasive squamous cell carcinoma or squamous
cell carcinoma detected.
Results
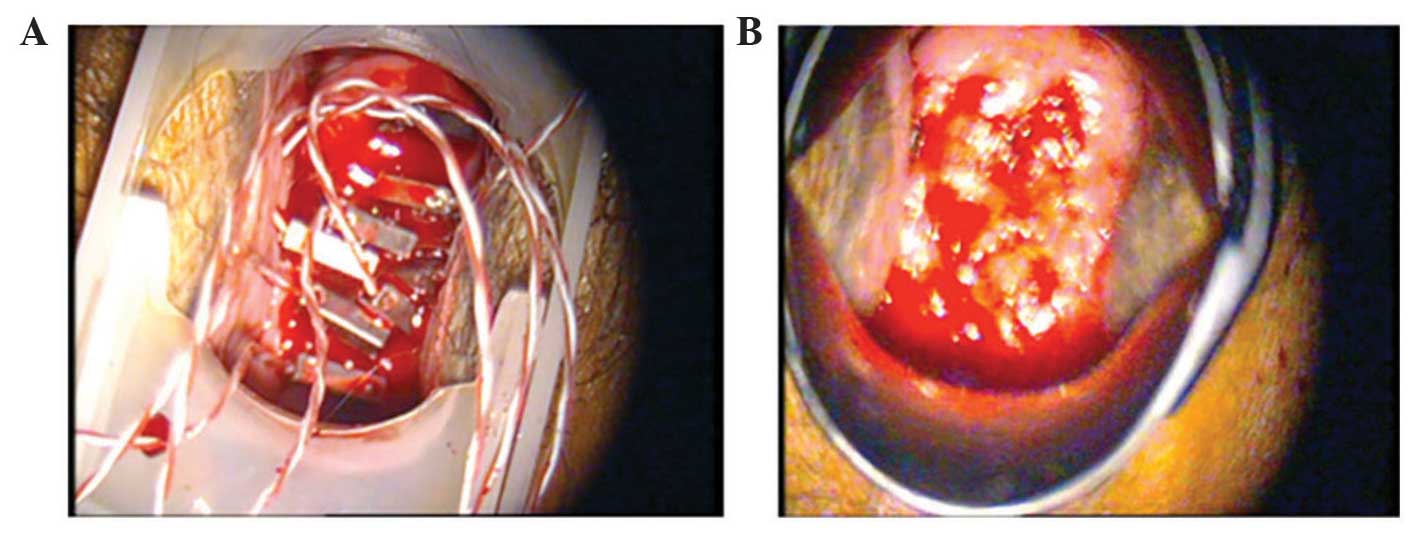
The treatment was administered to four patients who
provided their consent (Table I). The
lesions were located and punctured with heating needles under
colposcopy (Fig. 3). No anesthesia
was required; intraoperative pain and heat sensations were mild and
required no additional intervention. Postoperative bleeding
following the removal of the needles was minimal and stopped by
gauze compression (Fig. 4).
 | Table I.Patient characteristics, high-risk HPV
test results, histopathological results following conization and
efficacy assessment. |
Table I.
Patient characteristics, high-risk HPV
test results, histopathological results following conization and
efficacy assessment.
| Case | Age | Pregnancy/delivery
historya | High-risk HPV | Cytology (5 weeks
following treatment) | Histopathology
(conization, 6 weeks following treatment) | Efficacy
assessment |
|---|
| 1 | 36 | G0P0 | Type 16 → type
16 | NILM | CIN1 | Moderate
improvement |
| 2 | 34 | G1P1 | Type 52 → type
52 | NILM | CIN1 | Moderate
improvement |
| 3 | 32 | G1P1 | Type 16, 58 →
negative | NILM | CIN1 | Moderate
improvement |
| 4 | 35 | G3P1 | Type 16 →
negative | NILM | CIN1 | Moderate
improvement |
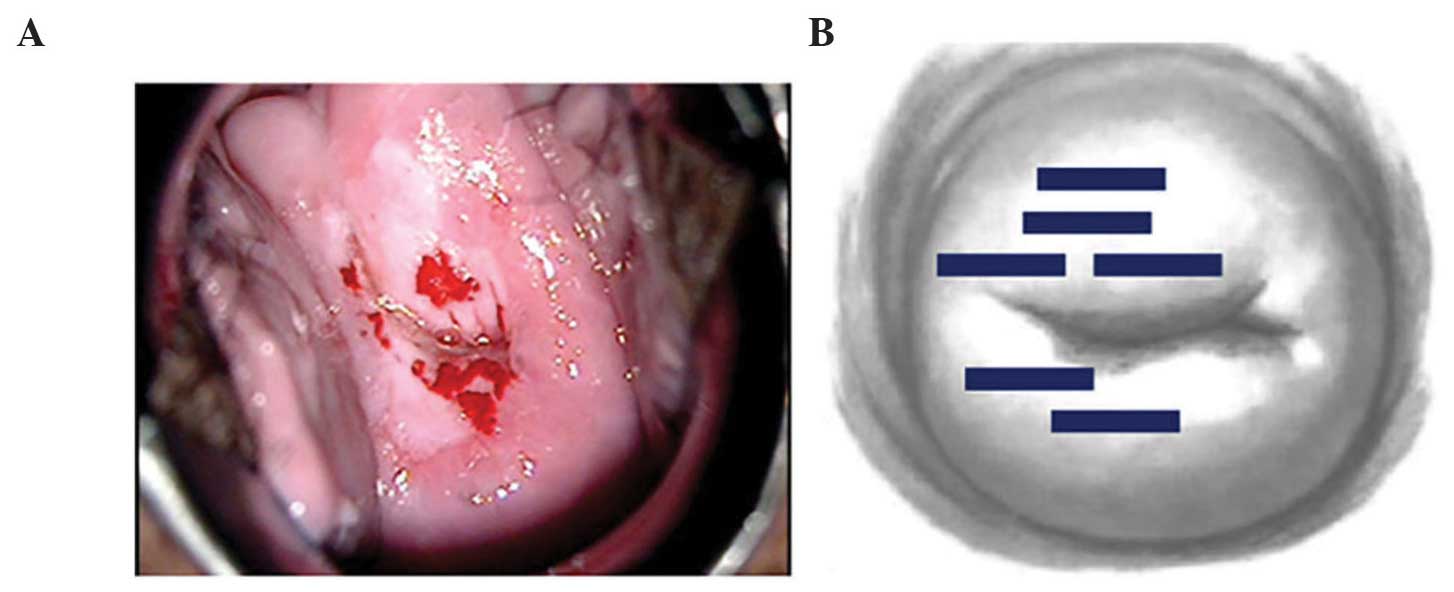
With respect to chronological changes observed via
colposcopy, the vaginal portion of the cervix had turned white by
postoperative day 3, and exhibited erosion with a partial white
coating by postoperative day 10 in all cases. At postoperative day
31, the erosion had diminished, with overall epithelialization
under way (Fig. 5).
No clear symptoms other than minor bleeding were
reported following the treatment. At approximately 4 weeks
following the treatment, high-risk HPVs had disappeared from two of
the four patients (Table I).
A colposcopy performed approximately 5 weeks after
treatment revealed no apparent abnormal findings in any of the
patients. The results of the cytology were negative for
intraepithelial lesions and malignancies (NILM) in all four
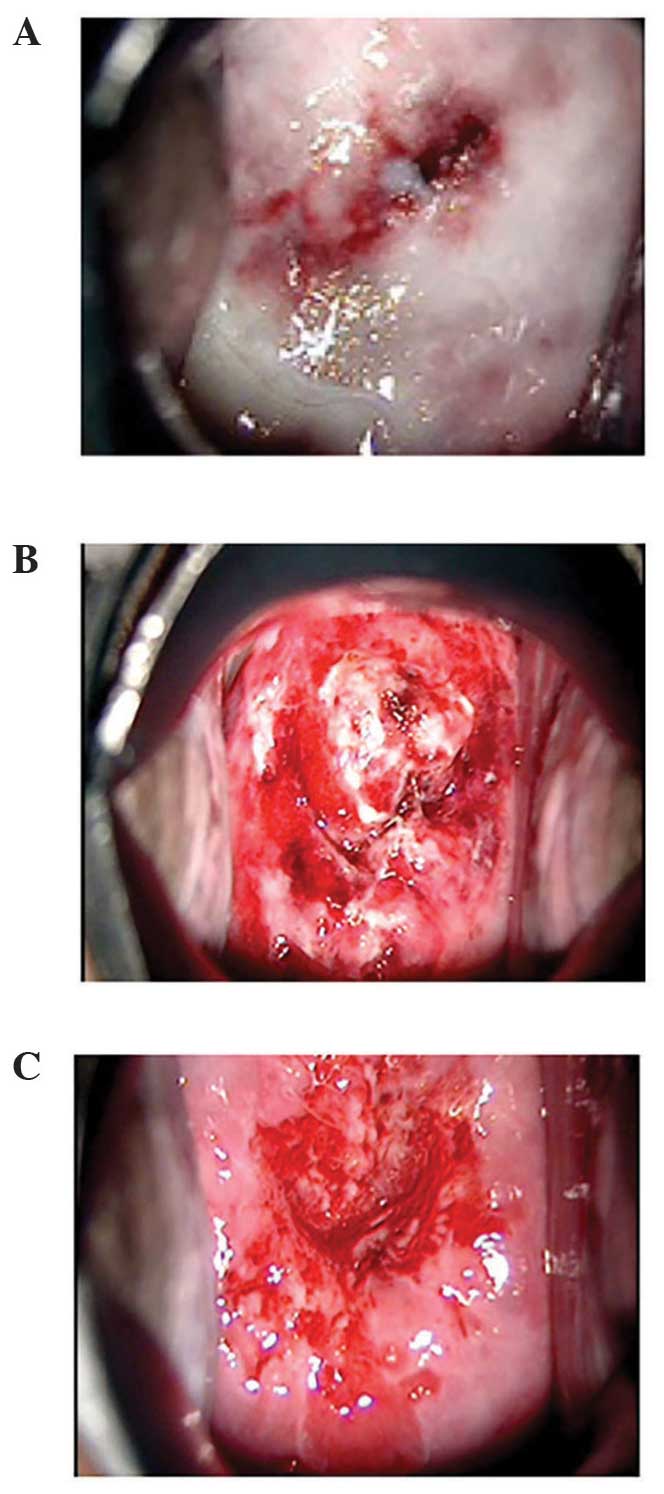
patients. However, histopathological examination of the conization
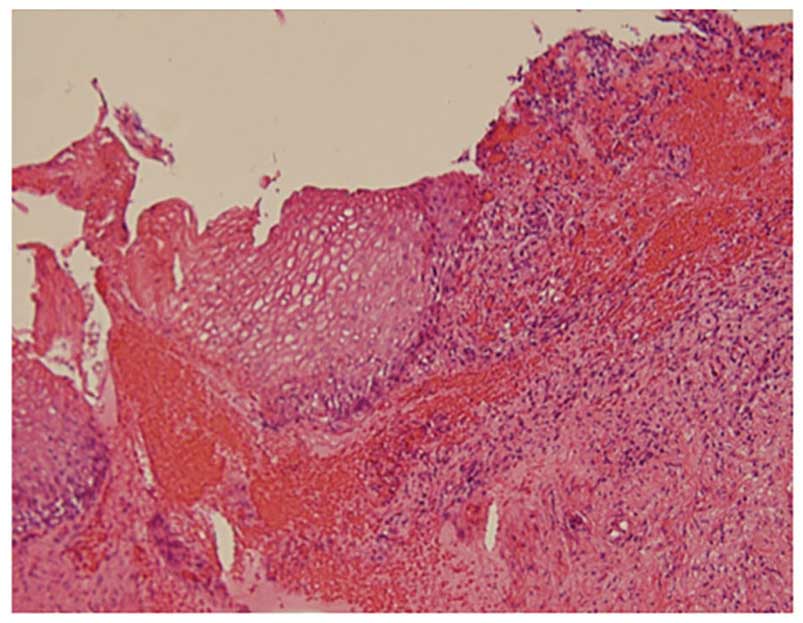
specimens revealed koilocytosis in the surface layers, with
atypical cells in parts of the lower third of the epithelium in all
four patients, leading to a diagnosis of CIN1 (Fig. 6). The efficacy assessment was moderate
improvement in all four patients (Table
I).
With respect to safety analysis, one case had mild
pain following the treatment, although no serious adverse events
associated with the treatment were observed (Table II). Device malfunctions or defects
emerged with two patients. The first instance involved a pinhole on
the probe cover discovered following treatment. Examination of the
affected area revealed no abnormalities, and the patient was
unaffected. As a countermeasure, the use of the probe covers was
discontinued, and the enrolment of new patients was halted until a
preventive measure had been implemented. The second instance
involved disconnection of the needle portion of the
temperature-measuring/heating needle during treatment preparations.
This issue arose prior to commencing the treatment, and a spare
temperature-measuring/heating needle was used instead, treating the
patient as planned, although without any effects on the patient. As
a countermeasure, the use of the temperature-measuring/heating
needles was discontinued and the enrolment of new patients was
halted until a preventive measure had been implemented (Table II).
 | Table II.Safety analysis. |
Table II.
Safety analysis.
| Occasion of defect
malfunction | Occurrence | Number (%) |
|---|
| 1 | Occurrences of
related serious adverse events |
|
|
| Number of occurrences
(incidence) | 0/4 (0.0%) |
| 2 | Occurrences of
malfunctions or defects |
|
|
| Number of occurrences
(incidence) | 2/4 (50.0%) |
|
| Description of
malfunction or defect |
|
|
| A pinhole was found
on the probe cover | 1 (25.0%) |
|
| The needle part of a
temperature-measuring/heating needle was disconnected | 1 (25.0%) |
Discussion
To the best of our knowledge, this is the first time
that a report has been published of studies undertaken on the basis
of the principle applied in the present treatment method. The
efficacy of treatment applied to treat HG-CIN with a hyperthermia
by the transaction magnetic field induction heating device,
AMTC400, in the present investigation was deemed to constitute a
moderate improvement. Conization specimens from all four patients
were found to have CIN1. Colposcopy performed prior to conization
revealed no apparent abnormalities in any of the four patients,
since they were all in the process of healing from the changes
resulting from hyperthermia at that time. Although the results of
cytology were NILM for all four patients, histopathological
examination of conization specimens revealed koilocytosis in the
surface layers, with atypical cells in parts of the lower third of
the epithelium in all four patients, leading to a diagnosis of
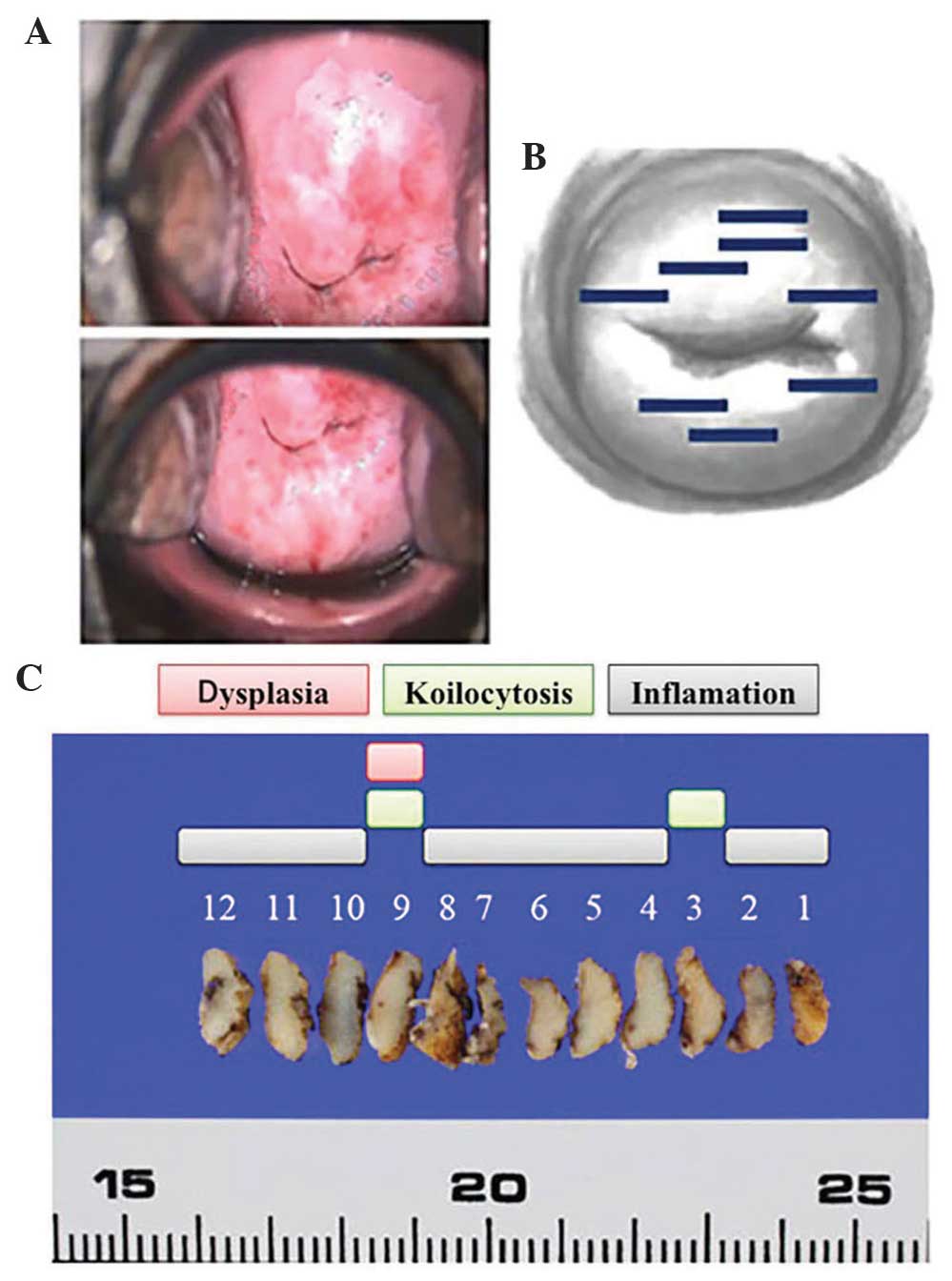
CIN1. Comparing these areas with areas punctured with heating
needles as part of the treatment revealed that the former had not
been punctured with needles, since the colposcopy performed prior
to the treatment had resulted in an assessment that abnormalities
were absent (Fig. 7 and Table III). In other words, the heating
applied may have been inadequate, leading to CIN1 being present
prior to treatment, which persisted following treatment. Possible
future improvements in this procedure would involve puncturing the
entire vaginal area of the cervix with heating needles without
relying on the findings from colposcopy; this may lead to improved
therapeutic effects.
 | Table III.Heating needle insertion sites and
CIN1 sites. |
Table III.
Heating needle insertion sites and
CIN1 sites.
|
| Case 1 | Case 2 | Case 3 | Case 4 |
|---|
|
|
|
|
|
|
|---|
| Direction | Heating needle
insertion | Dysplasia | Heating needle
insertion | Dysplasia | Heating needle
insertion | Dysplasia | Heating needle
insertion | Dysplasia |
|---|
| 1 | − | − | + | − | + | − | + | − |
| 2 | − | − | + | − | − | CIN1 | − | CIN1 |
| 3 | − | − | − | − | − | CIN1 | − | − |
| 4 | − | − | + | − | − | − | − | − |
| 5 | + | − | + | − | + | − | − | − |
| 6 | + | − | + | − | + | − | + | − |
| 7 | + | − | + | − | + | − | + | − |
| 8 | − | − | − | − | + | − | + | − |
| 9 | − | − | − | CIN1 | − | − | + | − |
| 10 | − | CIN1 | + | − | − | − | + | − |
| 11 | − | CIN1 | + | − | + | − | + | − |
| 12 | − | − | + | − | + | − | + | − |
As for the analysis of treatment safety, no serious
adverse events were observed other than mild pain and bleeding. Two
instances of device malfunctions or defects emerged, but neither
was detrimental to the patients, and both were addressed and later
rectified.
In conclusion, we consider that the treatment method
explored in the present study may prove beneficial to younger
patients who wish to preserve their fertility. The method does not
involve trachelectomy, despite room for improvement with respect to
the areas punctured with heating needles. Since it requires no
anesthesia and the burden on the patients is small, our
consideration is that this treatment may be provided on an
outpatient basis. The method requires improvements with respect to
the areas punctured with heating needles, and future studies should
accumulate additional case studies.
In conclusion, the thermotherapy administered with
the transaction magnetic field induction heating device, AMTC400,
appears to be safe and effective in terms of treating HG-CIN in
women of fertile age, although further improvements are required
with respect to puncture needle sites. Further studies are also
required to confirm long-term efficacy and reproductive
outcomes.
References
|
1
|
McCredie MR, Sharples KJ, Paul C, Baranyai
J, Medley G, Jones RW and Skegg DC: Natural history of cervical
neoplasia and risk of invasive cancer in women with cervical
intraepithelial neoplasia 3: A retrospective cohort study. Lancet
Oncol. 9:425–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soutter WP, Sasieni P and Panoskaltsis T:
Long-term risk of invasive cervical cancer after treatment of
squamous cervical intraepithelial neoplasia. Int J Cancer.
118:2048–2055. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strander B, Andersson-Ellström A, Milsom I
and Sparén P: Long term risk of invasive cancer after treatment for
cervical intraepithelial neoplasia grade 3: Population based cohort
study. BMJ. 335:10772007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbert A: Cervical screening in England
and Wales: Its effect has been underestimated. Cytopathology.
11:471–479. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paraskevaidis E, Kitchener HC, Miller ID,
Mann E, Jandial L and Fisher PM: A population-based study of
microinvasive disease of the cervix - a colposcopic and cytologic
analysis. Gynecol Oncol. 45:9–12. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fallani MG, Penna C, Fambrini M and
Marchionni M: Laser CO2 vaporization for high-grade cervical
intraepithelial neoplasia: A long-term follow-up series. Gynecol
Oncol. 91:130–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathevet P, Chemali E, Roy M and Dargent
D: Long-term outcome of a randomized study comparing three
techniques of conization: Cold knife, laser, and LEEP. Eur J Obstet
Gynecol Reprod Biol. 106:214–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueda M, Ueki K, Kanemura M, Izuma S,
Yamaguchi H, Nishiyama K, Tanaka Y, Terai Y and Ueki M: Diagnostic
and therapeutic laser conization for cervical intraepithelial
neoplasia. Gynecol Oncol. 101:143–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Hamont D, van Ham MA, Struik-van der
Zanden PH, Keijser KG, Bulten J, Melchers WJ and Massuger LF:
Long-term follow-up after large-loop excision of the transformation
zone: Evaluation of 22 years treatment of high-grade cervical
intraepithelial neoplasia. Int J Gynecol Cancer. 16:615–619. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitchener HC, Cruickshank ME and Farmery
E: The 1993 British society for colposcopy and cervical
pathology/national coordinating network united kingdom colposcopy
survey. Comparison with 1988 and the response to introduction of
guidelines. Br J Obstet Gynaecol. 102:549–552. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prendiville W, Cullimore J and Norman S:
Large loop excision of the transformation zone (LLETZ). A new
method of management for women with cervical intraepithelial
neoplasia. Br J Obstet Gynaecol. 96:1054–1060. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arbyn M, Kyrgiou M, Simoens C, Raifu AO,
Koliopoulos G, Martin-Hirsch P, Prendiville W and Paraskevaidis E:
Perinatal mortality and other severe adverse pregnancy outcomes
associated with treatment of cervical intraepithelial neoplasia:
Meta-analysis. BMJ. 337:a12842008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bevis KS and Biggio JR: Cervical
conization and the risk of preterm delivery. Am J Obstet Gynecol.
205:19–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kyrgiou M, Koliopoulos G, Martin-Hirsch P,
Arbyn M, Prendiville W and Paraskevaidis E: Obstetric outcomes
after conservative treatment for intraepithelial or early invasive
cervical lesions: Systematic review and meta-analysis. Lancet.
367:489–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sadler L, Saftlas A, Wang W, Exeter M,
Whittaker J and McCowan L: Treatment for cervical intraepithelial
neoplasia and risk of preterm delivery. JAMA. 291:2100–2106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inaba K, Nagasaka K, Kawana K, Arimoto T,
Matsumoto Y, Tsuruga T, Mori-Uchino M, Miura S, Sone K, Oda K, et
al: High-risk human papillomavirus correlates with recurrence after
laser ablation for treatment of patients with cervical
intraepithelial neoplasia 3: A long-term follow-up retrospective
study. J Obstet Gynaecol Res. 40:554–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghaem-Maghami S, Sagi S, Majeed G and
Soutter WP: Incomplete excision of cervical intraepithelial
neoplasia and risk of treatment failure: A meta-analysis. Lancet
Oncol. 8:985–993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skjeldestad FE, Hagen B, Lie AK and
Isaksen C: Residual and recurrent disease after laser conization
for cervical intraepithelial neoplasia. Obstet Gynecol. 90:428–433.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Insinga RP, Glass AG and Rush BB:
Diagnoses and outcomes in cervical cancer screening: A
population-based study. Am J Obstet Gynecol. 191:105–113. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Keltner L, Christophersen J, Zheng
F, Krouse M, Singhal A and Wang SS: New technology for deep light
distribution in tissue for phototherapy. Cancer J. 8:154–163. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kikkawa H: Anti-tumor effects of induced
hyperthermia using a MgFe2O4 needle in rat tumor models. Ehime
Igaku. 24:156–164. 2005.
|