Introduction
T-cell acute lymphoblastic leukemia (ALL), which is
an aggressive hematological malignancy occurring as a consequence
of malignant transformation of T-cell progenitors, accounts for
~25% of all adult cases of ALL (1,2). Although
CD1a, CD2, CD3 (membranous as well as cytoplasmic), CD4, CD5, CD7
and CD8 are all T-cell antigens, cytoplasmic CD3 is considered as
an indicator of T-cell lineage (2).
In addition to T-cell markers, myeloid-associated antigens CD33,
CD117 (2–4), CD34 (2,5) and CD56
(6,7)
have been found to be expressed by blasts in T-cell ALL. However,
concomitant expression of all these markers in the same patient has
not been previously reported.
We herein present a case of T-cell ALL exhibiting
aberrant expression of CD34, CD56, CD33 and CD117, in addition to
T-cell markers, which did not respond to induction treatment.
Case report
A 55-year-old woman was admitted to our hospital
with a sore throat unresponsive to medication for 1 month. On
physical examination, the tonsils and oropharynx were found to be
hyperemic, without any other remarkable findings. The laboratory
tests revealed a hemoglobin level of 9.1 g/dl, platelet count of
1.95×109/l, leukocyte count of 1.97×109/l and lactate dehydrogenase
level of 205 U/l. The peripheral blood smear examination revealed
blast cells. Bone marrow biopsy and aspiration were performed. The
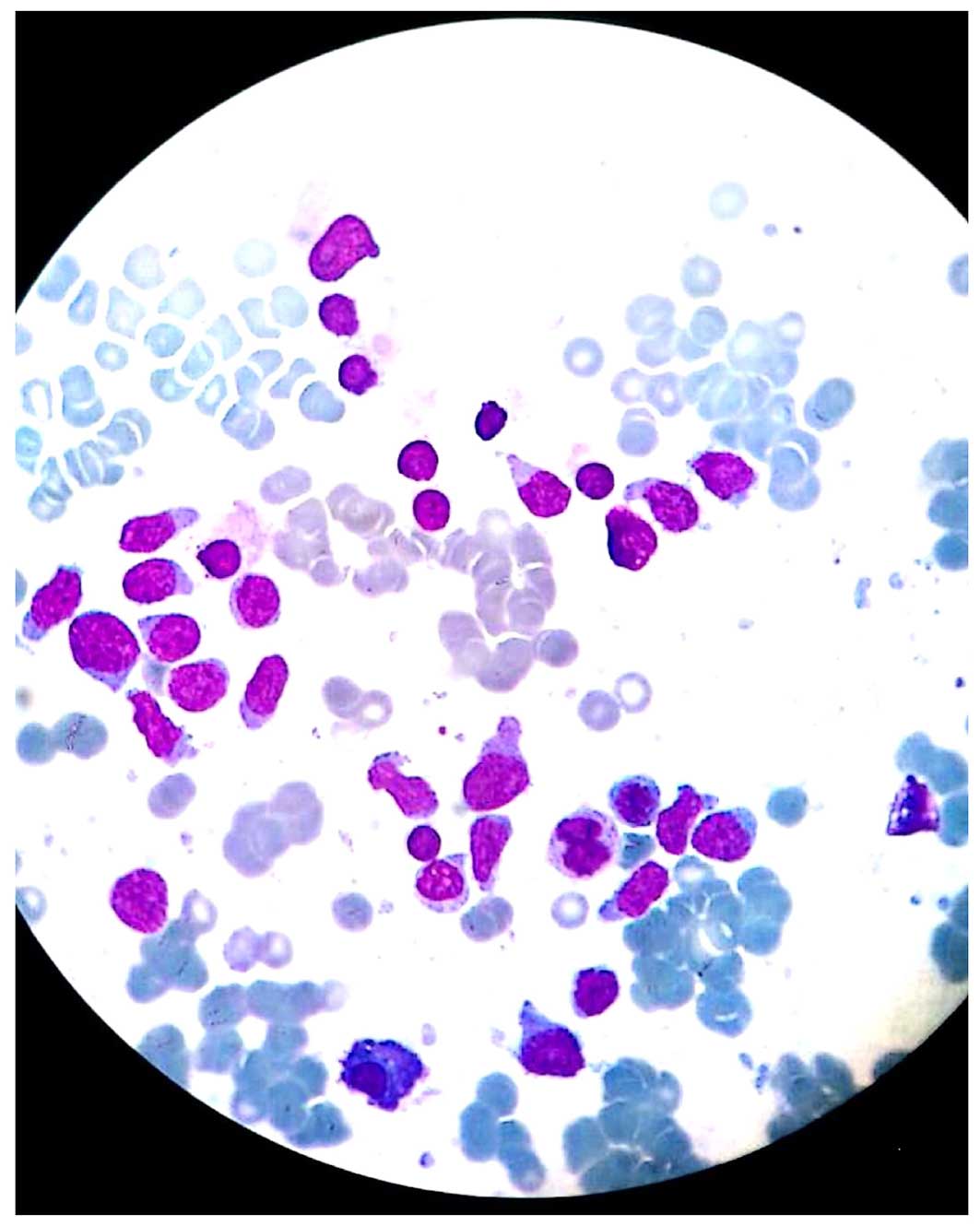
bone marrow aspiration showed hand mirror-shaped blast cells
(Fig. 1). On conventional cytogenetic
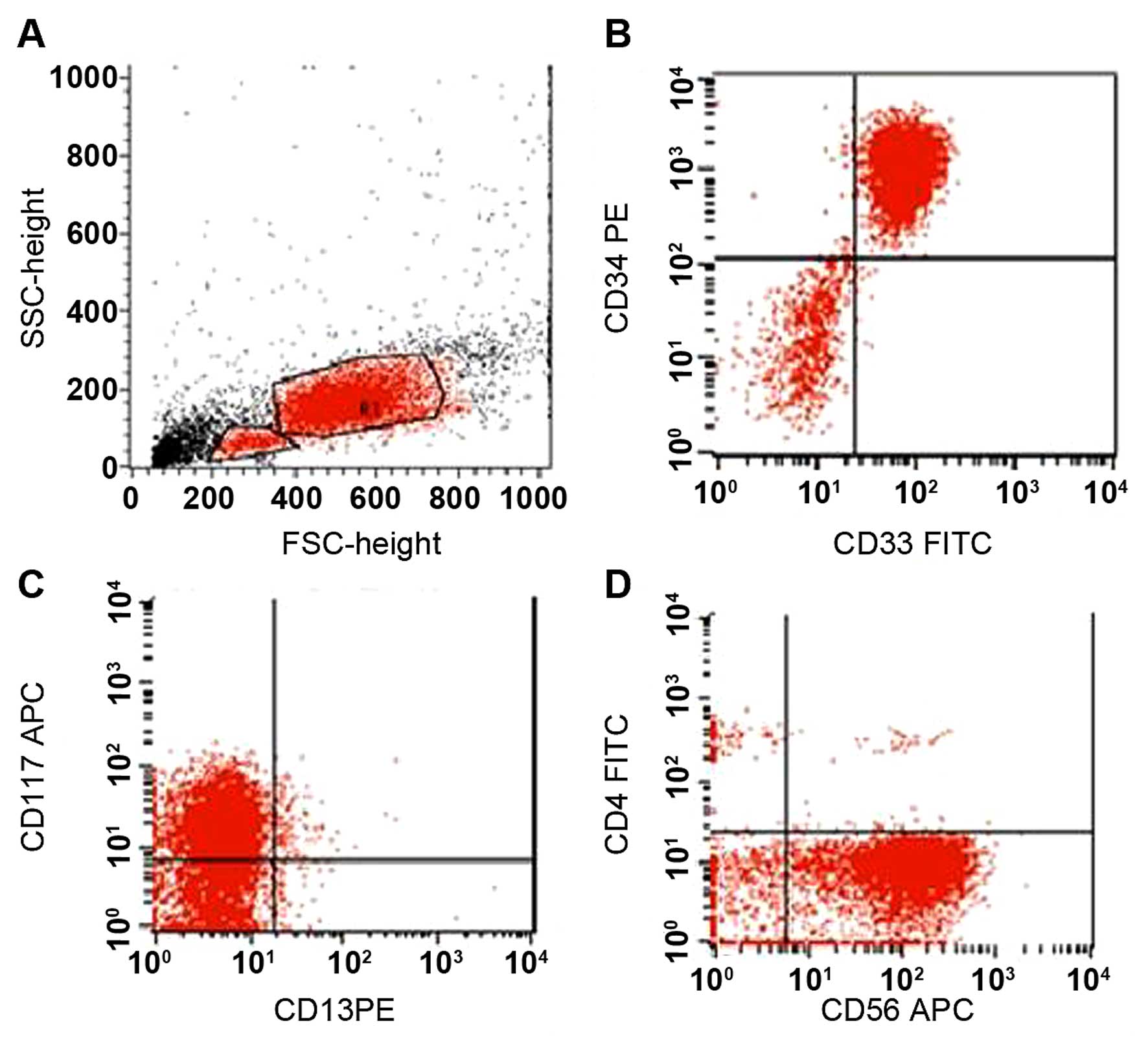
study, there was no metaphase for assessment. On flow cytometric
analysis, the blast cells were found to be positive for cytoplasmic
CD3, CD2, CD5, CD7, CD34, CD56, CD33 and CD117, whereas they were
negative for myeloperoxidase, CD13, CD11b, CD15, CD19, CD79a, CD22
and CD10 (Fig. 2). The patient was
diagnosed with T-cell ALL according to the World Health
Organisation 2008 criteria (8), and
was administered cyclophosphamide, vincristine, adriamycin and
dexamethasone (hyper-CVAD 1A) therapy. After the first cycle of
treatment, bone marrow aspiration revealed 10% blast cells.
Therefore, methotrexate and cytarabine (hyper-CVAD 1B) treatment
was initiated. On the 18th day of therapy, the patient deteriorated
clinically and subsequently developed septic shock. The patient was
intubated and admitted to the intensive care unit; however, she
succumbed to the disease on day 20 of the hyper-CVAD 1B
treatment.
Discussion
Although T-cell ALL develops due to malignant
transformation of T-cell progenitors, aberrant myeloid marker
expression on blast cells, particularly of CD13, CD33 and rarely
CD117, has been previously described (2–4). In
myeloid antigen-expressing T-cell ALL patients, the frequency of
CD34 expression, a transmembrane glycoprotein that is expressed on
hematopoietic stem cells and early thymic T-cell precursors
(9), was also found to be increased
(5). There is an ongoing debate
regarding the clinical relevance of myeloid marker expression in
T-cell ALL and whether their expression is a predictor of poor
outcome (3–5,10,11). Furthermore, the effect of CD34
expression on clinical outcome in T-cell ALL is controversial
(9,10)
and remains to be elucidated.
Another surface antigen associated with poor
prognosis in T-cell ALL is the CD56 (also referred to as neural
cell adhesion molecule) surface antigen. CD56 is a marker for
natural killer (NK) cells, but is also expressed in neoplastic
myeloid, lymphoid, plasmacytoid dendritic and myeloma cells, as
well as in a minority of T cells (7).
Co-expression of myeloid markers together with CD34 and CD56 in
T-cell ALL is seen more frequently (6,7). To the
best of our knowledge, this is the first report of aberrant
co-expression of the NK cell marker CD56, the myeloid cell markers
CD117 and CD33 and the stem cell marker CD34 in a patient with
T-cell ALL. This appears to be associated with an unfavorable
outcome, despite the use of intensive chemotherapy.
References
|
1
|
Van Vlierberghe P and Ferrando A: The
molecular basis of T-cell acute lymphoblastic leukemia. J Clin
Invest. 122:3398–3406. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gamal AH: Classification of Acute
Leukemia. University of Aden/Hematology unit. Yemen: 2011.
|
|
3
|
Supriyadi E, Veerman AJ Sutaryo, Purwanto
I, Vd Ven PM and Cloos J: Myeloid antigen expression in childhood
acute lymphoblastic leukemia and its relevance for clinical outcome
in indonesian ALL-2006 protocol. J Oncol. 2012:1351862012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suggs JL, Cruse JM and Lewis RE: Aberrant
myeloid marker expression in precursor B-cell and T-cell leukemias.
Exp Mol Pathol. 83:471–473. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uckun FM, Sather HN, Gaynon PS, Arthur DC,
Trigg ME, Tubergen DG, Nachman J, Steinherz PG, Sensel MG and
Reaman GH: Clinical features and treatment outcome of children with
myeloid antigen-positive acute lymphoblastic leukemia: A report
from the Children's cancer group. Blood. 90:28–35. 1997.PubMed/NCBI
|
|
6
|
Montero I, Rios E, Parody R, Perez-Hurtado
JM, Martin-Noya A and Rodriguez JM: CD56 in T-cell acute
lymphoblastic leukaemia: A malignant transformation of an early
myeloid-lymphoid progenitor? Haematologica. 88:ELT262003.PubMed/NCBI
|
|
7
|
Fischer L, Gökbuget N, Schwartz S,
Burmeister T, Rieder H, Brüggemann M, Hoelzer D and Thiel E: CD56
expression in T-cell acute lymphoblastic leukemia is associated
with non-thymic phenotype and resistance to induction therapy but
no inferior survival after risk-adapted therapy. Haematologica.
94:224–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A and Bloomfield CD: The 2008
revision of the world health organization (WHO) classification of
myeloid neoplasms and acute leukemia: Rationale and important
changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pui CH, Hancock ML, Head DR, Rivera GK,
Look AT, Sandlund JT and Behm FG: Clinical significance of CD34
expression in childhood acute lymphoblastic leukemia. Blood.
82:889–894. 1993.PubMed/NCBI
|
|
10
|
Vitale A, Guarini A, Ariola C, Mancini M,
Mecucci C, Cuneo A, Pane F, Saglio G, Cimino G, Tafuri A, et al:
Adult T-cell acute lymphoblastic leukemia: Biologic profile at
presentation and correlation with response to induction treatment
in patients enrolled in the GIMEMA LAL 0496 protocol. Blood.
107:473–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhushan B, Chauhan PS, Saluja S, Verma S,
Mishra AK, Siddiqui S and Kapur S: Aberrant phenotypes in childhood
and adult acute leukemia and its association with adverse
prognostic factors and clinical outcome. Clin Exp Med. 10:33–40.
2010. View Article : Google Scholar : PubMed/NCBI
|