Post‑transplant lymphoproliferative disorder presenting with skin ulceration in a renal transplant recipient who achieved sustained remission with rituximab therapy: A case report
- Authors:
- Published online on: September 19, 2016 https://doi.org/10.3892/mco.2016.1024
- Pages: 610-612
Abstract
Introduction
Post-transplant lymphoproliferative disorder (PTLD) is a known complication of both solid organ and stem cell transplantation. The majority of PTLD cases occur within the first year after the transplant and ~90% of the cases are Epstein-Barr virus (EBV)-related, CD20-positive B-cell neoplasms (1). PTLD is associated with a variety of clinical presentations, but is rarely found in the skin (2). Reduction of immunosuppression is currently the preferred initial treatment for PTLD. Due to comorbid diseases and chronic immunosuppression, high morbidity and mortality rates are observed in PTLD patients treated with cytotoxic drugs. The risk of infection is also high.
We herein report a case of PTLD in a renal transplant recipient, presenting with skin ulceration that was complicated with cryptococcal infection following treatment with chemotherapy. Antifungal agents were administered to control the fungal infection. The patient later developed recurrence of the lymphoma and was successfully treated with single-agent rituximab.
Case report
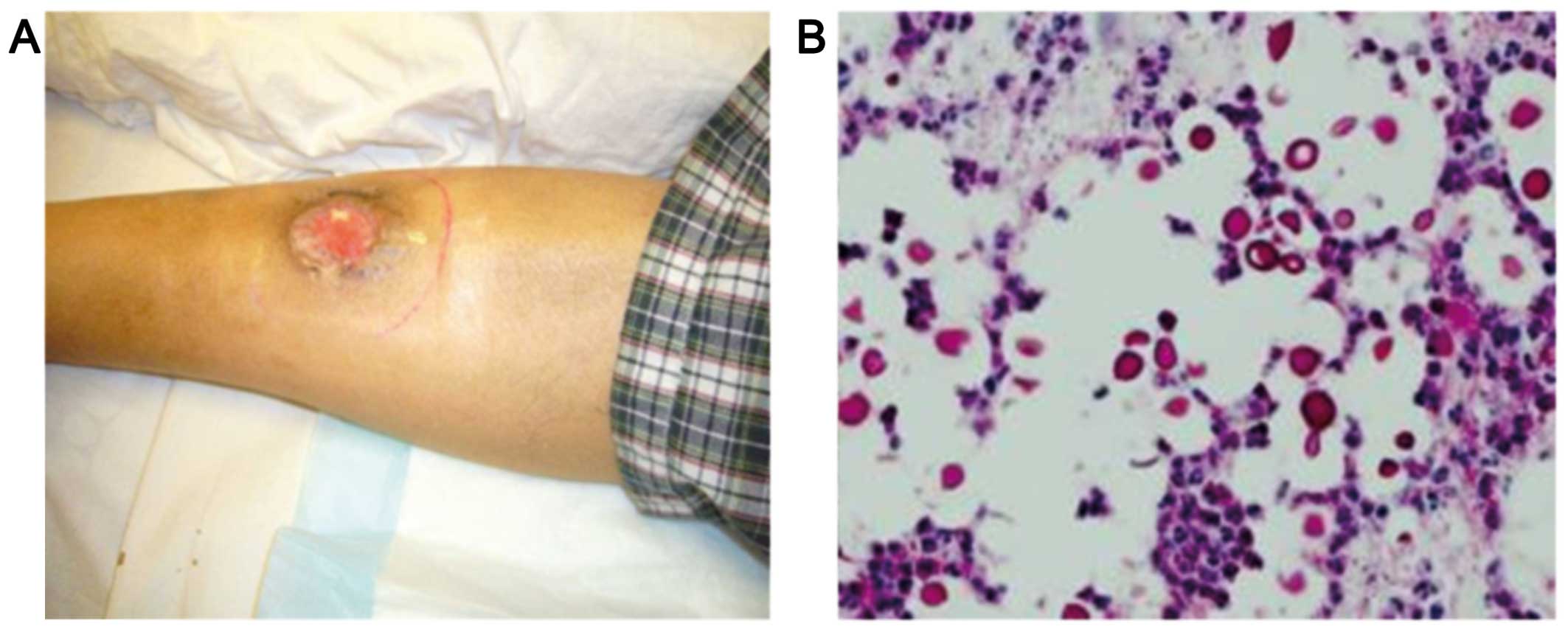
A 41-year-old man with a past history of hepatitis B and chronic renal failure of unknown etiology had received a cadaveric renal transplant in 1997. The maintenance immunosuppressive therapy included prednisolone, mycophenolate mofetil and tacrolimus. The patient presented to the Tuen Mun Hospital (Hong Kong, China) in June, 2009 with an ulcer in the right leg 12 years after receiving the renal transplant (Fig. 1A). There was no fever, night sweats or weight loss. There was also no lymphadenopathy or hepatosplenomegaly. Laboratory investigations revealed a white blood cell count of 5.2×109/l, a haemoglobin level of 9.9 g/dl, a platelet count of 151×109/l and a creatinine level of 340 µmol/l. The lactate dehydrogenase level was elevated to 1,063 U/l (normal, 106–218 U/l). A computed tomography scan of the neck, thorax, abdomen and pelvis did not reveal any enlarged lymph nodes and bone marrow examination did not show any evidence of lymphoma. A biopsy of the leg ulcer revealed the presence of ulcerated skin tissue with diffuse infiltration of the dermis by monomorphous lymphoid cells, associated with large areas of necrosis. The cells were medium to large in size, with irregular nuclei, several of which contained large prominent nucleoli. The mitotic activity was brisk. The lymphoid cells were positive for CD20, CD30, CD79a, B-cell lymphoma (Bcl)-2, Bcl-6 and multiple myeloma oncogene 1, and negative for CD3, CD4, CD5, CD8, CD10, CD43, CD56, cyclin D1 and anaplastic lymphoma kinase. Epstein Barr virus (EBV)-encoded RNA in situ hybridization and latent membrane protein-1 were both positive. The pathological diagnosis was post-transplant lymphoproliferative disorder, namely diffuse large B-cell lymphoma.
Following the diagnosis of PTLD, the patient was treated with immunosuppression reduction, but the skin ulcer persisted. He was then administered two courses of CEOP chemotherapy, consisting of cyclophosphamide 750 mg/m2 intravenously (i.v.) on day 1, epirubicin 50 mg/m2 i.v. on day 1, vincristine 1.4 mg/m2 i.v. on day 1, and prednisolone 40 mg/m2 per os on days 1–5. The patient also received antibiotic prophylaxis with levofloxacin. The circulating EBV-DNA level was measured at diagnosis and serially every 2 months after treatment initiation using quantitative polymerase chain reaction. The results were expressed as copies/ml of total EBV DNA calculated using a standard curve, and log scale interpretation was used.
The patient developed cellulitis of the right leg 1 week after the second course of chemotherapy. A biopsy of the affected area revealed cryptococcal infection (Fig. 1B). Chemotherapy was discontinued and the patient was administered a course of antifungal agents (amphotericin B followed by fluconazole). Debridement of the infected tissue was also performed and the cellulitis gradually subsided. There was recurrence of leg ulcer 2 months later and the plasma EBV-DNA was elevated. The patient was then administered four courses of rituximab 375 mg/m2 delivered i.v. once a week for 4 weeks. The skin ulcer healed and the plasma EBV-DNA titre decreased to undetectable levels. The patient remains well 6 years after treatment, with no evidence of disease relapse at the last follow-up in April, 2016.
Discussion
Post-transplant lymphoproliferative disorder is a well-known complication of solid organ or allogenic stem cell transplantation. The risk of PTLD depends on the type of transplant, with a likely direct association with the intensity and duration of the immunosuppression (1). Approximately 90% of the cases are EBV-related CD20-positive B-cell neoplasms, which proliferate in an environment of impaired T-cell immunity. EBV induces uncontrolled B-cell proliferation, resulting in increased levels of interleukin and tumour growth factors. The dysregulated and uncontrolled B-cell proliferation ultimately causes PTLD (3).
The World Health Organization (WHO) classifies PTLDs into four major categories: i) Early lesions, which encompass reactive plasmacytic hyperplasia and infectious mononucleosis-like lesions; ii) polymorphous PTLD; iii) monomorphic PTLD, which is classified according to the WHO classification of lymphoma; and iv) classical Hodgkin lymphoma-like PTLD (4).
The risk factors for developing PTLD include EBV serostatus (higher risk of developing PTLD when an EBV seronegative organ recipient receives an organ from a seropositive donor), type of organ transplant, intensity of immunosuppression and age: There is a higher incidence of PTLD among extrarenal transplant recipients (5–7); the incidence of PTLD is highest with haploidentical hematopoietic stem cell transplant (HSCT), heart/lung and multivisceral transplants (≤20%), followed by liver (4.5%), combination heart-lung (2.5%), pancreas (2%), kidney (1–1.5%) and matched related and unrelated HSCT (0.5–1%) (7,8); in addition, pediatric patients are at greater risk compared with adults (5).
The presentation of PTLD is variable, and early symptoms may be non-specific, such as fever, malaise and weight loss. Extranodal involvement by PTLD is also common, but it rarely affects the skin. Skin lesions in PTLD may be solitary or multiple papules, nodules, plaques with ulceration, comedo-like lesions, localized alopecia and follicular keratotic papules (2). It was reported that cutaneous T-cell PTLD was more common compared with cutaneous B-cell PTLD, with mycosis fungoides as the most common cutaneous T-cell lymphoma subtype. EBV-associated cutaneous B-cell PTLD predominates among organ transplant recipients (5). Our patient presented with a leg ulceration and awareness of this rare presentation is crucial for early diagnosis. Our patient also had late-onset PTLD, 12 years after the renal transplant. Relative to early-onset PTLD, late-onset PTLD is often associated with more monoclonal lesions and a worse prognosis.
Treatment modalities for PTLD include reduction of immunosuppression, antiviral agents, chemotherapy and monoclonal antibodies. Reduction of immunosuppression is the preferred initial management of PTLD. The goal is to restore EBV-specific cellular immunity without graft rejection. Chemotherapy is used to treat patients who do not respond to reduction of immunosuppression. Cytotoxic drugs are usually effective and have a rapid response rate, but at a considerable cost. Due to comorbid diseases and chronic immunosuppression, high morbidity and mortality rates are observed in PTLD patients compared with non-Hodgkin lymphoma patients treated with similar regimens. There is also a higher risk of infection in PTLD patients. Cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP)-based therapy was shown to achieve a complete remission rate of 50% and a 5-year progression free survival of 43%, but the treatment-related mortality was high at 31% (9).
Additional treatment options include immunotherapy with the anti-CD20 monoclonal antibody rituximab. Single-agent rituximab therapy, following failure of immunosuppression reduction, achieved overall response rates of 44–70.5% and complete response rates of 28–53% (10,11). Sequential immunochemotherapy with rituximab followed by chemotherapy was also proven to be safe and effective in adult B-cell and Burkitt PTLD. Trappe et al reported an overall response rate of 90%, a complete remission rate of 68% and a median overall survival of 6.6 years after sequential therapy with rituximab followed by chemotherapy in patients with adult B-cell PTLD (12).
Quantification of the plasma EBV-DNA level may facilitate the monitoring of treatment response and it has been found to be effective in different types of lymphoproliferative diseases (13–15). For cases responding to treatment, circulating EBV-DNA falls to undetectable levels when complete remission is achieved (13). The disease activity in our patient correlated well with the plasma EBV-DNA level.
In conclusion, skin lesions are a rare manifestation of PTLD and awareness of this presentation is crucial. Physicians must be aware of infective complications if cytotoxic chemotherapy is used for the treatment of PTLD. Rituximab appears to be a feasible option for treatment of PTLD in patients who do not respond to reduction of immunosuppression.
References
|
Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, Horowitz MM, Jaffe ES, Kingma DW, Travis LB, et al: Risk factors for lymphoprolipherative disorders after allogeneic hematolopoietic cell transplantation. Blood. 113:4992–5001. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Seçkin D, Barete S, Euvrard S, Francès C, Kanitakis J, Geusau A, Del Marmol V, Harwood CA, Proby CM, Ali I, et al: Primary cutaneous posttransplant lymphoproliferative disorders in solid organ transplant recipients: A multicenter European case series. Am J Transplant. 13:2146–2153. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Tanner JE and Alfieri C: The Epstein-Barr virus and post-transplant lymphoproliferative diseases: Interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transpl Infect Dis. 3:60–69. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Swerdlow SH, Webber SA, Chadburn A and Ferry JA: Post-transplant lymphoproliferative disordersWHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL, Jafffe ES, Pileri SA, Stein H, Thiele J and Vardiman JW: 4th. International Agency for Research on Cancer; Lyon: pp. 343–349. 2008 | |
|
Trofe J, Beebe TM, Buell JF, Hanaway MJ, First MR, Alloway RR, Gross TG and Woodle ES: Posttransplant malignancy. Prog Transplant. 14:193–200. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Nassi L and Gaidano G: II. Challenges in the management of post-transplant lymphoproliferative disorder. Hematol Oncol. 33(Suppl 1): S96–S99. 2015. View Article : Google Scholar | |
|
Dierickx D, Tousseyn T and Gheysens O: How I treat posttransplant lymphoproliferative disorders. Blood. 126:2274–2283. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, Brepoels L, Kuypers D, Vanhaecke J, Nevens F, et al: Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: Incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 54:2433–2440. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Choquet S, Trappe R, Leblond V, Jäger U, Davi F and Oertel S: CHOP-21 for the treatment of post-transplant lymphoproliferative disorders (PTLD) following solid organ transplantation. Haematologica. 92:273–274. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa AM, Vandenberghe P, Fischer A, Morschhauser F, Salles G, Feremans W, et al: Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: Results of a prospective multicenter phase 2 study. Blood. 107:3053–3057. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Oertel SH, Verschuuren E, Reinke P, Zeidler K, Papp-Váry M, Babel N, Trappe RU, Jonas S, Hummel M, Anagnostopoulos I, et al: Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). Am J Transplant. 5:2901–2906. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Salles G, et al: Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): The prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 13:196–206. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Au WY, Pang A, Choy C, Chim CS and Kwong YL: Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. 104:243–249. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Gulley ML and Tang W: Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol Rev. 23:350–366. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Law MF, Poon WL, Ng KS, Chan HN, Lai HK, Ha CY, Ng C, Yeung YM and Yip SF: Quantification of plasma Epstein-Barr virus DNA for assessing treatment response in a patient with plasmablastic lymphoma. Ann Hematol. 91:789–791. 2012. View Article : Google Scholar : PubMed/NCBI |